Abstract
The Bcl-2 family member Mcl-1 is essential for macrophage survival. However, the mechanisms that contribute to the expression of Mcl-1 in these cells have not been fully characterized. The present study focused on the role of signal transducer and activator of transcription 3 (STAT3) in regulation of Mcl-1 in macrophages. Sodium salicylate (NaSal) treatment induced apoptotic cell death in primary human macrophages in a dose- and time-dependent fashion. Incubation with NaSal resulted in the loss of mitochondrial transmembrane potential, the release of cytochromecand second mitochondria-derived activator of caspase/direct IAP binding protein with low pH of isoelectric point (pI) from the mitochondria, and the activation of caspases 9 and 3. Western blot analysis and reverse transcription—polymerase chain reaction demonstrated that NaSal down-regulated the expression of Mcl-1. Electrophoretic mobility shift assay and Western blot analysis for phosphorylated STAT3 demonstrated that STAT3 was constitutively activated in macrophages and that this STAT3 activation was suppressed by NaSal. The activation of STAT3 in macrophages was dependent on Ser727 phosphorylation, in the absence of detectable Tyr705phosphorylation. Ectopic expression of STAT3 in murine RAW264.7 macrophages rescued the inhibition of Mcl-1 promoter-reporter gene activation and the cell death induced by NaSal treatment, while a dominant-negative STAT3 resulted in cell death. To confirm its role in primary macrophages, STAT3 antisense (AS) oligodeoxynucleotides (ODNs) were employed. STAT3 AS, but not control, ODNs decreased STAT3 and Mcl-1 expression and resulted in macrophage apoptosis. These observations demonstrate that the STAT3-mediated expression of Mcl-1 is essential for the survival of primary human in vitro differentiated macrophages. (Blood. 2003;102:344-352)
Introduction
Mcl-1 was first cloned from ML-1 cells, a human myeloid leukemia cell line, as an early-induction gene.1,2 Structurally and functionally, Mcl-1 belongs to the prosurvival Bcl-2 family.3 Mice transgenic for Mcl-1 demonstrated the enhanced viability of a wide range of hematopoietic cell types, including B and T lymphocytes, and CD 11b—positive myeloid cells including monocytes, macrophages, and polymorphonuclear leukocytes (PMNs) at both immature and mature stages of differentiation.4 Mcl-1 is essential for the survival of certain hematopoietic cells because apoptosis is rapidly triggered by antisense (AS) depletion of Mcl-1 in primary human macrophages, in differentiating U937 cells, a human monocytic cell line, and in human polymorphonuclear cells (PMNs).5-7
The cell type—specific mechanisms that contribute to the regulation of Mcl-1 are controversial. PI3K/Akt signaling pathway mediated Mcl-1 expression in interleukin-3 (IL-3)—induced TF-1 leukemia cells8 and IL-6—stimulated human hepatoma cell line Hep3B.9 In contrast, in human myeloma cells, the IL-6—induced expression of Mcl-1 was mediated through the JAK (Janus kinase)/STAT (signal transducer and activator of transcription) pathway.10 Recent observations employing human PMNs and leukemic large granular lymphocytes (LGLs) demonstrated that the JAK/STAT3 pathway was responsible for the regulation of Mcl-1 expression.11,12 Our recently published observations demonstrated that the PI3K/Akt-1 pathway was critical for the expression of Mcl-1 in primary human macrophages.7 Functional analysis of the murine Mcl-1 promoter revealed a STAT binding SIE (serum inducible element) at position -86 to -93 and a CRE (cAMP response element) at -70 to -76, which contributed to IL-3 stimulation of Mcl-1 gene expression.8 Putative SIE and CRE sites have also been identified in human Mcl-1 promoter region,13 however, the mechanisms by which they may contribute to the regulation of Mcl-1 expression remain to be determined.
The JAK/STAT pathway is used by numerous cytokines and nonimmune mediators.14 Located in the cytoplasm in an inactive state, upon tyrosine phosphorylation, STATs dimerize and migrate into the nucleus, promoting gene activation.15,16 STAT proteins also may undergo further activation by serine phosphorylation, which may be mediated through the MAPK (mitogen activated protein kinase) pathway.16 Activation of STAT3 has been associated with hematologic malignancies,11,12,17-19 while inhibition of STAT3 resulted in apoptosis of the malignant cells, suggesting a causal relationship.11,12,17,18 Recent data demonstrated that STAT3 was highly activated in LGL leukemic cells, and inhibition of STAT3 by antisense oligonucleotides and AG490, a specific inhibitor of JAK2, resulted in down-regulation of Mcl-1 and apoptotic cell death.11
In this study, the role of STAT3 in the regulation of Mcl-1 and apoptosis of primary, normal, in vitro—differentiated human macrophages was examined. STAT3 was constitutively activated by several criteria. The activated STAT3 was phosphorylated on Ser727 but not Tyr705. Activated STAT3 was essential for maintaining cell survival through the regulation of the expression of Mcl-1. Although constitutively activated Akt-1 also was necessary for the expression of Mcl-1 and macrophage survival, the ectopic expression of activated Akt-1 did not prevent the apoptotic cell death induced by suppression of STAT3 activation. These observations demonstrate a novel role for Ser727-phosphorylated STAT3 in normal macrophages.
Materials and methods
Materials
Sodium salicylate (NaSal) and polymyxin B sulfate were obtained from Sigma (St Louis, MO) and dissolved in phosphate-buffered saline. Lipofectamine was from Gibco (Gaithersburg, MD). Propidium iodide (PI) was from Roche Molecular Biochemicals (Indianapolis, IN), and rhodamine 123 (Rh123) was from Molecular Probes (Eugene, OR).
Cell isolation and culture
Human monocytes were isolated by countercurrent elutriation from commercially obtained buffy coats as previously described.20 Monocytes were differentiated in vitro for 7 days in RPMI containing 20% heat-inactivated fetal bovine serum (FBS), 1 μg/mL polymyxin B sulfate. RAW 264.7 cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in Dulbecco modified Eagle medium with 10% heat-inactivated FBS.
Adenovirus infection of human macrophages
Differentiated macrophages were infected for 2 hours in serum-free RPMI at the indicated multiplicity of infection (moi) with replication-defective adenoviruses expressing either β-galactosidase (Adβgal), green fluorescence protein (AdGFP), activated Akt-1 (AdMyrAkt-1), Bcl-xL (AdBcl-xL), and NIK (AdNIK) (kindly provided by Prof Harris Perlman, St Louis University Medical School, MO) as previously described.20 Infection of macrophages with AdGFP at 100 to 200 moi resulted in 70%-80% GFP-positive cells determined by flow cytometry.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared, as previously described,21 from macrophages incubated in control medium or medium containing 20 mM NaSal for 24 hours. The radiolabeled probe employed represents the murine Mcl-1 SIE (5′-TTTCCCCTTTTACGGGAAGTCCT-3′).8,11 In order to determine the specificity of the binding to the radiolabeled probe, 100-fold unlabeled murine Mcl-1 SIE oligonucleotide was added to the reaction. Antibodies to STAT3 (ST3) and STAT1 (ST1) (Santa Cruz Biotechnology, Santa Cruz, CA) and an irrelevant control antibody also were used to characterize the bound complex.
Promoter activity assay
RAW 264.7 cells were transiently transfected employing lipofectamine with 0.5 μg human Mcl-1 promoter-reporter plasmid (kindly provided by Dr Steven W. Edwards, University of Liverpool, United Kingdom)13 plus 2.5 μg of a plasmid expressing activated STAT3 or dominant-negative STAT3 (STAT3 DN)22 (both activated STAT3 and STAT3 DN were provided by Dr J. E. Darnell Jr, The Rockefeller University, New York, NY) or a control plasmid. In some experiments, 4 hours after the transient transfection RAW264.7 cells were treated with LY294002 or NaSal for another 18 hours, then cells were harvested for the luciferase activity assay. Promoter activity is presented as relative light units (RLUs) normalized for protein concentration (RLU/μg protein).
Viability assays
Propidium iodide (PI) exclusion was employed to assess macrophage viability. PI was added just prior to analysis by flow cytometry and the data are presented as the percentage of cell death (PI + cells) in each culture. Additionally, transient cotransfections were employed to determine if expression of Mcl-1 or STAT3 was capable of rescuing RAW264.7 cells from cell death induced by NaSal, employing a previously described assay.7,20,23 The rationale behind the assay was to determine if cotransfection of the experimental plasmid (expressing Mcl-1 or STAT3), compared to a control plasmid, will protect the GFP-expressing cells from death induced by NaSal or LY294002. In these experiments the transfection efficiency, determined as GFP-positive cells, ranged from 3% to 10% in the absence of treatment. RAW 264.7 cells were cotransfected with 0.5 μg cytomegalovirus—enhanced green fluorescence protein (CMV-EGFP) expression plasmid plus 2.5 μg control plasmid or plasmids expressing Mcl-1 or STAT3. Following transfection, cultures were incubated for 24 hours and then were treated with 20 mM NaSal for an additional 24 hours. RAW 264.7 cells were collected, and GFP-expressing cells were quantified by flow cytometry.
Determination of subdiploid DNA content
At the indicated time points, cultures were stained with propidium iodide, as previously described.24 The subdiploid DNA peak (< 2N DNA), immediately adjacent to the Go/G1 peak (2N DNA), represents apoptotic cells and was quantified by flow cytometry.
Determination of mitochondrial permeability transition
Western blot analysis
Western blot analysis was conducted as previously described20 using the indicated antibodies: rabbit anti—phospho-Tyr705 STAT3 (Cell Signaling, Beverly, MA, and BD PharMingen, San Diego, CA), rabbit anti—phospho-Ser727, and total STAT3 (Cell Signaling), rabbit anti—phospho-Akt-1 or total Akt-1 (New England Biolabs), rabbit anti—caspase 9 (Calbiochem, San Diego, CA), rabbit anti—Mcl-1 (Santa Cruz Biotechnology), mouse anti—caspase 3, rabbit anti—Bcl-xL (Transduction Laboratories, Lexington, KY), or mouse antitubulin (Calbiochem). To examine cytochrome c and Smac/DIABLO, the cytosolic fraction was isolated, as previously described by us,25 and probed with a monoclonal anti—cytochrome c antibody (PharMingen) or a rabbit anti-Smac antibody provided by Prof Xiaodong Wang (University of Texas Southwestern Medical Center at Dallas).26
RT-PCR
Total cellular RNA was extracted and was used for reverse transcription—polymerase chain reaction (RT-PCR) as previously described.20 The cDNA was amplified using specific glyceraldehyde phosphate dehydrogenase (GAPDH) or Mcl-1. We used the following sequences for Mcl-1: 5′-CGGCAGTCGCTGGAGATTAT-3′ (526-545, sense) and 5′-GTGGTGGTGGTTGGTTA-3′ (1062-1081, antisense).27 The primers for G3PDH were purchased from Clontech (Palo Alto, CA). The primers for human A1 are 5′-CAGCACATTGCCTCAACAGC-3′ (sense) and 5′-TGCAGATAGTCCTGAGCCAGC-3′ (antisense), as previously described.20
STAT3 antisense
The antisense oligonucleotide (AS ODN) to STAT3 (STAT3 AS) and the 5-base mismatch control ODN (STAT3 5bp) were synthesized and provided by Isis Pharmaceuticals (Carlsbad, CA). The sequences employed were synthesized using phosphorothioate chemistry and are as follows: STAT3 AS, 5′-GCTCCA GCA TCT GCT GCTTC-3′, and STAT3 5bp, 5-GCTCCA ATA CCC GTT GCTTC-3′. To increase stability and hybridization affinity, the oligonucleotides were synthesized with 2′-O-methoxyethyl modification of the 5 terminal nucleotides (underlined; Monia et al28 ). Primary macrophages were transfected as previously described,28 except that lipofectamine was used instead of lipofectin. The final concentration of control and AS ODNs was 400 nM, and macrophages were treated for 48 hours, then harvested and examined for STAT3 and Mcl-1 expression by Western blot analysis and apoptosis by determination of subdiploid DNA content.
Statistical analysis
Results were expressed as the mean plus or minus standard error. Significance was determined by Student t test for paired samples.
Results
Sodium salicylate treatment of human macrophages results in the loss of Δψm and apoptotic cell death in a dose- and time-dependent fashion. Sodium salicylate (NaSal) has been shown to inhibit tumor necrosis factor α (TNFα)—induced production of inflammatory cytokines through inhibition of nuclear factor-κB (NF-κB), extra-cellular signal-regulated kinase (ERK), and c-Jun NH2-terminal kinase (JNK) activation in fibroblasts.29-31 It has also been shown to suppress Mcl-1 expression and induce apoptosis of monocytic cell line U937,32 but the mechanisms contributing to the suppression of Mcl-1 remain unknown. Our previous observations demonstrated that the PI3K/Akt-1 pathway was essential for maintaining the expression of Mcl-1 and cell survival in primary human macrophages. Therefore, NaSal was employed as a tool to determine if it suppressed Mcl-1 and induced apoptosis in macrophages and to determine if a mechanism other than the PI3k/Akt-1 pathway was involved. Treatment of monocyte-differentiated macrophages with NaSal (1 mM to 20 mM) resulted in a dose- and time-dependent loss of mitochondrial transmembrane potential (Δψm, determined by decrease of Rho123 retention in mitochondria, Figure 1A), cell death (determined by loss of PI exclusion, Figure 1B), and apoptotic phenotype (defined by DNA fragmentation, Figure 1C). NaSal at the concentration of 20 mM also induced the loss of mitochondrial transmembrane potential and apoptotic cell death of the murine macrophage cell line RAW264.7 determined by DNA fragmentation (data not shown), suggesting that a similar pathway is shared by human macrophages and RAW264.7 cells. In contrast, NaSal at 20 mM did not induce apoptotic cell death in normal fibroblasts (data not shown), suggesting that the effect of NaSal was cell type—specific and not due to a generalized toxicity of the reagent.
NaSal treatment results in the loss of Δψmand apoptosis in primary human macrophages. Normal human in vitro—differentiated macrophages were incubated with NaSal (1 mM to 20 mM) for 24 or 48 hours as indicated. (A) The effect of NaSal on Δψm (retention of Rh123, mean fluorescence [% of control Rh123]). (B) Cell death induced by NaSal, defined by PI uptake. (C) The effects of NaSal on apoptosis, determined by analysis of subdiploid DNA (< 2N). No indicates no NaSal, or medium control. * indicates P < .05, ** indicates P < .01 determined by Student t test for paired samples compared with no treatment. The results in each panel are presented as the mean ± 1 SE of an experiment performed in triplicate, which is representative of 3 independent experiments.
NaSal treatment results in the loss of Δψmand apoptosis in primary human macrophages. Normal human in vitro—differentiated macrophages were incubated with NaSal (1 mM to 20 mM) for 24 or 48 hours as indicated. (A) The effect of NaSal on Δψm (retention of Rh123, mean fluorescence [% of control Rh123]). (B) Cell death induced by NaSal, defined by PI uptake. (C) The effects of NaSal on apoptosis, determined by analysis of subdiploid DNA (< 2N). No indicates no NaSal, or medium control. * indicates P < .05, ** indicates P < .01 determined by Student t test for paired samples compared with no treatment. The results in each panel are presented as the mean ± 1 SE of an experiment performed in triplicate, which is representative of 3 independent experiments.
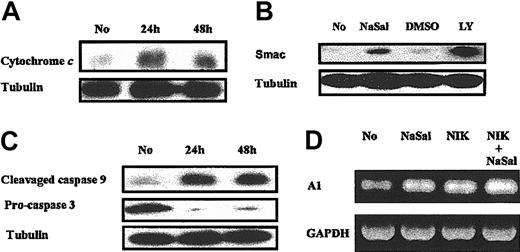
The effects of NaSal are mediated through the mitochondria. Further studies were performed in order to characterize the mechanism(s) of the apoptosis induced by NaSal. Incubation of macrophages with NaSal resulted in the release of cytochrome c (Figure 2A) and Smac/DIABLO (second mitochondria-derived activator of caspase/direct IAP binding protein with low pH of isoelectric point [pI]) from the mitochondria (Figure 2B). As a control, inhibition of the PI3K/Akt-1 pathway with LY294002, which we previously demonstrated resulted in the loss of Δψm,7 also resulted in the release of Smac/DIABLO from the mitochondria. Additionally, caspases 9 and 3 were activated following treatment with NaSal (Figure 2C). Caspases 9 and 3 also were activated in RAW264.7 cells following NaSal treatment determined by measuring caspase 9— and caspase 3—like activities employing a fluorometric assay (data not shown). To determine the role of death receptors, the broad specificity caspase inhibitor zVAD.fmk, which protects against death receptor—mediated apoptosis,20,23 was employed. Incubation of macrophages with zVAD.fmk, which protected against Fas-mediated monocyte caspase 8 activation and apoptosis,23 did not protect against the loss of Δψm or cell death induced by NaSal, although NaSal-induced activation of caspases 9 and 3 was suppressed and DNA fragmentation was significantly (P < .02) reduced (data not shown). These observations suggest that NaSal-mediated apoptosis was induced by the loss of Δψm, which was caspase independent and resulted in release of cytochrome c and Smac/DIABLO, and the activation of caspases 9 and 3.
NaSal treatment results in release of cytochromecand Smac and caspase activation, but not the reduction of A1. (A-B) Monocyte-differentiated macrophages were treated with NaSal (20 mM) or LY294002 (50 μM) for indicated periods in panel A and for 48 hours in panel B, then cytosolic fraction of cells were used for Western blot assay using anti—cytochrome c antibody and anti-Smac antibody. (C) Macrophages were treated with NaSal (20 mM) for 24 or 48 hours. Cell lysates were collected and Western blot assays were conducted using the indicated antibodies. (D) Macrophages were infected with adenoviral vector (AdNIK, 100 moi) expressing NR-κB—inducing kinase for 24 hours as described in “Materials and methods.” Then cells were treated with NaSal at 20 mM for another 24 hours. Cells were harvested and total RNA was extracted for RT-PCR using human A1 and GAPDH-specific primers as was previously described by us.20 No indicates normal macrophages without NaSal treatment.
NaSal treatment results in release of cytochromecand Smac and caspase activation, but not the reduction of A1. (A-B) Monocyte-differentiated macrophages were treated with NaSal (20 mM) or LY294002 (50 μM) for indicated periods in panel A and for 48 hours in panel B, then cytosolic fraction of cells were used for Western blot assay using anti—cytochrome c antibody and anti-Smac antibody. (C) Macrophages were treated with NaSal (20 mM) for 24 or 48 hours. Cell lysates were collected and Western blot assays were conducted using the indicated antibodies. (D) Macrophages were infected with adenoviral vector (AdNIK, 100 moi) expressing NR-κB—inducing kinase for 24 hours as described in “Materials and methods.” Then cells were treated with NaSal at 20 mM for another 24 hours. Cells were harvested and total RNA was extracted for RT-PCR using human A1 and GAPDH-specific primers as was previously described by us.20 No indicates normal macrophages without NaSal treatment.
The effects of NaSal are not mediated through NF-κB in macrophages
NaSal has been shown to inhibit TNFα-induced activation of the NF-κB in fibroblasts.29-31 We previously demonstrated that inhibition of the constitutive activation of NF-κB in macrophages induced apoptosis, which was due to suppression of the expression of the Bcl-2 family member A1.20 The addition of NaSal to macrophages failed to reduce the expression of A1 (Figure 2D), suggesting that the inhibition of the constitutive activation of NF-κB was not responsible for the effects of NaSal. Since the constitutive activation of NF-κB in macrophages is weaker than that observed following activation, NF-κB was activated in macrophages by the ectopic expression of NF-κB—inducing kinase (NIK), employing an adenoviral vector (AdNIK). The expression of NIK strongly induced NF-κB activation determined by EMSA compared with the control adenoviral vector (data not shown), but failed to protect macrophages from NaSal-induced apoptotic cell death (data not shown). Further, employing AdNIK-infected macrophages, NaSal did not suppress the expression of the A1 (Figure 2D). These observations demonstrate that although NaSal may suppress TNFα-induced NF-κB activation, the suppression of A1, which we have shown is mediated by the constitutive activation of NF-κB in macrophages, was not responsible for the apoptotic death observed following treatment with NaSal.
Effects of NaSal are mediated through Mcl-1
The effect of NaSal on the expression of Mcl-1 was examined. Incubation with NaSal suppressed the expression of Mcl-1 at the transcriptional level, since both mRNA and protein were decreased (Figure 3A-B). In contrast, NaSal did not suppress the expression of other Bcl-2 family members, Bcl-xL (Figure 3A), or A1 (Figure 2D). NaSal treatment also suppressed Mcl-1 expression in RAW264.7 cells (data not shown). Therefore, experiments were performed to determine the role of Mcl-1 in apoptosis induced by NaSal employing RAW264.7 cells. NaSal induced cell death of the RAW264.7 macrophage cell line (Figure 3C). The ectopic expression of Mcl-1 protected the RAW264.7 macrophage-like cell line from NaSal-induced cell death (Figure 3C), suggesting that the reduction of Mcl-1 may be responsible for the apoptotic cell death induced by NaSal.
NaSal treatment down-regulates the expression of Mcl-1 and suppresses the activation of the Mcl-1 promoter. (A) Effect of NaSal on Mcl-1 and Bcl-xL expression. Monocyte-differentiated macrophages were treated with NaSal (20 mM) for 12 to 48 hours, then cell lysates were collected and Western blot analysis performed using the antibodies indicated. (B) Effect of NaSal on Mcl-1 mRNA expression. Macrophages were treated with NaSal for the times indicated, then total RNA was extracted and used for RT-PCR using primers specific to Mcl-1. No indicates normal macrophages without NaSal treatment. (C) Mcl-1 expression protects RAW264.7 cells from NaSal-induced death; 0.5 μg of GFP-expressing plasmid and a total of 3 μg plasmid were included in each arm of the experiment. Control or Mcl-1—expressing plasmid (2.5 μg) were transfected where indicated. After 24 hours, the RAW264.7 cells were treated with NaSal (20 mM) for another 24 hours. GFP-positive cells were detected by fluorescence-activated cell-sorter, as previously described.7 (D) Effect of NaSal on Mcl-1 promoter activation. 1 μg Mcl-1 promoter-reporter construct was transiently transfected into RAW264.7 cells, which were then treated with NaSal (20 mM), LY294002 (50 μM), and control (dimethyl sulfoxide [DMSO]) as indicated for 24 hours. Cells were harvested, and luciferase activity was detected, and the values corrected for protein concentration. * indicates P < .05 and ** indicates P < .01 as determined by Student t test for paired samples compared with control (No indicating no NaSal [medium alone] or DMSO), as indicated. The results in panels C and D are presented as the mean ± 1 SE of experiments performed in triplicate, which are representative of 3 independent experiments.
NaSal treatment down-regulates the expression of Mcl-1 and suppresses the activation of the Mcl-1 promoter. (A) Effect of NaSal on Mcl-1 and Bcl-xL expression. Monocyte-differentiated macrophages were treated with NaSal (20 mM) for 12 to 48 hours, then cell lysates were collected and Western blot analysis performed using the antibodies indicated. (B) Effect of NaSal on Mcl-1 mRNA expression. Macrophages were treated with NaSal for the times indicated, then total RNA was extracted and used for RT-PCR using primers specific to Mcl-1. No indicates normal macrophages without NaSal treatment. (C) Mcl-1 expression protects RAW264.7 cells from NaSal-induced death; 0.5 μg of GFP-expressing plasmid and a total of 3 μg plasmid were included in each arm of the experiment. Control or Mcl-1—expressing plasmid (2.5 μg) were transfected where indicated. After 24 hours, the RAW264.7 cells were treated with NaSal (20 mM) for another 24 hours. GFP-positive cells were detected by fluorescence-activated cell-sorter, as previously described.7 (D) Effect of NaSal on Mcl-1 promoter activation. 1 μg Mcl-1 promoter-reporter construct was transiently transfected into RAW264.7 cells, which were then treated with NaSal (20 mM), LY294002 (50 μM), and control (dimethyl sulfoxide [DMSO]) as indicated for 24 hours. Cells were harvested, and luciferase activity was detected, and the values corrected for protein concentration. * indicates P < .05 and ** indicates P < .01 as determined by Student t test for paired samples compared with control (No indicating no NaSal [medium alone] or DMSO), as indicated. The results in panels C and D are presented as the mean ± 1 SE of experiments performed in triplicate, which are representative of 3 independent experiments.
In order to define the mechanism by which NaSal suppressed Mcl-1 expression in human Mφs, a 296—base pair human mcl-1 promoter-reporter construct13 was transiently transfected into the RAW264.7 cells. This promoter reporter possesses a potential STAT binding site, referred to as a serum inducible element (SIE), as well as a serum response element (SRE), a cyclic AMP response element (CRE), and an Sp-1 binding site.13 NaSal treatment of RAW264.7 cells repressed the constitutive activation of the mcl-1 promoter (Figure 3D). We previously demonstrated that the constitutive activation of the PI3K/Akt-1 pathway in macrophages was important in regulating the expression of Mcl-1.7 Therefore, additional studies were performed to determine the role of the PI3K/Akt-1 pathway in mcl-1 promoter activation. Inhibition of the PI3K/Akt-1 pathway with LY294002 also suppressed the activation of mcl-1 promoter (Figure 3D). These observations suggest that in macrophages, inhibition of the PI3K/Akt-1 pathway and treatment with NaSal each suppressed the transcriptional activation of the human mcl-1 promoter in RAW264.7 macrophages.
The effects of NaSal on macrophage viability are independent of Akt-1
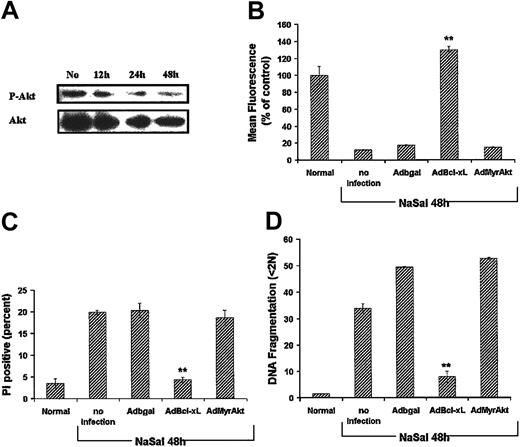
Experiments were performed to determine if the effects of NaSal were mediated through Akt-1. NaSal resulted in a reduction of constitutively activated Akt-1 in macrophages (Figure 4A). However, total Akt-1 was comparably reduced, suggesting that NaSal may reduce the expression of Akt-1 rather than directly affect its activation. In order to determine if the reduction of Akt-1 was responsible for the inhibition of Mcl-1 expression by NaSal, an adenoviral vector expressing constitutively activated Akt-1 (AdMyrAkt-1) was employed. The expression of MyrAkt-1 failed to rescue macrophages from the apoptotic cell death induced by NaSal (Figure 4B-D). In contrast, MyrAkt-1 protected the macrophages from the apoptotic cell death induced by LY294002 (data not shown), as previously documented by us.7 Furthermore, the ectopic expression of another Bcl-2 family member Bcl-xL protected against NaSal-induced apoptotic death (Figure 4), supporting the role of mitochondrial dysfunction as a proximal event in the induction of apoptosis. These observations suggest that the effects of NaSal are either downstream of Akt-1 or that a pathway independent of PI3K/Akt-1 is involved.
Activated Akt-1 does not protect against NaSal-induced apoptosis. (A) Effect of NaSal on Akt-1 expression and activation. Monocyte-differentiated macrophages were treated with NaSal (20 mM) for 12 to 48 hours, then cell lysates were collected and Western blot analysis performed using the antibodies to phosphorylated and total Akt-1. No indicates no NaSal added. (B-D) Seven-day differentiated macrophages were untreated (Normal) or infected with adenoviral vectors (100 moi) expressing the control β-galactosidase, Bcl-xL, or activated Akt-1 (MyrAkt-1) for 24 hours. Where indicated, NaSal (20 mM) was added for an additional 48 hours. The cells were harvested and analyzed for Δψm (mean fluorescence), cell death (PI-positive cells), or apoptosis (DNA fragmentation). Values represent the mean ± 1 SE and were compared for statistical significance by Student t test for paired samples. ** indicates P < .01 compared with control Adβgal. Shown is a representative of 2 experiments performed in triplicate.
Activated Akt-1 does not protect against NaSal-induced apoptosis. (A) Effect of NaSal on Akt-1 expression and activation. Monocyte-differentiated macrophages were treated with NaSal (20 mM) for 12 to 48 hours, then cell lysates were collected and Western blot analysis performed using the antibodies to phosphorylated and total Akt-1. No indicates no NaSal added. (B-D) Seven-day differentiated macrophages were untreated (Normal) or infected with adenoviral vectors (100 moi) expressing the control β-galactosidase, Bcl-xL, or activated Akt-1 (MyrAkt-1) for 24 hours. Where indicated, NaSal (20 mM) was added for an additional 48 hours. The cells were harvested and analyzed for Δψm (mean fluorescence), cell death (PI-positive cells), or apoptosis (DNA fragmentation). Values represent the mean ± 1 SE and were compared for statistical significance by Student t test for paired samples. ** indicates P < .01 compared with control Adβgal. Shown is a representative of 2 experiments performed in triplicate.
NaSal treatment suppresses the constitutive activation of STAT3 in macrophages
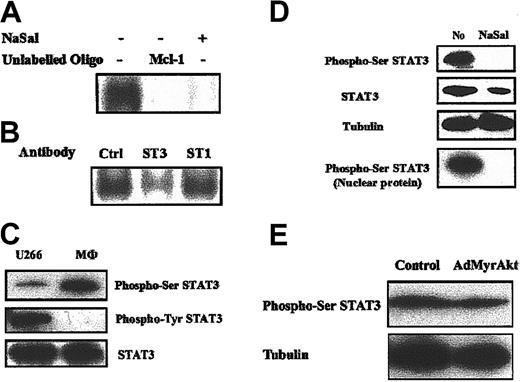
These observations suggest that NaSal regulates Mcl-1 expression in human macrophages through a pathway independent of the PI3K/Akt-1 pathway. A recent report demonstrated that the STAT3 pathway was involved in the regulation of Mcl-1 expression in LGL leukemia.11,12 Therefore, we examined STAT3 activation and the effects of NaSal employing nuclear extracts from primary, in vitro differentiated, macrophages. EMSA, employing a murine Mcl-1 SIE oligodeoxynucleotide (ODN), demonstrated that STAT3 was constitutively activated in primary human macrophages (Figure 5A). The specificity of binding to the Mcl-1 SIE ODN was confirmed by the inhibition of the binding with unlabeled murine Mcl-1 SIE ODN (Mcl-1, Figure 5A). STAT3, but not STAT1, was identified in the bound complex, by reduction of the complex, employing monospecific antibodies (Figure 5B). NaSal treatment dramatically suppressed STAT3 activation determined by EMSA employing primary human macrophages (Figure 5A). These observations suggest that the constitutive activation of STAT3 in macrophages was suppressed by NaSal.
STAT3 is constitutively activated in human macrophages and is suppressed by NaSal treatment. (A-B) Primary human macrophages were treated with control medium or NaSal (20 mM), where indicated, for 48 hours. Cells were harvested and nuclear proteins were extracted and used for the EMSA. The radiolabeled probe employed represents the murine Mcl-1 SIE (5′-TTTCCCCTTTTACGGGAAGTCCT-3′).8,11 Added to the reaction mixture to define the specificity of the bound proteins were 100-fold unlabeled probe of murine Mcl-1 (Mcl-1) (A) and antibodies to STAT3 (ST3) or STAT1 (ST1) and control antibody (Ctrl) (B). (C) The STAT3 is constitutively phosphorylated at the Ser727 site and not the Tyr705 site in primary human macrophages. Nuclear proteins (30 μg) extracted from primary human macrophages or U266 cells were employed to detect phospho-Ser727 and phospho-Tyr705 STAT3 by Western blot analysis employing monospecific antibodies. (D) NaSal treatment inhibits the constitutive Ser727 phosphorylation of STAT3 in human macrophages. Macrophages were treated with NaSal (20 mM) for 48 hours. Cell lysates were collected and Western blot assays were conducted using indicated antibodies to phospho-serine STAT3, total STAT3, and tubulin. Nuclear proteins also were used to detect the effect of NaSal on phospho-serine STAT3, where indicated. (E) Akt-1 does not regulate STAT3 phosphorylation in human macrophages. Human macrophages were infected with adenoviral vectors (100 moi) expressing the control or activated Akt-1 (MyrAkt-1) for 24 hours. Cell lysates were collected and Western blot assays were conducted using indicated antibodies to phospho-serine STAT3 and tubulin.
STAT3 is constitutively activated in human macrophages and is suppressed by NaSal treatment. (A-B) Primary human macrophages were treated with control medium or NaSal (20 mM), where indicated, for 48 hours. Cells were harvested and nuclear proteins were extracted and used for the EMSA. The radiolabeled probe employed represents the murine Mcl-1 SIE (5′-TTTCCCCTTTTACGGGAAGTCCT-3′).8,11 Added to the reaction mixture to define the specificity of the bound proteins were 100-fold unlabeled probe of murine Mcl-1 (Mcl-1) (A) and antibodies to STAT3 (ST3) or STAT1 (ST1) and control antibody (Ctrl) (B). (C) The STAT3 is constitutively phosphorylated at the Ser727 site and not the Tyr705 site in primary human macrophages. Nuclear proteins (30 μg) extracted from primary human macrophages or U266 cells were employed to detect phospho-Ser727 and phospho-Tyr705 STAT3 by Western blot analysis employing monospecific antibodies. (D) NaSal treatment inhibits the constitutive Ser727 phosphorylation of STAT3 in human macrophages. Macrophages were treated with NaSal (20 mM) for 48 hours. Cell lysates were collected and Western blot assays were conducted using indicated antibodies to phospho-serine STAT3, total STAT3, and tubulin. Nuclear proteins also were used to detect the effect of NaSal on phospho-serine STAT3, where indicated. (E) Akt-1 does not regulate STAT3 phosphorylation in human macrophages. Human macrophages were infected with adenoviral vectors (100 moi) expressing the control or activated Akt-1 (MyrAkt-1) for 24 hours. Cell lysates were collected and Western blot assays were conducted using indicated antibodies to phospho-serine STAT3 and tubulin.
To further characterize the constitutively activated STAT3 in human macrophages, Western blot analysis was employed. Generally, Tyr705 phosphorylation is necessary for STAT3 activation by inducing STAT3 to dimerize, translocate to the nucleus, and bind DNA.33-35 Unexpectedly, phospho-Tyr705 STAT3 was not detected in macrophages (Figure 5C), employing monospecific antibodies obtained from 2 sources (Cell Signaling and BD PharMingen). However, Tyr705-phosphorylated STAT3 was readily detected in extracts from unstimulated U266 cells, as previously described.17 In contrast, Ser727-phosphorylated STAT3 was readily detected in macrophages and to a lesser degree in U266 cells (Figure 5C). Supporting its importance, Ser727-phosphorylated STAT3 was highly expressed in macrophage nuclear extracts (Figure 5D). Total and nuclear localized Ser727-phosphorylated STAT3 were reduced following treatment with NaSal (Figure 5D). Similar to primary macrophages, Ser727-phosphorylated STAT3 was present in RAW264.7 cells, and its expression was suppressed by NaSal (data not shown). These observations suggest that the constitutive activation of STAT3 by phosphorylation of Ser727 but not Tyr705 may be important in the regulation of Mcl-1 and the survival of macrophages. In order to study the possible regulation of STAT3 Ser727 phosphorylation by Akt-1, active Akt-1 (MyrAkt-1) was ectopically expressed in human macrophages, employing an adenoviral vector (AdMyrAkt-1). Even though MyrAkt-1 was strongly expressed (data not shown), it failed to influence the phosphorylation of STAT3 at Ser727 (Figure 5E). These data demonstrated that STAT3 was not regulated by Akt-1 in primary human macrophages.
Importance of Ser727 phosphorylation of STAT3 in Mcl-1 expression and macrophage survival
In order to determine if Ser727 phosphorylation was important in survival and the expression of Mcl-1, macrophages were incubated with the serine kinase inhibitor H7. Incubation of macrophages with H7 resulted in the loss of Δψm (Figure 6A), apoptosis defined by DNA fragmentation (Figure 6B), and cell death defined by PI uptake (data not shown). Additionally, H7 suppressed the phosphorylation of Ser727 on STAT3 (Figure 6C) and reduced the expression of Mcl-1 in macrophages (Figure 6C). Experiments were performed to determine the mechanism responsible for the Ser727 phosphorylation of STAT3. The ERK inhibitor PD98059 and JNK inhibitor SP600125 failed to inhibit the phosphorylation of STAT3 Ser727 and failed to induce macrophage cell death (data not shown). Although the mechanism remains to be defined, these observations suggest that Ser727-phosphorylated STAT3, in the absence of detectable Tyr705 phosphorylation, may be functionally active in macrophages and may contribute to the regulation of Mcl-1 expression.
H7, an inhibitor of serine kinases, induces apoptosis and the reduction of activated STAT3 and Mcl-1 expression in macrophages. (A-B) Macrophages were treated with H7 (50 μM) for 24 hours. The cells were collected and analyzed for mitochondrial integrity by Rh123 retention (mean fluorescence, % control, panel A), and apoptosis defined by DNA fragmentation (% < 2N DNA, panel B). ** indicates P < .01 determined by Student t test for paired samples compared with control. The results (mean ± 1 SE) of a single experiment, performed in triplicate, are presented, which are representative of 2 experiments. (C) H7 treatment inhibits STAT3 Ser727 phosphorylation (Phospho-Ser STAT3) and Mcl-1 expression in primary macrophages. Macrophages were treated with H7 (50 μM) for 24 hours. Cells were harvested for Western blot analysis, probing with antibodies to Ser727-phosphorylated STAT3 and to Mcl-1.
H7, an inhibitor of serine kinases, induces apoptosis and the reduction of activated STAT3 and Mcl-1 expression in macrophages. (A-B) Macrophages were treated with H7 (50 μM) for 24 hours. The cells were collected and analyzed for mitochondrial integrity by Rh123 retention (mean fluorescence, % control, panel A), and apoptosis defined by DNA fragmentation (% < 2N DNA, panel B). ** indicates P < .01 determined by Student t test for paired samples compared with control. The results (mean ± 1 SE) of a single experiment, performed in triplicate, are presented, which are representative of 2 experiments. (C) H7 treatment inhibits STAT3 Ser727 phosphorylation (Phospho-Ser STAT3) and Mcl-1 expression in primary macrophages. Macrophages were treated with H7 (50 μM) for 24 hours. Cells were harvested for Western blot analysis, probing with antibodies to Ser727-phosphorylated STAT3 and to Mcl-1.
The constitutive activation of STAT3 contributes to the maintenance of cell survival through regulation of Mcl-1 expression in macrophages
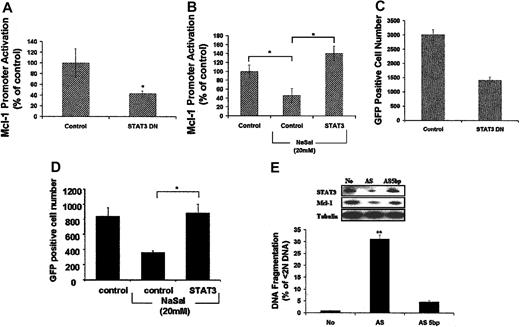
In order to determine if STAT3 regulates the expression of Mcl-1 in macrophages, transient transfection using the mcl-1 promoter-reporter construct was performed in RAW264.7 cells. The ectopic expression of dominant-negative (DN) STAT3 suppressed the constitutive activation of the mcl-1 promoter in RAW264.7 cells (Figure 7A). Additionally, the ectopic expression of DN STAT3 significantly decreased the number of GFP-positive RAW264.7 cells, consistent with the induction of cell death (Figure 7C). Further, the ectopic expression of activated STAT3 rescued the suppression of mcl-1 promoter activation induced by NaSal treatment (Figure 7B) and protected against the cell death induced by NaSal (Figure 7D). These observations suggest that in macrophages, STAT3 activation regulates the expression of Mcl-1.
STAT3 regulated the transcription of Mcl-1 promoter and is essential to cell survival of macrophages. (A-B) STAT3 regulates Mcl-1 promoter activation. Mcl-1 (0.5 μg) promoter-report construct plus 2.5 μg of STAT3 dominant-negative (DN) (A) or activated STAT3 (B) expression plasmids or control plasmid were transiently transfected into RAW264.7 cells. After 24 hours, cells were harvested for luciferase assay (A) or treated by NaSal (20 mM) for an additional 24 hours before luciferase assay (B). Luciferase activity was corrected for protein concentration as described above. (C-D) STAT3 controls survival in the RAW 264.7 macrophage cell line. Cell death assays were performed employing RAW 264.7 cells transfected with a GFP-expressing plasmid (0.5 μg) plus 2.5 μg STAT3 DN (C), or activated STAT3-expressing plasmids (D), or control plasmid. Cells were harvested 24 hours after transfection for detection of GFP-positive cells by FACS (C) as described7 or cells were treated with NaSal (20 mM) for another 24 hours before the detection of GFP-positive cells (D). (E) STAT3 regulates Mcl-1 expression and macrophage survival. Differentiated primary human macrophages were transfected with 12 μg/mL lipofectamine plus 400 nM AS STAT3 ODN or the control ODN (AS, 5 bp) in OptiMem medium as described in “Materials and methods.” Cells were harvested after 48 hours and apoptosis was determined by detection of subdiploid DNA (DNA fragmentation, % < 2N DNA). Cell lysates also were employed for Western blot analysis to detect the expression of STAT3 and Mcl-1 using monospecific antibodies. * indicates P < .05, ** indicates P < .01 determined by Student t test for paired samples compared with control. The results in each panel are presented as the mean ± 1 SE of experiments performed in triplicate, which are representative of 3 independent experiments.
STAT3 regulated the transcription of Mcl-1 promoter and is essential to cell survival of macrophages. (A-B) STAT3 regulates Mcl-1 promoter activation. Mcl-1 (0.5 μg) promoter-report construct plus 2.5 μg of STAT3 dominant-negative (DN) (A) or activated STAT3 (B) expression plasmids or control plasmid were transiently transfected into RAW264.7 cells. After 24 hours, cells were harvested for luciferase assay (A) or treated by NaSal (20 mM) for an additional 24 hours before luciferase assay (B). Luciferase activity was corrected for protein concentration as described above. (C-D) STAT3 controls survival in the RAW 264.7 macrophage cell line. Cell death assays were performed employing RAW 264.7 cells transfected with a GFP-expressing plasmid (0.5 μg) plus 2.5 μg STAT3 DN (C), or activated STAT3-expressing plasmids (D), or control plasmid. Cells were harvested 24 hours after transfection for detection of GFP-positive cells by FACS (C) as described7 or cells were treated with NaSal (20 mM) for another 24 hours before the detection of GFP-positive cells (D). (E) STAT3 regulates Mcl-1 expression and macrophage survival. Differentiated primary human macrophages were transfected with 12 μg/mL lipofectamine plus 400 nM AS STAT3 ODN or the control ODN (AS, 5 bp) in OptiMem medium as described in “Materials and methods.” Cells were harvested after 48 hours and apoptosis was determined by detection of subdiploid DNA (DNA fragmentation, % < 2N DNA). Cell lysates also were employed for Western blot analysis to detect the expression of STAT3 and Mcl-1 using monospecific antibodies. * indicates P < .05, ** indicates P < .01 determined by Student t test for paired samples compared with control. The results in each panel are presented as the mean ± 1 SE of experiments performed in triplicate, which are representative of 3 independent experiments.
In order to determine the role of STAT3 in primary human macrophages directly, antisense (AS) ODNs to STAT3 were employed. STAT3 AS ODNs resulted not only in decreased STAT3 expression, but also reduced Mcl-1 expression, compared with control ODNs (AS, 5 bp) (Figure 7E). The decrease of STAT3 and Mcl-1 induced by STAT3 AS ODNs resulted in macrophage apoptosis, as determined by DNA fragmentation (Figure 7E). Since Tyr705-phosphorylated STAT3 was not detected, these observations suggest that the constitutive activation of Ser727-phosphorylated STAT3 in macrophages contributes to the transcriptional regulation of Mcl-1, which is essential to maintain macrophage survival.
Discussion
Previously we demonstrated that the constitutive activation of the PI3K/Akt-1 pathway in primary human macrophages was necessary for Mcl-1 expression and cell survival.7 The current study extends these observations, identifying an additional pathway, mediated through STAT3, which is also essential for the expression of Mcl-1 and the survival of macrophages. Suppression of the constitutive activation of STAT3, identified by Ser727 phosphorylation, in macrophages by either NaSal or the Ser/Thr kinase inhibitor H7 resulted in the reduction of Mcl-1 and apoptosis. Additionally, STAT3 obtained from the nucleus of macrophages bound to the mcl-1 promoter SIE binding site, suggesting that STAT3 was capable of directly activating the expression of mcl-1. Further documenting its importance, suppression of STAT3 employing AS ODNs also inhibited the expression of Mcl-1 and resulted in macrophage apoptosis. The effects of activated STAT3 were mediated through the transcriptional regulation of mcl-1 and a dominant-negative STAT3 suppressed mcl-1 promoter activation.
The data presented demonstrate that 2 pathways, one involving STAT3 and the other Akt-1, are essential for the expression of Mcl-1 and the survival of macrophages. The data suggest that each pathway may independently activate the mcl-1 promoter. The failure of zVAD.fmk to protect against the NaSal-induced loss of Δψm and cell death, while ectopic expression of Bcl-xL prevented apoptotic cell death, suggests the primary role of the mitochondria in the NaSal-induced apoptosis. Since Akt-1 is a Ser/Thr kinase and since NaSal partially reduced the expression of Akt-1 in macrophages, experiments were performed to determine if Akt-1 was responsible for the activation of STAT3 in macrophages. However, activated Akt-1 did not protect against the apoptotic cell death induced by NaSal, and the activation of Akt-1 had no effect on the Ser727 phosphorylation of STAT3 in macrophages, suggesting that STAT3 was not activated by Akt-1 in macrophages. Consistent with these observations, inhibition of the PI3K pathway by pretreatment with LY294002 had no effect on STAT3 Ser727 phosphorylation in UV-A—irradiated mouse epidermal cells.36 We have not excluded the possibility that activated STAT3 might contribute to the activation of Akt-1. The translocation of the ets transcription factor TEL with JAK2 results not only in STAT activation, including STAT3, but also the activation of Akt-1.37 However, this mechanism of activation of Akt-1 seems unlikely in macrophages since we previously demonstrated that PI3K was responsible for the constitutively activated Akt-1 observed in macrophages.7 Additionally, Akt-1 remained partially activated, following the treatment with NaSal. Activated Akt-1 may regulate Mcl-1 expression through cyclic AMP responsive element binding protein (CREB). Previous studies have demonstrated that Akt-1 activated CREB, which bound to the CRE in the mcl-1 promoter, and regulated Mcl-1 expression.8,38 Together, these observations suggest that the binding of complexes containing both STAT3 and CREB may be necessary for the expression of Mcl-1 in macrophages.
The effects of NaSal were not mediated through the inhibition of NF-κB, even though NaSal is capable of suppressing lipopolysaccharide (LPS) and TNFα-induced NF-κB activation.29,39,40 Direct examination of NF-κB activation by EMSA demonstrated that the inhibition by NaSal did not suppress NF-κB activation below the basal or constitutive level.29 We previously demonstrated that the constitutive activation of NF-κB was essential for macrophage survival and that this was mediated through the NF-κB—regulated expression of A1.20 However, consistent with its inability to fully suppress NF-κB activation,29 NaSal failed to suppress the expression of A1 in macrophages, suggesting that inhibition of NF-κB did not contribute to the apoptosis induced by NaSal. Consistent with this observation, NaSal did not suppress the activation of other downstream cellular targets of NF-κB below the baseline, following LPS stimulation in monocytes.39 Additionally, the adenoviral-mediated ectopic expression of NIK, which strongly activated the NF-κB in macrophages (data not shown), failed to protect macrophages from NaSal-induced apoptosis. These observations demonstrate that even if NaSal is capable of suppressing TNFα or LPS-induced NF-κB activation, in monocytes or macrophages the downstream targets regulated by the basal or constitutive activation of NF-κB were not suppressed by NaSal.
The mechanisms of apoptosis induced by nonsteroidal antiinflammatory drugs (NSAIDs) is controversial. Our observations demonstrate that NaSal, which is a weak prostaglandin inhibitor, suppressed the expression of total and activated Akt-1 comparably, and the ectopic expression of activated Akt-1 did not protect the macrophages. These observations suggest that the reduction of Akt-1 following NaSal treatment may have been due to caspase-dependent cleavage, as previously described.41 Another NSAID, celecoxib, a cyclooxygenase 2 inhibitor, suppressed phosphorylated but not total Akt-1 in colon and prostate cancer cell lines.42,43 In each case, the ectopic expression of activated Akt-1 demonstrated only marginal protection (maximum, 42%) against cell death.42,43 The ectopic expression of phosphoinositide-dependent kinase 1 (PDK1), which is also activated by PI3K and contributes to the activation of Akt-1, was more effective than activated Akt-1 in promoting cell survival in the presence of celecoxib.42 These observations suggest that other mechanisms activated by PDK1, such as the PKC or p70S6k pathways, may have been affected by celecoxib. Although the role of inhibition of cyclooxygenase 2 itself by NSAIDs in the induction of apoptosis is controversial,44 cycolooxygenase 2 overexpression induced Mcl-1 expression.45 Even though our data do not exclude the possibility that NaSal might affect Mcl-1 expression by inhibiting PDK1 activation, an inhibitor of mTOR/p70S6 kinase failed to suppress STAT3 phosphorylation in macrophages (data not shown). Therefore, it is possible that the effects of NSAIDs, including NaSal, may be mediated in part through PDK1, which might affect PKC-mediated STAT3 activation.46,47
A novel observation of this study is the constitutive activation of STAT3 in normal macrophages, which previously had been described in hematopoeitic tumor cells or Src-transformed cells.18,33,48,49 The data presented document the importance of STAT3 in the expression of Mcl-1 and in macrophage viability. The mechanism for the constitutive activation of STAT3 in macrophages was not examined. Although IL-6 may contribute to STAT3 activation,17,50 this is unlikely because IL-6 was not detected by enzyme-linked immunosorbent assay (ELISA) in macrophage culture supernatants (data not shown). It is possible that factors potentially present in the fetal calf serum such as granulocyte-macrophage colony-stimulating factor (GM-CSF) or leptin may be responsible for activating STAT3.51,52 The endogenous production of reactive oxygen species by macrophages, which has been shown to activate the JAK-STAT pathway,53 might also contribute to the constitutive STAT3 activation. Recently, a novel type I cytokine receptor that can activate STAT3 was found specifically expressed on monocyte and macrophages, although its ligand is not known.54 Regardless of the responsible mechanism, the activation of STAT3 in unstimulated macrophages was not expected.
Although it is generally accepted that the Tyr705 phosphorylation of STAT3 is a prerequisite for homodimerization, nuclear localization, and transactivation,55 our observations demonstrate the importance of nuclear localized STAT3, phosphorylated on Ser727, in the absence of Tyr705 phosphorylation. The Ser727-phosphorylated STAT3 was functional, defined in several ways. The nuclear localized STAT3 bound to Mcl-1 SIE ODNs. Additionally, inhibition of Ser727 phosphorylation by NaSal or H7 resulted in reduced Mcl-1 expression and apoptotic cell death. Finally, suppression of STAT3, which was phosphorylated on Ser727 but not Tyr705, by specific AS ODNs also suppressed the expression of Mcl-1 and resulted in apoptosis. Nonetheless, our data cannot exclude a role for Tyr705 phosphorylation in the constitutive activation of STAT3 in macrophages. It is possible that STAT3-phosphorylated Tyr705 was not sensitively detected by the antibodies employed. This seems unlikely since STAT3 phosphorylated Tyr705 was readily detected in the control cell line. It is also possible that Tyr705 phosphorylation may have been responsible for the nuclear translocation, followed by subsequent dephosphorylation. Supporting this possibility, strong STAT3 Ser727 phosphorylation may negatively regulate Tyr705 phosphorylation.56 Regardless of the mechanism, the data are consistent with the functional signifi-cance of STAT3 phosphorylated on Ser727 in the absence of Tyr705 phosphorylation.
Supporting the potential relevance of our observations, under certain conditions, including stimulation by insulin, GM-CSF, and IL-3, STAT3 phosphorylated on Ser727 in the absence of Tyr705 phosphorylation was observed.36,57-61 Furthermore, nuclear localized STAT3 has been detected in some tumor and primary cells, which were independent of tyrosine phosphorylation.62 Additionally, STAT3 may form stable homodimers in the absence of Tyr705 phosphorylation.63 However, to our knowledge, this is the first demonstration that STAT3 phosphorylated on Ser727 but not on Tyr705 contributes to the regulation of a functional and essential gene, Mcl-1, in primary human macrophages. Activated macrophages are critical to the pathogenesis of chronic inflammatory conditions such as rheumatoid arthritis and in tumor surveillance and infection. Further studies will be needed to define differences in STAT3 activation and Mcl-1 expression between cell types and between normal or malignant cells to develop new therapeutic strategies that may be effective at preventing or treating malignancies or inflammatory conditions, such as rheumatoid arthritis.
Prepublished online as Blood First Edition Paper, March 13, 2003; DOI 10.1182/blood-2002-11-3396.
Supported by awards from the National Institute of Health (N01-AR62221 and 1R01 AR049217) and The Veterans Administration Research Service (Merit Review) from the National Arthritis Foundation (R.M.P.).
J.K. has declared a financial interest in Isis Pharmaceuticals Inc, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr J. E. Darnell Jr from The Rockefeller University, New York, NY, for providing plasmids expressing activated STAT3 and STAT3 DN; and Dr Xiaodong Wang from University of Texas Southwestern Medical Center at Dallas for providing the anti-Smac antibody. We also thank Dr Steven W. Edwards from University of Liverpool, United Kingdom, for providing the 296—base pair human mcl-1 promoter-reporter construct. We also thank Mary Paniaqua for the flow cytometry conducted at the Robert H. Lurie Comprehensive Cancer Center, Flow Cytometry Core Facility of the Northwestern University Feinberg School of Medicine, Chicago, IL.

![Figure 1. NaSal treatment results in the loss of Δψmand apoptosis in primary human macrophages. Normal human in vitro—differentiated macrophages were incubated with NaSal (1 mM to 20 mM) for 24 or 48 hours as indicated. (A) The effect of NaSal on Δψm (retention of Rh123, mean fluorescence [% of control Rh123]). (B) Cell death induced by NaSal, defined by PI uptake. (C) The effects of NaSal on apoptosis, determined by analysis of subdiploid DNA (< 2N). No indicates no NaSal, or medium control. * indicates P < .05, ** indicates P < .01 determined by Student t test for paired samples compared with no treatment. The results in each panel are presented as the mean ± 1 SE of an experiment performed in triplicate, which is representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/1/10.1182_blood-2002-11-3396/5/m_h81334552001.jpeg?Expires=1767790166&Signature=m4GNmCSohY5qW0c-ECrn5mfEMLQhZtr~ccH-t~Y2f~bNXEOPqI95yTl2~nHutz17L76t3qjJiTXcvtZY~TTxVlJNXNFjmZ9eSarlyotZqEPdpKrw7ndt1VxwbRhnBjSbtM7Ooq9fo1HS5293JZRLbHxH7p-k-A9vLg8-TSJxxD~I20hQnxu0c6vgpkhuO3b728l1sWfImzDVm4HRLMhA4aNrwSnSMwsL~a52azKuviS09E6n0gWmwg-naYi1vQ2n~VPVMK64S7QqJwCw-iMYhrtTHPbyw~D26wu9iYC8y6a2Iv8Z2tLoTeK5bNsNJ9wvT2gjj1QzvRfRE9UiQsUPRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. NaSal treatment down-regulates the expression of Mcl-1 and suppresses the activation of the Mcl-1 promoter. (A) Effect of NaSal on Mcl-1 and Bcl-xL expression. Monocyte-differentiated macrophages were treated with NaSal (20 mM) for 12 to 48 hours, then cell lysates were collected and Western blot analysis performed using the antibodies indicated. (B) Effect of NaSal on Mcl-1 mRNA expression. Macrophages were treated with NaSal for the times indicated, then total RNA was extracted and used for RT-PCR using primers specific to Mcl-1. No indicates normal macrophages without NaSal treatment. (C) Mcl-1 expression protects RAW264.7 cells from NaSal-induced death; 0.5 μg of GFP-expressing plasmid and a total of 3 μg plasmid were included in each arm of the experiment. Control or Mcl-1—expressing plasmid (2.5 μg) were transfected where indicated. After 24 hours, the RAW264.7 cells were treated with NaSal (20 mM) for another 24 hours. GFP-positive cells were detected by fluorescence-activated cell-sorter, as previously described.7 (D) Effect of NaSal on Mcl-1 promoter activation. 1 μg Mcl-1 promoter-reporter construct was transiently transfected into RAW264.7 cells, which were then treated with NaSal (20 mM), LY294002 (50 μM), and control (dimethyl sulfoxide [DMSO]) as indicated for 24 hours. Cells were harvested, and luciferase activity was detected, and the values corrected for protein concentration. * indicates P < .05 and ** indicates P < .01 as determined by Student t test for paired samples compared with control (No indicating no NaSal [medium alone] or DMSO), as indicated. The results in panels C and D are presented as the mean ± 1 SE of experiments performed in triplicate, which are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/1/10.1182_blood-2002-11-3396/5/m_h81334552003.jpeg?Expires=1767790166&Signature=I31GyRRFtvqF11o104H7Igz8h5VuEaBRrqJHzE-Kroq4IDUEoi7ddcbUiYvK4uwUwodYUpffJXxja~8tWu6rhNBboc89GvrH4WwAxymo5qNkTeH7JgBEhVdtOqJiD-5ruQKFj2ixBv9jozFbBAfoNprXT4DVaZO7LaaTxgYJ96m61FpTBcVwYbn3sm-ka~fc48jaUAhXQ5SmbIv41gO8wQv~GFXJRbpnJlko2TMJAIBI2WyCbWR6xirPImJUZ1cUDtkUQclhKUYczTEwpb6g7qEgvjpvcepiKLlMMzgJO0V4J0TL6STyO93qoim3Jh9e1vHq8ZfET8b~BsJ4cR6SHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal