Acquired factor VIII (FVIII) inhibitors can cause life-threatening bleeding. Rapid restoration of coagulation is vital. Therapeutic approaches include factor substitution,1,2immunosuppression (eg, steroids, cyclophosphamide3), and plasmapheresis.4 A novel treatment option is rituximab, a chimeric monoclonal antibody targeting the CD20 antigen and blocking proliferation of normal B cells.5
Recently, Wiestner et al reported on the reduction of acquired FVIII inhibitors in 4 patients by an immunosuppressive regimen including rituximab.6 Patients presented with FVIII activity (FVIIIc) ranging from less than 1% to 4% (normal range, 70%-200%) and inhibitor titers ranging from 5 to 60 Bethesda units (BU). In 3 patients, FVIIIc normalized after the first of 1 to 4 treatment courses. The inhibitor became undetectable within 3 to 12 weeks. Plasmaphereses were not necessary.
Here, we describe the clinical course of a patient suffering from acquired idiopathic FVIII inhibitors with extraordinarily high titer. The 71-year-old male was admitted for the development of a large painful mass in his left gluteal region. He had received an intramuscular injection for lumbalgia 4 days prior. Patient history included years of chronic obstructive pulmonary disease but was otherwise unremarkable, particularly for allergic diathesis. There was no family history of autoimmune diseases, bleeding disorders, or neoplasias. Clinical examination revealed a large painful mass in his left gluteal region and diffuse mucosal bleeding. Respiratory sounds were slightly prolonged; liver and spleen were not enlarged. Laboratory work-up demonstrated pathologic coagulation studies with a markedly prolonged activated partial thromboplastin time (aPTT) of 80 seconds, decreased FVIIIc of less than 1%, and high FVIII inhibitor titers of 633 BU. Extensive laboratory exams did not reveal further pathologic results.
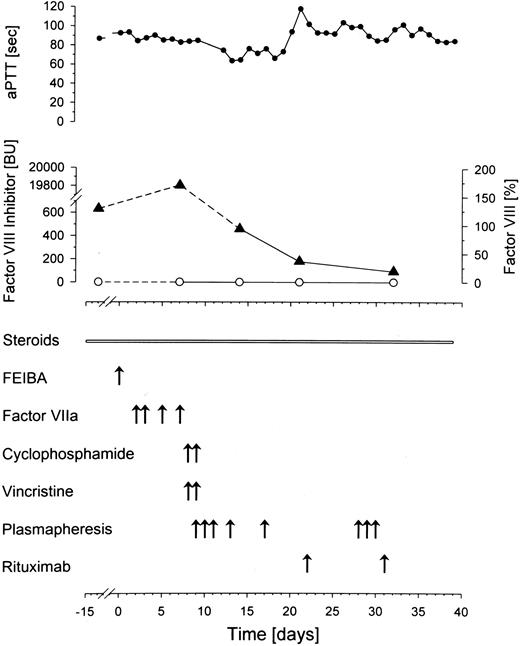
After a 2-week treatment with steroids he was transferred to our unit with persistent bleeding (day 0, Figure1). Here, the patient received one dose of FVIII inhibitor bypassing activity (FEIBA, Baxter BioScience, Heidelberg, Germany), followed by recombinant FVIIa (NovoSeven, NovoNordisk, Mainz, Germany) given for 3 days, which did not improve the clinical course. In need of rapid intervention, cyclophosphamide and vincristine were applied twice. At this point, as inhibitor titer had even increased to 19 800 BU, plasmapheresis was started. A dramatic decline in inhibitor titers was observed immediately thereafter (Figure 1). Nevertheless, the clinical parameters worsened (hematuria, hematemesis, and multiple cutaneous suggilations). aPTT remained extensively elevated and FVIIIc was still below the detection level, when rituximab (375 mg/m2) was added to the treatment regimen on days 22 and 31 after admission. Treatment was well tolerated. At one week after the first application, B lymphocytes were already reduced to 13/μL (normal range, 74-394/μL). Despite multimodal treatment, the patient died on day 39 with one of numerous large hematomas occluding the upper airways. No further pathologic findings were reported in postmortem examination, particularly no clonal B-cell disorder.
Multimodal treatment of acquired factor VIII inhibitor.
Time course of activated partial thromboplastin time (aPTT, ●), factor VIII (○), and factor VIII inhibitor (▴). A rapid decline in factor VIII inhibitor is accomplished by plasmapheresis. FEIBA indicates factor VIII inhibitor bypassing activity.
Multimodal treatment of acquired factor VIII inhibitor.
Time course of activated partial thromboplastin time (aPTT, ●), factor VIII (○), and factor VIII inhibitor (▴). A rapid decline in factor VIII inhibitor is accomplished by plasmapheresis. FEIBA indicates factor VIII inhibitor bypassing activity.
In 30% of patients, spontaneous resolution of acquired FVIII inhibitors has been described after an average of 21 months.7 However, in the case of bleeding and high antibody titers, rapid restoration of coagulation is required. This often is not achieved by current immunosuppressive regimen. With regard to novel treatment options, the successful application of 2-chloro-deoxyadenosine has recently been reported.8 Here, the median time to reach nadir inhibitor titers was 137 days; the median time for a 50% increase in FVIIIc was 117 days. Concerning efficacy of rituximab, data of Wiestner and colleagues suggest a faster FVIII recovery (3-12 weeks). Despite the promising treatment results with rituximab in several immunoglobulin-mediated disorders,9 it remains a concern whether the nadir of FVIII inhibitors can be achieved fast enough in high-risk cases.
To maximize treatment efficacy in our critically ill patient, we combined standard immunosuppressive therapy with plasmapheresis and rituximab. Plasmapheresis was intended to rapidly reduce autoantibody levels and allow for infusion of large amounts of plasma with procoagulant activities. Indeed, we experienced a decline in inhibitor titers after initiation of plasmapheresis. Within 25 days, a 200-fold reduction of inhibitors was achieved. Yet, it is of note that the remaining FVIII inhibitor titer of 94 BU still was high enough to cause fatal bleeding.
As the number of B cells at that time had already been markedly reduced, half-life of autoantibodies should be investigated.10 Whereas the combination of rituximab and plasmapheresis was effective in significantly reducing FVIII inhibitor titer, the autoimmune process with its enormous initial inhibitor burden was not overcome. Given the efficacy of combining rituximab with plasmapheresis, however, we strongly suggest its implementation in the very early clinical course in patients with extremely high antibody titers, when rapid elimination of antibodies is required to prevent fatal bleeding. This combined approach may be one way to solve the clinical problem of life-threatening bleeding upon FVIII inhibitors in the future.
Rituximab in the treatment of acquired factor VIII inhibitors
The letter by Fischer et al highlights the clinical challenge presented by patients with acquired factor VIII (FVIII) inhibitors. Fatal bleeding remains a dreaded complication despite the availability of several hemostatic agents and a choice of immunosuppressive drugs. Their patient had an extremely high FVIII inhibitor titer and was treated initially with prednisone alone for 2 weeks, followed by combination chemotherapy, plasmapheresis, and 2 doses of rituximab. While there was a significant decline in inhibitor titer, the patient succumbed to bleeding complications 4 weeks after the start of polychemotherapy and 2 weeks after the initiation of rituximab. The 4 patients with autoimmune hemophilia that we reported had lower inhibitor titers (5 to 60 Bethesda units [BU]) at presentation. Following treatment with rituximab and prednisone, plus cyclophosphamide in the patient with the highest titer, all had rapid clinical improvement and complete resolution within 3 to 12 weeks.1-1 The responses have been durable, lasting to date + 17 to + 22 months without any maintenance treatment. A comparable experience has been reported by Kain et al1-2 in a patient who had autoimmune hemophilia for 10 years who was refractory to standard immunosuppressive drugs. Their patient's high titer inhibitor (268 BU) resolved over a 4-month period following 4 weekly doses of rituximab (375 mg/m2) alone and remained less than 1 BU for + 7 months.
What should the role of rituximab be in the treatment of patients with FVIII inhibitors? Unfortunately it is unlikely that controlled studies will be possible in this rare disease. Perhaps experiences with this agent in the more common autoimmune disorders, for example immune thrombocytopenic purpura (ITP)1-3 and rheumatoid arthritis (RA),1-4 may serve to guide treatment decisions in patients with acquired FVIII inhibitors. Similar to the responses in our FVIII inhibitor patients, clinical improvement appears often surprisingly rapid in these autoimmune diseases and does not fully correlate with the resolution of antibody titers. While there are numerous reports that rituximab alone may suffice, it appears, at least for patients with RA, that combination therapy with cyclophosphamide may be superior.1-4 Rituximab is not effective in all RA patients and relapse is frequent, occurring typically following B-cell recovery 6 to 9 months from the start of therapy. Patients who relapse after an initial response may respond to second courses of rituximab. The question of maintenance therapy has hardly been addressed.
At this time we would certainly agree with Fischer et al that rituximab should be considered early in the management of patients with active bleeding and/or high titer FVIII inhibitors. In patients with very high antibody burden, it seems appropriate to use combination chemotherapy including prednisone, cyclophosphamide, and rituximab at the time of diagnosis. It is well known that FVIII inhibitors can resolve spontaneously in up to 30% of patients,1-5 and prednisone alone or in combination with cyclophosphamide will effect remissions in a substantial proportion of patients.1-6 However time to resolution of the antibody with these agents is usually slow, taking months, and prolonged treatment with prednisone and cyclophosphamide may be associated with significant side effects. If the response rate to rituxmab continues to be confirmed, it is likely to be shown cost-effective in those patients who require factor replacement. A full course of 4 weekly doses of rituximab is less expensive than one day of replacement therapy with recombinant FVIII and a fraction of the cost of FVIIa (NovoSeven). In patients with low-titer inhibitors it may not even be necessary to give a full course of 4 doses of rituximab once a clear improvement has been detected.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal