CD22 is a membrane glycophosphoprotein found on nearly all healthy B-lymphocytes and most B-cell lymphomas. Recent in vitro studies have identified several anti-CD22 monoclonal antibodies (mAbs) that block the interaction of CD22 with its ligand. One of these mAbs, HB22.7, has been shown to effectively induce apoptosis in several B-cell lymphoma cell lines. Lymphoma xenograft studies with Raji-xenograft mice were used to assess the toxicity and efficacy of HB22.7 alone and with combined modality immunotherapy (CMIT) with yttrium 90Y-DOTA-peptide-Lym-1 radioimmunotherapy (RIT). The effect of the sequence of these agents on the combined treatment was assessed by administering HB22.7 24 hours before, simultaneously with, or 24 hours after RIT. Within the groups treated with RIT alone or with RIT and HB22.7 (CMIT), the reduction in tumor volume was the greatest when HB22.7 was administered simultaneously with and 24 hours after RIT, and in the RIT treatment groups, this translated into the greatest overall response and survival, respectively. Overall survival rates at the end of the 84-day CMIT trial were 67% and 50% in the groups treated with HB22.7 simultaneously and 24 hours after RIT, respectively. This compared favorably with the untreated and the RIT alone groups, which had survival rates of 38% and 43% at the end of the trial. Surprisingly, when compared with untreated controls and all other treatment groups, the greatest cure and overall survival rates were observed in the group treated with HB22.7 alone, with 47% cured and 76% surviving at the end of the 84-day trial. RIT clearance was not affected by treatment with HB22.7. When compared with RIT alone, there was no significant additional hematologic (white blood cell, red blood cell, or platelet count) toxicity when HB22.7 was added to RIT. Nonhematologic toxicity (assessed as change in body weight) was also unchanged when HB22.7 was added to RIT. Thus the anti-CD22 ligand-blocking antibody HB22.7 has independent lymphomacidal properties and augments the efficacy of90Y-DOTA-peptide-Lym-1 in lymphoma xenografts without significant toxicity.
Introduction
Despite effective chemotherapy for non-Hodgkin lymphoma (NHL), more than two thirds of patients do not achieve long-term, disease-free survival. New therapeutic options are needed. RIT is systemic anticancer therapy that makes use of tumor-specific monoclonal antibodies (mAbs) to deliver cytotoxic radionuclides specifically to widespread sites of NHL, thus sparing healthy tissue from excessive radiation and the associated toxicity. RIT has proven especially effective for NHL because of the radiosensitivity of NHL, the abundance of target-specific antigens on lymphocyte membranes, and the vascular accessibility of these types of malignancies.1-7
The therapeutic potential of RIT in patients with NHL has been shown by a number of investigators using numerous B-cell–specific targets including CD20, CD19, CD22, and HLA-DR10 (Lym-1).8-15Lym-1 has high affinity against a discontinuous epitope on the beta chain of the HLA-DR10 antigen on the surfaces of malignant B-lymphocytes.16 Because of its greater avidity for malignant rather than healthy B cells, Lym-1 preferentially targets malignant lymphocytes. Multiple preclinical and clinical studies with sodium iodine-131(131I), copper-67 (67Cu), and yttrium-90 (90Y)–labeled Lym-1 have demonstrated significant efficacy in relapsed and refractory NHL.8,9,17-20 In an MTD trial in which heavily pretreated NHL patients were treated with 131I-Lym-1, 85% had tumor regression, and 19% achieved complete response (CR).8
Combined modality therapy (CMT) consists of the concurrent or sequential use of chemotherapy and external beam radiation. CMT has become an increasingly frequent maneuver for the treatment of solid tumors and provides an example applicable to RIT for NHL. At least 2 concepts are involved in CMT: radiosensitization of cancer cells by drugs and the direct cytotoxic effect of chemotherapy. A randomized study in aggressive but early-stage NHL showed superior results with cyclophosphamide, hydroxydaunomycin/doxorubicin, Oncovin, prednisone (CHOP) plus involved field radiation versus CHOP alone.21In addition to demonstrating that the combination of external beam radiation and chemotherapy can be beneficial for patients with NHL, it also illustrates a challenge—external beam radiation, though effective, can only be delivered in high doses to a limited region of the body, whereas NHL most frequently is widespread. Although RIT has proved to be an effective strategy for the delivery of tumor-specific radiation, to date, the higher response rates with RIT have not translated into longer overall survival rates compared with treatment using the parent naked mAb. The efficacy of RIT is limited by toxicity, particularly myelosuppression.7,22-25 We have taken several approaches to improve the therapeutic index of RIT. Newly developed linkers used in the conjugation of the mAb and the radiometal chelator selectively degrade in the liver. One biodegradable linker, DOTA peptide, has demonstrated a favorable biodistribution profile when used with the chimeric L6 mAb in breast xenograft studies26 and in a phase 1 clinical trial.27Another approach to improve efficacy involves the enhancement of tumoricidal effects of RIT by combining RIT with chemotherapeutic agents or, as described herein, antibodies.19 28-30
Conventional CMT has proven clinically useful for locally advanced malignancies.31-35 Combined modality immunotherapy (CMIT) goes one step further by pairing the specific delivery of systemic radiation (90Y-DOTA-peptide-Lym-1) to NHL with the systemic radiation-sensitizing effects of an additional agent (mAb). CMIT is further enhanced by its ability to provide continuous radiation at the site of the malignancy—the ultimate in hyperfractionation. Because the radiation is delivered continuously, cancer cells that are hypoxic are more likely to pass through the radiosensitive G2/M phase of the cell cycle during the course of treatment, making cure more likely. The benefit of CMIT is provided by the specific targeting of NHL by RIT and by the timing of the radiosensitizing agent. This allows for the radiation sensitizer to potentially synergize only at the sites targeted by RIT, thus maximizing efficacy and minimizing toxicity. In several different previous xenograft studies, synergy has been demonstrated when the radiation sensitizer (Taxol) was given 24 to 48 hours after RIT.20 36
CD22 is a membrane glycophosphoprotein found on nearly all B-lymphocytes and most B-cell lymphomas. Cross-linking CD22 triggers CD22 tyrosine phosphorylation and assembles a complex of effector proteins that activate the stress-activated protein kinase (SAPK) pathway. In conjunction with interleukins, antigen receptor cross-linking, or CD40 cross-linking, CD22 cross-linking provides a costimulatory signal in primary B cells and a proapoptotic signal in neoplastic B cells.37-40 In addition, CD22 is thought to be a B-cell–receptor (BCR) modulator, with recent reports demonstrating positive and negative effects on BCR-mediated signaling.41 Recent studies have revealed that several anti-CD22 mAbs (termed HB22.7, HB22.23, and HB22.33) block the interaction of CD22 with its ligand and have distinct functional properties.42 Cross-linking CD22 on several B-cell lymphoma cell lines with these mAbs resulted in a 3- to 5-fold induction of SAPK activity and efficient and effective induction of apoptosis. Based on these finding we have proposed that the blocking mAb HB22.7, when given in the appropriate sequence, enhances the efficacy of RIT. Presented herein are the results of Raji lymphoma xenograft trials that were designed to assess the toxicity and to compare the efficacy of RIT (90Y-DOTA-peptide-Lym-1) alone, the combination of RIT and HB22.7 administered in 3 different sequences (24 hours before, simultaneously with, and 24 hours after RIT), HB22.7 alone, and no treatment.
Materials and methods
Reagents
Carrier-free 90Y (Pacific Northwest National Laboratory, Richland, WA) and indium-111 (111In; Nordion, Kanata, ON, Canada) were purchased as chlorides in dilute HCl. Lym-1 (Techniclone, Tustin, CA) is an immunoglobulin (IgG2a) mAb generated in mice immunized with human Burkitt lymphoma cell nuclei. Lym-1 recognizes a cell surface 31- to 35-kDa antigen on malignant B cells and reacts with more than 80% of human B-cell NHL. Lym-1 purity was assessed according to the specifications that required more than 95% pure monomeric IgG by polyacrylamide gel electrophoresis.90Y-DOTA-peptide-Lym-1 was prepared as previously described.43 Assessment by high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), and cellulose acetate electrophoresis revealed that90Y-DOTA-peptide-Lym-1 was prepared to 98% radiochemical purity with less than 5% aggregate content.
The anti-CD22 mAb HB22.7 was prepared as previously described,42 using a Protein A Sepharose Fast Flow column (Pharmacia, Piscataway, NJ). HB22.7 purity was determined by HPLC and flow cytometry and was found to be more than 95% pure. Physiologic properties were determined by flow cytometric–based analysis of apoptotic induction (Apo-Tag; Pharmacia) and were found to be consistent with previous published results.42 Endotoxin removal was achieved using an ActiClean ETOX column (Sterogene, Carlsbad, CA), with final endotoxin levels determined to be less than 0.15 endotoxin units (EU)/mg mAb (BioWhittaker, Walkerville, MD). The Lym-1 and HB22.7 mAbs met mouse antibody production (MAP) guidelines for murine, viral, mycoplasma, fungal, and bacterial contamination and for endotoxin, pyrogen, and DNA content and general safety testing in animals.
Cell lines and Scatchard analysis
Raji and Ramos Burkitt lymphoma cell lines were purchased from American Type Culture Collection (ATCC, Gaithersburg, MD). Both cell lines stained for CD22 expression by flow cytometric methods using the HB22.7 mAb, as described previously.42 Cell lines were maintained in RPMI 1640 supplemented with 10% fetal calf serum at 0.5 × 106 cells/mL. Scatchard analysis using Raji and Ramos cells was performed as described previously.44Briefly, HB22.7 was labeled with iodide I 125 by the chloramine T method (specific activity of 1.1 μCi/μg [0.037 MBq/μg]). A competitive binding assay was performed using serially diluted, unlabeled HB22.7.
Mouse studies
Female athymic BALB/c nu/nu mice (Harlan Sprague-Dawley), 7 to 9 weeks of age, were maintained according to University of California, Davis animal care guidelines on a normal diet ad libitum and under pathogen-free conditions. Five mice were housed per cage. Raji or Ramos cells were harvested in logarithmic growth phase; 2.5 to 5.0 × 106 cells were injected subcutaneously into both sides of the abdomen of each mouse. Studies were initiated 3 weeks after implantation, when tumors measured 28 to 328 mm.3Groups consisted of untreated mice and of mice treated with 125 μCi (4.625 MBq) RIT alone, 1.4 mg HB22.7 alone, or combined RIT and HB22.7, with HB22.7 administered 24 hours before, simultaneously with, or 24 hours after RIT. To minimize ambient radiation, bedding was changed daily for 1 week after treatment with90Y-DOTA-peptide-Lym-1 and twice weekly thereafter.
Tumoricidal effect
Tumor volume was calculated as described by the formula for hemiellipsoids.45 Initial tumor volume was defined as the volume on the day before treatment. Mean tumor volume was calculated for each group on each day of measurement; tumors that had completely regressed were considered to have a volume of zero. Tumor responses were categorized as follows: C indicated cure (tumor disappeared and did not regrow by the end of the 84-day study), CR indicated complete regression (tumor disappeared for at least 7 days but later regrew), and PR indicated partial regression (PR) (tumor volume decreased by 50% or more for at least 7 days, then regrew).
Statistical analysis
Differences in response among treatment groups were evaluated using the Kruskal-Wallis rank sum test, with the response ordered as none, PR, CR, and cure. Survival time was also evaluated using the Kruskal-Wallis test. Tumor volume was compared at 3 time points: month 1 (days 26-29), month 2 (days 55-58), and at the end of the study (day 84). If an animal was killed because of tumor-related causes, the last volume was carried forward and used in the analysis of later time points. Analysis of variance was used to test for differences among treatment groups. Tests for P values were 2-tailed and represented the nominal values. Protection for multiple comparison was provided by testing only within subsets of groups found to be statistically significantly different.
Results
Scatchard analysis
Scatchard analysis was used to assess the binding affinity of HB22.7 and the number of CD22 receptors on Ramos and Raji cells. Cells were assayed for maximum binding percentage (Bmax), disassociation constant (Kd), and number of antibodies bound per cell. Results are the average of 2 experiments.
Scatchard analysis (Table 1) revealed a nearly 2.5-fold increase in the number of HB22.7 antibodies bound per cell, Bmax, and a 2-fold increase inKd for Raji cells versus Ramos cells, respectively.
Scatchard analysis
| Parameter . | Cell lines . | |
|---|---|---|
| Raji . | Ramos . | |
| Bmax,% | 53.5 ± 0.9 | 21.0 ± 1.3 |
| R2 | 0.954 | 0.926 |
| Kd | 1.3 ± 0.08 × 109 | 5.95 ± 1.0 × 108 |
| Antibody/cell | 118 000 | 43 000 |
| Parameter . | Cell lines . | |
|---|---|---|
| Raji . | Ramos . | |
| Bmax,% | 53.5 ± 0.9 | 21.0 ± 1.3 |
| R2 | 0.954 | 0.926 |
| Kd | 1.3 ± 0.08 × 109 | 5.95 ± 1.0 × 108 |
| Antibody/cell | 118 000 | 43 000 |
Whole body autoradiography
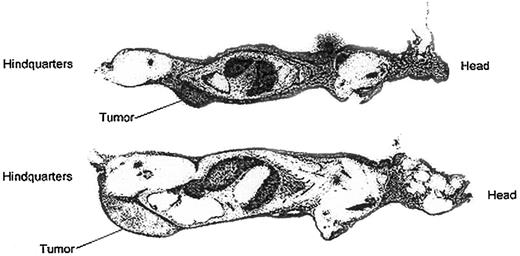
To assess HB22.7-specific tumor targeting, whole body autoradiography of tumor-bearing nude mice injected with111In-2IT-BAD–anti-CD22 (HB22.7) was performed. Forty-eight hours after injection mice were killed, sectioned, and autoradiographed (Figure 1), as previously described.46 Autoradiography revealed intense tumor localization in the Raji-tumor mice and moderate localization in the Ramos-tumor mice. This targeting study is consistent with the Scatchard analysis revealing that less HB22.7 was bound per Ramos cell than Raji cell. However, the rapid growth of Ramos tumors, and likely central necrosis, might also have contributed to the apparent inferior targeting of Ramos cells.
Whole body autoradiography of Raji and Ramos tumor-bearing nude mice injected with 111In-2IT BAD anti-CD22 (HB22.7).
Mice were killed and autoradiographed 48 hours after injection. Upper image is Raji-tumor mouse; lower image is Ramos-tumor mouse.
Whole body autoradiography of Raji and Ramos tumor-bearing nude mice injected with 111In-2IT BAD anti-CD22 (HB22.7).
Mice were killed and autoradiographed 48 hours after injection. Upper image is Raji-tumor mouse; lower image is Ramos-tumor mouse.
Efficacy of RIT and CMIT
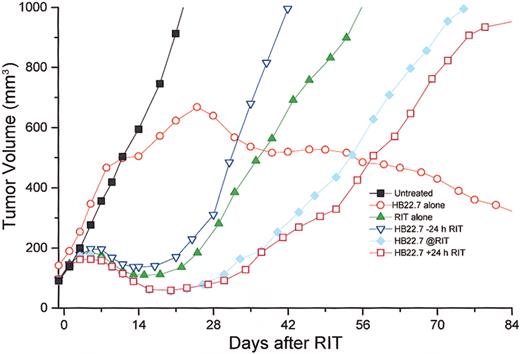
The initial trial (081 500) used 125 μCi (4.625 MBq)90Y-DOTA- peptide-Lym-1 alone or in combination with HB22.7 (1.4 mg) given either 24 hours before, simultaneously with, or 24 hours after RIT. Each group in this trial had 5 mice, with the exception of the group treated with RIT alone, which had 9 mice and 5 untreated controls (mouse numbers are tabulated in Table2). As predicted from similar Raji xenograft studies with 90Y-2IT-BAD-Lym-1, RIT alone resulted in maximal mean tumor volume reduction by day 21, with increasing tumor volume thereafter. Xenografts treated with90Y-2IT-BAD-Lym-1 (RIT) and HB22.7 (CMIT) demonstrated greater and more sustained mean tumor volume reduction, which was greatest when HB22.7 was administered simultaneously with and 24 hours after RIT. Surprisingly, HB22.7 administered alone resulted in the stabilization of mean tumor volume by 2 to 3 weeks, then a gradual and sustained tumor volume reduction. Several additional replicate trials were conducted with highly reproducible results (Table 2). The data from all trials were compiled and revealed results highly consistent with the initial study (Figure 2).
Xenograft trials/mouse numbers
| Trial . | Treatment groups* . | |||||
|---|---|---|---|---|---|---|
| No treatment . | HB22.7 . | RIT . | −24 . | @RIT . | +24 . | |
| 081500 | 5 | 4 | 9 | 5 | 5 | 5 |
| 101600 | 5 | 6 | 5 | 5 | 3 | 5 |
| 011601 | — | 5 | 4 | — | 9 | 7 |
| 032701 | — | 5 | 2 | — | 3 | 12 |
| 052401 | 3 | — | 3 | — | — | — |
| 060401 | 5 | 5 | — | — | — | — |
| 071701 | 7 | 5 | — | — | — | 4 |
| 092101 | 4 | — | — | — | — | — |
| 102401 | 13 | — | — | — | — | — |
| Total | 42 | 30 | 23 | 10 | 20 | 33 |
| Trial . | Treatment groups* . | |||||
|---|---|---|---|---|---|---|
| No treatment . | HB22.7 . | RIT . | −24 . | @RIT . | +24 . | |
| 081500 | 5 | 4 | 9 | 5 | 5 | 5 |
| 101600 | 5 | 6 | 5 | 5 | 3 | 5 |
| 011601 | — | 5 | 4 | — | 9 | 7 |
| 032701 | — | 5 | 2 | — | 3 | 12 |
| 052401 | 3 | — | 3 | — | — | — |
| 060401 | 5 | 5 | — | — | — | — |
| 071701 | 7 | 5 | — | — | — | 4 |
| 092101 | 4 | — | — | — | — | — |
| 102401 | 13 | — | — | — | — | — |
| Total | 42 | 30 | 23 | 10 | 20 | 33 |
Treatment groups consisted of HB22.7 alone; RIT alone; and HB22.7 administered 24 h prior to (−24), simultaneous with (@RIT), or 24 h after (+24) administration of RIT.
Temporal assessment of tumor volume in Raji-xenografted mice that were untreated or treated with 125 μCi (4.625 MBq)90Y-DOTA-peptide-Lym-1 (RIT) alone, anti-CD22 alone (HB22.7), or 3 different sequences of RIT and HB22.7 (CMIT) in all trials and include HB22.7 administered 24 h prior to RIT (−24), simultaneous with RIT (@RIT), or 24 h after RIT (+24).
Tumor volume was assessed 3 times per week. Mouse numbers for each treatment group are tabulated (Table 2).
Temporal assessment of tumor volume in Raji-xenografted mice that were untreated or treated with 125 μCi (4.625 MBq)90Y-DOTA-peptide-Lym-1 (RIT) alone, anti-CD22 alone (HB22.7), or 3 different sequences of RIT and HB22.7 (CMIT) in all trials and include HB22.7 administered 24 h prior to RIT (−24), simultaneous with RIT (@RIT), or 24 h after RIT (+24).
Tumor volume was assessed 3 times per week. Mouse numbers for each treatment group are tabulated (Table 2).
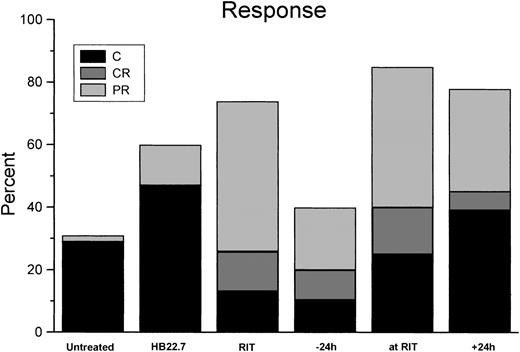
Using analysis of variance, when examining all treatment groups at day 30 the differences were highly significant (P < .001). Although analysis of volume reduction in all treatment groups at day 60 did not demonstrate significant differences (P = .39), the differences at day 84 again were significant (P = .003). Results graphically revealed that the difference in volume reduction in the RIT/CMIT groups was highly reproducible and different in HB22.7 alone and untreated control; however, comparison of volume reductions only in only RIT treatment groups (including CMIT) at all time points assessed (days 30, 60, and 84) did not reveal significant differences (P ≥ .5). Additional CMIT trials were conducted, with HB22.7 administered 48 and 72 hours after RIT. The extended interval between the administration of RIT and HB22.7 did not result in improved tumor volume reduction when compared with trials in which HB22.7 was given simultaneously with and 24 hours after RIT (data not shown). Response and cure rates were consistent with the effects of treatment on tumor volume (Figure 3).
Response and cure rates for Raji-xenografted mice that were treated as described in Figure 2.
Tumor responses were categorized as follows: C indicates cure (tumor disappeared and did not regrow by the end of the 84-day study); CR, complete regression (tumor disappeared for at least 7 days but later regrew); PR, partial regression (tumor volume decreased by 50% or more for at least 7 days, then regrew). Data represent results of all independent trials.
Response and cure rates for Raji-xenografted mice that were treated as described in Figure 2.
Tumor responses were categorized as follows: C indicates cure (tumor disappeared and did not regrow by the end of the 84-day study); CR, complete regression (tumor disappeared for at least 7 days but later regrew); PR, partial regression (tumor volume decreased by 50% or more for at least 7 days, then regrew). Data represent results of all independent trials.
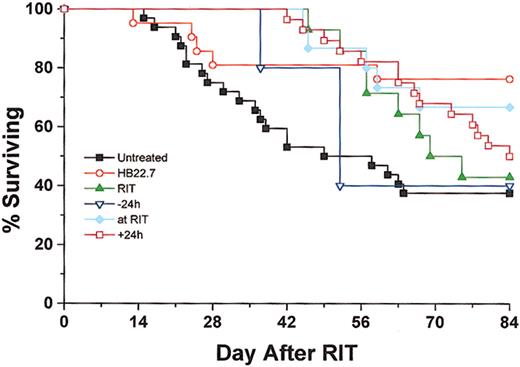
Treatment with 90Y-DOTA-peptide-Lym-1 alone produced 48% PR, 13% CR, and 13% cure rates. In the CMIT groups, the overall response rate was maximized when HB22.7 and RIT were administered simultaneously, generating 45% PR, 15% CR, and 25% cure. However, in the CMIT groups the cure rate was the greatest (39%) when HB22.7 was administered 24 hours after RIT, which compared favorably with the cure rates observed in the untreated (29%), RIT alone (13%), 24 hours before (10%), and simultaneous (25%) treatment groups. When examining the degree of response (ranking cure better than CR, better than PR) in all treatment groups using the Kruskal-Wallis test, the differences were statistically significant (P = .01). Individual comparisons against untreated controls were all statistically significant (P < .05), with the exception of RIT alone (P = .06) and HB22.7 given 24 hours before RIT (P = .16). Although comparison of only active treatment groups (RIT alone, CMIT, and HB22.7) was not significantly different (P = .18), the CMIT groups treated with HB22.7 simultaneously with and after 24 hours had the best observed pattern of response. Interestingly, the group treated with HB22.7 alone had the highest cure rate (47%), which was a significant improvement when compared with the untreated controls (P < .05). Tumor volume regression and cure rates translated to a similar pattern of survival. At the end of the 84-day study period, 38% and 42%, respectively, of the untreated and RIT alone groups were alive (Figure4).
Overall survival was assessed for Raji xenografted mice that were treated as described in Figure 2.
Mice were killed when the tumor burden exceeded 2000 mg or at the end of the 84-day trial. Data represent results of all independent trials.
Overall survival was assessed for Raji xenografted mice that were treated as described in Figure 2.
Mice were killed when the tumor burden exceeded 2000 mg or at the end of the 84-day trial. Data represent results of all independent trials.
In the CMIT treatment groups, survival rates increased to 67% and 50% when HB22.7 was administered simultaneously with and 24 hours after RIT, respectively. Analysis of survival using Kruskal-Wallis was significant (P < .05) for comparison of all groups. Similar to the response rate analysis, comparison of survival in the RIT groups only did not reveal significant differences (P = .41). However, the best survival in these groups was consistently observed when HB22.7 was administered either simultaneously with or 24 hours after RIT. The best overall survival rate, 76%, was observed in the group treated with HB22.7 alone, a significant difference when compared with the untreated control (P = 0.02).
Toxicity
Hematologic and nonhematologic toxicity levels were assessed by blood counts (Figure 5A-C) and mouse weights, respectively. White blood cell (WBC) and platelet nadirs in the RIT treatment groups were at 14 to 20 and 10 to 14 days, respectively. WBC and platelet recovery was approximately 28 and 21 days after treatment, respectively. The WBC and platelet nadirs were consistent with observations in previous studies that used 150 μCi (5.55 MBq) 90Y-2IT-BAD-Lym-1. Hematologic toxicity of RIT was not altered by the coadministration of HB22.7. No hematologic toxicity was detected in mice treated with HB22.7 alone. Analysis of mononuclear cell counts in all treatment groups revealed that HB22.7 had no effect on RIT-mediated mononuclear cell nadirs (data not shown). Nonhematologic toxicity, as assessed by changes in mouse weight, was found to be equivalent in all treatment groups (data not shown). There were no deaths caused by toxicity in any treatment group.
90Y-DOTA-peptide-Lym-1 pharmacokinetics
Blood and whole body clearances of90Y-DOTA-peptide-Lym-1 in Raji-tumor mice with or without HB22.7 were similar (Figure 6). The blood biological T1/2 α was 1.4 hours for the RIT alone group and 2.2, 2.4, and 2.0 hours for the 24 hours before, simultaneous with, and 24 hours after groups, respectively. The blood biological T1/2 β was 127 hours for the RIT alone group and 133, 87, and 103 hours for the 24 hours before, simultaneous with, and 24 hours after groups, respectively. Whole body T1/2 was 246 hours for the RIT alone group and 207, 207, and 196 hours for the 24 hours before, simultaneous with, and 24 hours after groups, respectively. The addition of HB22.7 to RIT did not change the pharmacokinetics of90Y-DOTA-peptide-Lym-1.
Hematologic toxicity was assessed by measuring WBC, RBC, and platelet counts twice weekly in the Raji-xenografted mice that were treated as described in Figure 2.
When compared with RIT alone, there was no difference in hematologic toxicity in the CMIT groups. In addition, no hematologic toxicity was observed in the mice treated with HB22.7 alone.
Hematologic toxicity was assessed by measuring WBC, RBC, and platelet counts twice weekly in the Raji-xenografted mice that were treated as described in Figure 2.
When compared with RIT alone, there was no difference in hematologic toxicity in the CMIT groups. In addition, no hematologic toxicity was observed in the mice treated with HB22.7 alone.
RIT clearance was assessed by measuring radioactivity in whole body (WB) and blood daily for 5 days after the initiation of treatment with RIT.
Results were reported after adjusting for decay based on the T1/2 of 90Y. There were no significant differences in RIT clearance in any of the CMIT treatment groups.
RIT clearance was assessed by measuring radioactivity in whole body (WB) and blood daily for 5 days after the initiation of treatment with RIT.
Results were reported after adjusting for decay based on the T1/2 of 90Y. There were no significant differences in RIT clearance in any of the CMIT treatment groups.
Discussion
Raji xenograft studies were designed to determine whether the anti-CD22 mAb (HB22.7) would generate additive or synergistic effects when combined with RIT to enhance apoptosis or DNA damage induced by low-dose–rate radiation. The Raji xenograft nude mouse model has proven useful when used to assess toxicity and efficacy of RIT using90Y-2IT-BAD-Lym-1 RIT alone.19 Responses in this preclinical model translated into significant efficacy in human clinical trials.20 47
In the study described herein, the addition of the anti-CD22 mAb HB22.7 to 90Y-DOTA-peptide-Lym-1 (125 μCi [4.625 MBq]) enhanced the efficacy of RIT without any change in toxicity level. Previous Raji xenograft studies with 150 and 200 μCi (5.55 and 7.4 MBq) 90Y-2IT BAD Lym-1 generated response and cure rates that were comparable to those observed in the present study.19 The 125 μCi (4.625 MBq) dose of90Y-DOTA-peptide-Lym-1 was chosen based on these previous studies with the 2IT-BAD linker. Although the previous studies with 2IT-BAD demonstrated greatest efficacy with the 200 μCi (7.4 MBq) dose, the choice of 125 μCi (4.625 MBq) was based on the hypothesis that HB22.7 would be synergistic or additive with RIT and that the lower dose would allow for better assessment of these effects. This study used a novel linker (DOTA-peptide) that has not been previously examined in lymphoma xenograft models. The DOTA-peptide linker was designed for enhanced hepatic degradation of unbound radiopharmaceuticals, thereby leading to a more favorable biodistribution. Although tumor-specific uptake was not assessed in detail in this study, the toxicity profile observed with 125 μCi (4.625 MBq) 90Y-DOTA-peptide-Lym-1 alone was acceptable, with no treatment-related deaths and with predictable leukocyte and platelet nadirs.
HB22.7 was chosen based on in vitro studies demonstrating proapoptotic and signaling effects.42 The treatment dose of HB22.7 was empiric, but it was based on the amount shown to be effective at inducing apoptosis in vitro and extrapolating this to the mouse model. In addition, when formulating the dose of HB22.7, consideration was given to the equivalent (when adjusted for body surface area differences in humans versus mice) dose of rituximab used in human clinical trials. Approximation to the rituximab dose was based on the fact that this is the only naked mAb available that has demonstrated efficacy for the treatment of lymphoma (the optimal dose of rituximab is currently undefined).
The study was designed to assess the efficacy of HB22.7 alone, of RIT and HB22.7, and of the effect of 3 different sequence combinations. The tumor volume reduction observed with 90Y-DOTA-peptide-Lym-1 alone was consistent with previous studies with 90Y-2IT BAD Lym-1 in terms of timing, magnitude, and duration of response.19 RIT alone resulted in approximately 50% reduction in tumor volume 14 days after therapy. When assessing at the approximate point of maximal volume reduction (days 21-30), the addition of HB22.7 to RIT significantly enhanced the magnitude of response in a sequence-specific manner. It appears that the addition of HB22.7 was most effective when administered simultaneously with or 24 hours after RIT. The distinctive pattern of volume reduction was highly reproducible. Independent replicate trials demonstrated similar patterns and magnitude of tumor volume reduction. Improved reductions in tumor volume translated into superior response and survival rates. RIT alone generated 13% CR and 13% cures. The addition of HB22.7 increased the cure rate to 25% when administered simultaneously with RIT and to 39% when HB22.7 was administered 24 hours after RIT.
The mechanism by which HB22.7 augments the tumoricidal effects of RIT is an area of active investigation. The mechanism by which apoptotic signals are transferred from the membrane to the nucleus is only partially known. In some cell types this mechanism has been shown to involve the stress-activated protein kinase (SAPK/JNK) system.42 The SAPK/JNK cascade is a generic signaling system that becomes activated by a variety of stimuli and cellular stress.48 Previous studies have shown that exposure of various cell lines to ionizing and ultraviolet radiation activated the SAPK cascade downstream of ceramide.49,50 These data suggest that the SAPK/JNK cascade couples membrane and nuclear elements of the apoptotic pathways, which become activated by ionizing radiation and other cellular stresses. Previous studies have demonstrated that the proapoptotic effects of the mAb HB22.7 are mediated through the SAPK pathway42 and that the SAPK pathway is a known apoptotic mediator in B-cell lymphomas. We have hypothesized that the sequence dependence of RIT and HB22.7 is likely caused by augmented SAPK activation by HB22.7 and subsequent downstream proapoptotic effects. SAPK activation mediated by ionizing radiation can occur up to 4 hours after irradiation. Given the time from the administration of RIT to optimal binding and SAPK induction, augmentation of SAPK activation by HB22.7 24 hours after RIT administration would account for the additive tumoricidal and clinical effects observed with this sequence. In addition, though in vitro studies have demonstrated the proapoptotic potential of HB22.7 in lymphoma cell lines, there is no direct evidence that this occurs in vivo in this xenograft model. However, the observed lymphomacidal effects are potentially consistent with in vivo proapoptotic effects. This is the first time that a second monoclonal antibody has been combined with RIT, and it demonstrates the potential of using monoclonal antibodies or other agents with well-defined physiologic properties that may augment efficacy without increasing toxicity.
Surprisingly, the mice treated with HB22.7 alone had impressive tumor volume reduction and superior cure and survival rates when compared with all other treatment groups. Again, several independent trials generated highly consistent results with delayed initial tumor volume stabilization and then tumor volume reduction beginning approximately 14 days after treatment. This translated into the best cure and overall survival rates observed in any of the treatment groups. The high cure and survival rates observed with HB22.7 alone are under active investigation. Although the possibility exists that the residual natural killer (NK) cell function known to be present in nude mice could account for host immune responses that mediate antibody-dependent cell-mediated cytotoxicity (ADCC), this becomes less likely given that the mice are irradiated before tumor implantation. In addition, published xenograft studies that use unconjugated or naked murine mAbs have not reported similar findings.
Although the data clearly demonstrate that HB22.7 and90Y-DOTA-peptide-Lym-1 have independent lymphomacidal effects, the data also show that the pattern of response to these 2 agents is different. RIT generates prompt tumor shrinkage followed by regrowth of NHL xenografts, whereas the antitumor response to HB22.7 was slower in onset but was sustained. All groups treated with RIT had tumor volume reductions at day 14, a time when the group treated with HB22.7 alone still had increasing tumor volume similar to the untreated control. However, at day 28, the group treated with HB22.7 alone had begun a pattern of consistent tumor shrinkage, whereas all the groups treated with RIT (with or without HB22.7) had increasing tumor volumes. The data also show that the administration of HB22.7 concurrent with, or 24 hours after, RIT augmented the antitumor response from RIT, as demonstrated by a longer time to tumor progression compared with RIT alone. However, the addition of RIT to HB22.7 did not improve the overall survival or CR rates, which was best with HB22.7 alone. This suggests that these 2 antibodies act by an invocation of different cellular mechanisms that may involve synergistic and antagonistic effects.
The pattern of response to these 2 agents suggests that HB22.7 required time to work but continued to exert antitumor effects over the 84-day study period. Therefore, mice that had rapid and substantial early tumor shrinkage secondary to RIT subsequently also benefited from the later effects of HB22.7. This is similar clinically to induction followed by consolidation therapy for human hematologic malignancies. This is also a useful combination because the late therapeutic effect of HB22.7 does not carry with it additional myelosuppression. Conversely, there was no long-term additional efficacy from the addition of RIT to HB22.7 because all the positive effects of RIT occurred early, within the first 42 days.
That tumor volume reduction and survival rates were better for the HB22.7 alone group than for the CMIT group at the end of the 84-day study suggested that HB22.7 and 90Y-DOTA-peptide-Lym-1 have different mechanisms of antitumor efficacy and that there may be some negative interactions as well. Unlabeled Lym-1 has little, if any, antitumor effect by itself, though the data are limited on its physiologic effects. Studies examining the effects of co–cross-linking CD22 and class 2 have revealed that co–cross-linking of these 2 receptors can attenuate class 2-mediated signals.51 Lym-1 binds a class 2 subtype, HLA-DR10. Based on the difference observed when HB22.7 was added to RIT and the previously established signaling/proapoptotic properties of HB22.7, one could postulate that radiolabeled Lym-1 inhibits an apoptotic pathway that would otherwise have been activated by HB22.7 while still having independent lymphomacidal activity. Further study is required to elucidate the physiologic mechanisms involved in the interaction of anti-CD22 and anti-HLA DR antibodies in lymphoma therapy and would validate the need to understand the physiologic properties of mAb binding for rational design of combination therapies.
Prepublished online as Blood First Edition Paper, January 2, 2003; DOI 10.1182/blood-2002-08-2629.
Supported by grants from the Veterans Administration (821/HEMA-015-49F) and the National Institutes of Health (CA 81776, CA 54464, PO1 CA 47828-12).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joseph M. Tuscano, Department of Internal Medicine, University of California, Davis Medical Center, Sacramento, CA 95817; e-mail:joseph.tuscano@ucdmc.ucdavis.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal