Abstract
Hereditary hemochromatosis is a common iron-loading disorder found in populations of European descent. It has been proposed that mutations causing loss of function of HFE gene result in reduced iron incorporation into immature duodenal crypt cells. These cells then overexpress genes for iron absorption, leading to inappropriate cellular iron balance, a persistent iron deficiency of the duodenal mucosa, and increased iron absorption. The objective was to measure duodenal iron content in Hfe knock-out mice to test whether the mutation causes a persistent decrease in enterocyte iron concentration. In both normal and Hfe knock-out mice, duodenal nonheme iron content was found to correlate with liver iron stores (P < .001, r = 0.643 and 0.551, respectively), and this effect did not depend on dietary iron levels. However, duodenal iron content was reduced in Hfe knock-out mice for any given content of liver iron stores (P < .001).
Introduction
Genetic hemochromatosis is a common hereditary defect in human beings that can lead to massive tissue iron loading with associated pathology1 due to inappropriate intestinal iron absorption.2 Body iron levels in mammals are normally tightly controlled by regulation of intestinal iron absorption. In populations of Northern European descent, 80% of genetic hemochromatosis is related to a Cys282Tyr mutation in theHFE gene. In both humans and mice, null mutations and Cys282Tyr mutation cause iron-loading phenotypes,3-6 suggesting that the function of HFE gene product is required for iron homeostasis.
Mice with targeted mutations in Hfe gene are an important tool for understanding such genetic disorders, and several examples of mice with disrupted Hfe expression have been studied.4,5 A similar phenotype also occurs in β2-microglobulin knock-out mice, which fail to express cell surface Hfe protein.7 All these mice develop increased liver iron, considered to be a characteristic of hemochromatosis, although the degree of increase varies between mouse genotypes.5 The best hypothesis to explain the inappropriate iron absorption, seen when HFE function is disrupted, suggests that plasma membrane HFE protein interacts with transferrin receptor and β2-microglobulin to determine iron levels in the duodenal crypt.8,9 HFE is expressed in the crypt,10 but not on the villus, where dietary iron uptake and iron absorption genes are expressed.11-13 A recent study has shown that uptake of plasma radioiron into intestine (presumed to be mediated via transferrin receptor, which is expressed mainly in crypt cells) is indeed reduced in Hfe knock-out (KO) mice.14 If the iron supply from the plasma to the immature crypt cells were a key signal to gear intestinal iron absorption to body iron stores,15 16 such inappropriately low iron uptake should change the set point of this feedback loop and thereby explain its dysregulation in hemochromatosis. To further test this hypothesis, we set out to measure steady-state duodenal and hepatic iron in sex- and age-matched Hfe KO and wild-type controls fed various levels of dietary iron.
Study design
Hfe KO breeders (originally mixed 129/Ola—C57BL/6 background strain4,17; donated by Susan Gilfillan, Department of Immunology, Washington University, St Louis, MO) were mated with C57BL/6 and subsequently genotyped by polymerase chain reaction (PCR).4 Wild-type and Hfe KO homozygote breeders were established to produce age-matched male mice for experimental study. Mice at 7 weeks of age were maintained on either an iron-adequate (180 mg iron per kilogram) or iron-deficient (6 mg iron per kilogram) diet ad libitum for 5 more weeks as reported previously.4 Mice were killed by pentobarbitone overdose, and the duodenum was removed and rinsed in saline. All experiments were carried out under the authority of the United Kingdom Home Office. Tissue nonheme iron content was determined as described previously.18 Correlation was examined by linear regression.
Results and discussion
Deletion of the Hfe gene had no effect on body weight but increased body iron stores as measured by liver nonheme iron (Table1, P < .001). Iron absorption remains sufficiently well regulated in Hfe KO mice with this genetic background, so the high iron overload characteristic of human hemochromatosis is not seen in them.17 This combined with the large variation seen in these animals (M.-P. Roth, personal communication, September 2002) is useful because it means that correlations can be informative with some overlap between the KO and wild-type genotype.
Body weights and tissue nonheme iron content in mice
| Group . | Body weight, g . | Liver nonheme iron, nmol/mg tissue . | Duodenum nonheme iron, nmol/mg tissue . |
|---|---|---|---|
| Wt iron-deficient | 27.5 ± 1.2 | 0.40 ± 0.04 | 0.29 ± 0.06 |
| Wt iron-adequate | 26.2 ± 0.3 | 1.11 ± 0.12 | 0.49 ± 0.08 |
| Hfe iron-deficient | 27.9 ± 0.7 | 2.29 ± 0.42 | 0.22 ± 0.04 |
| Hfe iron-adequate | 26.9 ± 0.3 | 6.19 ± 1.00 | 0.38 ± 0.04 |
| Group . | Body weight, g . | Liver nonheme iron, nmol/mg tissue . | Duodenum nonheme iron, nmol/mg tissue . |
|---|---|---|---|
| Wt iron-deficient | 27.5 ± 1.2 | 0.40 ± 0.04 | 0.29 ± 0.06 |
| Wt iron-adequate | 26.2 ± 0.3 | 1.11 ± 0.12 | 0.49 ± 0.08 |
| Hfe iron-deficient | 27.9 ± 0.7 | 2.29 ± 0.42 | 0.22 ± 0.04 |
| Hfe iron-adequate | 26.9 ± 0.3 | 6.19 ± 1.00 | 0.38 ± 0.04 |
Data are means ± SEM for 12 to 26 mice.
Wt indicates wild-type; Hfe, Hfe knock-out.
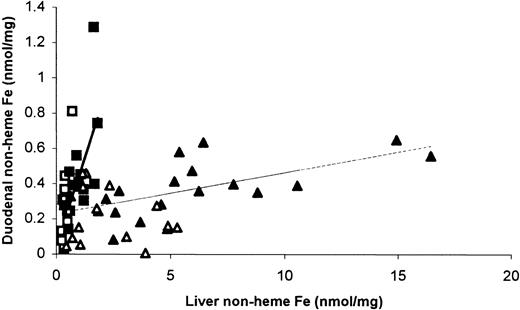
Duodenal iron was found to be correlated with liver iron in both wild-type and Hfe KO mice (wild-type,r = 0.587, P < .05, n = 12; HfeKO, r = 0.643, P < .01, n = 15) fed an iron-adequate diet. However, the slope of the linear regression line differed significantly for the 2 genotypes (P < .05). To extend the range of iron stores studied and to investigate the impact of dietary iron, mice were fed an iron-deficient diet in place of an iron-adequate diet for 5 weeks, and the relationship between duodenal and liver nonheme iron was determined. Both genotypes showed no significant difference in the slope of the regression lines on an iron-deficient—compared with an iron-adequate—diet, demonstrating a steady state in body iron distribution in both cases and that this effect was not due to dietary iron level. It must therefore represent a property of body iron metabolism, and data from both diets for each genotype were combined (Figure 1). Mice of both genotypes fed with the iron-deficient diet supplemented with 25 g/kg carbonyl iron had greatly elevated liver and duodenal nonheme iron values (not shown) in line with previous findings.4
Duodenal and liver iron content in mice fed an iron-adequate or iron-deficient diet.
Data points are wild-type (▪, ■) and Hfe KO (▴, ▵) mice fed iron-adequate (▪, ▴) or iron-deficient (■, ▵) diets. The lines are linear regression fits to data for both diets combined with the following slopes: wild-type (bold line), 0.36 ± 0.09,P < .001, r = 0.643, n = 24;Hfe knock-out (faint line), 0.025 ± 0.007,P < .001, r = 0.551, n = 33. Comparison of regression slopes: P < .001.
Duodenal and liver iron content in mice fed an iron-adequate or iron-deficient diet.
Data points are wild-type (▪, ■) and Hfe KO (▴, ▵) mice fed iron-adequate (▪, ▴) or iron-deficient (■, ▵) diets. The lines are linear regression fits to data for both diets combined with the following slopes: wild-type (bold line), 0.36 ± 0.09,P < .001, r = 0.643, n = 24;Hfe knock-out (faint line), 0.025 ± 0.007,P < .001, r = 0.551, n = 33. Comparison of regression slopes: P < .001.
It has been known for some years that duodenal iron changes when body iron stores are manipulated (reviewed by Turnbull19). There has been debate about the cellular location of this iron, but work in mice showed that changes in villus enterocyte iron reflected changes in the whole mucosa.20 A relationship between iron stores and duodenal iron is in line with the hypothesis for iron absorption regulation proposed by Conrad and Crosby.15 It is reported that hemochromatosis patients have duodenal iron content in the normal range, despite their increased iron stores,21and that their enterocytes are functionally iron deficient.22
The present study found that wild-type mice fed an iron-adequate diet exhibited a correlation between hepatic and duodenal iron. The 5-week diet feeding regime ensured that a steady state in liver and duodenal iron was reached.16 The measurements did not require perturbation of iron metabolism prior to killing the mice. A correlation was also found in Hfe KO mice; however, the slope of the line differed between the 2 genotypes. This has several implications; first, mice must have a mechanism that relates iron stores to duodenal iron, and Hfe KO mice retain such a mechanism. Second, Hfe gene product acts to modulate this relationship and is presumably necessary to ensure the correct “set point”23 for duodenal iron. Third, villus enterocytes must retain a “memory” of the iron levels experienced when they developed, and in mice lacking Hfe protein duodenal iron is reduced compared with wild-type mice with the same iron stores. Recently, hepcidin24 has been implicated as a potential signaling mechanism linking liver iron stores to duodenal iron absorption, presumably operating in parallel with the crypt cells' own ability to sense plasma iron.16 The present data suggest that such mechanisms still function in Hfe KO mice, although inappropriately.
The question of how plasma iron changes are “remembered” by the enterocytes as they develop remains a central issue in the regulation of iron absorption.1,8,9,23 Interestingly, a recent study by Oates et al25 provided evidence that small changes in crypt cell iron are amplified as the enterocytes develop and move along the villus, the function of divalent metal transporter 1 (DMT1) being necessary for this process by supplying iron from the intestinal lumen. Schümann et al16 showed that altered iron regulatory protein (IRP) activity could form part of this memory. The work of Trinder et al14 points toward reductions in iron uptake by the crypt being caused by loss of Hfe function. These changes presumably lead to a setting of the balance between uptake of luminal iron and its release into the plasma, which then persistently determines enterocyte iron.
Prepublished online as Blood First Edition Paper, December 5, 2002; DOI 10.1182/ blood-2002-10-3112.
Supported by the Wellcome Trust, Sir Jules Thorn Charitable Trust, and United Kingdom Medical Research Council (UK MRC). S.B.'s laboratory is supported by Ministère de la Recherche and INSERM of France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
R. J. Simpson, Department of Life Sciences, King's College London, Franklin Wilkins Building, Stamford St, London SE1 9NN, England; e-mail:robert.simpson@kcl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal