Abstract
Essential thrombocythemia (ET) and polycythemia vera (PV) are clonal myeloproliferative disorders that are often difficult to distinguish from other causes of elevated blood cell counts. Assays that could reliably detect clonal hematopoiesis would therefore be extremely valuable for diagnosis. We previously reported 3 X-chromosome transcription-based clonality assays (TCAs) involving the G6PD, IDS, and MPP1 genes, which together were informative in about 65% of female subjects. To increase our ability to detect clonality, we developed simple TCA for detecting the transcripts of 2 additional X-chromosome genes: Bruton tyrosine kinase (BTK) and 4-and-a-half LIM domain 1 (FHL1). The combination of TCA established the presence or absence of clonal hematopoiesis in about 90% of female subjects. We show that both genes are subject to X-chromosome inactivation and are polymorphic in all major US ethnic groups. The 5 TCAs were used to examine clonality in 46 female patients along with assays for erythropoietin-independent erythroid colonies (EECs) and granulocyte PRV-1 mRNA levels to discriminate polycythemias and thrombocytoses. Of these, all 19 patients with familial polycythemia or thrombocytosis had polyclonal hematopoiesis, whereas 22 of 26 patients with clinical evidence of myeloproliferative disorder and 1 patient with clinically obscure polycythemia were clonal. Interestingly, interferon α therapy in 2 patients with PV was associated with reversion of clonal to polyclonal hematopoiesis. EECs were observed in 14 of 14 patients with PV and 4 of 12 with ET, and increased granulocyte PRV-1 mRNA levels were found in 9 of 13 patients with PV and 2 of 12 with ET. Thus, these novel clonality assays are useful in the diagnosis and follow-up of polycythemic conditions and disorders with increased platelet levels.
Introduction
The concept of clonality in hematology contributed greatly to our understanding of hematopoiesis and its hierarchy.1,2 The myeloproliferative disorders (MPDs), chronic myelogenous leukemia, polycythemia vera (PV), essential thrombocythemia (ET), and agnogenic myeloid metaplasia, are characterized by clonal hematopoiesis of the myeloid cells.1 3-5 Thus, the demonstration of clonal hematopoiesis has a role in their differential diagnosis and evaluation of their response to therapy.
The Lyon-Beutler hypothesis of random X-chromosome inactivation provided the basis for assessing clonality of hematopoietic cells.6,7 Either the maternal or the paternal X chromosome is randomly inactivated in each cell at an early stage of embryogenesis.6 Therefore, clonal tissue will consist of a population of cells all containing the same active X chromosome, whereas a mixture of paternally and maternally inherited active X chromosomes is present in nonclonal tissue. Methods for determining X-chromosome inactivation have been devised at the protein,8 DNA,9-14 and RNA levels.15-19 The original clonality assay was an analysis of electrophoretic differences among human glucose-6-phosphate dehydrogenase (G6PD) isoenzyme patterns in G6PD heterozygous females of African descent8; however, its application was limited to one particular racial group. Subsequent clonality assays exploited the differences in DNA methylation between the active and inactive X chromosome–encoded genes, such as phosphoglycerate kinase (PGK),9,11 hypoxanthine phosphoribosyl transferase (HPRT),10 the DXS255locus (M27β),12,14 and the human androgen receptor (HUMARA).13 Although these assays increased the rate of heterozygosity (informativeness), methylation differences may be also affected by nongenetic factors and carcinogenesis,20 and may vary from gene to gene,15 and an incomplete DNA digestion may lead to an unreliable clonality result.21 Finally, these assays cannot be used to study nonnucleated cells, such as platelets and reticulocytes. Therefore, we searched for other approaches that could circumvent these shortcomings. The initial assays were based on differentiating the active X chromosome by its transcript (transcriptional clonality assay [TCA]) using reverse transcription–polymerase chain reaction followed by ligase detection reaction (rtPCR-LDR) to identify the transcribed alleles of the exonic polymorphism of G6PD (C/T at coding sequence, cds, no. 1311, dbSNP: 2230037).16,22 The second TCA exploited an exonic polymorphism of membrane palmitoylated protein 1 (MPP1; also known as P55; G/T at cds no. 358, dbSNP: 1126762).15,17 Although these methods were sensitive, reproducible, quantitative, and useful for nonnucleated cells, they were time consuming and laborious and required a large amount of radioactivity and only about 50% of the female subjects were informative for these polymorphisms. To overcome these disadvantages, another method, allele-specific polymerase chain reaction (ASPCR), was used to detect 3 exonic polymorphisms of X-chromosome genesG6PD, MPP1, and another exonic X-chromosome polymorphism studied previously by El-Kassar and colleagues23 iduronate-2-sulfatase (IDS, C/T at cds no. 438, dbSNP: 1141608).18,19,24 Although these methods were less laborious and technically demanding, they were not quantitative and 35% of female subjects were still not informative19; in addition, Southeast Asian individuals are not polymorphic at the IDS locus.19Clearly, increased informativeness and simplification of transcriptional-based clonality assays would enhance their general usefulness.
We report on 2 additional exonic X-chromosome polymorphisms and the use of single-stranded conformation polymorphism (SSCP) analysis for genotyping and TCA of BTK, FHL1, and 3 previously used polymorphic X-chromosome genes. The gene frequencies of BTKand FHL1 polymorphisms in the common US ethnic groups and the clinical applicability of TCA in studies of the patients diagnosed with clinical thrombocythemic or polycythemic disorders are shown. These novel TCAs are compared with other laboratory tests previously used for discrimination of PV and ET, that is, in vitro formation of erythropoietin-independent erythroid colonies (EECs)25,26and an increased expression of PRV-1 mRNA in granulocytes.27-29 The results of these tests are now reported to demonstrate their relative value and clinical usefulness for differential diagnosis of polycythemias and thrombocytoses.
Patients, materials, and methods
Patients
All patients with a clinical diagnosis of PV and ET fulfilled the clinical diagnostic criteria of PV as defined in a widely used hematology text.30 Most of the studied subjects were seen and examined by one of the authors (J.T.P.). Nineteen other patients were referred to us by other hematologists; 9 of 19 were subsequently seen and examined by one of us (J.T.P.). These patients were referred for the establishment or confirmation of diagnosis or additional diagnostic and research assays not routinely available. Acid-citrate-dextrose (ACD)–anticoagulated peripheral blood samples were obtained from the individuals with proven or suspected MPD. The study was approved by the Baylor College of Medicine (BCM) institutional review board; all studied subjects participated in the studies after giving informed consent. DNA samples from unrelated healthy women of 4 major US ethnic groups (white, Hispanic, African American, and Southeast Asian) were kindly provided by the BCM Polymorphism Resource Core.
Cell fractionation
Platelets and granulocytes were separated by differential centrifugation and isopyknic density gradient separation using standard protocols.2 T lymphocytes were isolated from 5 mL whole blood using RosetteSep antibody cocktail (Stem Cell Technologies, Vancouver, BC, Canada) following the manufacturer's protocol.
Preparation of DNA and RNA and PCR for genotype determination
gDNA was extracted from 200 μL peripheral blood by using QIAamp DNA blood mini kit (Qiagen, Valencia, CA). RNA was prepared from platelets, granulocytes, and T lymphocytes using TRI reagent (Molecular Research Center, Cincinnati, OH) in combination with RQ1 RNase-free DNase (Promega, Madison, WI) and RNeasy mini kit (Qiagen) according to the manufacturers' protocols to avoid DNA contamination.
PCR was used for genotype determination. We used a rapid PCR amplification program to amplify the polymorphisms of 4 genes (BTK, FHL1, MPP1, and G6PD) at the same time, that is, a touch-down program that consisted of 3 minutes at 94°C, 23 cycles of 30 seconds at 94°C, 40 seconds at 65°C, −0.5°C/cycle, 40 seconds at 72°C, then 25 cycles of 30 seconds at 94°C, 40 seconds at 54°C, and 40 seconds at 72°C, and a final 10-minute extension at 72°C in a PTC-200 Peltier thermal cycler (MJ Research, Waltham, MA). The PCR products were labeled with 32P by 0.1 μL (1 μCi [0.037 MBq]) of [α-32P]dCTP (Amersham Pharmacia Biotech, Piscataway, NJ) in 10 μL PCR mixture. The sequences of the primers and PCR conditions are shown in Table 1. TheIDS genotyping was performed by PCR-restriction enzyme digestion method as previously reported.23
Primers and PCR conditions for determination of genotype and TCA of 5 X-chromosome markers
| Gene . | Primer . | Primer sequence (5′-3′) . | MgCl2, mM . | DMSO, % . | PCR product, bp . |
|---|---|---|---|---|---|
| BTK | D-For | GTGAGACTGCTGAACACATTG | 3 | 5 | 156 |
| D-Rev | GCTAAATGGGCAAGTAGATTC | ||||
| R-For | GTGAGACTGCTGAACACATTG | 1 | 0 | 172 | |
| R-Rev | GCTCAGGATTCTTCATCCATG | ||||
| FHL1 | D-For | AGCCCCCTC AGATGTTCC | 3 | 10 | 149 |
| D-Rev | CTACGTTACAA GGGATTCAACC | ||||
| R-For | AGCCCCCTC AGATGTTCC | 1 | 0 | 149 | |
| R-Rev | CTACGTTACAA GGGATTCAACC | ||||
| MPP1 | D-For | TCTAAAATA ACCTCAGAA AGAG | 3 | 0 | 130 |
| D-Rev | GGATCATGCCACCA TGAAGA | ||||
| R-For | GACAGGAGGTGCGGAAAGTG | 1 | 0 | 129 | |
| R-Rev | GGATCATGCCACCATGAAGA | ||||
| G6PD | D-For | CAGTGGCATCAGCAAGACAC | 1.5 | 5 | 151 |
| D-Rev | CATAGCCCA CAGGTATGCAGG | ||||
| R-For | CTGGACCTGACCTACGGCA | 1 | 0 | 147 | |
| R-Rev | CAGTGGGGTGAAAATACGC | ||||
| IDS | R-For | GGAGAATGGCTATGTGACC | 1 | 0 | 159 |
| R-Rev | TCTCCATCTGGCCCTCGAC |
| Gene . | Primer . | Primer sequence (5′-3′) . | MgCl2, mM . | DMSO, % . | PCR product, bp . |
|---|---|---|---|---|---|
| BTK | D-For | GTGAGACTGCTGAACACATTG | 3 | 5 | 156 |
| D-Rev | GCTAAATGGGCAAGTAGATTC | ||||
| R-For | GTGAGACTGCTGAACACATTG | 1 | 0 | 172 | |
| R-Rev | GCTCAGGATTCTTCATCCATG | ||||
| FHL1 | D-For | AGCCCCCTC AGATGTTCC | 3 | 10 | 149 |
| D-Rev | CTACGTTACAA GGGATTCAACC | ||||
| R-For | AGCCCCCTC AGATGTTCC | 1 | 0 | 149 | |
| R-Rev | CTACGTTACAA GGGATTCAACC | ||||
| MPP1 | D-For | TCTAAAATA ACCTCAGAA AGAG | 3 | 0 | 130 |
| D-Rev | GGATCATGCCACCA TGAAGA | ||||
| R-For | GACAGGAGGTGCGGAAAGTG | 1 | 0 | 129 | |
| R-Rev | GGATCATGCCACCATGAAGA | ||||
| G6PD | D-For | CAGTGGCATCAGCAAGACAC | 1.5 | 5 | 151 |
| D-Rev | CATAGCCCA CAGGTATGCAGG | ||||
| R-For | CTGGACCTGACCTACGGCA | 1 | 0 | 147 | |
| R-Rev | CAGTGGGGTGAAAATACGC | ||||
| IDS | R-For | GGAGAATGGCTATGTGACC | 1 | 0 | 159 |
| R-Rev | TCTCCATCTGGCCCTCGAC |
PCR primers for genotyping (D-For, D-Rev); RT-PCR primers for TCA (R-For, R-Rev).
One-step RT-PCR
One-step reverse transcription–PCR (RT-PCR) was performed for the TCA. It was carried out with Access RT-PCR System (Promega) in one reaction following the manufacturer's protocol. The reactions were subjected to a PCR condition consisting of 45 minutes at 47°C, 2 minutes at 92°C, then 35 cycles of 30 seconds at 94°C, 30 seconds at 62°C, and 45 seconds at 68°C, and a final 5-minute extension at 68°C according to the manufacturer's recommendations. The products of one-step RT-PCR were labeled with 32P in the same manner as for genotype determination. The sequences of the primers for 5 polymorphic markers (BTK, FHL1, MPP1, IDS, andG6PD) are depicted in Table 1.
SSCP analysis
SSCP analysis was used for both genotype determination and TCA.32P-labeled products of PCR or one-step RT-PCR were used directly for SSCP analysis. Two microliters of 32P-labeled product mixed with 2 μL sequencing loading dye were heated for 3 minutes at 95°C to denature and then snap-cooled on ice. The samples were then loaded and run on a nondenaturing sequencing 0.5 × mutation detection enhancement (MDE) polyacrylamide gel (Biowhittaker Molecular Applications, Rockland, ME) with constant power (6 W) at room temperature for 16 hours. After electrophoresis, the gel was dried for 1 hour at 80°C and exposed to an x-ray film at −80°C for autoradiography.31
Sequencing analysis
To confirm the different genotypes of BTK andFHL1 in SSCP analysis, unlabeled PCR products were purified with QIAquick gel extraction kit (Qiagen). Five microliters of the eluate was used as the template for cycle sequencing with a DNA sequencing kit (BigDye Terminator Cycle Sequencing Ready Reaction, Applied Biosystems, Foster City, CA) in the PTC-200 Peltier thermal cycler. Sequences for both the coding and the complementary strands were analyzed on the ABI Prism 377 DNA Sequencer (Applied Biosystems) according to the manufacturer's protocol.
In vitro assay of erythroid responsiveness to Epo
In vitro assay of erythroid responsiveness to erythropoietin (Epo) was performed as previously described.32 Briefly, mononuclear cells from peripheral blood were isolated on Histopaque 1077 (Sigma, St Louis, MO) density gradient and cultured at a final concentration of 3 × 105 cells/mL in Methocult H-4531 medium (Stem Cell Technologies) in 35-mm Petri dishes in the presence of 0, 30, 60, 125, 250, and 3000 mIU/mL Epo. Cultures were maintained in a humidified atmosphere of 5% carbon dioxide at 37°C. Erythroid colonies were scored at 14 days by standard criteria.32
Quantification of PRV1 mRNA expression in granulocytes
Real-time RT-PCR was used to quantify PRV-1 mRNA in total RNA isolated from peripheral blood granulocytes using TaqMan one-step RT-PCR master mix reagents kit and ABI Prism 7000 sequence detection system (Applied Biosystems). The primers and TaqMan MGB probes designed by using Primer Express software (Applied Biosystems) were: PRV-1-1158F 5′-CAACCTTCCAGCTTCTTGTTGA-3′, PRV-1-1219R 5′-TTCTCACGCGCAGAGAAGATC-3′, and probe: PRV-1-1182T 5′-CACACCAGACAAATC-3′ labeled with the FAM fluorophore. The PRV-1 amplicon was based on the assay designed by Dr Pahl (University Hospital Freiburg, Germany; personal information, January 17, 2002). To normalize the PRV-1 expression level GAPDH mRNA and 18S ribosomal RNA were used as reference standards (the primers were GAPDH-284F 5′-ATGGAAATCCCATCACCATCTT-3′, GAPDH-340R 5′-CGCCCCACTTGATTTTGG-3′, h18S-542F 5′-TCGAGGCCCTGTAATTGGAA-3′, h18S-602R 5′-CCCTCCAATGGATCCTCGTT-3′; the probes were GAPDH-307T 5′-CAGGAGCGAGATCC-3′ labeled with the VIC fluorophore and h18S-564T 5′-AGTCCACTTTAAATCCTT-3′ labeled with the FAM fluorophore). The primers were used at 900 nM and the probes at 100 nM concentrations. We analyzed the expression of each gene (PRV-1, GAPDH, and 18S) in separate reactions. We used the RNA amount giving the linear range of response for PRV-1, GAPDH, and 18S, typically 10 ng RNA/reaction for PRV-1 and GAPDH, and 0.1 ng for 18S. We used a universal RT-PCR protocol recommended by the manufacturer, that is, reverse transcription at 48°C for 30 minutes, denaturation and polymerase activation at 95°C for 10 minutes, followed by 45 cycles of denaturation at 92°C for 15 seconds and annealing/extension/plate reading at 60°C for 1 minute. RT-PCRs for each sample and each gene were done in triplicate. PCR without reverse transcriptase was performed for each sample to control for the possible interference from gDNA contamination. The threshold amplification cycles (CT) at the normalized reporter signal minus the baseline signal level of 0.2 for PRV-1, GAPDH, and 18S rRNA were determined and their differences ΔCT(GAPDH-PRV1) and ΔCT(18S-PRV1) were calculated. The cutoff for normal PRV-1 mRNA level was established as the 95% upper confidence limit (mean + 1.96 SDs) of ΔCT(GAPDH-PRV1) and ΔCT(18S-PRV1) analyzed in neutrophil RNA samples from 33 healthy volunteer donors. The PRV-1/GAPDH ratio was calculated as 2ΔCT(GAPDH-PRV1).
Results
BTK and FHL1 exonic genotypes and TCA
To improve the usefulness of TCA, we searched for new X-chromosome exonic genomic polymorphisms based on the following criteria: (1) exonic polymorphisms of sufficient frequency that make them suitable for transcriptional clonality assays; (2) their location on the X chromosome is at a significant distance from the 3 polymorphic genes previously used for TCA (G6PD, IDS, and MPP1located on chromosome Xq28) to be in linkage equilibrium; (3) they are subject to X-chromosome inactivation; and (4) they are expressed in hematopoietic cells.
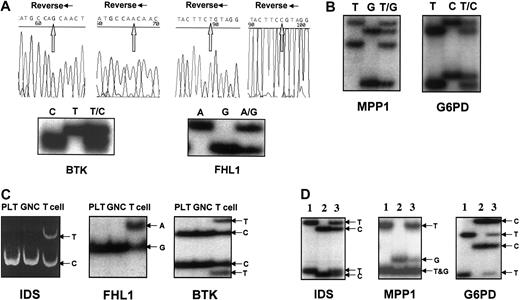
We examined 2 additional exonic X-chromosome polymorphic genes: Bruton tyrosine kinase (BTK: C/T at cds no. 1899, dbSNP: 1135363) and 4-and-a-half LIM domain1 (FHL1: G/A at cDNA no. 1958, 3′-UTR, dbSNP: 9018). BTK is located at Xq21.3-q22,33 and FHL1 at Xq27.2.34 The genotypes for BTK andFHL1 were first determined on 20 female subjects who were randomly chosen. Three different SSCP patterns were obtained on bothBTK and FHL1 genes corresponding to the 3 different genotypes: homozygous T or C, or heterozygous T/C forBTK; homozygous A or G, or heterozygous A/G forFHL1 (Figure 1A). The genotypes of the 6 women (all with a distinct SSCP pattern) were then confirmed by direct sequencing of the PCR products.
Genotyping and expression of 5 X-chromosome exonic polymorphic genes.
(A) Genotype determination and sequence analysis of BTK andFHL1. Sequencing using reverse primers is shown. (B) Genotypes of MPP1 and G6PD. (C) FHL1and BTK are subject to X-chromosome inactivation. Clonal expression of IDS, FHL1, and BTK(shown here patients no. 38 and no. 37) was detected in platelets (PLT) and granulocytes (GNC); the weak upper band of IDSexpression in GNC was 3.35% of the major band, which is clearly outside the normal range,41 whereas T lymphocytes were polyclonal. (D) IDS, MPP1, and G6PDexpression patterns using SSCP analysis (the RNA mixture of PLT and GNC was used). Lanes 1 and 2, expression patterns of homozygosity inIDS, MPP1 and G6PD; lane 3, expression pattern of heterozygosity.
Genotyping and expression of 5 X-chromosome exonic polymorphic genes.
(A) Genotype determination and sequence analysis of BTK andFHL1. Sequencing using reverse primers is shown. (B) Genotypes of MPP1 and G6PD. (C) FHL1and BTK are subject to X-chromosome inactivation. Clonal expression of IDS, FHL1, and BTK(shown here patients no. 38 and no. 37) was detected in platelets (PLT) and granulocytes (GNC); the weak upper band of IDSexpression in GNC was 3.35% of the major band, which is clearly outside the normal range,41 whereas T lymphocytes were polyclonal. (D) IDS, MPP1, and G6PDexpression patterns using SSCP analysis (the RNA mixture of PLT and GNC was used). Lanes 1 and 2, expression patterns of homozygosity inIDS, MPP1 and G6PD; lane 3, expression pattern of heterozygosity.
We optimized the PCR-SSCP method for MPP1 andG6PD genes. DNA from 20 female subjects with known genotypes for MPP1 and G6PD were subjected to the PCR-SSCP analysis. Identical results were obtained with PCR-SSCP (Figure 1B) and PCR-restriction enzyme digestion assays of MPP1 andG6PD genes23 (data not shown).
Subsequently, we developed RT-PCR-SSCP for detecting the allelic transcripts of all 5 polymorphic genes FHL1, BTK,MPP1, IDS, and G6PD (Figure 1C-D).
FHL1 and BTK genes are subjects to X-chromosome inactivation
To demonstrate that FHL1 and BTK genes are X-chromosome inactivated, we tested the RNAs from platelets, granulocytes, and T lymphocytes of 4 subjects with PV or ET who were also heterozygous for both IDS and BTK/FHL1exonic polymorphisms and were previously shown to be clonal using theIDS assay (RT-PCR/restriction enzyme digestion method; Figure 1C). RT-PCR-SSCP analyses of BTK or FHL1genes on the same patients demonstrated that only one allele of theFHL1 gene (Figure 1C) and the BTK gene (Figure1C) were expressed in their platelets and granulocytes, whereas both alleles were expressed in T lymphocytes. This confirmed thatBTK and FHL1 genes are subject to X-chromosome inactivation.
Polymorphic frequency of BTK and FHL1 genes in different US ethnic groups
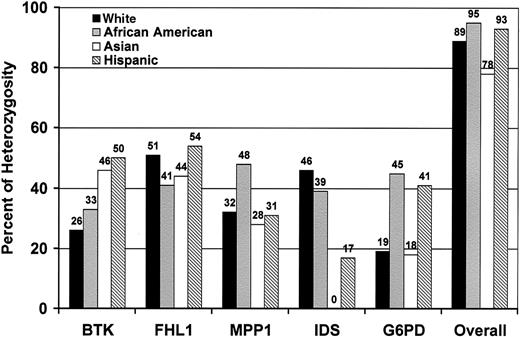
The applicability of X-chromosome polymorphisms for TCA depends on their informativeness. Therefore, we studied DNA from 174 unrelated healthy women. We found that BTK andFHL1 genes are highly polymorphic among all of the common US ethnic groups (white, Hispanic, African American, and Southeast Asian). The frequency of heterozygosity as depicted in Figure2 was found to be 26% to 50% forBTK and 41% to 54% for FHL1. These 2 new polymorphisms in combination with previous markers—MPP1,IDS, and G6PD—rendered about 90% of the female subjects informative for clonality studies.
Distribution of heterozygosity for X-chromosome exonic polymorphisms in common US ethnic groups.
White (n = 47), African American (n = 42), Asian (n = 40), Hispanic (n = 45). Overall: percentage of females heterozygous for at least one marker.
Distribution of heterozygosity for X-chromosome exonic polymorphisms in common US ethnic groups.
White (n = 47), African American (n = 42), Asian (n = 40), Hispanic (n = 45). Overall: percentage of females heterozygous for at least one marker.
Sensitivity, linearity, and reproducibility of the BTKand FHL1 RT-PCR-SSCP assays
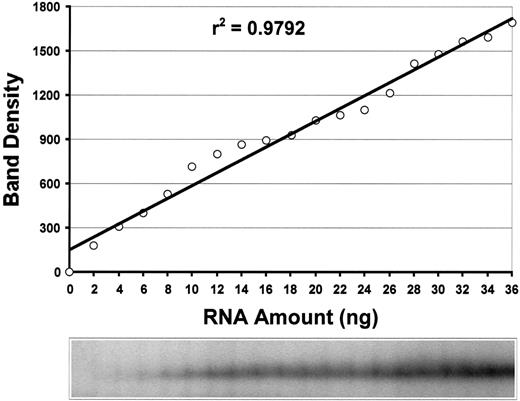
The sensitivity and linearity of the methods were tested by using serial dilutions of RNA from a healthy homozygous female subject. As shown in Figure 3, the assay was linear for the FHL1 RT-PCR-SSCP assayed in the range of 2 to 36 ng total RNA/sample (r2 = 0.98). Similar data were generated for BTK assay (data not shown). The results were highly reproducible with intertest variation of 4%.
Sensitivity and linearity of
FHL1 TCA. The RT-PCR-SSCP analysis ofFHL1 showed a linear response in the range of 2 to 36 ng total RNA/reaction (r2 = 0.98). As little as 2 ng total RNA can be detected.
Sensitivity and linearity of
FHL1 TCA. The RT-PCR-SSCP analysis ofFHL1 showed a linear response in the range of 2 to 36 ng total RNA/reaction (r2 = 0.98). As little as 2 ng total RNA can be detected.
Studies of women with polycythemic and thrombocythemic disorders
We used 5 exonic X-chromosome polymorphic markers (BTK, FHL1, MPP1, IDS, and G6PD) to analyze the clonality of hematopoiesis on 46 informative patients. The T-lymphocyte fraction was used as a control nonclonal tissue for the X-chromosome inactivation patterns, because previous studies had shown that T lymphocytes are not involved in MPDs35 36; the data are shown in Table 2.
Clonality analysis, PRV-1 mRNA expression, and the in vitro response of erythroid progenitors to Epo in patients with polycythemias and thrombocytosis
| Patient no. . | Clonality . | PRV-1/GAPDH . | BFU-E response to Epo . | Hb . | Plt . | Age, y . | Duration of disease, y . | Therapy at time of analysis . | Therapy prior to analysis . | Diagnosis . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Clonal | ND | EEC | H | H | 62 | 13 | HU, P | HU, P | PV |
| 2 | Clonal | 20.32* | EEC | H | H | 58 | 5 | HU, P | A, P | PV |
| 3 | Clonal | 16.62* | EEC | H | H | 71 | 6 | P | P | PV |
| 4 | Clonal | 14.88* | EEC | H | H | 56 | 10 | HU | HU | PV |
| 5 | Clonal | 23.10* | EEC | H | H | 64 | 1 | A, P | — | PV |
| 6 | Clonal | 15.08* | EEC | H | H | 74 | 1 | No | No | PV |
| 7 | Clonal | 6.04* | EEC | H | H | 54 | 5 | A | HU, B | PV |
| 8 | Clonal | 4.71* | EEC | H | H | 47 | 8 | P | P | PV |
| 9 | Clonal | 5.52* | EEC | H | H | 55 | 9 | HU, P | HU, P | PV |
| 10 | Clonal | 4.17* | EEC | H | H | 62 | 8 | P | P | PV |
| 11 | Clonal | 1.85 | EEC | H | H | 62 | 5 | HU | HU | PV |
| 12 | Clonal | 1.18 | EEC | H | H | 36 | 4 | No | No | PV |
| 13 | Clonal | 1.55 | EEC | H | H | 56 | 22 | P | P | PV |
| 14 | Clonal | 0.76 | EEC | H | H | 51 | 5 | A, P | HU, P | PV |
| 15 | Clonal | 0.03 | Normal | H | H | 47 | 15 | No | HU, P | UP |
| 16 | Poly | 4.59* | HS | H | N | 43 | 1 | HU | No | UP |
| 17 | Poly | 0.24 | HS | H | N | 8 | 5 | P | P | UP |
| 18 | Poly | ND | HS | H | N | 24 | 24 | P | P | CFP |
| 19 | Poly | 4.00* | HS | H | N | 5 | 5 | No | P | CFP |
| 20 | Poly | 0.86 | HS | H | N | 23 | 2 | P | No | CFP |
| 21 | Poly | 1.25 | HS | H | N | 53 | 53 | P | P | CFP |
| 22 | Poly | 0.48 | HS | H | N | 71 | >5 | No | No | CFP |
| 23 | Poly | 0.44 | HS | H | N | 22 | 22 | P | P | CFP |
| 24 | Poly | 0.44 | HS | H | N | 54 | 22 | No | No | CFP |
| 25 | Poly | 0.17 | HS | H | N | 9 | 9 | No | No | CFP |
| 26 | Poly | 0.22 | HS | H | N | 17 | 12 | P | P | CFP |
| 27 | Poly | 0.02 | HS | H | N | 38 | >2 | No | — | CFP |
| 28 | Poly | 0.11 | HS | H | N | 50 | — | No | No | CFP |
| 29 | Poly | 0.07 | HS | H | N | 7 | 7 | P | No | CFP |
| 30 | Poly | 0.8 | Normal | H | N | 59 | — | No | No | CFP? UP |
| 31 | Poly | 0.03 | Normal | H | N | 6 | 6 | P | P | CFP |
| 32 | Poly | 0.19 | Normal | H | N | 39 | >30 | P | HU, P | CFP |
| 33 | Clonal | 8.51* | EEC | N | H | 63 | 4 | HU, A | HU | ET |
| 34 | Clonal | 1.65 | EEC | N | H | 23 | 6 | No | No | ET |
| 35 | Clonal | 1.40 | EEC | N | H | 42 | 4 | A | A | ET |
| 36 | Clonal | 0.21 | EEC | N | H | 60 | 17 | HU | HU | ET |
| 37 | Clonal | 35.38* | Normal | N | H | 42 | 11 | No | No | ET |
| 38 | Clonal | 1.29 | Normal | N | H | 55 | 2 | A | HU, A | ET |
| 39 | Clonal | 0.44 | Normal | N | H | 31 | 15 | A | HU | ET |
| 40 | Clonal | 0.02 | Normal | N | H | 51 | 11 | No | No | ET |
| 41 | Poly | 0.06 | HS | N | H | 47 | 5 | HU | HU | ET |
| 42 | Poly | 0.80 | HS | N | H | 46 | 14 | HU | HU | ET |
| 43 | Poly | 0.07 | Normal | N | H | 41 | 2 | A | No | ET |
| 44 | Poly | 1.8 | Normal | N | H | — | 3 | A | HU | ET |
| 45 | Poly | 0.07 | HS | N | H | 7 | 1 | No | No | FT |
| 46 | Poly | ND | HS | N | H | — | — | IFN | HU | FT |
| Patient no. . | Clonality . | PRV-1/GAPDH . | BFU-E response to Epo . | Hb . | Plt . | Age, y . | Duration of disease, y . | Therapy at time of analysis . | Therapy prior to analysis . | Diagnosis . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Clonal | ND | EEC | H | H | 62 | 13 | HU, P | HU, P | PV |
| 2 | Clonal | 20.32* | EEC | H | H | 58 | 5 | HU, P | A, P | PV |
| 3 | Clonal | 16.62* | EEC | H | H | 71 | 6 | P | P | PV |
| 4 | Clonal | 14.88* | EEC | H | H | 56 | 10 | HU | HU | PV |
| 5 | Clonal | 23.10* | EEC | H | H | 64 | 1 | A, P | — | PV |
| 6 | Clonal | 15.08* | EEC | H | H | 74 | 1 | No | No | PV |
| 7 | Clonal | 6.04* | EEC | H | H | 54 | 5 | A | HU, B | PV |
| 8 | Clonal | 4.71* | EEC | H | H | 47 | 8 | P | P | PV |
| 9 | Clonal | 5.52* | EEC | H | H | 55 | 9 | HU, P | HU, P | PV |
| 10 | Clonal | 4.17* | EEC | H | H | 62 | 8 | P | P | PV |
| 11 | Clonal | 1.85 | EEC | H | H | 62 | 5 | HU | HU | PV |
| 12 | Clonal | 1.18 | EEC | H | H | 36 | 4 | No | No | PV |
| 13 | Clonal | 1.55 | EEC | H | H | 56 | 22 | P | P | PV |
| 14 | Clonal | 0.76 | EEC | H | H | 51 | 5 | A, P | HU, P | PV |
| 15 | Clonal | 0.03 | Normal | H | H | 47 | 15 | No | HU, P | UP |
| 16 | Poly | 4.59* | HS | H | N | 43 | 1 | HU | No | UP |
| 17 | Poly | 0.24 | HS | H | N | 8 | 5 | P | P | UP |
| 18 | Poly | ND | HS | H | N | 24 | 24 | P | P | CFP |
| 19 | Poly | 4.00* | HS | H | N | 5 | 5 | No | P | CFP |
| 20 | Poly | 0.86 | HS | H | N | 23 | 2 | P | No | CFP |
| 21 | Poly | 1.25 | HS | H | N | 53 | 53 | P | P | CFP |
| 22 | Poly | 0.48 | HS | H | N | 71 | >5 | No | No | CFP |
| 23 | Poly | 0.44 | HS | H | N | 22 | 22 | P | P | CFP |
| 24 | Poly | 0.44 | HS | H | N | 54 | 22 | No | No | CFP |
| 25 | Poly | 0.17 | HS | H | N | 9 | 9 | No | No | CFP |
| 26 | Poly | 0.22 | HS | H | N | 17 | 12 | P | P | CFP |
| 27 | Poly | 0.02 | HS | H | N | 38 | >2 | No | — | CFP |
| 28 | Poly | 0.11 | HS | H | N | 50 | — | No | No | CFP |
| 29 | Poly | 0.07 | HS | H | N | 7 | 7 | P | No | CFP |
| 30 | Poly | 0.8 | Normal | H | N | 59 | — | No | No | CFP? UP |
| 31 | Poly | 0.03 | Normal | H | N | 6 | 6 | P | P | CFP |
| 32 | Poly | 0.19 | Normal | H | N | 39 | >30 | P | HU, P | CFP |
| 33 | Clonal | 8.51* | EEC | N | H | 63 | 4 | HU, A | HU | ET |
| 34 | Clonal | 1.65 | EEC | N | H | 23 | 6 | No | No | ET |
| 35 | Clonal | 1.40 | EEC | N | H | 42 | 4 | A | A | ET |
| 36 | Clonal | 0.21 | EEC | N | H | 60 | 17 | HU | HU | ET |
| 37 | Clonal | 35.38* | Normal | N | H | 42 | 11 | No | No | ET |
| 38 | Clonal | 1.29 | Normal | N | H | 55 | 2 | A | HU, A | ET |
| 39 | Clonal | 0.44 | Normal | N | H | 31 | 15 | A | HU | ET |
| 40 | Clonal | 0.02 | Normal | N | H | 51 | 11 | No | No | ET |
| 41 | Poly | 0.06 | HS | N | H | 47 | 5 | HU | HU | ET |
| 42 | Poly | 0.80 | HS | N | H | 46 | 14 | HU | HU | ET |
| 43 | Poly | 0.07 | Normal | N | H | 41 | 2 | A | No | ET |
| 44 | Poly | 1.8 | Normal | N | H | — | 3 | A | HU | ET |
| 45 | Poly | 0.07 | HS | N | H | 7 | 1 | No | No | FT |
| 46 | Poly | ND | HS | N | H | — | — | IFN | HU | FT |
PRV-1/GAPDH is the ratio of PRV-1 to GAPDH mRNA in granulocytes; values marked with an asterisk are increased above the 95% CI based on 33 healthy controls (mean PRV-1/GAPDH and 95% CI for the 33 normal controls: 0.33, 0.023-3.45, respectively). The diagnosis is the presumed clinical diagnosis.
BFU-E indicates erythroid burst-forming units; Hb, hemoglobin; Plt, platelet; ND, not done; EEC, endogenous erythroid colonies growing in the absence of added Epo; H, high value at diagnosis; HU, hydroxurea; P, phlebotomy; PV, polycythemia vera; A, anagrelide; No, no treatment; B, busulfan; UP, unexplained polycythemia; Poly, polyclonal; HS, hypersensitivity of erythroid progenitors manifesting as the growth of erythroid colonies in the presence of 30 and 60 mlU Epo/mL culture; N, normal value at diagnosis; CFP, congenital or familial polycythemia; ET, essential thrombocythemia; IFN, interferon; and FT, familial thrombosis.
PRV-1 mRNA expression in granulocytes
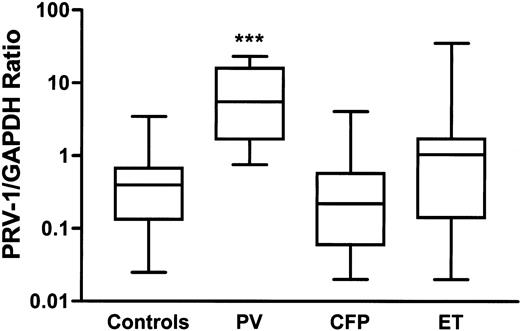
PRV-1 expression in granulocytes was assessed to test its diagnostic value in PV. Linear response between 100 ng and 0.1 ng RNA/20 μL reaction was observed for PRV-1, GAPDH, and 18S rRNA by real-time RT-PCR analysis of RNA isolated from granulocytes of a typical PV patient. The RT-PCR results were highly reproducible; the mean intertest SD calculated from 105 repeated assays was 0.8 amplification cycle (data not shown). GAPDH RNA as a normalizer gave a lower variance in the PRV-1 expression values in 33 control samples; the SD was 2.3 amplification cycles for ΔCT(18S–PRV-1) and 1.7 cycles for ΔCT (GAPDH-PRV1). Therefore, we used the GAPDH normalizer to calculate the PRV-1 expression level in the patients (Figure4).
PRV-1 mRNA expression in granulocytes.
PV indicates polycythemia vera; CFP, congenital or familial polycythemia; ET, essential thrombocythemia. The boxes represent the interquartile range that contains 50% of values. The bars are lines that extend from the box to the highest and lowest values. A line across the box indicates the median. PRV-1/GAPDH expression ratio (calculated as 2ΔCT(GAPDH-PRV1)) was significantly increased in patients with PV (***P < .0001) when compared with Mann-Whitney nonparametric test to 33 healthy controls or CFP patients.
PRV-1 mRNA expression in granulocytes.
PV indicates polycythemia vera; CFP, congenital or familial polycythemia; ET, essential thrombocythemia. The boxes represent the interquartile range that contains 50% of values. The bars are lines that extend from the box to the highest and lowest values. A line across the box indicates the median. PRV-1/GAPDH expression ratio (calculated as 2ΔCT(GAPDH-PRV1)) was significantly increased in patients with PV (***P < .0001) when compared with Mann-Whitney nonparametric test to 33 healthy controls or CFP patients.
Surprisingly, 4 of 13 patients with PV did not have an elevated PRV-1 mRNA level in their granulocytes. Clinical and laboratory characteristics of these patients are shown in Table3.
PV patients with low PRV-1 mRNA level in granulocytes and the diagnostic criteria for PV30
| Patient no. . | Clinical and laboratory criteria for diagnosis of PV3-150 . | ||||||
|---|---|---|---|---|---|---|---|
| Elevated red cell mass . | Normal arterial oxygen saturation . | Splenomegaly . | Thrombocytosis and leukocytosis . | Bone marrow hypercellularity . | Low serum Epo level . | EEC . | |
| 11 | ND | + | + | + | + | + | + |
| 12 | + | + | + | + | + | + | + |
| 13 | + | + | + | + | + | + | + |
| 14 | + | + | 0 | + | + | + | + |
| Patient no. . | Clinical and laboratory criteria for diagnosis of PV3-150 . | ||||||
|---|---|---|---|---|---|---|---|
| Elevated red cell mass . | Normal arterial oxygen saturation . | Splenomegaly . | Thrombocytosis and leukocytosis . | Bone marrow hypercellularity . | Low serum Epo level . | EEC . | |
| 11 | ND | + | + | + | + | + | + |
| 12 | + | + | + | + | + | + | + |
| 13 | + | + | + | + | + | + | + |
| 14 | + | + | 0 | + | + | + | + |
The presence of elevated red cell mass and any 3 additional criteria are diagnostic for PV.
Correlation of the TCA data with the in vitro assay of erythroid progenitor responsiveness to Epo and PRV-1 mRNA expression in granulocytes
The TCA data were correlated with PRV-1 mRNA neutrophil expression and the in vitro assay of erythroid progenitor responsive-ness to Epo. The results are summarized in Table 2. All patients with PV had clonal hematopoiesis and endogenous erythroid colonies (EECs) growing without added Epo. PRV-1 expression in granulocytes was significantly increased in the group of PV patients (P < .0001; Figure 4); however, not all PV patients had increased PRV-1. Normal level of PRV-1 expression in granulocytes was detected in 4 of 13 patients (31%) with PV. Clonal hematopoiesis was detected in 8 of 12 patients with ET, EECs were observed in 4 of 12, and increased PRV-1 expression was seen in 2 of 12 ET patients. All 15 patients with congenital or familial polycythemia had polyclonal hematopoiesis, 12 of 15 had erythroid progenitors hypersensitive to Epo (responding to low concentrations of Epo in vitro), whereas 1 of 15 of these patients had a slightly increased expression of PRV-1. Polyclonal hematopoiesis was found in 2 of 2 patients with familial thrombocytosis, both patients had erythroid progenitors hypersensitive to Epo.
Discussion
We previously reported TCA for the X-chromosome genesG6PD, MPP1, and IDS detected either by RTPCR-LDR or ASPCR.15-19 Although they are valuable, these methods have limitations. They were laborious and time consuming and their informativeness was limited by their lower degree of heterozygosity. To increase the percentage of informative female subjects, we searched for new X-chromosome polymorphic markers.BTK and FHL1 were 2 candidates because they both have exonic polymorphisms, which make them suitable for clonality assays, and they both are at a distant location on the X chromosome compared with the 3 previously used X-chromosome markers—G6PD, IDS, and MPP1, thus these polymorphisms would be expected to be at the linkage equilibrium. Importantly, BTKand FHL1 are expressed in hematopoietic cells.34,37 We chose to circumvent the technical difficulties encountered by the previous tests and after experimenting with various alternate approaches developed SSCP-based analysis for genotype determination and evaluation of their allelic transcripts. SSCP analysis is a simple method,38,39 which has the potential to discriminate single base pair differences between DNA fragments. This method is based on the principle that the electrophoretic mobility of a single-stranded DNA molecule in a nondenaturing gel is dependent not only on its length and molecular weight but also on its structure (ie, conformation). A point mutation or polymorphism at a particular site in the primary sequence can change the conformation of the molecule, which alters its mobility during electrophoresis.40 Moreover, the use of a touch-down program PCR in the genotype determination and the same one-step RT-PCR conditions allowed simultaneous discrimination of the genotypes and determination of their transcribed alleles. We report the accuracy, sensitivity (as little as 2 ng total RNA can be detected), and simplicity of these improved TCA methods; the addition of the assays for detection of BTK and FHL1 exonic polymorphisms greatly increased the proportion of female subjects informative for clonality studies. A possible shortcoming of these new TCA assays might be the limitation of their polymorphism to only some of the US ethnic groups. For example, the IDS exonic polymorphism has been the most informative transcriptionally based clonality assay for white and African women but was entirely uninformative for Southeast Asian women.19 We report here the results of an analysis of 174 unrelated healthy female subjects for BTK no. 1899 (at cds) and FHL1 no. 1958 (at cDNA, 3′-UTR) polymorphisms and show the high frequencies of heterozygosity in all 4 common US ethnic groups (Figure 2), combining with 3 previously established TCAs,G6PD, MPP1, and IDS, the percentage of informative female subjects is increased from about 65% to about 90%.
To be useful for clonality studies, the BTK andFHL1 genes must be subject to X-chromosome inactivation, that is, only one allele of the gene is detected in a clonal cell population; for BTK gene this was already suggested by previous studies of mothers of boys with X-linked agammaglobulinemia.41 42 We confirmed that BTKand FHL1 genes are subject to X-chromosome inactivation. Thus, these 2 exonic polymorphisms of BTK andFHL1 genes are suitable for clonality studies.
The diagnosis of an MPD such as PV and ET can be difficult. Polycythemic disorders include a large variety of disorders, of variable prognostic and therapeutic implications. Whereas PV is characterized by a clonal hematopoiesis, other polycythemic disorders such as familial and congenital polycythemias (PFCPs) or secondary polycythemias are polyclonal.43 We have used the assays for 5 exonic X-chromosome polymorphisms to determine the clonality of easily separated hematopoietic progenies—platelets and granulocytes in 46 informative female subjects who were referred to us for evaluation of polycythemia and thrombocytosis. The demonstration of clonal hematopoiesis supported a diagnosis of MPD in 22 patients with acquired polycythemia or thrombocytosis.
Evaluation of subjects with polycythemic disorders
Of 14 patients with PV, all had EECs and were clonal, and 9 of 13 patients had elevated PRV-1 expression in granulocytes. One subject (no. 15), a heavy smoker, was found polycythemic at hospitalization for gastrointestinal bleeding with a hematocrit value of 53, white blood cell count of 16 000/mm3, platelet counts of 580 000/mm3, and mild hepatosplenomegaly at the age of 32 years, and was diagnosed with PV. Now 15 years later she has normal liver and spleen size and normal blood count without any therapy. She has no EECs and normal PRV-1 expression, but her granulocytes and platelets are clonal. It is speculative whether the clonality data represent an acquired clonal hematopoietic disorder with a transient polycythemic phenotype, “pseudoclonality,”44 or “somatic selection after X inactivation” such as seen in female carriers of some X-linked congenital disorders.45,46Nineteen patients with congenital or familial polycythemic or thrombocythemic disorder had all polyclonal hematopoiesis. Of 17 subjects with congenital or familial polycythemia, 7 had elevated or inappropriately normal Epo level for their hematocrit value, 5 of 7 were analyzed for mutation of von Hippel Lindau (VHL) gene; 2 of 5 had VHL mutations.47 The remaining 8 subjects had low Epo levels and hypersensitive erythroid progenitors to Epo and most had familial history fulfilling the criteria for the diagnosis of PFCP.43 Interestingly, some of these polycythemic subjects with polyclonal hematopoiesis were previously diagnosed as PV while they had transient (presumably reactive) thrombocytosis or mild splenomegaly and other minor laboratory criteria of clinical diagnosis of PV.30
Although our study was not designed to prospectively follow patients, 2 patients with PV and clonal hematopoiesis were re-evaluated after treatment with interferon α (IFN-α), an agent successfully used in some patients with MPD.48 Although clinical remission after IFN-α therapy has been reported (decrease in the size of the spleen, stabilization of hemoglobin with decreased need for phlebotomies), the reversal of a clonal hematopoiesis has not. Two of our studied patients (nos. 6 and 14) paralleled their clinical response with reversion of clonal to polyclonal hematopoiesis.
Evaluation of subjects with thrombocytosis
Fourteen patients were referred for clarification of thrombocytosis; all but 2 with familial thrombocytosis were considered to have ET, and 8 were clonal. The fact that some patients with clinical diagnosis of ET are polyclonal was first reported by El Kassar et al23 and later also by others.49 In our view this fact underlies the difficulties in diagnosing ET and limitations of the clinical phenomenologic criteria as the sole diagnostic means. The development of specific laboratory tests for diagnosis of ET are sorely needed, albeit some are now being tested.50 Four clonal subjects with clinical diagnosis of ET (of 8) had EECs. It should be noted that Shih and Lee51 had studied a number of subjects with thrombocytosis presenting with normal hematocrit and EECs; most of these during several years of follow-up had eventually developed full PV phenotype. We have had a similar experience; at least 4 individuals with a similar clinical course had been followed in our clinic (J.T.P., unpublished observations). Six subjects with diagnosis of ET by their hematologist were polyclonal; of these, 4 had significant thrombotic complications, 2 had familial history of thrombocytosis, and 4 of 6 subjects had hypersensitive erythroid progenitors to Epo. Another patient (no. 42) in our series with a presumed diagnosis of ET had been taking hydroxyurea (HU) for more than 5 years and was found to have polyclonal hematopoiesis. Although there is a possibility that this patient underwent a complete remission on HU treatment, we know of no other patients where a reversal of clonal hematopoiesis occurred after HU therapy. However, this conclusion is speculative because her peripheral blood was not assayed for clonality prior to the HU therapy.
PRV-1 mRNA expression
We confirmed the observations that the increased PRV-1 mRNA expression level in granulocytes is a useful diagnostic marker for PV27-29; however, it was not entirely specific for PV and some of our PV patients were found to have normal levels of PRV-1 expression. We compared 2 different internal standards for quantification of PRV-1 mRNA: GAPDH mRNA used in the PRV-1 original studies28,29 and 18S rRNA. The usefulness of GAPDH mRNA as a normalizer for gene expression studies has been challenged because its expression can be affected by many factors52 including hypoxia,53 a disturbed response to which is a pathophysiologic basis of some polycythemic disorders.43,54 On the other hand, the 100- to 1000-fold variation of the 18S rRNA content has been reported in early hematopoietic progenitors CD34+CD38− cells where GAPDH mRNA levels in these cells provided a better reference.55 We show that both 18S rRNA and the GAPDH mRNA levels can be used as a reliable reference for normalization of PRV-1 expression; the relative advantage of GAPDH mRNA as normalizer is that its level is close to the level of PRV-1 mRNA and the same dilution of RNA can be used for determining the expression of both genes.
We have carefully considered reasons for finding in our studied samples PRV-1 assay to be less specific for PV than reported by Dr Pahl's group.28 It is possible that some of our patients did not represent a typical PV picture, because a significant number of our subjects are the patients who have been referred to us by other hematologists, admittedly because they were considered a diagnostic challenge. Our laboratory has had extensive experience with erythroid progenitor assays for more than 2 decades and the availability of standardized commercial reagents in the last decade made this assay when rigorously performed very reproducible and reliable. In addition, we have had 3 decades of experience with various clonality assays, many developed by our group. Other patients were referred for additional studies including Epo receptor analyses, clonality studies, searching for VHL gene mutations, and so forth. However, as shown in Table 3, the clinical and routine laboratory characteristics of the 4 PV subjects with normal PRV-1 mRNA level fulfilled PV diagnostic criteria.
The comparison of various diagnostic assays reported to be useful for discrimination of polycythemic disorders and thrombocytoses demonstrated that the clonality assays significantly contribute to the clarification of the diagnostic dilemma posed for the practicing hematologist. Our concomitant performance of PRV-1 assay, erythroid progenitor assay, and clonality and PRV-1 analyses for differential diagnoses of difficult to classify polycythemic and thrombocytosis conditions augments the specificity and diagnostic usefulness of these diagnostic tests.
We thank Baylor Human Polymorphism Resource for providing us with samples from unrelated healthy women.
Prepublished online as Blood First Edition Paper, December 19, 2002; DOI 10.1182/blood-2002-07-2287.
Supported by grant P60HL 58418 from the National Institutes of Health National Heart, Lung and Blood Institute, MPD Foundation, and Friends of ET Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Josef T. Prchal, Baylor College of Medicine, One Baylor Plaza, 802E, Houston, TX 77030; e-mail:jprchal@bcm.tmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal