Abstract
Here we demonstrate that treatment with SAHA (suberoylanilide hydroxamic acid), a known inhibitor of histone deacetylases (HDACs), alone induced p21 and/or p27 expressions but decreased the mRNA and protein levels of Bcr-Abl, which was associated with apoptosis of Bcr-Abl–expressing K562 and LAMA-84 cells. Cotreatment with SAHA and imatinib (Gleevec) caused more down-regulation of the levels and auto-tyrosine phosphorylation of Bcr-Abl and apoptosis of these cell types, as compared with treatment with either agent alone (P < .05). This finding was also associated with a greater decline in the levels of phospho-AKT and Bcl-xL. Significantly, treatment with SAHA also down-regulated Bcr-Abl levels and induced apoptosis of CD34+ leukemia blast progenitor cells derived from patients who had developed progressive blast crisis (BC) of chronic myelocytic leukemia (CML) while receiving therapy with imatinib. Taken together, these findings indicate that cotreatment with SAHA enhances the cytotoxic effects of imatinib and may have activity against imatinib-refractory CML-BC.
Introduction
In chronic myelocytic leukemia (CML) cells the activity of the Bcr-Abl tyrosine kinase (TK) in the cytosol activates several molecular mechanisms known to inhibit apoptosis.1-3 These mechanisms include increased expression of the antiapoptotic Bcl-xL protein and increased activity of AKT kinase that confers resistance to apoptosis through several known mechanisms.4-7 Treatment with the Bcr-Abl TK inhibitor imatinib mesylate (Gleevec, formerly known as STI571) was shown to selectively inhibit the growth and induce apoptosis of Bcr-Abl–expressing leukemia cells.8-10Although demonstrating impressive clinical activity against chronic-phase CML, in the accelerated and blastic phases of CML (CML-BC) the outcome after imatinib therapy is unacceptably poor.11 This finding emphasizes the need to identify novel anti–Bcr-Abl therapies for the advanced stages of CML. SAHA (suberoylanilide hydroxamic acid) is a known inhibitor of the histone deacetylases (HDACs).12 HDACs catalyze the deacetylation of the amino terminal lysine residues of the core nucleosomal histones, an activity implicated in chromatin remodeling and the transcriptional regulation of cell-cycle and differentiation regulatory genes, eg, p21WAF1 (p21), in human leukemia and cancer cells.12-14 Would treatment with SAHA exert significant antileukemia effects and potentiate imatinib-induced apoptosis of Bcr-Abl–expressing leukemia cells? This treatment had heretofore not been reported and was the focus of the present studies.
Study design
Reagents
Cells
CML blast-crisis K562 and LAMA-84 cells were cultured, as previously described.10,15 Bone marrow and/or peripheral blood samples from 3 patients with Bcr-Abl–positive CML-BC, who had relapsed while receiving imatinib mesylate (Gleevec), were obtained after informed consent as a protocol study sanctioned by the local institutional review board (IRB). Mononuclear cells were isolated and used to purify CD34+ leukemia blasts, as previously described.15
Western blot analyses
Northern blot analysis
Total cellular RNA was purified, and Northern blot analysis of bcr-abl mRNA was performed, using a previously reported method.17
Autophosphorylation of Bcr-Abl
Apoptosis assessment
Real-time RT-PCR for estimation of bcr-ablmRNA
Total RNA was isolated, reverse transcribed, and amplified using the reaction mixture in the Taqman reverse transcriptase–polymerase chain reaction (RT-PCR) reagent kit and ABI7700 thermocycler from Perkin-Elmer (Boston, MA), as previously described.15Histone H1 primers, reverse primer, and a probe, as well as the p210bcr-abl (b3a2) forward primer, reverse primer, and a probe were synthesized by PE-Applied Biosystems (Branchburg, NJ), as previously described.15
Statistical analyses
Data were expressed as mean ± SEM. Comparisons used Student t test or analysis of variance (ANOVA), as appropriate. A P value less than .05 was assigned significance.
Results and discussion
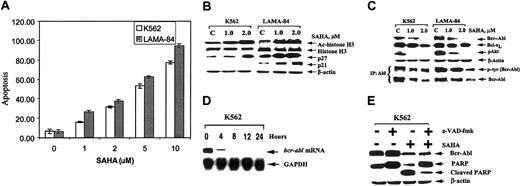
Exposure to 1.0 to 10.0 μM SAHA for 48 hours induced a dose-dependent increase in apoptosis of K562 and LAMA-84 cells (Figure1A). This increase was also confirmed by morphologic assessment of the cells (data not shown). Treatment with 1.0 to 5.0 μM SAHA for 48 hours increased the percentage of K562 cells in the G1 phase of the cell cycle in a dose-dependent manner, which was not seen in LAMA-84 cells (K562, from 38.4% to 62.2% versus LAMA-84, from 57.9% to 60.5%, values represent a mean of 3 experiments). SAHA is known to induce the accumulation of the acetylated histones in the chromatin associated with thep21 gene, as well as induce the expression of p21 mRNA and protein.13 Consistent with this finding, exposure to SAHA increased the acetylation of histone H3 and expression of p21 in LAMA-84 cells (Figure 1B). However, in K562 cells, SAHA did not induce p21 expression, despite causing increased acetylation of histone H3. This finding suggests that SAHA-mediated cell cycle G1arrest or apoptosis of K562 cells is not dependent on the induction of p21. Indeed, a previous report had also documented that p21 induced by SAHA was not required for SAHA-induced apoptosis of human leukemia cells.18 Treatment with SAHA induced p27 expression in both K562 and LAMA-84 cells (Figure 1B). SAHA has been previously shown not to induce acetylation of histones of the chromatin associated withp27 gene or increase its transcription.13Hence, it may be possible that SAHA up-regulates the expression of p27 specifically by modulating the expression and/or TK activity of Bcr-Abl in K562 and LAMA-84 cells, because Gesbert et al19demonstrated that Bcr-Abl down-regulates p27 levels through the activity of phosphatidylinositol 3 kinase (PI3K)/AKT.19 Indeed, treatment with SAHA down-regulated the levels of Bcr-Abl (≤ 10% of control cells) in K562 and LAMA-84 cells (Figure 1C). This down-regulation was associated with the attenuation of auto-tyrosine phosphorylation of Bcr-Abl by approximately 52% in K562 and 35% in LAMA-84 cells, following treatment with 2.0 μM SAHA for 48 hours (Figure 1C). Bcl-xL and p-AKT levels also declined in SAHA-treated cells (Figure 1C). A real-time quantitative RT-PCR estimation of the ratio of the mRNA transcripts of bcr-abl and histone H1genes in K562 cells treated with 2.0 μM SAHA demonstrated an attenuation of this ratio from 2.6 to 0.9 in a time-dependent manner for up to 24 hours. Northern analysis confirmed that exposure intervals as short as 8 hours to 2.0 μM SAHA resulted in a near complete loss of the bcr-abl message in K562 cells (Figure 1D). This loss occurred prior to an appreciable decline in the Bcr-Abl protein levels (data not shown).
SAHA down-regulates Bcr-Abl levels and induces apoptosis of K562 and LAMA-84 cells.
(A) Cells were treated with the indicated concentrations of SAHA for 48 hours. Following this treatment, the percentage of annexin V–stained apoptotic cells was determined by flow cytometry. (B) Western blot analyses of acetylated (Ac) histone 3, histone H3, p21, and p27 in the cell lysates from K562 and LAMA-84 cells, following treatment with the indicated concentrations of SAHA for 48 hours. The levels of β-actin served as the loading control. (C) Western blot analyses of Bcr-Abl, Bcl-xL, and phospho(p)-AKT in the cell lysates from K562 and LAMA-84 cells, following treatment with the indicated concentrations of SAHA for 48 hours. Alternatively, immunoprecipitates with anti-Abl antibody were probed and immunoblotted with anti-phosphotyrosine (p-tyr) antibody. (D) Treatment with SAHA reduces bcr-abl mRNA in K562 cells. Samples of 15 μg total cellular RNA were isolated from K562 cells treated with SAHA (2 μM) for the indicated exposure intervals and analyzed by Northern blotting. A 1000-bp probe from the b3-a2 junction sequence of bcr-abl was used as a probe, which detected a 615-Kb transcript. The same membrane was stripped and rehybridized with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe. (E) Cotreatment with z-VAD-fmk (25 μM) PARP cleavage activity of caspases without affecting the SAHA-mediated decline in Bcr-Abl levels. K562 cells were exposed to SAHA and/or z-VAD-fmk for 48 hours. Following this exposure, immunoblot analyses of Bcr-Abl and PARP, and the cleavage product, were performed on the cell lysates. The levels of β-actin served as the loading control.
SAHA down-regulates Bcr-Abl levels and induces apoptosis of K562 and LAMA-84 cells.
(A) Cells were treated with the indicated concentrations of SAHA for 48 hours. Following this treatment, the percentage of annexin V–stained apoptotic cells was determined by flow cytometry. (B) Western blot analyses of acetylated (Ac) histone 3, histone H3, p21, and p27 in the cell lysates from K562 and LAMA-84 cells, following treatment with the indicated concentrations of SAHA for 48 hours. The levels of β-actin served as the loading control. (C) Western blot analyses of Bcr-Abl, Bcl-xL, and phospho(p)-AKT in the cell lysates from K562 and LAMA-84 cells, following treatment with the indicated concentrations of SAHA for 48 hours. Alternatively, immunoprecipitates with anti-Abl antibody were probed and immunoblotted with anti-phosphotyrosine (p-tyr) antibody. (D) Treatment with SAHA reduces bcr-abl mRNA in K562 cells. Samples of 15 μg total cellular RNA were isolated from K562 cells treated with SAHA (2 μM) for the indicated exposure intervals and analyzed by Northern blotting. A 1000-bp probe from the b3-a2 junction sequence of bcr-abl was used as a probe, which detected a 615-Kb transcript. The same membrane was stripped and rehybridized with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe. (E) Cotreatment with z-VAD-fmk (25 μM) PARP cleavage activity of caspases without affecting the SAHA-mediated decline in Bcr-Abl levels. K562 cells were exposed to SAHA and/or z-VAD-fmk for 48 hours. Following this exposure, immunoblot analyses of Bcr-Abl and PARP, and the cleavage product, were performed on the cell lysates. The levels of β-actin served as the loading control.
Notably, increased acetylation of histones or transcription factors has also been previously demonstrated to repress transcription.14 Also, treatment with HDAC inhibitors is known to inhibit the expression of other tyrosine kinases, such as Her-2-neu.20 21 SAHA-mediated decline in Bcr-Abl protein levels appeared not to be due to the processing of Bcr-Abl during SAHA-induced apoptosis. Cotreatment with the caspase inhibitor z-VAD-fmk, although blocking the poly(ADP-ribose) polymerase (PARP) cleavage activity of caspases, had no effect on SAHA-mediated decline in the Bcr-Abl levels in K562 cells (Figure 1E).
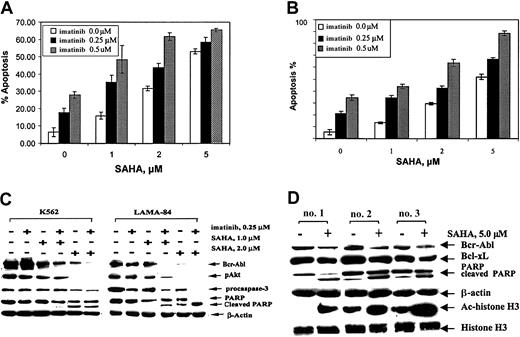
Agents that lower Bcr-Abl levels have been shown to enhance the apoptotic effects of imatinib against CML-BC cells.22Figure 2A-B demonstrates that, as compared with the treatment with either agent alone, combined treatment with SAHA and imatinib for 48 hours induced more apoptosis of K562 and LAMA-84 cells. Treatment with 0.25 μM imatinib plus 1.0 μM SAHA for 48 hours was associated with a greater decline in the levels of Bcr-Abl and p-AKT (Figure 2C). Treatment with this combination also caused down-regulation of Bcl-xL and increased expression of p27 in K562 and LAMA-84 cells (data not shown). Shorter exposure intervals to SAHA and imatinib induced less effect on Bcr-Abl levels and apoptosis of these cells (data not shown).
Cotreatment with SAHA and imatinib causes more decline in Bcr-Abl levels and apoptosis.
K562 (A) and LAMA-84 (B) cells were treated with the indicated concentration of SAHA and/or imatinib for 48 hours. Following this treatment, the percentage of annexin V–stained apoptotic cells was determined by flow cytometry. (C) Western blot analyses of Bcr-Abl, p-AKT, procaspase-3, PARP, and its cleaved product in the cell lysates from K562 and LAMA-84 cells, following treatment with the indicated concentrations of SAHA and/or imatinib for 48 hours. The levels of β-actin served as the loading control. (D) Western blot analyses of Bcr-Abl, Ac-histone H3, histone H3, PARP, and its cleaved product in the cell lysates from CD34+ leukemia progenitor cells, from patients who had developed imatinib-refractory CML-BC, following treatment with the indicated concentrations of SAHA for 24 hours.
Cotreatment with SAHA and imatinib causes more decline in Bcr-Abl levels and apoptosis.
K562 (A) and LAMA-84 (B) cells were treated with the indicated concentration of SAHA and/or imatinib for 48 hours. Following this treatment, the percentage of annexin V–stained apoptotic cells was determined by flow cytometry. (C) Western blot analyses of Bcr-Abl, p-AKT, procaspase-3, PARP, and its cleaved product in the cell lysates from K562 and LAMA-84 cells, following treatment with the indicated concentrations of SAHA and/or imatinib for 48 hours. The levels of β-actin served as the loading control. (D) Western blot analyses of Bcr-Abl, Ac-histone H3, histone H3, PARP, and its cleaved product in the cell lysates from CD34+ leukemia progenitor cells, from patients who had developed imatinib-refractory CML-BC, following treatment with the indicated concentrations of SAHA for 24 hours.
We next determined the effects of SAHA on CD34+leukemia progenitor cells isolated from 3 peripheral blood or bone marrow samples from patients who had developed imatinib-refractory CML-BC. Figure 2D demonstrates that exposure to SAHA increased the acetylated histone H3 levels, as well as produced a decline in the levels of Bcr-Abl and Bcl-xL in each of the samples of the CD34+ leukemia progenitor cells. This decline in Bcr-Abl in sample no. 1 was 43%, sample no. 2 was 54%, and sample no. 3 was 49%, following treatment with 5 μM SAHA for 24 hours (Figure 2D). This finding correlated with the induction of the PARP cleavage activity of caspase-3. SAHA-induced apoptosis ranged between 53.6% and 65.0%, as was also determined by morphologic evaluation of Wright-stained samples of these cells (data not shown). Because of the limited sample size, it was not feasible to determine whether the mechanism of resistance to imatinib in these progenitor cells was gene amplification and overexpression of Bcr-Abl or one of the point mutations in the ATP binding region of Bcr-Abl.23 24 Yet, treatment with SAHA clearly down-regulated Bcr-Abl levels and induced apoptosis of the imatinib-refractory CD34+ leukemia progenitor cells. Collectively, these findings generate the rationale to investigate the clinical efficacy of the combined treatment with SAHA and imatinib against the advanced phases of CML as well as test the antileukemia effects of SAHA against imatinib-refractory CML-BC.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-08-2675.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kapil Bhalla, Moffitt Cancer Center & Research Institute, 12902 Magnolia Dr, MRC 3 East, Room 3056, Tampa, FL 33612; e-mail: bhallakn@moffitt.usf.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal