Abstract
The therapeutic effects of intravenous immunoglobulins (IVIGs) in several autoimmune diseases are characterized by a decrease in pathologic autoantibody levels. Although little direct evidence has been reported in humans, the large repertoire of natural immunoglobulin G (IgG) antibodies in IVIGs is expected to be involved in the regulation of autoreactive B lymphocytes. In normal adult mice, IVIGs have been reported to modulate immature B cells as well as peripheral B lymphocytes through V-region connections. Studies with human serum also indicated that anti-idiotypic antibodies, present in IVIG preparations, could recognize both natural and pathologic autoantibodies. We have used an in vitro culture system to characterize the direct effect of IVIGs on human B lymphocytes. This in vitro culture system involves CD40 activation of B lymphocytes by its ligand CD154 in the presence of cytokines. In this system, addition of IVIGs decreased by 50% to 80% the expansion of B lymphocytes. This reduced expansion was due to a decrease in the proliferation rate. In addition, a portion of B lymphocytes was differentiated into IgG-secreting cells in the presence of IVIGs and the secreted IgGs were reactive with antigens such as nucleoprotamine, dsDNA, tetanus toxin, and human IgG F(ab′)2 fragments. These observations indicate that IVIGs can have direct effects on B lymphocytes and suggest that such IVIG regulation of B lymphocytes could be involved in the therapeutic effects of IVIGs in autoimmune diseases.
Introduction
Intravenous immunoglobulins (IVIGs) are concentrated immunoglobulin G (IgG) solutions prepared from the pooled plasma of 3000 to 15 000 healthy donors and contain antibodies reacting against a large repertoire of self and non-self antigens.1,2 IVIGs have been used in the treatment of many inflammatory and autoimmune diseases (reviewed in Kazatchkine and Kaveri1 and Sacher and Panel2) for more than 20 years. Although the origin of autoimmune diseases remains enigmatic, defects in immunoregulatory mechanisms are assumed to play a central role3-5 and many authors consider that a failure in V-region connectivity is responsible for the emergence of pathologic autoantibodies.6-10 Following treatment with IVIGs, the improvement in the patient's health is often characterized by a diminution of the level and activity of pathologic autoimmune antibodies.11-13 Many mechanisms have been proposed to explain the beneficial effects of IVIGs but their exact mode of action remains unclear in most diseases. Therefore, the clarification of IVIG mechanisms of action in autoimmune diseases would help to validate clinical practices and to define suitable substitutes.
Studies have shown that the Fc region of IVIGs inhibits phagocytosis of opsonized platelets by negative signaling through the FcγRIIB receptor,14 and accelerates clearance of autoimmune antibodies by saturation of the IgG transport receptor (FcRn).15,16 Other studies have demonstrated that the presence of IgG dimers in IVIGs contributes to the immune regulation of autoantibodies by blocking low-affinity Fcγ receptor.17 Sundblad and colleagues also reported that IVIGs could activate peripheral B lymphocytes, increase secretion of IgM and IgG, and modulate the antibody repertoire in mice.7,18,19 They also showed that IVIGs could eliminate and modify emergent B-cell clones in bone marrow through the specific binding of variable regions of surface immunoglobulins.7,19 These observations indicated that autoimmunity could be modulated through connections between anti-idiotypic antibodies in IVIGs and idiotypes of surface immunoglobulins.19 Furthermore, it has been reported that anti-idiotypic antibodies present in IVIG preparations were able to recognize both natural and pathologic autoantibodies in humans.20-25 Anti-idiotypic antibodies are also present in the serum of healthy individuals and participate in the maintenance of homeostasis in tolerance and autoimmunity.26,27 Therefore, anti-idiotypic antibodies present in IVIGs have been proposed to restore homeostasis in patients with autoimmune diseases.1,10,19,28 In fact, many autoimmune diseases are characterized by dominant idiotypes, present on pathologic IgG,29 which are suitable targets for anti-idiotypes present in IVIGs. Finally, sera of patients in remission contained anti-idiotypic antibodies specific for autoantibodies and showed a decreased level of pathologic antibodies.11,12,22,30 31All these observations strongly suggest that in addition to Fc receptors, the idiotypic network is important in IVIGs beneficial effects in autoimmune diseases.
Most of the above studies on IVIGs have been done using mouse models and human serum. The few studies done with normal human cells have reported that IVIGs were inhibitory for immunoglobulin secretion.32-34 In this study, we have investigated the effect of IVIGs on purified human B lymphocytes. We have used an in vitro culture system that reproduces T-dependent activation of B lymphocytes through the binding of CD40 on B lymphocytes.35,36 This system allows the proliferation of human B lymphocytes for up to one month as well as the differentiation into antibody-secreting cells.37 We have observed that the addition of IVIGs reduced expansion and stimulated the differentiation of peripheral B lymphocytes into IgG-secreting cells. Moreover, we have observed that the secreted IgG antibodies were reactive with antigens such as nucleoprotamine, dsDNA, tetanus toxin, and human IgG F(ab′)2 fragments.
Materials and methods
Intravenous immunoglobulin preparations
Intravenous immunoglobulins (IVIGs) Gammar P I-V, containing 50 mg/mL IgG, 30 mg/mL human albumin, and 50 mg/mL sucrose, was obtained from Aventis Behring Canada (Ottawa, ON, Canada). IVIGs Gamimune N, containing 100 mg/mL IgG and 160 mM to 240 mM glycine, was obtained from Bayer (Toronto, ON, Canada). IVIGs Gamimune N preparations were dialyzed against 10 mM potassium/sodium phosphate with 136 mM NaCl (Gibco/BRL, Grand Island, NY) containing 40 mM glycine pH 4.5 (Sigma, Oakville, ON, Canada). IgG content in dialyzed IVIGs (2 lots) was 75 mg/mL and 100 mg/mL as determined by a standard enzyme-linked immunosorbent assay (ELISA). To verify that dialysis had not altered the protein profile of IVIGs, nondialyzed and dialyzed IVIG preparations were compared by capillary electrophesis (Paragon CZE 2000; Beckman, Palo Alto, CA). Dialyzed and nondialyzed IVIGs showed monomeric IgG contents ranging from 93% to 95% of total protein as reported by the manufacturer for the commercial IVIGs. Human serum albumin, sucrose, glycine (all from Sigma), and bovine serum albumin (Gibco/BRL) were added to control cultures of human B lymphocytes at concentrations equivalent to the ones present in the tested IVIGs.
Isolation of peripheral B lymphocytes
Blood samples were collected from healthy individuals after informed consent. Whole blood samples were collected in heparinized tubes (Vacutainer; Becton Dickinson, Franklin Lakes, NJ), pooled, and diluted in one volume phosphate-buffered saline (PBS) (10 mM potassium/sodium phosphate buffer with 136 mM NaCl, pH 7.4, Dulbecco PBS; Gibco/BRL). Peripheral blood mononuclear cells (PBMCs) were prepared by density centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech, Baie D'Urfé, QC, Canada). Red blood cells were removed by lysis with 0.83% (wt/vol) NH4Cl and platelets by a second centrifugation over Ficoll-Paque diluted 1:2 with PBS. B lymphocytes were purified by negative selection using the StemSep CD19 cocktail following the manufacturer's instructions (Stem Cell Technologies, Vancouver, BC, Canada). Purified human B lymphocytes were 90% to 95% CD19+ as determined by flow cytometry analysis.
Culture of human B lymphocytes
Purified B cells were seeded at 3.75 × 105cells/mL in Primaria plates (Becton Dickinson) in the presence of 0.5 × 105 cells/cm2 γ-irradiated L4.5 expressing CD154.36 Cells were cultured in Iscove modified Dulbecco medium (IMDM) supplemented with 10% heat inactivated ultra-low IgG fetal bovine serum (FBS; Gibco/BRL), 5 μg/mL bovine insulin, 5 μg/mL bovine transferrin, antibiotics (Sigma), and 100 U/mL interleukin 4 (IL-4) (R&D Systems, Minneapolis, MN) or 50 ng/mL interleukin 10 (IL-10) (Endogen, Cedarlane Laboratories, Hornby, ON, Canada). Cultures were fed by replacing half of the culture medium every 2 to 3 days, while L4.5 cells were renewed every 4 to 5 days. IVIGs were added at indicated concentrations. Cell counts and viability were evaluated in triplicate by trypan blue exclusion using a hemacytometer. At indicated days, cultured lymphocytes were harvested, washed 5 times using PBS, and cultured without IVIGs for 6 hours or 22 hours to secrete immunoglobulins. For all secretion assays, the IgG concentration in the fifth washing supernatant was found to be below 60 ng/mL.
Flow cytometry analysis
Allophycocyanin (APC)–, fluorescein isothiocyanate (FITC)–, or phycoerythrin (PE)–conjugated anti-CD19, FITC-conjugated anti-CD38, PE-conjugated anti-CD45, PE- or FITC-conjugated anti-IgG, and APC-, PE-, and FITC-conjugated isotypic controls were used in double or triple staining procedures. IgG and Ki-67 intracellular staining was done using PE- or FITC-conjugated anti-IgG and PE-conjugated anti–Ki-67 with cells permeabilized with 0.5% Triton (Sigma). Cells were extensively washed before staining. All antibodies were IgG1 mouse monoclonal antibodies obtained from BD Pharmingen (Oakville, ON, Canada). All stainings were done with 1 μg of each antibody for 1 × 106 cells at 4°C and cells were fixed with 2% paraformaldehyde (Sigma). In all analyses, more than 95% of cells were double-negative with markers set according to isotype-matched negative control staining. The regions containing dead cells and debris were excluded from analysis using 7-actinomycin D (Sigma) staining. All analyses were done by gating 5000 to 10 000 living cells according to 7-amino-actinomycin-D (7AAD) staining following manufacturer instructions (BD Pharmingen). Analyses were done with a FACSCalibur flow cytometer and the CellQuest software (Becton Dickinson).
Apoptosis level
Apoptosis level, based on annexin V and propidium iodine staining, was evaluated with the Vybrant apoptosis assay following the manufacturer instructions (Molecular Probes, Eugene, OR). A negative control was prepared by incubating the cells with 2 mM EGTA (ethylene glycol tetraacetic acid; Sigma). All analyses were done after collecting 10 000 events without gating.
IgG and IgM concentrations
IgG and IgM concentrations in culture and washing supernatants were determined in a standard ELISA using 96-well plates and plastic-adsorbed goat–affinity-purified antibodies specific to human γ and μ chains. The bound antibodies were revealed with peroxidase-conjugated goat anti–human Ig antibodies. All antibodies were from Jackson Laboratories, Mississauga, ON, Canada. O-phenylene diamine-HCL (Abbott Laboratories, North Chigaco, IL) was used as substrate and optical densities were measured at 490 nm.
Determination of the number of Ig-secreting cells
The number of cells secreting IgG or IgM was determined by a standard ELISPOT assay38 using the same antibody sandwich described for ELISA. The cultured cells were washed thoroughly and incubated in serial dilutions (10 000 to 156 cells/well) in the culture medium without IL-4 and IVIGs in 96-well flat-bottom plates for 18 hours at 37°C in CO2 incubator. Spots were revealed by addition of alkaline phosphatase–conjugated goat anti–human IgG or IgM specific for IgG Fc fragment and mu chain respectively (Jackson Laboratories). A solution of 1.5 M BCiP (5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt) prepared in 2-amino-2-methyl-1-propanol (Sigma) was used as substrate. The spots were counted under a microscope at a × 100 magnification.
35S-labeling of secreted IgG
At indicated days, cells were harvested, washed as indicated above, and incubated at 37°C for 18 hours in modified Eagle medium (MEM) without methionine (Gibco/BRL) supplemented with 100 μCi (3.7 MBq) of a mix of 35S-methionine and35S-cysteine (70:30) (Redivue Pro-mix L-[35S]; Amersham Pharmacia Biotech). IgGs in culture supernatants were captured by overnight incubation in microplates coated with goat anti–human IgG Fc-specific antibodies (Jackson). After washing, bound IgGs were eluted by incubation at 37°C for 30 minutes with PBS containing 2% (wt/vol) sodium dodecyl sulfate (SDS) and 1% (wt/vol) 2-mercaptoethanol. 35S-IgG contents were measured by counting the eluted material diluted in 4 mL of Liquid Scintillation (Beckman) in a 1217 RackBeta liquid Scintillation Counter (LKB Wallac, Baie d'Urfé, QC, Canada).
Reactivity against a panel of antigens
Reactivity of IgG in culture supernatants and in IVIGs was evaluated by ELISA using plastic adsorbed with human IgG F(ab′)2 or Fc fragments, nucleoprotamine, dsDNA, histone, actin, insulin, myoglobulin, lipopolysaccharide (LPS) (E coli 055:B5), LPS (E coli 026:B6), tetanus toxin, and keyhole limpet hemocyanin (KLH) at 10 μg/mL. All antigens were from Sigma, except purified human IgG-F(ab′)2 and Fc fragments obtained from Jackson Laboratories and tetanus toxin (IAF-BioVac, Laval, Canada). Adsorption was done in 100 mM carbonate buffer, pH 9.6 for all antigens except dsDNA, which was adsorbed in 100 mM citrate buffer, pH 6.0.39 All bound antibodies were revealed with peroxidase-conjugated goat anti–human IgG Fc-specific antibodies (Jackson Laboratories) except for Fc reactivities, which were revealed with peroxidase goat anti–human anti–kappa/lambda antibodies (Jackson Laboratories). O-phenylene diamine-HCL was used as substrate. A commercial human anti–dsDNA autoimmune serum (The Binding Site, Birmingham, England) was used as positive control. Serial dilutions of culture supernatants and of commercial IVIGs were used to evaluate their reactivity with dsDNA and F(ab′)2fragments.
Results
IVIGs reduce expansion of CD40-activated human B lymphocytes
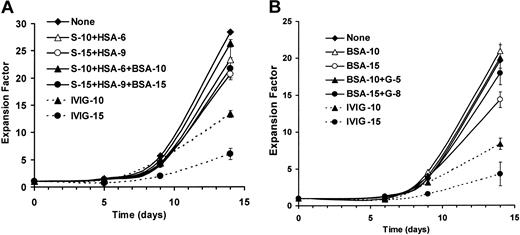
Patients with autoimmune diseases can receive up to 2.0 g/kg of IVIGs,10 corresponding to a serum IVIG level of 5 mg/mL to 25 mg/mL.33 40 We used IVIG concentrations within this range to study its effects on human B lymphocytes cultured in the presence of CD154 and IL-4. IVIGs were added at 10 mg/mL or 15 mg/mL from the beginning of the culture and maintained for 14 days (Figure 1). IVIG preparations contain stabilizers, such as human serum albumin (HSA) and sucrose (S) (Figure1A) or glycine (G) in dialyzed IVIGs (Figure 1B), which were added in controls at concentrations equivalent to each IVIG preparation. In addition, to evaluate the effect of higher protein content resulting from IVIG addition, bovine serum albumin (BSA) was used in controls at 10 mg/mL or 15 mg/mL (Figure 1A-B).
IVIGs reduce B-lymphocyte expansion in CD40-CD154 system.
Purified human B lymphocytes were cultured with irradiated L4.5 cells in complete medium containing IL-4 without (diamond, plain line) or with (dashed lines) 10 mg/mL IVIGs (triangle) or 15 mg/mL IVIGs (circle) for 14 days. Cell expansion was based on seeding cell number and viable cell count evaluated in triplicate at the indicated day by trypan blue exclusion. (A) Culture controls were done with stabilizers sucrose (S) and human serum albumin (HSA) (open symbols, filled line), and stabilizers plus bovine serum albumin (BSA) (filled symbols, plain line) in concentrations corresponding to 10 mg/mL IVIGs (triangle) and 15 mg/mL IVIGs (circle) as indicated. (B) Culture controls were done as described above with 5.3 mM and 8 mM glycine (G) and BSA (filled symbols, filled plain line), and with BSA only (open symbols, plain line) in both cases BSA was in concentrations corresponding to 10 mg/mL dialyzed IVIGs (triangle) and 15 mg/mL dialyzed IVIGs (circle) as indicated. A and B were done with the B lymphocytes from 2 donors. Error bars, showing standard deviation for triplicates, might be smaller than symbols. These results are representative of 3 independent experiments.
IVIGs reduce B-lymphocyte expansion in CD40-CD154 system.
Purified human B lymphocytes were cultured with irradiated L4.5 cells in complete medium containing IL-4 without (diamond, plain line) or with (dashed lines) 10 mg/mL IVIGs (triangle) or 15 mg/mL IVIGs (circle) for 14 days. Cell expansion was based on seeding cell number and viable cell count evaluated in triplicate at the indicated day by trypan blue exclusion. (A) Culture controls were done with stabilizers sucrose (S) and human serum albumin (HSA) (open symbols, filled line), and stabilizers plus bovine serum albumin (BSA) (filled symbols, plain line) in concentrations corresponding to 10 mg/mL IVIGs (triangle) and 15 mg/mL IVIGs (circle) as indicated. (B) Culture controls were done as described above with 5.3 mM and 8 mM glycine (G) and BSA (filled symbols, filled plain line), and with BSA only (open symbols, plain line) in both cases BSA was in concentrations corresponding to 10 mg/mL dialyzed IVIGs (triangle) and 15 mg/mL dialyzed IVIGs (circle) as indicated. A and B were done with the B lymphocytes from 2 donors. Error bars, showing standard deviation for triplicates, might be smaller than symbols. These results are representative of 3 independent experiments.
During the first 9 days, the addition of sucrose and human serum albumin (S+HSA) with or without BSA (Figure 1A), and BSA alone or glycine and BSA (Figure 1B), did not affect the cellular expansion when compared with control cultures without addition. On day 14, a decrease in cellular expansion was observed in the presence of the highest concentration of sucrose and human serum albumin with (23%) or without (27%) BSA (Figure 1A) or BSA alone (12%) (Figure 1B). However, when BSA and stabilizers were used in amounts equimolar to IVIGs at 10 mg/mL, we observed a less than 10% decrease in cellular expansion. We also observed in similar experiments that glycine at 2.6 mM and 13.3 mM increased the total expansion by 16% and 6%, respectively. In contrast to the effect of stabilizers, the cellular expansion in the presence of IVIGs is already reduced by 15% to 50% within the first week. On day 14, the cellular expansion was reduced by 53% and 79% when IVIGs were added at 10 mg/mL and 15 mg/mL, respectively (Figure 1A), and by 48% and 54% when dialyzed IVIGs were added at 10 mg/mL and 15 mg/mL, respectively (Figure 1B). Taking into account the stabilizers and BSA effects, IVIGs were responsible for 70% to 90% of the observed decrease in cellular proliferation. In addition, we have not observed any negative or positive effect of dialyzed IVIGs (5 mg/mL to 25 mg/mL) on L4.5 viability and proliferation rate as well as no binding of IVIGs to L4.5 cells as determined by flow cytometry (data not shown). These data are representative of independent experiments done with the B lymphocytes from 3 donors.
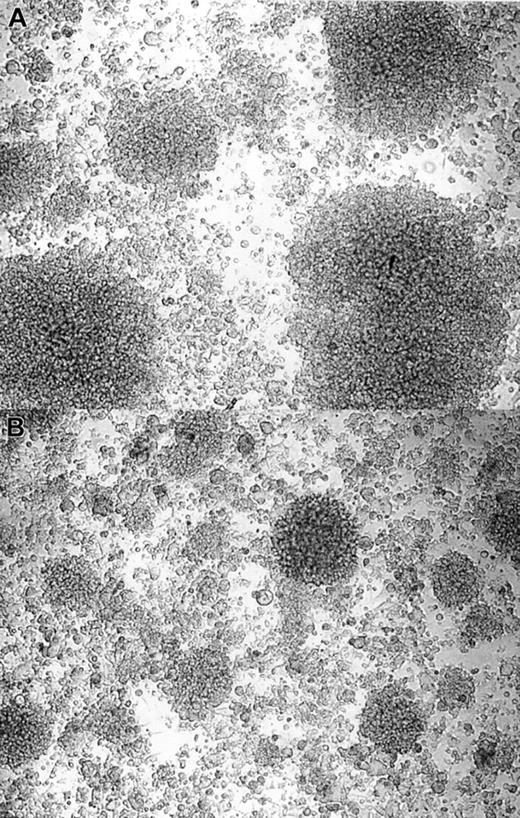
When cultured in vitro, B lymphocytes develop in characteristic clusters formed by hundreds to thousands of aggregated cells.36 We have observed that the addition of IVIGs reduced the formation of B-lymphocyte clusters (Figure2), resulting in smaller and fewer clusters when compared with control cultures with stabilizers and BSA. In addition, we observed that stabilizers with or without BSA permitted the formation of clusters in number and size similar to culture without addition (data not shown). Both IVIG preparations had the same effect on cluster formation. All together, these results indicated that the IgG in IVIGs reduced the in vitro expansion of human B lymphocytes.
IVIGs reduce B-lymphocyte aggregation in the culture system.
Purified human B lymphocytes were cultured in the presence of irradiated L4.5 and IL-4 with 4 mM glycine and 10 mg/mL BSA (A) or with 10 mg/mL dialyzed IVIGs (B). Fibroblastic L4.5 single cells in the background are supporting the growth of B lymphocytes in large or small clusters; pictures were taken on day 8 at a × 100 magnification. These results are representative of 3 independent experiments with both IVIG preparations.
IVIGs reduce B-lymphocyte aggregation in the culture system.
Purified human B lymphocytes were cultured in the presence of irradiated L4.5 and IL-4 with 4 mM glycine and 10 mg/mL BSA (A) or with 10 mg/mL dialyzed IVIGs (B). Fibroblastic L4.5 single cells in the background are supporting the growth of B lymphocytes in large or small clusters; pictures were taken on day 8 at a × 100 magnification. These results are representative of 3 independent experiments with both IVIG preparations.
To get closer to blood environment, we have used autologous human serum instead of fetal bovine serum. Human serum was much less efficient (10%-50%) than FBS to support the growth of human B lymphocytes for 14 days, while no such difference was observed for the first 5 days. We also observed high donor-to-donor variation. We attributed the lower efficiency of adult human serum versus fetal bovine serum to its low level of growth factors, although it is possible that autologous IgG (< 2 mg/mL) can play a role. In fact, we also observed that purified autologous IgG (10 mg/mL) added to FBS also decreased cellular expansion by 30% (data not shown). These observations, suggesting that autologous IgG can affect cellular expansion, need to be further investigated. In addition, as for FBS, we observed a more than 50% decrease in cellular expansion when IVIGs were added to B lymphocytes cultured with human serum (data not shown).
Continuous presence of IVIGs increases its negative effect on expansion
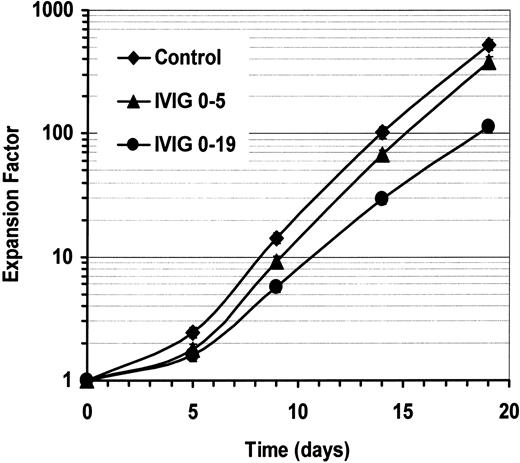
To determine if IVIGs had to be present during the entire culture period, we have evaluated the effect of IVIGs on B-lymphocyte expansion when IVIGs were present for 5 or 19 days during a 19-day period. IVIGs were used at 10 mg/mL and control cultures were done in parallel (Figure3). The addition of IVIGs for the first 5 days decreased cellular expansion by 10% to 25% on day 5, and about 27% on day 19. After removal of IVIGs on day 5, the cells started to expand at the same rate as the control cells. In contrast, when IVIGs were maintained during the 19-day period, the negative effect was much more important, showing a decrease of 61% to 78% of the cellular expansion. Thus, the continuous presence of IVIGs were required to maintain the reduction of human B-lymphocyte expansion.
Maintenance of IVIGs increases its negative effect on expansion.
Purified human B lymphocytes were cultured as previously described with IL-4 and irradiated L4.5 cells. Cells were cultured with 10 mg/mL BSA and 5.3 mM glycine (♦) or with 10 mg/mL dialyzed IVIGs from day 0 to day 5 (▴) or from day 0 to day 19 (●). Total cell expansion on days 0, 5, 9, 14, and 19 has been evaluated in triplicate as indicated in Figure 1. Error bars, showing standard deviation for triplicates, might be smaller than symbols. Both IVIG preparations showed similar effect.
Maintenance of IVIGs increases its negative effect on expansion.
Purified human B lymphocytes were cultured as previously described with IL-4 and irradiated L4.5 cells. Cells were cultured with 10 mg/mL BSA and 5.3 mM glycine (♦) or with 10 mg/mL dialyzed IVIGs from day 0 to day 5 (▴) or from day 0 to day 19 (●). Total cell expansion on days 0, 5, 9, 14, and 19 has been evaluated in triplicate as indicated in Figure 1. Error bars, showing standard deviation for triplicates, might be smaller than symbols. Both IVIG preparations showed similar effect.
IVIGs reduce the proportion of proliferating cells
The negative effect of IVIGs on cellular expansion could result from an increase in cell death, or a decrease in proliferation rate, or both. To evaluate the contribution of cell death to the IVIGs negative effect, the viability and apoptosis levels were measured using annexin V binding and PI staining of DNA, and trypan blue exclusion during the first culture days (Table1). Viability and apoptosis were monitored during the first culture days using IVIGs at 15 mg/mL and equimolar amounts of sucrose and human serum albumin without BSA (Figure 1). In both conditions, most cells showed high viability by annexin V and PI staining (82%-99%), as well as trypan blue exclusion (80%-96%). No significant increase in cell death was observed in the presence of IVIGs at 15 mg/mL, indicating that the IVIGs negative effect on cellular expansion did not result from increased cell death. Similar results were obtained for 14-day culture period (data not shown).
Addition of IVIGs to cultured B lymphocytes did not affect cell viability
| Time, days . | Apoptotic cells and cell viability . | |||
|---|---|---|---|---|
| Control . | IVIGs . | |||
| Annexin V/PI, % . | Viable cells, % . | Annexin V/PI, % . | Viable cells, % . | |
| 0 | 98.7 | 96 | 98.7 | 96 |
| 1 | 83.6 | 92 | 89.9 | 86 |
| 2 | 86.0 | 87 | 93.1 | 90 |
| 3 | 92.0 | 88 | 92.8 | 90 |
| 4 | 82.4 | 89 | 89.4 | 80 |
| Time, days . | Apoptotic cells and cell viability . | |||
|---|---|---|---|---|
| Control . | IVIGs . | |||
| Annexin V/PI, % . | Viable cells, % . | Annexin V/PI, % . | Viable cells, % . | |
| 0 | 98.7 | 96 | 98.7 | 96 |
| 1 | 83.6 | 92 | 89.9 | 86 |
| 2 | 86.0 | 87 | 93.1 | 90 |
| 3 | 92.0 | 88 | 92.8 | 90 |
| 4 | 82.4 | 89 | 89.4 | 80 |
Purified human B lymphocytes were cultured with IL-4 and L4.5 cells in the presence of human serum albumin (9 mg/mL) and sucrose (15 mg/mL) or IVIGs (15 mg/mL). Apoptosis and necrosis levels were determined with annexin V and PI staining. Viable cell proportions were determined by viable cell count using trypan blue exclusion.
Since Ki-67 is expressed during all active phases of the cell cycle,41 we measured its expression in B lymphocytes to evaluate the proportion of cycling cells in the presence of IVIGs (Table 2). In this culture system, B lymphocytes are quiescent for about 48 hours (< 5% Ki-67+cells) before progressively entering the cell cycle from day 3 to 5 (29%-89% Ki-67+ cells). In the presence of IVIGs, the proportion of B lymphocytes expressing Ki-67 was reduced on day 4 and day 5 (by 20%-40%), and the mean fluorescence intensity (MFI) for Ki-67 expression was lower. This general decrease in Ki-67 expression in the B lymphocytes cultured in the presence of IVIGs indicated that the reduced expansion resulted from a lower number of cycling cells rather than increased cell death.
Addition of IVIGs to cultured B lymphocytes reduced the number of cycling cells
| Time, days . | Intracellular expression of Ki-67 . | |||
|---|---|---|---|---|
| Control . | IVIGs . | |||
| % . | MFI . | % . | MFI . | |
| 0 | 1.5 | 2.5 | 1.5 | 2.5 |
| 1 | 0.6 | 2.4 | 0.7 | 2.3 |
| 2 | 3.4 | 4.0 | 3.2 | 3.4 |
| 3 | 29.0 | 6.1 | 17.7 | 6.7 |
| 4 | 79.0 | 34.3 | 65.0 | 20.3 |
| 5 | 88.9 | 41.9 | 70.3 | 23.2 |
| Time, days . | Intracellular expression of Ki-67 . | |||
|---|---|---|---|---|
| Control . | IVIGs . | |||
| % . | MFI . | % . | MFI . | |
| 0 | 1.5 | 2.5 | 1.5 | 2.5 |
| 1 | 0.6 | 2.4 | 0.7 | 2.3 |
| 2 | 3.4 | 4.0 | 3.2 | 3.4 |
| 3 | 29.0 | 6.1 | 17.7 | 6.7 |
| 4 | 79.0 | 34.3 | 65.0 | 20.3 |
| 5 | 88.9 | 41.9 | 70.3 | 23.2 |
Purified human B lymphocytes were cultured with IL-4 and L4.5 cells in the presence of human serum albumin (9 mg/mL) and sucrose (15 mg/mL) or IVIGs (15 mg/mL). Expression of intracellular Ki-67 was analyzed in permeabilized B lymphocytes with anti-Ki-67-PE as described in “Materials and methods.” All flow cytometry analyses were done with the FACScalibur cytometer using Cell Quest software.
IVIGs stimulate B-lymphocyte differentiation
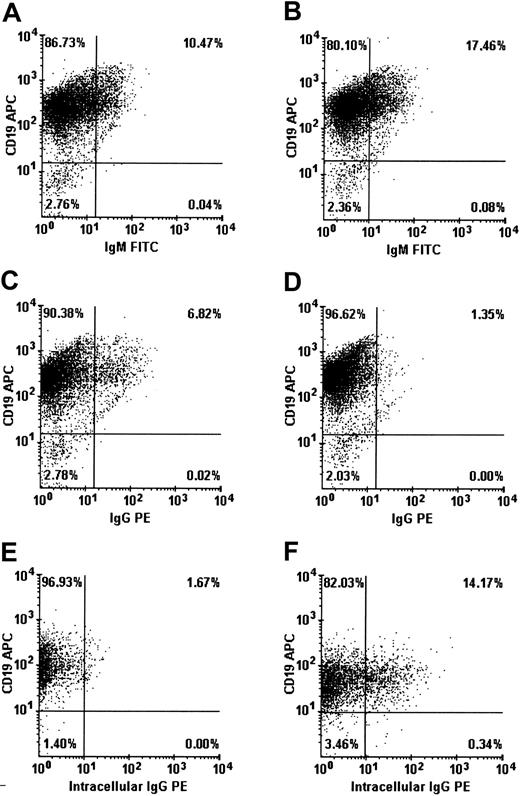
In the absence of cell death, reduction of proliferation might indicate that the cells have been driven to differentiate. We have done flow cytometry analyses to determine if differentiation was affected when B lymphocytes were cultured in the presence of IVIGs. Cellular expression of CD19, IgG, IgM, and intracellular IgG were evaluated in B lymphocytes on day 8 (Figure 4). In the presence of IVIGs, the population of IgM+ B lymphocytes was increased from 10.5% to 17.5% while the population of IgG+ B lymphocytes was reduced from 6.8% to about 1%. However, the proportion of B lymphocytes expressing intracellular IgG was increased by more than 8-fold, from 1.7% to more than 14.2% in the presence of IVIGs. Most of the B lymphocytes expressing intracellular IgG (14%) were not expressing surface IgG (1%). We have also evaluated the expression of CD11a, CD21, CD23, CD38, and CD40 on B lymphocytes cultured with or without IVIGs. We did not observe significant differences except for CD38 expression, which was increased by about 1.3-fold (70%-90%) in the presence of IVIGs (data not shown). This increase in the proportion of CD38-expressing cells suggested higher activation and differentiation levels in the B-lymphocyte populations.42 43 Taken together, these results indicated that B lymphocytes in the presence of IVIGs were stimulated to differentiate into Ig-secreting cells.
IVIG addition stimulates B-lymphocyte differentiation.
Purified human B lymphocytes were cultured with IL-4 and irradiated L4.5 during 14 days with 4 mM glycine and 10 mg/mL BSA (A,C,E) or with 10 mg/mL dialyzed IVIGs (B,D,F). On day 8, cells were collected, washed, and stained for CD19 and IgM (A-B), CD19 and IgG (C-D), or CD19 and intracellular IgG expression (E-F). Both IVIG preparations induced similar patterns during the 14-day culture period.
IVIG addition stimulates B-lymphocyte differentiation.
Purified human B lymphocytes were cultured with IL-4 and irradiated L4.5 during 14 days with 4 mM glycine and 10 mg/mL BSA (A,C,E) or with 10 mg/mL dialyzed IVIGs (B,D,F). On day 8, cells were collected, washed, and stained for CD19 and IgM (A-B), CD19 and IgG (C-D), or CD19 and intracellular IgG expression (E-F). Both IVIG preparations induced similar patterns during the 14-day culture period.
IVIGs increase Ig secretion and Ig-secreting cells
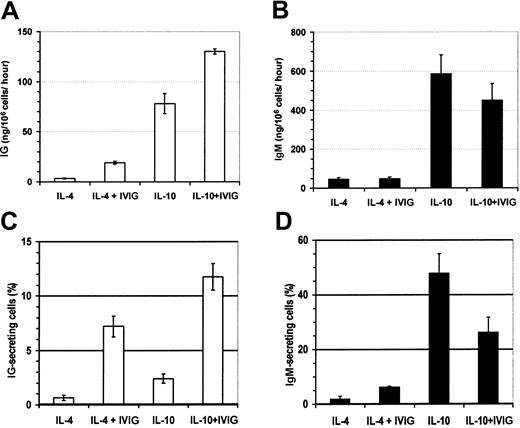
To confirm the effect of IVIGs on B-lymphocyte differentiation, we have measured the secreted IgG and IgM as well as the proportion of IgG- and IgM-secreting cells. IL-10 has been shown to stimulate Ig secretion in vitro44 and was used in parallel with IL-4 to study the IVIG effects. Human B lymphocytes were cultured during 19 days in the presence of IL-4 or IL-10 with or without IVIGs (Figure 5). On day 19, the cells were extensively washed and cultured without IVIGs for the evaluation of Ig secretion and Ig-secreting cells as described in “Materials and methods.” The addition of IVIGs resulted in a positive effect on B-lymphocyte differentiation, as shown by 1.7- and 5.4-fold increases in the IgG secretion in cells cultured with IL-10 or IL-4, respectively (Figure 5A). IgM secretion was not significantly affected by the presence of IVIGs in both conditions (Figure 5B). In parallel, the proportion of IgG- and IgM-secreting cells was evaluated by ELISPOT assay. As observed for IgG secretion, the addition of IVIGs increased the proportion of IgG-secreting cells by 5- and 10-fold in B lymphocytes cultured with IL-10 or IL-4, respectively (Figure 5C). The proportion of IgM-secreting cells was increased by 3-fold in culture with IL-4 but decreased by about 50% when IL-10 was present (Figure 5D). These observations indicated that the addition of IVIGs increased the number of IgG-secreting cells corresponding to higher IgG secretion in the presence of IL-4 or IL-10.
B lymphocyte IgG secretion is increased in the presence of IVIGs.
Purified human B lymphocytes were cultured with IL-4 or IL-10 and irradiated L4.5 during 19 days with 4 mM glycine and 10 mg/mL BSA or with 10 mg/mL dialyzed IVIGs. Cells were extensively washed and transferred without IVIGs in complete culture medium and allowed to secrete for 6 hours in ELISPOT assay and 6 hours (IL-10) or 22 hours (IL-4) for ELISA evaluation of IgG and IgM contents. Secreted IgG (A) and IgM (B) amounts are expressed in nanograms per 1 × 106 cells per hour, and the proportion of cells secreting IgG (C) and IgM (D). Error bars show standard deviation for 2 dilutions in triplicate. Results are representative of 3 independent experiments. Both IVIG preparations showed similar results.
B lymphocyte IgG secretion is increased in the presence of IVIGs.
Purified human B lymphocytes were cultured with IL-4 or IL-10 and irradiated L4.5 during 19 days with 4 mM glycine and 10 mg/mL BSA or with 10 mg/mL dialyzed IVIGs. Cells were extensively washed and transferred without IVIGs in complete culture medium and allowed to secrete for 6 hours in ELISPOT assay and 6 hours (IL-10) or 22 hours (IL-4) for ELISA evaluation of IgG and IgM contents. Secreted IgG (A) and IgM (B) amounts are expressed in nanograms per 1 × 106 cells per hour, and the proportion of cells secreting IgG (C) and IgM (D). Error bars show standard deviation for 2 dilutions in triplicate. Results are representative of 3 independent experiments. Both IVIG preparations showed similar results.
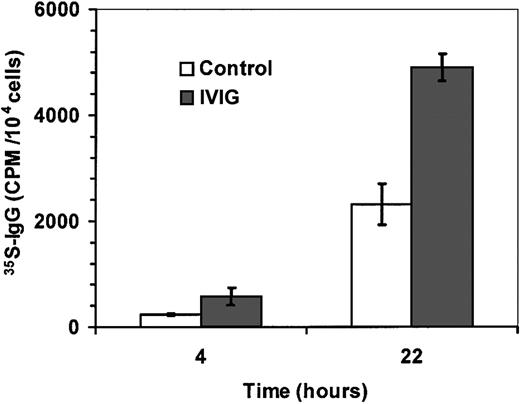
To rule out the possibility that IgG secretion was the result of IVIGs recycling by the B lymphocytes through mechanisms such as the neonatal Fc receptor,45 we have labeled synthesized immunoglobulins with 35S-methionine and35S-cysteine as described in “Materials and methods.”35S-IgG was measured in supernatants from B lymphocytes on day 14 after 4 hours and 22 hours (Figure6). The amount of 35S-IgG in supernatants from B lymphocytes treated with IVIGs was 2-fold higher than in control supernatants, indicating that the increase of secreted IgG, as shown in Figure 4, was the result of de novo IgG synthesis by the B lymphocytes. These results are representative of 3 independent experiments.
IVIGs increase de novo synthesis of IgG.
Purified human B lymphocytes were cultured with IL-4 and irradiated L4.5 during 14 days with 10 mg/mL sucrose and 6 mg/mL human serum albumin (control; ■) or with 10 mg/mL IVIGs (IVIGs; ▪). Cells were extensively washed and transferred without IVIGs in MEM without methionine as described in “Materials and methods.”35S-IgG content was evaluated in culture supernatants after 4 hours and 22 hours. Error bars are showing standard deviation for triplicates. Both IVIG preparations showed similar results. These results are representative of 3 independent experiments.
IVIGs increase de novo synthesis of IgG.
Purified human B lymphocytes were cultured with IL-4 and irradiated L4.5 during 14 days with 10 mg/mL sucrose and 6 mg/mL human serum albumin (control; ■) or with 10 mg/mL IVIGs (IVIGs; ▪). Cells were extensively washed and transferred without IVIGs in MEM without methionine as described in “Materials and methods.”35S-IgG content was evaluated in culture supernatants after 4 hours and 22 hours. Error bars are showing standard deviation for triplicates. Both IVIG preparations showed similar results. These results are representative of 3 independent experiments.
Reactivity of secreted antibodies
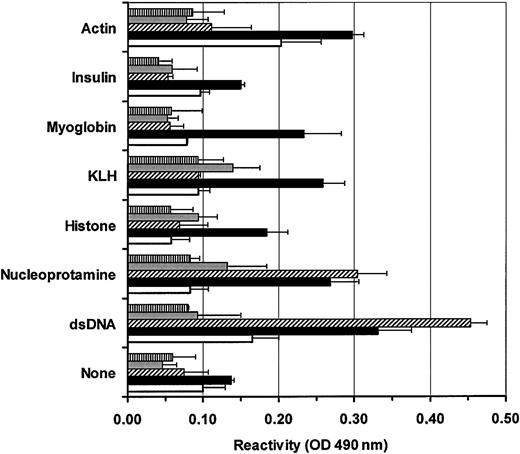
The increased secretion of IgG might result from the specific activation of some unique subset of B lymphocytes. To address this possibility, we have evaluated the reactivity of IVIG-induced IgG versus IgG secreted in controls on a panel of antigens. Supernatants from B lymphocytes cultured for 14 days with or without IVIGs were prepared as described in “Materials and methods.” IgG reactivity was measured in commercial IVIG preparation, in culture supernatants following incubation with or without IVIGs, and in a commercial anti-dsDNA autoimmune serum (Figure7). All samples were adjusted at 100 ng/mL for IgG content. The reactivity in commercial IVIGs was low (< 0.15) and often close to background under the ELISA conditions used. The commercial anti-dsDNA autoimmune serum showed, as expected, a high reactivity for dsDNA and nucleoprotamine. Surprisingly, the supernatants from B lymphocytes cultured with IVIGs contained IgG highly reactive for dsDNA, nucleoprotamine, myoglobin, and keyhole limpet hemocyanin (KLH). Moreover, the reactivity of IVIG-induced IgG for dsDNA and nucleoprotamine in B-lymphocyte supernatants was comparable to the levels measured in the commercial autoimmune anti-dsDNA serum. In contrast, only low reactivity for actin (about 0.20) was detected in culture supernatants from B lymphocytes cultured in the absence of IVIGs. In addition, we have observed very low reactivities (< 0.10) for all tested antigens even when using supernatants of B lymphocytes cultured without IVIGs for 21 days (data not shown). These results, representative of 3 independent experiments, indicated that IVIG addition can specifically stimulate B lymphocytes to secrete antibodies reacting against self-antigens.
B lymphocytes cultured in the presence of IVIGs secrete antibodies reacting with self-antigens.
All tested samples were adjusted to 100 ng/mL IgG. IgG reactivities for actin, insulin, myoglobin, KLH, histone, nucleoprotamine, and dsDNA were evaluated by ELISA as described in “Materials and methods.” IgG reactivities in commercial IVIGs (░), commercial human autoimmune anti-dsDNA serum (▧), and supernatants from B lymphocytes cultured with 10 mg/mL BSA and 4 mM glycine (■) or with 10 mg/mL dialyzed IVIGs (▪) are shown. Background reactivities (▤) for each antigen are also shown. Error bars show standard deviation for triplicates. Results are representative of 3 independent experiments and reactivity patterns are similar for both IVIG preparations.
B lymphocytes cultured in the presence of IVIGs secrete antibodies reacting with self-antigens.
All tested samples were adjusted to 100 ng/mL IgG. IgG reactivities for actin, insulin, myoglobin, KLH, histone, nucleoprotamine, and dsDNA were evaluated by ELISA as described in “Materials and methods.” IgG reactivities in commercial IVIGs (░), commercial human autoimmune anti-dsDNA serum (▧), and supernatants from B lymphocytes cultured with 10 mg/mL BSA and 4 mM glycine (■) or with 10 mg/mL dialyzed IVIGs (▪) are shown. Background reactivities (▤) for each antigen are also shown. Error bars show standard deviation for triplicates. Results are representative of 3 independent experiments and reactivity patterns are similar for both IVIG preparations.
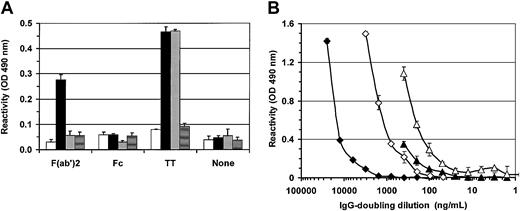
To verify if IVIG-induced IgG could interact with other IgG molecules, we have evaluated their reactivity for human IgG F(ab′)2 and Fc fragments (Figure8A). IgG reactivity for both IgG fragments was also determined in control supernatants, as well as in commercial IVIGs. No reactivity was observed for human IgG Fc fragments in all samples. In contrast, IVIG-induced IgG was highly reactive for human F(ab′)2 fragments while reactivities close to background were obtained in all other samples. These results indicated that IVIG-induced IgG can interact with other antibodies through F(ab′)2. We also evaluated if IVIG-induced IgG could react with non–self antigens such as tetanus toxin (TT) and LPS. No significant reactivity was observed for LPS 026:B6 (< background) and a slight increase of reactivity for LPS 055:B5 was observed only in IVIG-induced IgG (0.20 ± 0.02) compared with background (0.16 ± 0.01). In contrast, IVIG-induced IgG and commercial IVIGs showed similar reactivities for TT while no reactivity was observed in control (Figure 8A). We have also titrated the reactivity of IVIG-induced IgG for F(ab′)2 and dsDNA using commercial IVIGs as a reference (Figure 8B). The results showed that IVIG-induced IgG was more reactive (4-fold) for IgG-F(ab′)2 fragments than for dsDNA. All together, these results indicate that IVIGs can induce the secretion of IgG reacting against self- and non–self antigens.
IVIGs induce high IgG reactivities against dsDNA, F(ab′)2 and tetanus toxin.
(A) Supernatants from B lymphocytes cultured for 14 days with 4 mM glycine and 10 mg/mL BSA (■) or with 10 mg/mL dialyzed IVIGs (▪) and commercial IVIGs (░) were adjusted to 100 ng/mL IgG. Reactivities for human IgG F(ab′)2 and Fc fragments, and tetanus toxin (TT) were determined by ELISA as described in “Materials and methods.” Background reactivities of ELISA are shown for each Ag (▤) as are reactivities of each sample in the absence of coated Ag (none). (B) Dilutions of IVIG-induced IgG (triangle) and commercial IVIGs (diamond) were used in titration ELISA to quantify IgG reactivities for dsDNA (filled symbols) and human IgG F(ab′)2 fragments (open symbols) as described in “Materials and methods.” Both antigens were adsorbed at 10 μg/mL. Background reactivities in these ELISAs for dsDNA and F(ab′)2 fragments were 0.05 OD and 0.06 OD, respectively, at 490 nm. Error bars, showing standard deviation for triplicates, can be smaller than symbols.
IVIGs induce high IgG reactivities against dsDNA, F(ab′)2 and tetanus toxin.
(A) Supernatants from B lymphocytes cultured for 14 days with 4 mM glycine and 10 mg/mL BSA (■) or with 10 mg/mL dialyzed IVIGs (▪) and commercial IVIGs (░) were adjusted to 100 ng/mL IgG. Reactivities for human IgG F(ab′)2 and Fc fragments, and tetanus toxin (TT) were determined by ELISA as described in “Materials and methods.” Background reactivities of ELISA are shown for each Ag (▤) as are reactivities of each sample in the absence of coated Ag (none). (B) Dilutions of IVIG-induced IgG (triangle) and commercial IVIGs (diamond) were used in titration ELISA to quantify IgG reactivities for dsDNA (filled symbols) and human IgG F(ab′)2 fragments (open symbols) as described in “Materials and methods.” Both antigens were adsorbed at 10 μg/mL. Background reactivities in these ELISAs for dsDNA and F(ab′)2 fragments were 0.05 OD and 0.06 OD, respectively, at 490 nm. Error bars, showing standard deviation for triplicates, can be smaller than symbols.
Discussion
In the present study, we have shown that IVIGs can directly affect human B lymphocytes by reducing the proliferation while increasing the differentiation and IgG secretion. We have also shown that IVIGs were able to stimulate human B lymphocytes to secrete antibodies reactive with many antigens. All these observations were done using purified human B lymphocytes cultured in a well-defined culture system, therefore excluding any indirect effect from other cells or soluble factors. This quite simple in vitro culture system can assist in the study of IVIGs effect on normal human B lymphocytes but cannot replace clinical study. It is important to note that the results presented here have been obtained in an environment that mimics interaction between B and T lymphocytes without the normal restrictions present in germinal center, since CD154 and cytokine signals were continuously renewed during 14 days. Thus, the differences between this in vitro system and the in vivo environment are considered in the interpretation of our observations.
We suggest that all results reported in this study are converging to the same point: the enhancement by IVIGs of differentiation in a portion of peripheral B lymphocytes. The emergence of a higher proportion of B lymphocytes expressing intracellular IgG (Figure 4) as well as the increased IgG secretion and IgG-secreting cell numbers (Figures 5-6) characterize B-lymphocyte differentiation. In addition, the reduction of the cellular expansion rate can also indicate that more cells are engaged in differentiation (Figures 1-3; Table2). Finally, the emergence of IgG reactive with many antigens following IVIG addition (Figures 7-8) suggests that IVIGs were directly involved in activation of B-lymphocyte secretion. Although the exact meaning of these observations remains to be clarified by further studies, we believe that several hypotheses can be drawn from this work and could contribute to a better understanding of the long-term effects of IVIGs in autoimmune diseases.
The enhancement of IgG secretion by IVIGs was not fitting well with the possible involvement of the negative FcγIIb receptor (CD32)46 since this binding should result in apoptosis or anergy but not activation. However, we presently investigate whether the whole IgG molecule, or its Fc or F(ab′)2 regions in IVIGs, is essential for this positive effect of IVIGs. In addition, it could be interesting to determine whether monomeric, dimeric, or multimeric forms of IgG in IVIGs are involved in the direct effect on B lymphocytes.
High IVIG concentrations (22.5 mg/mL and 45 mg/mL) have been reported to induce apoptosis in CD40-activated tonsillar B lymphocytes in the absence of cytokines.47 Here, we have shown that IVIGs could not induce a significant level of apoptosis in the presence of CD154 and cytokines. In fact, Prasad and colleagues47 removed anti-CD40 before adding IVIGs, whereas we kept both signals during the experiments. The difference between their study and our data suggests that IVIG-induction of apoptosis could differ in function of the available microenvironment.
Our in vitro study suggests that IVIGs could interact with one or several B-lymphocyte receptors able to transform their binding into differentiation and IgG secretion. Sundblad and colleagues have previously described that IVIGs can stimulate the secretion of natural antibodies in mouse models,18,19 as we have shown here in vitro with human B lymphocytes. The authors postulated that an increased level of natural antibodies in patients with autoimmune diseases could interfere in the selection of autoreactive B-cell repertoire. IVIG-induced natural IgG antibodies could then neutralize and accelerate the elimination of self-antigens by phagocytosis as well as through the formation of immune complexes.48 The presence in IVIG-induced IgG of antibodies reacting with human IgG through the F(ab′)2 but not the Fc fragments suggests that these antibodies could interact with the variable regions of other antibodies as done by anti-idiotypic antibodies. For example, the balance between anti-F(ab′)2and anti-dsDNA antibodies in the serum of SLE patients has been reported to regulate the active and quiescent phases of the diseases.49 These anti-F(ab′)2 antibodies could thus be good candidates for the reestablishment of the homeostasis and idiotypic network, which is considered by many authors to be a key in the long-term effect of IVIGs (reviewed in Kazatchkine and Kaveri1 and Coutinho et al9).
The observation that only a subset of B lymphocytes (< 15%) was stimulated to differentiate and secrete IgG suggests that some restricted interactions were involved between IVIGs and B lymphocytes. Such specific interaction of IVIGs has already been described in T lymphocytes following activation with staphylococal enterotoxin B (SEB).50 In their study, the authors showed that IVIGs enhanced expansion of CD3+ blasts expressing Vβ3 and Vβ17 when activated with SEB. In the absence of SEB stimulation, IVIGs were considered to inhibit overall proliferation of T lymphocytes. Specific expansion of T lymphocytes in function of activation level and TCR Vβ family is in accordance with our data and indicates that IVIGs can selectively modulate a subset of lymphocytes. The authors also showed that IVIGs effect was V-region dependent using F(ab′)2 fragments prepared from IVIGs. On B lymphocytes, such restricted interaction could be done through the B-cell antigen receptor (BCR) by anti-idiotypic antibodies present in IVIGs.20
Since signals through BCR are pivotal for positive or negative regulation of B-lymphocyte maturation and differentiation,51 IVIGs interaction with this receptor could be important in immunomodulation of autoimmune diseases. One possibility is that, in opposition to in vitro, the in vivo binding of BCR by IVIGs could induce apoptosis in B lymphocytes. In mouse models, the injection of IVIGs has been shown to induce the deletion of immature B lymphocytes through the premature binding of BCR.7,18,19 It was also reported that prolonged stimulation of BCR on germinal center B lymphocytes can drive these cells to apoptosis even in the presence of a suitable microenvironment.52 The binding of BCR by IVIGs on immature, mature, and germinal center B lymphocytes could play a role in the deletion of self-reactive B lymphocytes. We suggest that in vivo the microenvironment essential to provide the second signal might become deficient if too many B lymphocytes are activated at the same time by IVIGs. For example, helper T cells or follicular dendritic cells could be overloaded and unable to rescue autoreactive B lymphocytes from apoptosis. Such mechanisms have been documented as competitive exclusion for niche occupancy, and were proposed to play an important role in the regulation of self-reactive B-cell repertoire.53 The deletion of autoreactive B lymphocytes could directly contribute to IVIGs long-term effects in autoimmune diseases. Obviously, second signals (CD154 and cytokines) were not limiting in the in vitro culture system, and this explains why we have been able to observe an increased B-lymphocyte differentiation in the presence of IVIGs.
From this work, we conclude that IVIGs can directly affect B lymphocytes and thus could modulate their repertoire in autoimmune diseases. It would therefore be of interest to characterize these IVIG-induced IgG and to further study how these could interact with the pathologic autoreactive B lymphocytes.
The authors thank Jean-Luc Boudreau, Marie Plamondon, and Hélène Guérin for IVIG analysis by capillarity electrophoresis. The authors also thank Drs Noëlla Deslauriers and André Darveau for stimulating discussion and Dr Daniel Jung for critical discussion and reading of the manuscript.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-06-1684.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sonia Néron, Recherche et Développement, Héma-Québec, 2535 boul Laurier, Sainte-Foy, Québec, Canada, G1V 4M3; e-mail:sneron@hema-quebec.qc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal