Abstract
Antithrombin (AT) supplementation in patients with severe sepsis has been shown to improve organ failures in which activated leukocytes are critically involved. However, the precise mechanism(s) for the therapeutic effects of AT is not well understood. We examined in rats whether AT reduces ischemia/reperfusion (I/R)–induced renal injury by inhibiting leukocyte activation. AT markedly reduced the I/R-induced renal dysfunction and histologic changes, whereas neither dansyl glutamylglycylarginyl chloromethyl ketone–treated factor Xa (DEGR-F.Xa), a selective inhibitor of thrombin generation, nor Trp49-modified AT, which lacks affinity for heparin, had any effect. Renal tissue levels of 6-keto-PGF1α, a stable metabolite of prostacyclin (PGI2), increased after renal I/R. AT enhanced the I/R-induced increases in renal tissue levels of 6-keto-PGF1α, whereas neither DEGR-F.Xa nor Trp49-modified AT had any effect. AT significantly inhibited I/R-induced decrease in renal tissue blood flow and the increase in the vascular permeability. Ischemia/reperfusion-induced increases in renal tissue levels of tumor necrosis factor-α, cytokine-induced neutrophil chemoattractant, and myeloperoxidase were significantly inhibited in animals given AT. Pretreatment of animals with indomethacin reversed the effects induced by AT. Iloprost, an analog of PGI2, produced effects similar to those induced by AT. These observations strongly suggest that AT reduces the I/R-induced renal injury by inhibiting leukocyte activation. The therapeutic effects of AT might be mainly mediated by PGI2released from endothelial cells through interaction of AT with cell surface glycosaminoglycans.
Introduction
Antithrombin (AT) is an important inhibitor of serine proteases, which are generated within the coagulation cascade.1 Interaction of AT with heparin-like glycosaminoglycans (GAGs) on endothelial cells is important for the acceleration of thrombin inhibition by AT.1 Thrombotic episodes have been frequently observed in patients with congenital AT deficiency and in those with variant AT that lacks affinity for heparin,2,3 suggesting that the interaction of AT with the endothelial cell surface heparin-like GAGs is important for regulation of the coagulation cascade by AT. AT has been shown to reduce the mortality of baboons challenged with Escherichia coli not only by inhibiting coagulation abnormalities but also by attenuating inflammatory responses in which activated leukocytes are critically involved.4 Furthermore, AT supplementation limited the inflammatory responses, thereby alleviating the respiratory failure, liver dysfunction, and renal failure in patients with severe sepsis.5,6 However, the anti-inflammatory mechanism of AT is still not well understood. We previously reported that AT promoted the endothelial release of prostacyclin (PGI2) in vivo by interacting with heparin-like GAGs on the cell surface.7PGI2 inhibits tumor necrosis factor-α (TNF-α) biosynthesis in monocytes by inhibiting its transcription through regulation of nuclear factor-κB (NF-κB) activation.8-10 Thus, AT might limit inflammatory responses in sepsis by inhibiting leukocyte activation through promotion of the endothelial release of PGI2. Consistent with this hypothesis, AT has been shown to inhibit pulmonary vascular injury and hypotension in rats challenged with lipopolysaccharide by inhibiting leukocyte activation through promotion of the endothelial release of PGI2.11,12 In contrast to these reports, AT has been demonstrated to inhibit leukocyte activation directly.13-15 These observations suggest that anti-inflammatory effects of AT may be mediated mainly by its direct inhibitory properties on leukocyte activation.
In the present study, we investigated the anti-inflammatory mechanism(s) of AT using a rat model of ischemia/reperfusion (I/R)–induced renal injury in which activated leukocytes are critically involved.16 Our results suggested that anti-inflammatory effects of AT were not mediated by its direct action but by mainly by the action of PGI2 released from the endothelial cells.
Materials and methods
Materials
AT was kindly provided by Mitsubishi-Welpharma (Osaka, Japan). AT was purified from heat-treated pooled human plasma by absorption on fixed heparin according to a modification of the technique of Miller-Andersson et al.17 The AT concentrate used in the experiment revealed a single band on sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Further characterization of the AT concentrate demonstrated that the heparin concentration was less than 0.01 U/mL and that it was pyrogen free. Dansyl glutamylglycylarginyl chloromethyl ketone (DEGR) was purchased from Calbiochem (San Diego, CA). Iloprost was kindly supplied by Eizai Pharmaceutical (Tokyo, Japan). Dimethyl-(2-hydroxy-5-nitrobenzyl) sulfonium bromide (DHNBSB), indomethacin (IM), Evans blue dye, and human neutrophil myeloperoxidase (MPO) were obtained from Sigma (St Louis, MO). All other reagents were of analytical grade.
Determination of the content of latent and cleaved form of AT in the AT concentrate
The AT concentrate has been shown to contain latent and cleaved form of AT, denoted as inactive form of AT, after heat treatment for pasteurization.18 Because the inactive form of AT has lower heparin affinity than that of intact AT, it can be separated using heparin-Sepharose affinity chromatography.18 To know what proportion of latent and cleaved form in the AT concentrate to use in the present study, we determined the content of the inactive form of AT using heparin-Sepharose affinity chromatography according to the previously described method.18 Consequently, 6.7% of total AT was found to be the inactive form in the AT concentrate. This result is consistent with the other reports showing that 3% to 14% of total AT in various AT concentrates was the inactive form.18 Thus, the major part of the AT concentrate employed in the present study consists of the active form of AT.
Preparation of DEGR-treated factor Xa
DEGR-treated factor Xa (DEGR-F.Xa), a selective inhibitor of thrombin generation,19 was prepared as described previously. Briefly, factor Xa was purified from human plasma and activated with Russell viper venom.20,21 Activated factor X was inactivated with a 20-fold molar excess of DEGR for 30 minutes at 25°C. The mixture was then subjected to extensive dialysis against a solution containing 20 mM tris(hydroxymethyl)aminomethane(Tris)–hydrochloric acid (pH 7.4) and 100 mM sodium chloride. DEGR-F.Xa competes with intact factor Xa for prothrombinase complex formation.22 DEGR-F.Xa generated as described above showed no clotting activity and a prolonged activated partial thromboplastin time in a concentration-dependent manner (0 to 300 μg/mL).
Preparation of Trp49-modified AT
The Trp49 residue of AT was chemically modified by a version of the method of Karp et al.23 In brief, DHNBSB was mixed with a continuously stirred solution containing 40 μM AT, 0.1 M Tris-HCl (pH 8.0), and 0.15 M NaCl. The final concentration of DHNBSB was calculated as 8 mM. After stirring for 15 minutes at 22°C, the insoluble hydroxynitrobenzyl alcohol, which formed as a hydrolysis product, was removed by centrifugation. The resulting solution was next subjected to chromatography on a column (2.6 × 60 cm) of Sephacryl S-200HR that had been equilibrated with 0.1 M Tris-HCl (pH 8.0) and 0.15 M NaCl and then subjected to chromatography on a column (3 × 6 cm) of heparin-Sepharose CL6B as previously described.7The extent of derivatization of AT was determined spectrophotometrically in 2 M NaOH at a wavelength of 410 nm (molar extinction coefficient, 1.85 × 104M−1).7 The progressive thrombin inhibitory activity of the Trp49-modified AT in the absence of heparin was virtually identical to that of intact AT (data not shown). The study of thrombin inhibition by Trp49-modified AT was performed as previously described.7
Animal model of renal I/R
The care and handling of the animals used in the present study was in accordance with the guidelines of the National Institutes of Health. All experimental procedures were approved by Kumamoto University and Oita Medical University Animal Care and Use Committee. Adult pathogen-free male Wistar rats (Nihon SLC, Hamamatsu, Japan) weighing 180 to 220 g were housed in a temperature-controlled (22°C) room with alternating 12-hour light/dark cycles and were given water but no food for 24 hours before the experiments. During surgery, core temperature was monitored using a rectal probe and maintained with a heating pad and a heating lamp at 35.5°C to 37°C. The renal I/R protocol was performed as described previously,24 with some modifications. In brief, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium 50 mg/kg (Abbott Laboratories, North Chicago, IL), followed by 20 mg/kg every hour for 4 hours and supplemented with an intraperitoneal injection of buprenorphine 2 mg/kg every 12 hours for 2 days. To perform nephrectomy, a midline incision was made in each rat and the left kidney was mobilized to allow the left renal vessels to be ligated. To cause ischemia, the right pedicle was clamped with a noncrushing microvascular clamp for 45 minutes. Ischemia was confirmed visually by blanching of the kidney. During the period of renal ischemia, the rats were covered with plastic wrap to prevent evaporation. After 45 minutes of ischemia, the clamp was removed and the wound was closed with a 3-0 silk suture. The animals were then returned to their cages and allowed free access to food and water. Sham-operated animals underwent the same operation but without clamping. At specified time points (before ischemia and 3, 6, 12, 24, and 48 hours after reperfusion, except for measurement of renal tissue level of 6-keto-PGF1α), the rats were anesthetized by intraperitoneal administration of pentobarbital (50 mg/kg) and killed. Blood samples were taken from the abdominal aorta. Blood was collected in tubes and centrifuged at 2000g for 10 minutes. Renal dysfunction was evaluated by measuring serum levels of blood urea nitrogen (BUN) and creatinine by standard urease assays and picric acid reactions.25Coagulation abnormality was evaluated by serum levels of fibrin degradation products (FDP(E)) measured with a latex agglutination assay as described previously.25 The kidneys were harvested for examination of histologic changes, renal tissue level of 6-keto-PGF1α, renal vascular permeability, assay of renal MPO activity, and renal tissue content of cytokines.
Experimental design
We initially examined the effects of various doses of AT (50, 100, and 250 U/kg intravenously) on renal dysfunction in rats with I/R injury. To elucidate the therapeutic mechanisms of AT, the rats were subjected to the following 9 experimental groups after left nephrectomy: sham-operated, vehicle-treated, AT-treated, DEGR-F.Xa–treated, Trp49-modified AT-treated, IM-treated, AT pretreated with IM-treated, iloprost-treated, and iloprost pretreated with IM-treated.
Administration of AT, DEGR-F.Xa, and Trp49-modified AT
We previously reported that the plasma concentration of 6-keto-PGF1α, a stable metabolite of PGI2, begins to increase 30 minutes after the intravenous administration of AT (250 U/kg) in intact rats.26 Therefore, AT was administered 30 minutes prior to reperfusion in the present study. DEGR-F.Xa (3 mg/kg) and Trp49-modified AT (250 U/kg) were also administered intravenously 30 minutes before reperfusion. At the dosage used in the present study, DEGR-F.Xa and Trp49-modified AT have anticoagulant activities comparable to that of 250 U/kg native AT.7
IM and iloprost administration
IM (20 mg/kg) was suspended in bicarbonate-buffered saline and administered subcutaneously 30 minutes prior to ischemia. Control animals received the same volume of bicarbonate-buffered saline instead of IM. Iloprost was dissolved in saline and continuously infused (100 ng/kg/min) via the right jugular vein for 3 hours after the onset of renal reperfusion. In other treated groups animals received continuous infusion of the same volume of saline instead of iloprost.
Histopathologic studies of the kidneys
The kidneys were removed 24 hours after reperfusion, fixed in 10% formalin, embedded in paraffin, cut into 5-μm–thick sections, and stained with hematoxylin and eosin. Samples were analyzed by a pathologist who was blinded to the experimental groups. Tubular necrosis, vascular congestion, and neutrophil accumulation in the outer medulla were evaluated in 20 random fields as described by Kelly et al27 and Chiao et al,28 with some modifications. Tubular necrosis was evaluated by determining the percentage of tubules in the outer medulla in which epithelial necrosis or necrotic debris was observed. Vascular congestion was assessed by counting the erythrocytes in the outer medulla. Neutrophil accumulation was quantified as numbers of neutrophils in the outer medulla. The cells were counted using an eyepiece graticule at a magnification of × 400.
Measurement of renal 6-keto-PGF1α levels
Renal 6-keto-PGF1α (a metabolite of PGI2) levels were determined in animals subjected to I/R before and 1, 3, 6, 12, 24, and 48 hours after reperfusion according to the methods described previously,29 with some modifications. In brief, the kidneys were weighed and then immediately homogenized in 5 mL of 0.1 M phosphate buffer (pH 7.4) containing 2 mM IM at 5°C. The homogenate was first centrifuged at 2000gfor 10 minutes to remove minute amounts of solid tissue debris, and the supernatant was then acidified with 1 M HCl. The 6-keto-PGF1α was extracted from the supernatant using columns packed with ethyl-bonded silica gel (ethyl C2; Amersham, Buckinghamshire, United Kingdom). The columns were prepared by washing them with 2 mL methanol followed by 2 mL water. The acidified supernatant was applied to the column, and this was followed by sequential washes with 5 mL water, 5 mL of 10% ethanol, and 5 mL hexane. The elution of 6-keto-PGF1α was performed with 5 mL methylformate, after which the solvent was evaporated under a stream of nitrogen gas. The concentration of 6-keto-PGF1α was assayed using a specific enzyme immunoassay kit (Amersham). This enzyme immunoassay was sensitive enough to detect 10 to 1280 pg/mL of 6-keto-PGF1α. The results are expressed as nanograms of 6-keto-PGF1α per gram of tissue. The cross-reactivities of this assay with prostaglandin E2 (PGE2), prostaglandin F2α, thromboxane B2, and arachidonic acid were 2.8%, 1.4%, 0.03%, and 0.01%, respectively, according to the manufacturer's data sheet. The maximum level of PGE2 in the liver of this animal model was about 4 ng per gram of tissue. The tissue level of PGE2 was assayed using a specific enzyme immunoassay kit (Amersham) as described previously.30
Measurement of renal tissue blood flow
Renal tissue blood flow was evaluated by measuring renal cortical blood flow using a laser-Doppler flow meter (ALF21N; Advance, Tokyo, Japan) as described previously.25 The Doppler probe was directed toward the cortex. Renal cortical blood flow was measured from 30 minutes before ischemia until 3 hours after reperfusion. Electrical signals from the probe were digitized and recorded in real time using MacLab software (ADInstruments, Castle Hill, Australia).24 Results are expressed as percentages of the preischemic levels.
Evaluation of renal vascular permeability
Renal vascular permeability was assessed by measuring extravasated dye as described.25 In brief, Evans blue dye (25 μg/kg) was injected intravenously 10 minutes before the rats were killed by exsanguination from the abdominal aorta. The kidneys were removed, weighed, and placed in 5 mL dimethylformamide (Wako, Osaka, Japan) for 7 days. After centrifugation (2000g for 10 minutes), the concentration of Evans blue dye extracted in the dimethylformamide was measured by a spectrophotometer (DU-54; Beckman, Irvine, CA) at a wavelength of 610 nm and compared with results obtained with standards. Evans blue concentrations are expressed in micrograms per gram of tissue.
Assay of renal MPO activity and tissue cytokine content
Accumulation of neutrophils in the kidney was evaluated by measuring renal MPO activity as described previously.25 31The rats were killed at various times points after reperfusion. The kidneys were removed, weighed, and homogenized (Physcotron; Niti-on, Tokyo, Japan) in 10% (wt/vol) 0.05 M phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide and sonicated for 20 seconds. After centrifugation (4500g for 20 minutes at 4°C), 0.1 mL of the supernatant was added to 0.55 mL of 0.1 M phosphate buffer (pH 6.0) containing 1.25 mg/mLo-dianisidine and 0.05% hydrogen peroxide. After 5 minutes, changes in absorbance at 460 nm were measured using a spectrophotometer. The activity of purified known human neutrophil MPO was used as the standard. Results are expressed as units of MPO activity per gram of tissue.
Renal levels of TNF-α and cytokine-induced neutrophil chemoattractant (CINC), the rat equivalent of human interleukin-8,32 were measured using enzyme-linked immunosorbent assay (ELISA) kits for rat TNF-α (Genzyme Corporation, Cambridge, MA) and CINC (Amersham), respectively. At specified times after reperfusion, the kidneys were removed, weighed, and homogenized using 0.1 M phosphate buffer (pH 7.4) containing 0.05% (wt/vol) sodium azide at 4°C. Homogenates were sonicated for 20 seconds and centrifuged (2000g for 10 minutes at 4°C). The resulting supernatants then were stored at −80°C until use. Results are expressed as picograms of cytokine per gram of tissue.
Statistical analysis
Data are expressed as means ± SDs. Differences between groups were examined for statistical significance using an unpairedt test for single comparison and analysis of variance followed by the Scheffé post hoc test for multiple comparison. AP value below .05 denoted the presence of a statistically significant difference.
Results
Effects of AT, DEGR-F.Xa, and Trp49-modified AT on I/R-induced renal injury
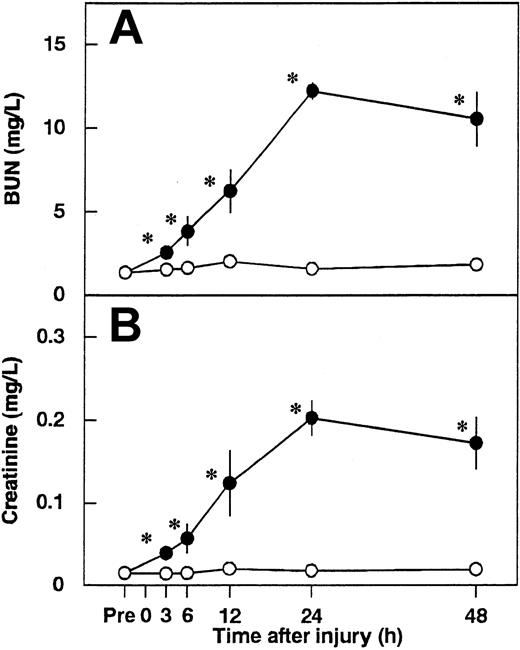
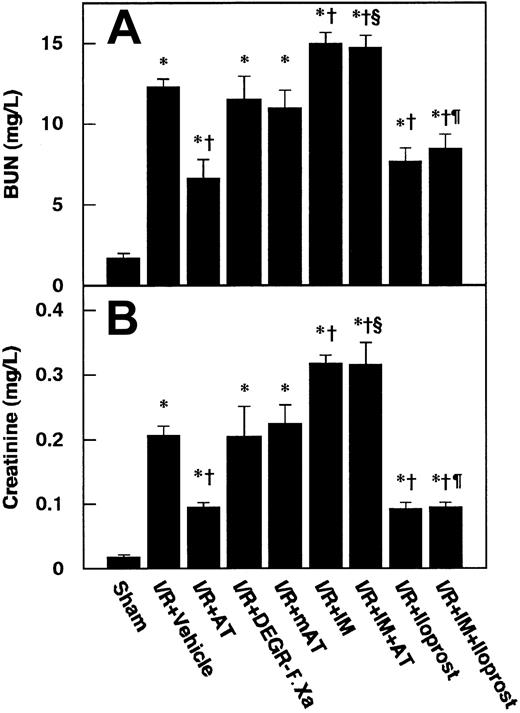
Serum levels of BUN and creatinine were significantly increased and reached a peak level at 24 hours after renal I/R in control animals (Figure 1). We determined the effects of 3 different dosages of AT on renal I/R-induced increases in serum levels of BUN and creatinine. AT, at a dosage of 250 U/kg, significantly inhibited these increases at 24 hours after reperfusion (Figure 2), whereas lower doses of AT (50 and 100 U/kg) had no effect (data not shown). Thus, all subsequent experiments were performed at the dosage of 250 U/kg of AT. Neither DEGR-F.Xa nor Trp49-modified AT had any effect on the renal injury (Figure 2).
Changes in serum levels of blood urea nitrogen (BUN) and creatinine in rats after renal ischemia/reperfusion (I/R).
Serum levels of BUN (A) and creatinine (B) were measured before ischemia (Pre) and at 3, 6, 12, 24, and 48 hours after reperfusion. Data are expressed as mean ± SD values of 6 animals. Open circle indicates sham-operated group; filled circle, vehicle-treated group. *P < .01 versus the sham-operated group.
Changes in serum levels of blood urea nitrogen (BUN) and creatinine in rats after renal ischemia/reperfusion (I/R).
Serum levels of BUN (A) and creatinine (B) were measured before ischemia (Pre) and at 3, 6, 12, 24, and 48 hours after reperfusion. Data are expressed as mean ± SD values of 6 animals. Open circle indicates sham-operated group; filled circle, vehicle-treated group. *P < .01 versus the sham-operated group.
Effects of antithrombin (AT) and other materials on renal ischemia/reperfusion (I/R)–induced increases in serum levels of BUN and creatinine.
Serum levels of BUN (A) and creatinine (B) were determined 24 hours after reperfusion. mAT indicates Trp49-modified AT. Data are expressed as means ± SDs from 6 animals. *P < .01 versus sham-operated group; §P < .05 versus vehicle-treated group; †P < .01 versus AT-treated group; ¶P < .01 versus IM-treated group.
Effects of antithrombin (AT) and other materials on renal ischemia/reperfusion (I/R)–induced increases in serum levels of BUN and creatinine.
Serum levels of BUN (A) and creatinine (B) were determined 24 hours after reperfusion. mAT indicates Trp49-modified AT. Data are expressed as means ± SDs from 6 animals. *P < .01 versus sham-operated group; §P < .05 versus vehicle-treated group; †P < .01 versus AT-treated group; ¶P < .01 versus IM-treated group.
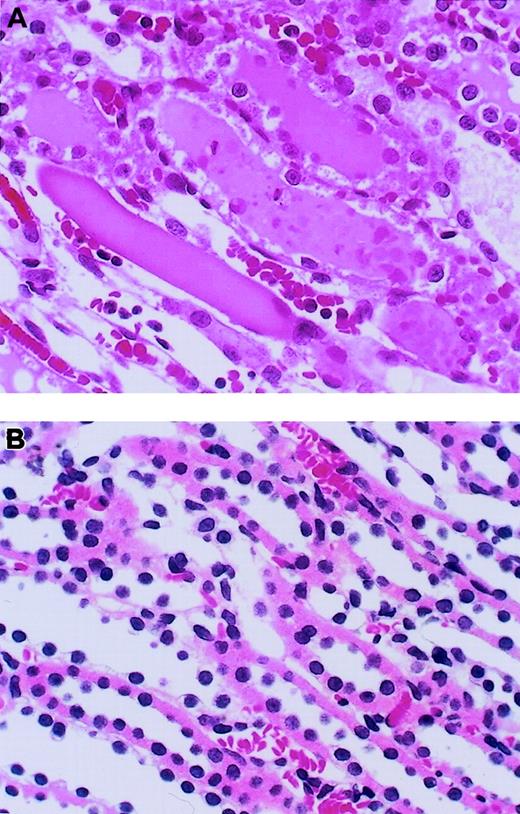
Histologic examinations of kidneys were performed at 24 hours after reperfusion (Figure 3). Microscopic assessment of the outer medulla of the kidney after I/R revealed severe tubular necrosis with cast formation, vascular congestion, and neutrophil accumulation in the vasa recta of the outer medulla (Figure3A). Fibrin deposition was not observed in kidneys from any group. Intravenous administration of AT resulted in a reduction of these histologic changes (Figure 3B), whereas neither DEGR-F.Xa nor Trp49-modified AT had any effects (data not shown). We also analyzed the histologic changes quantitatively using a scoring system for tubular necrosis, vascular congestion, and neutrophil accumulation. These changes were significantly fewer in rats treated with AT than in control animals and those given DEGR-F.Xa or Trp49-modified AT (Table1).
Effect of antithrombin (AT) on renal histopathologic features of outer medulla in rats with ischemia/reperfusion (I/R) injury.
Tissues were obtained 24 hours after reperfusion. (A) Vehicle-treated group; (B) AT-treated group. Original magnification × 400 (hematoxylin-and-eosin stain). Typical results from each group are shown.
Effect of antithrombin (AT) on renal histopathologic features of outer medulla in rats with ischemia/reperfusion (I/R) injury.
Tissues were obtained 24 hours after reperfusion. (A) Vehicle-treated group; (B) AT-treated group. Original magnification × 400 (hematoxylin-and-eosin stain). Typical results from each group are shown.
Tubular necrosis, vascular congestion, and neutrophil accumulation in the outer medulla in rats subjected to renal ischemia followed by reperfusion
| Treatment group . | Tubular necrosis, % . | Erythrocytes per HPF . | Neutrophil accumulation per HPF . |
|---|---|---|---|
| Sham-operated | 0.5 ± 0.8 | 77.7 ± 14.0 | 0.5 ± 0.8 |
| I/R + vehicle | 81.8 ± 6.0* | 332.7 ± 40.9* | 7.2 ± 3.9* |
| I/R + AT | 52.8 ± 11.1*,† | 169.7 ± 68.1*,† | 1.8 ± 1.3† |
| I/R + DEGR-F.Xa | 79.2 ± 5.0* | 299.0 ± 36.7* | 6.7 ± 2.3† |
| I/R + Trp49-modified AT | 78.3 ± 8.8* | 294.7 ± 38.8* | 6.2 ± 3.8* |
| I/R + IM | 86.5 ± 13.3* | 347.7 ± 74.0* | 8.2 ± 3.1* |
| I/R + IM + AT | 80.2 ± 11.0*,‡ | 304.4 ± 44.6*,‡ | 9.1 ± 3.0*,‡ |
| I/R + iloprost | 51.8 ± 16.4*,† | 158.5 ± 68.1*,† | 1.6 ± 1.0† |
| I/R + IM + iloprost | 54.5 ± 13.2*,†,1-153 | 166.3 ± 72.5*,†,1-153 | 1.9 ± 1.4†,1-153 |
| Treatment group . | Tubular necrosis, % . | Erythrocytes per HPF . | Neutrophil accumulation per HPF . |
|---|---|---|---|
| Sham-operated | 0.5 ± 0.8 | 77.7 ± 14.0 | 0.5 ± 0.8 |
| I/R + vehicle | 81.8 ± 6.0* | 332.7 ± 40.9* | 7.2 ± 3.9* |
| I/R + AT | 52.8 ± 11.1*,† | 169.7 ± 68.1*,† | 1.8 ± 1.3† |
| I/R + DEGR-F.Xa | 79.2 ± 5.0* | 299.0 ± 36.7* | 6.7 ± 2.3† |
| I/R + Trp49-modified AT | 78.3 ± 8.8* | 294.7 ± 38.8* | 6.2 ± 3.8* |
| I/R + IM | 86.5 ± 13.3* | 347.7 ± 74.0* | 8.2 ± 3.1* |
| I/R + IM + AT | 80.2 ± 11.0*,‡ | 304.4 ± 44.6*,‡ | 9.1 ± 3.0*,‡ |
| I/R + iloprost | 51.8 ± 16.4*,† | 158.5 ± 68.1*,† | 1.6 ± 1.0† |
| I/R + IM + iloprost | 54.5 ± 13.2*,†,1-153 | 166.3 ± 72.5*,†,1-153 | 1.9 ± 1.4†,1-153 |
Rats underwent 45 minutes of renal ischemia followed by reperfusion. Kidney samples were obtained 24 hours later. Data are expressed as mean ± SD values of 6 experiments.
HPF indicates high-power field; I/R, ischemia followed by reperfusion; AT, antithrombin; DEGR-F.Xa, dansyl glutamylglycylarginyl chloromethyl ketone-treated factor Xa; and IM, indomethacin.
P < .05 versus sham-operated group.
P < .05 versus vehicle-treated group.
P < .05 versus AT-treated group.
P < .05 versus IM-treated group.
Effects of AT, DEGR-F.Xa, and Trp49-modified AT on serum levels of FDP(E) in animals subjected to renal I/R
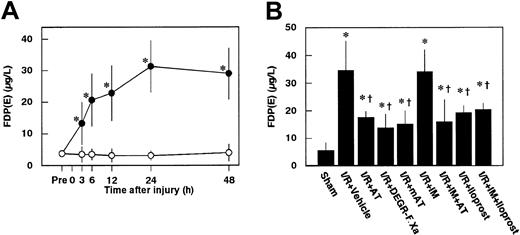
Serum levels of FDP(E) were increased after renal I/R, peaking at 24 hours after reperfusion in control animals (Figure4A). Increases in serum levels of FDP(E) at 24 hours after reperfusion were significantly inhibited by administration of AT, DEGR-F.Xa, and Trp49-modified AT (Figure4B).
Changes in serum levels of FDP(E) in rats subjected to renal ischemia/ reperfusion and effects of antithrombin (AT) and other materials on renal ischemia/reperfusion–induced increases in serum levels of FDP(E).
(A) Serum levels of FDP(E) were determined just before renal ischemia (Pre) and at indicated time points after reperfusion. (B) Serum levels of FDP(E) were determined 24 hours after reperfusion in rats subjected to renal ischemia/reperfusion (I/R). mAT indicates Trp49-modified AT. Data are expressed as mean ± SD values of 6 animals. Open circle indicates sham-operated group; filled circle, vehicle-treated group. *P < .05 versus sham-operated group; †P < .01 versus vehicle-treated group.
Changes in serum levels of FDP(E) in rats subjected to renal ischemia/ reperfusion and effects of antithrombin (AT) and other materials on renal ischemia/reperfusion–induced increases in serum levels of FDP(E).
(A) Serum levels of FDP(E) were determined just before renal ischemia (Pre) and at indicated time points after reperfusion. (B) Serum levels of FDP(E) were determined 24 hours after reperfusion in rats subjected to renal ischemia/reperfusion (I/R). mAT indicates Trp49-modified AT. Data are expressed as mean ± SD values of 6 animals. Open circle indicates sham-operated group; filled circle, vehicle-treated group. *P < .05 versus sham-operated group; †P < .01 versus vehicle-treated group.
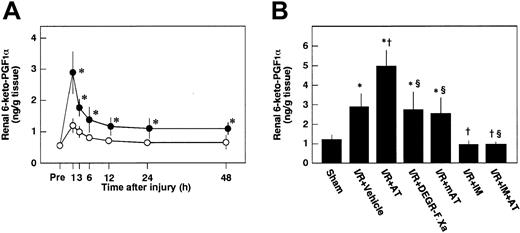
Effects of AT, DEGR-F.Xa, and Trp49-modified AT on renal levels of 6-keto-PGF1α
To examine whether AT enhances the release of PGI2from endothelial cells of the kidney after I/R by interacting with heparin-like GAGs on the endothelial cell surface, we analyzed the effects of AT and Trp49-modified AT on renal tissue levels of 6-keto-PGF1α after reperfusion. Renal tissue levels of 6-keto-PGF1α were increased after renal I/R in control animals, peaking at 1 hour after reperfusion (Figure5A). These levels were significantly higher than those of sham-operated animals (Figure 5A). AT significantly enhanced I/R-induced increases in renal tissue levels of 6-keto-PGF1α 1 hour after reperfusion (Figure 5B), whereas neither DEGR-F.Xa nor Trp49-modified AT had any effect on these changes (Figure 5B).
Changes in renal levels of 6-keto-PGF1α in rats subjected to renal ischemia/reperfusion and effects of antithrombin (AT) and other materials on renal ischemia/reperfusion–induced increases in renal levels of 6-keto-PGF1α.
(A) Renal levels of 6-keto-PGF1α were determined just before ischemia (Pre) and at indicated time points after reperfusion. (B) Renal levels of 6-keto-PGF1α were determined 1 hour after reperfusion in rats subjected to renal ischemia/reperfusion (I/R). mAT indicates Trp49-modified AT. Data are expressed as means ± SDs from 6 animals. *P < .01 versus sham-operated group; †P < .05 versus vehicle-treated group; §P < .01 versus AT-treated group.
Changes in renal levels of 6-keto-PGF1α in rats subjected to renal ischemia/reperfusion and effects of antithrombin (AT) and other materials on renal ischemia/reperfusion–induced increases in renal levels of 6-keto-PGF1α.
(A) Renal levels of 6-keto-PGF1α were determined just before ischemia (Pre) and at indicated time points after reperfusion. (B) Renal levels of 6-keto-PGF1α were determined 1 hour after reperfusion in rats subjected to renal ischemia/reperfusion (I/R). mAT indicates Trp49-modified AT. Data are expressed as means ± SDs from 6 animals. *P < .01 versus sham-operated group; †P < .05 versus vehicle-treated group; §P < .01 versus AT-treated group.
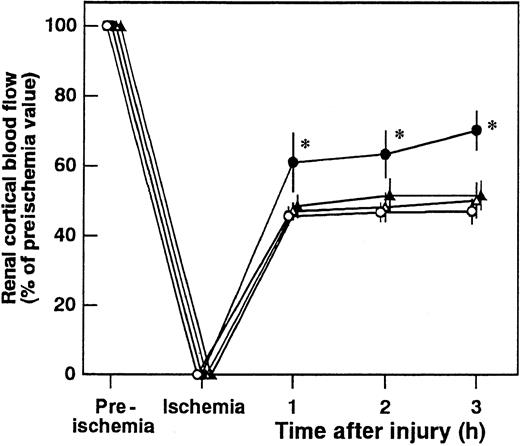
Effects of AT, DEGR-F.Xa, and Trp49-modified AT on renal cortical blood flow
Renal cortical blood flow decreased to approximately 40% of the preischemic level at 3 hours after reperfusion (Figure6). AT significantly inhibited the I/R-induced reduction in renal cortical blood flow. Neither DEGR-F.Xa nor Trp49-modified AT had any effect on the renal cortical blood flow (Figure 6).
Effect of antithrombin (AT), DEGR-F.Xa, and Trp49-modified AT on renal cortical blood flow during ischemia/reperfusion (I/R) injury in rats.
Renal cortical blood flow was measured from 30 minutes before ischemia until 3 hours after reperfusion. Data are expressed as means ± SDs of 5 experiments. Open circle indicates vehicle-treated group; filled circle, AT-treated group; open triangle, DEGR-F.Xa–treated group; filled triangle, Trp49-modified AT-treated group. *P < .05 versus vehicle-treated group.
Effect of antithrombin (AT), DEGR-F.Xa, and Trp49-modified AT on renal cortical blood flow during ischemia/reperfusion (I/R) injury in rats.
Renal cortical blood flow was measured from 30 minutes before ischemia until 3 hours after reperfusion. Data are expressed as means ± SDs of 5 experiments. Open circle indicates vehicle-treated group; filled circle, AT-treated group; open triangle, DEGR-F.Xa–treated group; filled triangle, Trp49-modified AT-treated group. *P < .05 versus vehicle-treated group.
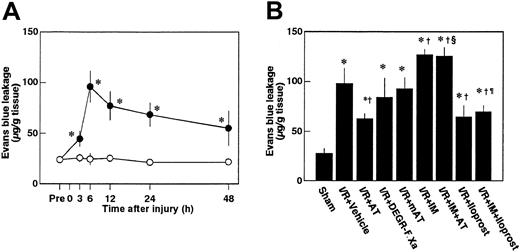
Effects of AT, DEGR-F.Xa, and Trp49-modified AT on renal vascular permeability
Renal vascular permeability, as assessed by Evans blue dye leakage, increased significantly after reperfusion, reaching a maximum level at 6 hours after injury (Figure7A). AT significantly inhibited the increases in renal vascular permeability 6 hours after reperfusion, whereas neither DEGR-F.Xa nor Trp49-modified AT inhibited these changes (Figure 7B).
Changes in renal vascular permeability in rats subjected to renal ischemia/reperfusion and effects of antithrombin (AT) and other materials on renal ischemia/reperfusion (I/R)–induced increases in renal vascular permeability.
(A) Renal vascular permeability was evaluated just before ischemia (Pre) and at indicated time points after reperfusion. (B) Renal vascular permeability was evaluated 6 hours after reperfusion in rats subjected to renal ischemia/reperfusion. mAT indicates Trp49-modified AT. Data are expressed as mean ± SD values of 6 animals. Open circle indicates sham-operated group; filled circle, vehicle-treated group. *P < .01 versus sham-operated group; †P < .05 versus vehicle-treated group; §P < .01 versus AT-treated group; ¶P < .01 versus IM-treated group.
Changes in renal vascular permeability in rats subjected to renal ischemia/reperfusion and effects of antithrombin (AT) and other materials on renal ischemia/reperfusion (I/R)–induced increases in renal vascular permeability.
(A) Renal vascular permeability was evaluated just before ischemia (Pre) and at indicated time points after reperfusion. (B) Renal vascular permeability was evaluated 6 hours after reperfusion in rats subjected to renal ischemia/reperfusion. mAT indicates Trp49-modified AT. Data are expressed as mean ± SD values of 6 animals. Open circle indicates sham-operated group; filled circle, vehicle-treated group. *P < .01 versus sham-operated group; †P < .05 versus vehicle-treated group; §P < .01 versus AT-treated group; ¶P < .01 versus IM-treated group.
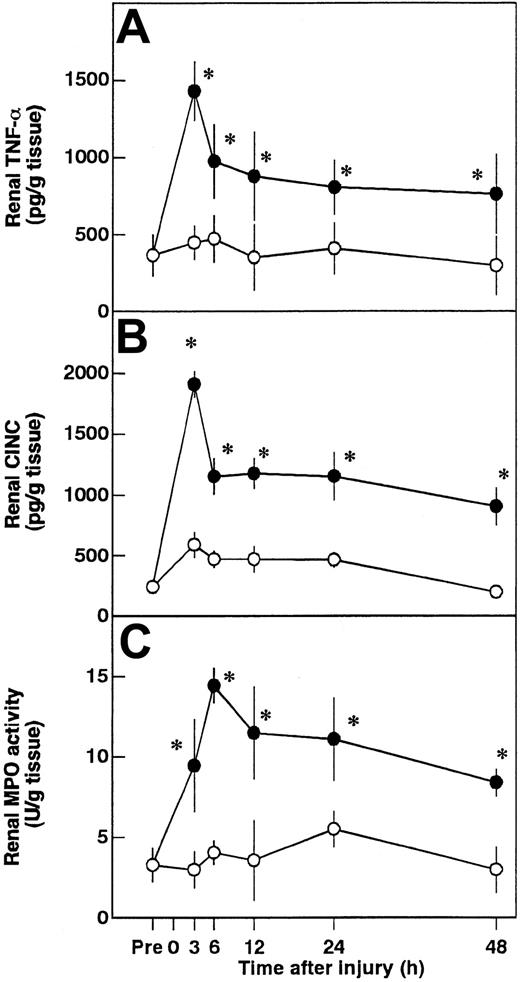
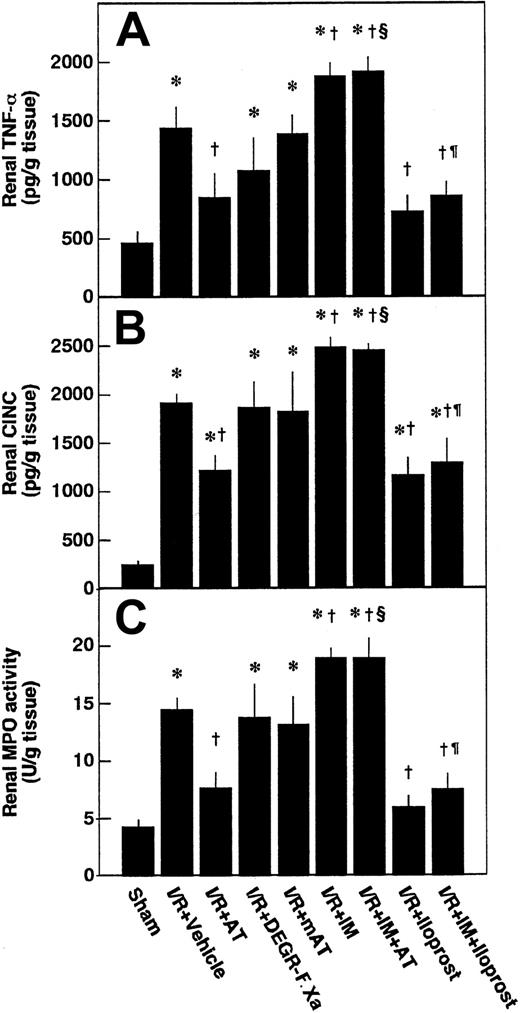
Effects of AT, DEGR-F.Xa, and Trp49-modified AT on renal tissue levels of TNF-α, CINC, and MPO after renal I/R
Renal tissue levels of TNF-α and CINC were increased after renal I/R, reaching peak levels at 3 hours after reperfusion and gradually decreasing thereafter (Figure8A-B). Accumulation of neutrophils in renal tissue was assessed by determination of renal MPO activity. Renal tissue levels of MPO were increased after renal I/R, reaching a maximum at 6 hours after reperfusion (Figure 8C). These variables reflecting leukocyte activation in animals subjected to renal I/R were significantly higher than those of sham-operated animals (Figure 9). Increases in renal tissue levels of TNF-α, CINC, and MPO were significantly inhibited by AT (Figure 9). Neither DEGR-F.Xa nor Trp49-modified AT had any effect on these changes (Figure 9).
Changes in renal tumor necrosis factor-α (TNF-α), cytokine-induced neutrophil chemoattractant (CINC), and myeloperoxidase (MPO) activity.
Renal tissue levels of TNF-α (A), CINC (B), and MPO (C) activity were measured before ischemia (Pre) and at 3, 6, 12, 24, and 48 hours after reperfusion. Data are mean ± SD values of 6 animals. Open circle indicates sham-operated group; filled circle, vehicle-treated group. *P < .05 versus sham-operated group.
Changes in renal tumor necrosis factor-α (TNF-α), cytokine-induced neutrophil chemoattractant (CINC), and myeloperoxidase (MPO) activity.
Renal tissue levels of TNF-α (A), CINC (B), and MPO (C) activity were measured before ischemia (Pre) and at 3, 6, 12, 24, and 48 hours after reperfusion. Data are mean ± SD values of 6 animals. Open circle indicates sham-operated group; filled circle, vehicle-treated group. *P < .05 versus sham-operated group.
Effects of antithrombin (AT) and other materials on renal ischemia/reperfusion (I/R)–induced increases in renal levels of tumor necrosis factor-α (TNF-α), cytokine-induced neutrophil chemoattractant (CINC), and myeloperoxidase (MPO) activity.
Renal levels of TNF-α and CINC were determined 3 hours after reperfusion and renal levels of MPO activity were determined at 6 hours after reperfusion. mAT indicates Trp49-modified AT. Data are expressed as mean ± SD values of 6 animals. *P < .01 versus sham-operated group; †P < .01 versus vehicle-treated group; §P < .01 versus AT-treated group; ¶P < .01 versus IM-treated group.
Effects of antithrombin (AT) and other materials on renal ischemia/reperfusion (I/R)–induced increases in renal levels of tumor necrosis factor-α (TNF-α), cytokine-induced neutrophil chemoattractant (CINC), and myeloperoxidase (MPO) activity.
Renal levels of TNF-α and CINC were determined 3 hours after reperfusion and renal levels of MPO activity were determined at 6 hours after reperfusion. mAT indicates Trp49-modified AT. Data are expressed as mean ± SD values of 6 animals. *P < .01 versus sham-operated group; †P < .01 versus vehicle-treated group; §P < .01 versus AT-treated group; ¶P < .01 versus IM-treated group.
Effects of IM pretreatment on AT-induced effects in renal I/R
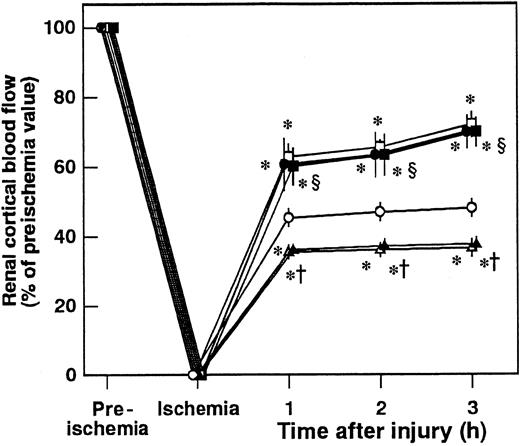
Pretreatment of animals with IM significantly prevented increases in renal tissue levels of 6-keto-PGF1α in control animals and those in animals given AT at 1 hour after reperfusion (Figure 5B). AT did not inhibit I/R-induced increases in serum levels of BUN and creatinine at 24 hours after reperfusion in animals pretreated with IM. IM itself also significantly exacerbated these increases at 24 hours after reperfusion compared with controls (Figure 2B). Pretreatment with IM reversed AT-induced effects on histologic findings in animals subjected to renal I/R (Table 1). AT significantly inhibited I/R-induced increase in serum levels of FDP(E) in animals pretreated with IM (Figure 4B). IM itself significantly reduced renal cortical blood flow and increased vascular permeability (Figures 7B and10). Effects of AT on I/R-induced changes in both renal cortical blood flow and renal vascular permeability were reversed by pretreatment with IM (Figures 7B and 10). AT did not reduce the I/R-induced increases in renal tissue levels of TNF-α, CINC, and MPO at 24 hours after reperfusion in animals pretreated with IM (Figure 9). IM itself significantly enhanced the I/R-induced increases in renal tissue levels of TNF-α, CINC, and MPO (Figure9).
Effects of antithrombin (AT), indomethacin (IM), and iloprost on renal cortical blood flow during ischemia/reperfusion (I/R) injury in rats.
Renal cortical blood flow was measured from 30 minutes before ischemia until 3 hours after reperfusion. Data are expressed as means ± SDs of 5 experiments. Open circle indicates vehicle-treated group; filled circle, AT-treated group; open triangle, IM-treated group; filled triangle, AT-treated group pretreated with IM; open square, iloprost-treated group; filled square, iloprost-treated group pretreated with IM. *P < .05 versus vehicle-treated group; †P < .01 versus AT-treated group; §P < .01 versus IM-treated group.
Effects of antithrombin (AT), indomethacin (IM), and iloprost on renal cortical blood flow during ischemia/reperfusion (I/R) injury in rats.
Renal cortical blood flow was measured from 30 minutes before ischemia until 3 hours after reperfusion. Data are expressed as means ± SDs of 5 experiments. Open circle indicates vehicle-treated group; filled circle, AT-treated group; open triangle, IM-treated group; filled triangle, AT-treated group pretreated with IM; open square, iloprost-treated group; filled square, iloprost-treated group pretreated with IM. *P < .05 versus vehicle-treated group; †P < .01 versus AT-treated group; §P < .01 versus IM-treated group.
Effects of iloprost on the I/R-induced renal changes
Iloprost, a stable derivative of PGI2, produced effects similar to those induced by AT. Iloprost significantly reduced renal dysfunction (Figure 2), inhibiting both reduction of renal cortical blood flow (Figure 10) and increase in renal vascular permeability (Figure 7B) in animals subjected to renal I/R. Increases in renal tissue levels of TNF-α, CINC, and MPO were significantly inhibited by iloprost (Figure 9). Furthermore, iloprost reversed the IM-induced exacerbation of renal changes in animals subjected to renal I/R (Figures 2,7B,9,10). Iloprost inhibited renal I/R-induced increases in serum levels of FDP(E) and those in animals pretreated with IM (Figure 4B).
Discussion
In the present study, we demonstrated that AT reduced renal dysfunction and histologic changes of the kidney in rats subjected to renal I/R independently of its anticoagulant activity, because DEGR-F.Xa did not reduce I/R-induced renal injury despite its anticoagulant activity comparable to that of AT.
Activated leukocytes are critically involved in the development of the I/R-induced renal injury.25 This notion was confirmed in the present study by the findings that renal tissue levels of TNF-α, CINC, and MPO were significantly increased after renal I/R. These variables reflecting leukocyte activation were significantly reduced by AT, suggesting that AT might reduce renal injury by inhibiting leukocyte activation.
AT inhibited I/R-induced reduction of renal cortical blood flow and increase in renal vascular permeability as shown in the present study. Endothelial cell injury induced by inflammatory mediators released from activated neutrophils such as neutrophil elastase and oxygen-free radicals are critically involved in the renal I/R-induced increase in vascular permeability, leading to the reduction of renal cortical blood flow.25 Our preliminary experiments demonstrating that ONO-5046, a specific inhibitor of neutrophil elastase,33 inhibited the reduction of renal cortical blood flow by inhibiting increase in renal vascular permeability. These observations strongly suggested that AT might inhibit I/R-induced decrease in renal cortical blood flow by inhibiting neutrophil activation.
Renal I/R-induced histologic changes included tubular necrosis, vascular congestion, and neutrophil accumulation in the outer medulla as shown in the present study. Because the main site of inflammation where neutrophils are activated is the postcapillary venules feeding proximal tubules in the kidney,34 activated neutrophil-induced endothelial cell injury might lead to ischemia of the proximal tubules in the outer medulla. Consequently, plug formation in the lumen of proximal tubules might lead to renal dysfunction after the renal I/R.35 Thus, AT might improve the I/R-induced renal dysfunction by reducing the tubular necrosis induced by activated neutrophils in the present study.
It is well known that neutrophils are activated by TNF-α and CINC, which are elaborated by monocytes stimulated with oxygen-free radicals generated during I/R.36 Because I/R-induced increases in renal tissue levels of TNF-α and CINC were significantly inhibited by AT, AT might reduce renal injury by inhibiting neutrophil activation through inhibition of production of these cytokines.
Recently, results of in vitro experiments raised the possibility that AT might inhibit leukocyte activation directly. Dunzendorfers et al13 reported that AT directly inhibited neutrophil chemotaxis by binding to the cell surface–heparan sulfate glycosaminoglycans. Souter et al14 demonstrated that AT directly inhibited interleukin-6 production by monocytes stimulated with lipopolysaccharide. Furthermore, Oelschläger et al15 demonstrated that AT directly inhibited TNF-α production by inhibiting NF-κB activation in lipopolysaccharide-stimulated monocytes. These observations suggest that AT might reduce I/R-induced renal injury by directly inhibiting leukocyte activation. However, this possibility seems less likely, because therapeutic effects and inhibition of leukocyte activation observed in animals given AT were not seen in animals pretreated with indomethacin. These observations also suggested that AT-induced inhibition of leukocyte activation is mainly mediated by prostaglandins. Because AT has been shown to promote the endothelial release of PGI2 by interacting with the cell surface heparin-like GAGs in vivo11 and PGI2 inhibits TNF-α production by inhibiting NF-κB activation,8-10it is possible that inhibition of leukocyte activation by AT is mainly mediated by PGI2. Consistent with this hypothesis are the observations in the present study that I/R-induced increases in the renal tissue levels of 6-keto-PGF1α, a stable metabolite of PGI2, were significantly enhanced by AT, but not by Trp49-modified AT, which is incapable of promoting the endothelial release of PGI2 due to the lack of affinity for heparin-like GAGs.11 Iloprost produced effects similar to those induced by AT, also supporting the above hypothesis.
Indomethacin inhibits activities of cyclooxygenases, thereby inhibiting production of some eicosanoids other than PGI2. Therefore, some other eicosanoids might be involved in both the IM-induced aggravation of I/R-induced renal injury and therapeutic mechanisms of AT in the present study. Because PGE2 as well as PGI2 have also been shown to inhibit leukocyte activation and both prostaglandins are synthesized from PGH2 in endothelial cells,9 37 it is likely that PGE2might be involved in therapeutic effects of AT in the present study. Consistent with this hypothesis are the results of our preliminary experiments showing that AT reduced the I/R-induced hepatic injury by promoting the I/R-induced increases in hepatic tissue levels of PGE2 in rats. This possibility should further be examined using this rat model of I/R-induced renal injury.
Singbartl et al38 demonstrated that platelets as well as neutrophils could also be critically involved in the development of I/R-induced renal injury by promoting neutrophil infiltration to the outer medulla of kidney, the most vulnerable region of the kidney after I/R. Because PGI2 potently inhibits platelet activation,37 AT might reduce the I/R-induced renal injury by inhibiting activation of platelets as well as neutrophils in the present study.
The molecular mechanism by which AT increases the endothelial production of PGI2 remains to be elucidated. We demonstrated that AT did not increase the production of PGI2 directly in cultured endothelial cells,39suggesting that some factors other than endothelial cells might be involved in the AT-induced endothelial production of PGI2in vivo. We recently demonstrated that activation of capsaicin-sensitive sensory neurons led to an increase in the endothelial production of PGI2 in rats subjected to hepatic I/R.40 We also reported briefly that AT reduced I/R-induced liver injury by increasing the hepatic tissue levels of 6-keto-PGF1α through activation of capsaicin-sensitive sensory neurons in rats.41 We are currently investigating the precise mechanisms by which AT enhances the endothelial production of PGI2 through this neuronal mechanism using this rat model of I/R-induced renal injury.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood- 2002-08-2406.
Supported in part by the departmental funds of Kumamoto University School of Medicine.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenji Okajima, Department of Laboratory Medicine, Kumamoto University School of Medicine, Honjo 1-1-1, Kumamoto, 860-0811, Japan; e-mail:whynot@kaiju.medic.kumamoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal