Abstract
The tetrapeptide acetyl-Ser-Asp-Lys-Pro (AcSDKP), purified from bone marrow and constitutively synthesized in vivo, belongs to the family of negative regulators of hematopoiesis. It protects the stem cell compartment from the toxicity of anticancer drugs and irradiation and consequently contributes to a reduction in marrow failure. This current work provides experimental evidence for another novel biologic function of AcSDKP. We report that AcSDKP is a mediator of angiogenesis, as measured by its ability to modulate endothelial cell function in vitro and angiogenesis in vivo. AcSDKP at nanomolar concentrations stimulates in vitro endothelial cell migration and differentiation into capillary-like structures on Matrigel as well as enhances the secretion of an active form of matrix metalloproteinase-1 (MMP-1). In vivo, AcSDKP promotes a significant angiogenic response in the chicken embryo chorioallantoic membrane (CAM) and in the abdominal muscle of the rat. Moreover, it induces the formation of blood vessels in Matrigel plugs implanted subcutaneously in the rat. This is the first report demonstrating the ability of AcSDKP to interact directly with endothelial cells and to elicit an angiogenic response in vitro and in vivo.
Introduction
The results of recent studies indicate that several cytokines and interleukins initially thought to be specific for the hematopoietic system are also capable of inducing responses in endothelial cells.1-5 Alternatively, the ability of angiogenic factors to affect several functions of hematopoietic cells has also been reported.6-8 Indeed, the responsiveness of both the hematopoietic and vascular system to the same factors probably reflects the common origin of hematopoietic and endothelial cells.9 10
The tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (AcSDKP), purified from bone marrow and present in blood at nanomolar concentrations, is recognized as a physiologic regulator of hematopoiesis.11 It inhibits the proliferation of normal hematopoietic stem cells and committed progenitors in vivo as well as in vitro11-14 and consequently reduces the damage to the stem cell compartment resulting from treatment with chemotherapeutic agents or ionizing radiation.15-18 We have previously shown that AcSDKP is degraded in the circulation by the angiotensin I–converting enzyme (ACE)19,20 and that administration of specific inhibitors of this protease (ACEIs) significantly reduces the proliferation of primitive hematopoietic cells.21-23 The antiproliferative ability of ACEIs, demonstrated both in vitro and in vivo, could be mediated by endogenous AcSDKP, concentrations of which are systematically increased following ACEI administration. Interestingly, ACEIs also modulate angiogenesis,24-26 although the mechanism of this activity remains unclear. It is therefore possible to hypothesize that the increased levels of AcSDKP following administration of ACEIs may contribute, at least in part, to the angiogenic activity of these compounds.
Our hypothesis that AcSDKP has angiogenic activity follows also results of recent studies on thymosin β4 (Tβ4), the potential metabolic precursor of the tetrapeptide. Indeed, it has been previously suggested that AcSDKP, which is constitutively produced by the bone marrow cells,27 may be generated from Tβ4, which contains an AcSDKP sequence at the N-terminus.28 Tβ4 is a 5-kDa ubiquitous polypeptide and has been identified as a major sequestering molecule of globular actin in motile and proliferating cells.29 It has been shown to share similar biologic properties with AcSDKP. It inhibits the proliferation of hematopoietic stem cells and protects mice against chemotoxic agents at the same concentrations as AcSDKP.30,31 These observations, together with the recent findings reporting the ability of Tβ4 to induce an angiogenic response both in vivo and in vitro,32 33 has raised the possibility that AcSDKP may also participate in the activation of endothelial cells and prompted us to investigate the role of AcSDKP in the regulation of angiogenesis.
Angiogenesis, the formation of new capillaries by the sprouting of pre-existing blood vessels, occurs both during embryonic development and in postnatal life. It is a critical process not only during normal growth, but also in pathologic situations, where inadequate vascular growth contributes to health complications. Angiogenesis is a complex multistep phenomenon. It involves the degradation of extracellular matrix and the subsequent migration, proliferation, and differentiation of endothelial cells. These cells form tubelike structures that subsequently organize into a capillary network. Angiogenesis is predominantly regulated by local factors that promote or inhibit neovascularization.34
The findings presented in this paper contribute to the emerging complex interactions of factors that regulate growth of the blood vessels. We report here that the tetrapeptide AcSDKP, a negative regulator of hematopoiesis, is a potent angiogenic factor both in vitro and in vivo. In vitro, AcSDKP promotes an angiogenic response of endothelial cells, namely, an increase in the secreted active form of matrix metalloproteinase-1 (MMP-1), and the stimulation of cell migration and differentiation into capillary-like structures on Matrigel. In vivo, AcSDKP induces neovascularization when applied on the surface of the chorioallantoic membrane (CAM) of the chick embryo and when injected in the abdominal muscle of the rat. Moreover, it stimulates vascular invasion in Matrigel plugs implanted subcutaneously in the rat. These data demonstrate the potential of AcSDKP as an angiogenesis promoter that may prove to be an effective agent for induction of therapeutic angiogenesis in the clinic.
Materials and methods
Peptides
The synthetic AcSDKP (molecular weight 487) was kindly provided by IPSEN-Biotech (Paris, France) and the inactive analog of the tetrapeptide, AcSDDKP, (l-Asp replaced byd-Asp) by Dr J. Thierry (ICSN, CNRS, Gif-sur-Yvette, France). Heparin affin regulatory peptide (HARP) was generously supplied by Dr J. Courty (University Paris XII, France). Basic fibroblast growth factor (bFGF) was from Sigma (St Louis, MO).
Animals
All in vivo experiments were carried out with adult male Sprague-Dawley (Janvier, Le Genest-St-Isle, France) or Wistar (IFFA CREDO, St Germain, France) rats. The animals were kept under specific pathogen-free conditions.
Cell culture
Immortalized EA.hy926 endothelial cells derived from fusion of human umbilical vein endothelial cells (HUVECs) with A549 lung carcinoma cells35 were generously provided by Dr E. C. J. Edgell (University of North Carolina, Chapel Hill, NC). Cells were grown in Dulbecco minimal essential medium (DMEM) containing 4.5 g/L glucose supplemented with 10% fetal calf serum (FCS), 1% glutamine, and HAT (100 μM hypoxantine, 0.4 μM aminopterin, 16 μM thymidine from Invitrogen; Cergy-Pontoise, France). Bovine brain capillary (BBC) endothelial cells (kindly provided by J. Courty, University Paris XII) were maintained in DMEM containing 4.5 g/L glucose supplemented with 10% FCS, 1% glutamine, and 1 ng/mL bFGF. HUVECs purchased from Biowhittaker Europe (Verviers, Belgium) were grown in M199 medium supplemented with 20% FCS, 15 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 15 mM sodium bicarbonate, and 1% glutamine. Lung human microvascular endothelial cells (HMVEC-Ls) purchased from Biowhittaker Europe were grown in EGM-2MV growth medium (Biowhittaker Europe) consisting of endothelial cell basal medium supplemented with human recombinant epidermal growth factor (10 ng/mL), hydrocortisone (1 μg/mL), gentamicin (50 μg/mL), amphotericin B (50 μg/mL), and fetal bovine serum (5% vol/vol). Cultures were maintained at 37°C and 5% CO2 in a humidified atmosphere. Cells were routinely harvested by trypsin treatment (0.5 g/L trypsin/0.2 g/L EDTA [ethylenediaminetetraacetic acid] from Invitrogen).
Detection and measurement of AcSDKP levels
AcSDKP concentration was measured using a highly specific competitive enzyme immunoassay (EIA) with acetylcholinesterase conjugate as a tracer (SPIbio, Massy, France). The quantitative limit of this assay was 0.5 pmol and the precision was characterized by a variation coefficient less than 10% in the 1- to 10-nM range. AcSDKP was quantified in the methanol extracts of EA.hy926 endothelial cells and in their serum-free conditioned media. Then 4 mL ice-cold methanol was added either to 1 mL sonicated cell suspension or to 1 mL conditioned medium both supplemented with lisinopril (Sigma) at 10−6 M final concentration to prevent AcSDKP degradation. Following 30 minutes of incubation at 4°C all samples were centrifuged for 15 minutes at 3500 rpm and the supernatants were evaporated to dryness under vacuum (Speed Vac Concentrator, Savant, Holbrook, NY). The extracts were then diluted in 0.25 mL EIA buffer and used for the dosage. The results are expressed in picomoles AcSDKP per milliliter supernatant or per 1 × 106 endothelial cells and are the mean of 4 experiments.
Matrix MMP enzyme-linked immunosorbent assay
EA.hy926 cells at 90% confluence were rinsed twice with serum-free DMEM and incubated for 24 hours in serum-free DMEM in the absence or presence of AcSDKP at various concentrations. The conditioned media were then collected, centrifuged at 1200 rpm for 10 minutes, and stored at −20°C until analyzed. The amounts of MMP-1 (EC 3.4.24.7), MMP-2 (EC 3.4.24.24), and MMP-9 (EC 3.4.24.25), as either free active or total (sum of active and proforms) enzyme, were determined in conditioned media using Biotrack cellular communication assays (Amersham Pharmacia Biotech, Orsay, France) according to the manufacturer's recommendations. Results are the mean of 6 independent experiments performed in triplicate.
Endothelial cell migration assay
In vitro endothelial cell chemotactic evaluation was performed using 24-well Boyden chambers (8-μm pore polycarbonate polyvinylpyrrolidone-free filters; Costar, Avon, France). The bottom chamber was filled with 0.6 mL serum-free DMEM containing 0.1% Albumax in the absence or the presence of various concentrations of AcSDKP or 1 ng/mL bFGF. EA.hy926 endothelial cells resuspended in serum-free DMEM containing 0.1% Albumax were loaded into the upper chamber of a Transwell insert (105 cells in 0.1 mL) and incubated at 37°C for 6 hours. The filters were then removed and the nonmigrated cells on the top of the membranes were removed using cotton swabs. The migrated cells attached to the bottom of the membrane were fixed with methanol and stained with Diff-Quick (Dade Behring, Düdingen, Swizerland). Cell migration was quantified by counting cells in 10 random microscopic fields per filter at × 20 magnification using an Olympus IMT-2 inverted phase-contrast microscope. Assays were performed in triplicate and repeated 3 times. Results are expressed as the fold increase over control values (basal migration toward medium alone).
Matrigel tube formation assay
The spontaneous formation of capillary-like structures by endothelial cells on a basement membrane matrix preparation (Matrigel, Becton Dickinson, Bedford, MA) was used to assess the angiogenic potential of AcSDKP. The 4-well plates (Lab-Tech, Nunc, Roskilde, Denmark) were coated with 300 μL Matrigel (13 mg/mL) that was allowed to solidify for 1 hour at 37°C. EA.hy926 cells (2 × 105 cells/mL/well) were plated onto the surface of the Matrigel in serum-free DMEM containing AcSDKP at different concentrations or 1 ng/mL bFGF. Medium alone was used as a control. After 2, 4, 6, and 18 hours of incubation at 37°C in a 5% CO2 humidified atmosphere, cellular organization into tubular structures was investigated using an inverted phase-contrast photomicroscope and photographs taken at × 4 and × 10 magnifications. Each assay was performed in triplicate and the results are the mean of 5 independent experiments. Results are expressed as the percentage increase in tube formation compared with control values.
In vivo Matrigel plug angiogenesis assay
An aliquot (0.5 mL) of Matrigel containing either saline or AcSDKP at different concentrations was injected subcutaneously into the mid-abdominal region of Sprague-Dawley rats. After 7 days, the animals were killed and the Matrigel plugs removed and fixed in 4% formaldehyde solution. Preparations were subsequently embedded in paraffin, sectioned, stained with hematoxylin-eosin-saffron, and examined for the in-growth of blood vessel. Ten random fields (31.4 mm2 total) localized on the border of plugs were analyzed for capillary growth. Photographs were taken at × 10 magnification. The experiment was repeated 3 times with 4 rats per condition in each experiment. Results are expressed as the percentage increase observed in the vessel counts (Matrigel containing AcSDKP) compared to control values (Matrigel alone).
Chicken embryo CAM assay
The in vivo chicken embryo CAM angiogenesis model was used to investigate the angiogenic potency of AcSDKP. Leghorn fertilized eggs (Pindos, Epirus, Greece) were incubated for 4 days at 37°C; thereafter, a window was opened on the eggshell, exposing the CAM. The window was covered with sterile tape and the eggs were returned to the incubator. At day 9 of embryo development, 20 μL distilled water containing different amounts of AcSDKP (0.002-200 pmol) was applied on an area of 1 cm2 of the CAM restricted by a plastic ring. Following 48 hours of subsequent incubation at 37°C, CAMs were fixed in situ, excised from the eggs, placed on slides, and left to air-dry. Photographs were taken using a stereoscope equipped with a digital camera. The total length of the vessels was measured as previously described.44 Water alone was used as a control. HARP, recognized as a potent angiogenic inducer in the chicken embryo CAM,36 was used as a positive control and AcSDDKP, a stereoisomer of AcSDKP, as a negative control. Each sample was tested 3 times using 10 to 20 eggs for each data point. Results are expressed as the percentage increase in the vessel length compared to control values.
In vivo effects of AcSDKP on vascularization of the abdominal muscle
To assess the effects of AcSDKP on normal vasculature, we performed local intramuscular injections into the abdominal wall of adult Wistar rats. All animals were approximately 300 g and injections were performed percutaneously using a 30-gauge needle, injecting 300 μL volume into the ventrolateral muscles of the abdomen. A dose of 5 μg AcSDKP/kg/injection was delivered twice a day for 5 consecutive days. Sterile saline was injected as an excipient. Rats were housed individually and allowed food and water ad libitum. Animals were analyzed at day 7 and day 28 to investigate vascularization of the ventrolateral muscles of the abdomen. Ketamine-xylazine (Centravet, Plancoet, France) anesthesia was administered intraperitoneally and a median abdominal incision was performed. Careful dissection of the skin allowed visualization of muscular layer of the abdominal wall. A small catheter was inserted into the descending aorta below the renal arteries and above the bifurcation of the iliac arteries. India ink was used to visualize the vasculature and to assay vascular permeability. Analysis focused on the epigastric arteries and the ascending branch of the lateral femoral arteries. All animals were killed by an excess dose of anesthetic agents. Three independent experiments with 4 animals per control and treated group (2 from day 7 and 2 from day 28) were included in this study.
Statistical analysis
All results were expressed as the mean ± SEM. Significance of differences was determined with the Student t test with significance at P < .05.
Results
Endothelial cells contain and secrete AcSDKP
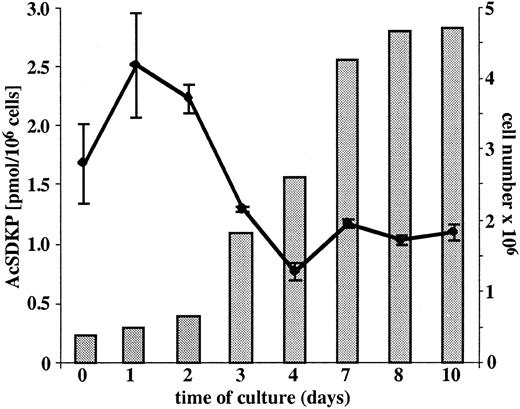
AcSDKP concentrations were measured in growing EA.hy926 endothelial cells and in the conditioned media of nonconfluent and confluent EA.hy926 cells. As shown in Figure1, the intracellular concentration of AcSDKP is related to the growth phase of the cells and varies from 0.78 ± 0.07 to 2.53 ± 0.44 pmol/106 cells. The concentrations of AcSDKP present in the serum-free supernatants of either nonconfluent or confluent endothelial cells were found to be between 1.03 ± 0.07 and 1.13 ± 0.06 pmol, respectively, at the second and seventh day of culture.
Concentration of AcSDKP in cultured EA.hy926 cells.
The intracellular concentration of AcSDKP (plotted line) was measured daily in EA.hy926 cells grown to confluence in 75-cm2 flasks. Following trypsin detachment, the cells were counted (full bars), washed twice with culture medium, sonicated, and AcSDKP extracted with methanol according to the procedure described in “Materials and methods.” The extracellular level of AcSDKP was evaluated in the supernatants collected from EA.hy926 cell layers grown in complete medium for 2 and 7 days and then cultured for 24 hours in the serum-free medium. The results are the mean ± SEM of 4 experiments.
Concentration of AcSDKP in cultured EA.hy926 cells.
The intracellular concentration of AcSDKP (plotted line) was measured daily in EA.hy926 cells grown to confluence in 75-cm2 flasks. Following trypsin detachment, the cells were counted (full bars), washed twice with culture medium, sonicated, and AcSDKP extracted with methanol according to the procedure described in “Materials and methods.” The extracellular level of AcSDKP was evaluated in the supernatants collected from EA.hy926 cell layers grown in complete medium for 2 and 7 days and then cultured for 24 hours in the serum-free medium. The results are the mean ± SEM of 4 experiments.
AcSKP induces directional migration of endothelial cells
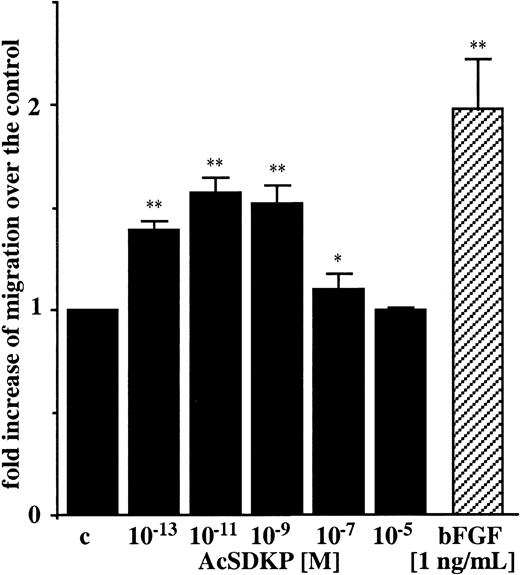
Regarding the ability of angiogenic factors to induce migration of endothelial cells, we tested different concentrations of AcSDKP, ranging from 10−13 M to 10−5 M using an in vitro chemotactic assay. As shown in Figure2, AcSDKP induced chemotaxis of EA.hy926 cells in a dose-dependent manner, with a typical bell-shaped curve. The maximal stimulation of EA.hy926 cell migration (1.57-fold increase) was observed with 10−11 M AcSDKP. This was about 20% lower than the chemotactic activity of bFGF (1.98-fold increase) included as a positive control and used at 5 × 10−11 M solution (1 ng/mL). Similar results were obtained with BBC cells with 10−9 M AcSDKP. A 1.63 ± 0.23-fold increase (P < .01) after 4 hours in migration was induced compared with untreated control cells.
AcSDKP is a chemoattractant for endothelial cells.
Chemotaxis of EA.hy926 cells toward different concentrations of AcSDKP was assayed in 24-well Boyden chambers for 6 hours and quantified as the number of migrated cells per 10 fields/filter. Results are expressed as the fold increase of migrated cells over the control values (c). Data are the means ± SEM of 3 independent experiments performed in triplicate. *P < .05; **P < .01.
AcSDKP is a chemoattractant for endothelial cells.
Chemotaxis of EA.hy926 cells toward different concentrations of AcSDKP was assayed in 24-well Boyden chambers for 6 hours and quantified as the number of migrated cells per 10 fields/filter. Results are expressed as the fold increase of migrated cells over the control values (c). Data are the means ± SEM of 3 independent experiments performed in triplicate. *P < .05; **P < .01.
AcSDKP induces endothelial cell differentiation on Matrigel
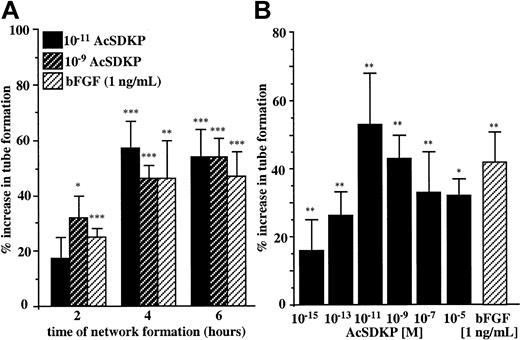
An important step in angiogenesis is the ability of endothelial cells to differentiate in response to specific stimuli and to migrate out of the existing blood vessels to organize new capillaries. To investigate the morphogenic potential of AcSDKP we used an in vitro assay that allowed the visualization of multicellular tubelike structures that resemble microvascular networks on Matrigel. We first evaluated the optimal time course for EA.hy926 endothelial cells to respond to AcSDKP used at 10−11 M and 10−9 M according to its previously determined maximal chemotactic activity. As shown in Figure 3A, the formation of tubelike structures (visible rings and cords) by EA.hy926 cells plated on Matrigel was significantly stimulated in the presence of 10−11 M and 10−9 M AcSDKP after 2 hours of incubation. The maximal stimulatory effect of the tetrapeptide (corresponding to 54% increase in tube formation) was measured after 6 hours of incubation. The data presented in Figure 3B indicate that AcSDKP stimulated tube formation by EA.hy926 cells in a dose-dependent manner. Stimulation was detectable at 10−15 M and reached its maximum when AcSDKP was present at 10−11 M. Higher concentrations of AcSDKP were less effective at inducing EA.hy926 cell morphogenesis, resulting in a bell-shaped dose-response curve similar to that observed for many angiogenic factors. The stimulatory effect of AcSDKP at 10−11 M was greater than the activity of bFGF assessed at 1 ng/mL (5 × 10−11 M). The accelerated formation of tubelike structures in the presence of AcSDKP was also observed for other endothelial cells. Thus, after 6 hours of treatment with 10−9 M AcSDKP, the increase in tube formation was 112% ± 20% for BBC cells, 33% ± 3% for HMVEC-Ls, and 31% ± 10% for HUVECs.
Morphogenic activity of AcSDKP.
(A) EA.hy926 cells were seeded on Matrigel and incubated for 2, 4, and 6 hours in the presence of 10−11 M and 10−9 M AcSDKP or 1 ng/mL bFGF. (B) EA.hy926 cells plated on Matrigel in the presence of various concentrations of AcSDKP or 1 ng/mL bFGF were cultured for 6 hours. Surface areas of the tubelike structure were evaluated by planimetry on at least 2 fields per well. Results are the means ± SEM of 5 independent experiments each performed in triplicate. *P < .05; **P < .01; ***P < .001.
Morphogenic activity of AcSDKP.
(A) EA.hy926 cells were seeded on Matrigel and incubated for 2, 4, and 6 hours in the presence of 10−11 M and 10−9 M AcSDKP or 1 ng/mL bFGF. (B) EA.hy926 cells plated on Matrigel in the presence of various concentrations of AcSDKP or 1 ng/mL bFGF were cultured for 6 hours. Surface areas of the tubelike structure were evaluated by planimetry on at least 2 fields per well. Results are the means ± SEM of 5 independent experiments each performed in triplicate. *P < .05; **P < .01; ***P < .001.
AcSDKP activates MMP-1
Angiogenic promoters induce several responses in endothelial cell cultures including the secretion of matrix MMPs, which are thought to play a critical role in endothelial cell migration during angiogenesis. To determine whether AcSDKP stimulated the secretion of MMPs, EA.hy926 cells were cultured for 24 hours in the presence of AcSDKP. Levels of secreted MMP-1, MMP-2, and MMP-9 were measured in the culture medium using a specific enzyme-linked immunosorbent assay (ELISA) that allows quantification of both total and active forms of these proteins. Taking into account the maximal chemotactic activity of AcSDKP observed at 10−9 M and 10−11 M, we used the tetrapeptide at these 2 concentrations. As shown in Table1, AcSDKP induces a significant increase in the active form of MMP-1, without modifying the secretion of the total amount of this protein with a maximal effect observed at 10−9 M (84% over the control). Secretion of MMP-2 was not increased in the presence of AcSDKP. MMP-9 was not detected under any of the experimental conditions.
AcSDKP enhances the secretion of active form of MMP-1
| . | MMP-1 . | MMP-2 . | ||
|---|---|---|---|---|
| . | A . | T . | A . | T . |
| Control | 36 ± 9 | 145 ± 23 | 22 ± 10 | 110 ± 31 |
| AcSDKP, 10−9M | 67 ± 9* | 164 ± 14 | 28 ± 6 | 120 ± 6 |
| AcSDKP, 10−11M | 50 ± 7† | 143 ± 9 | 21 ± 1 | 122 ± 7 |
| . | MMP-1 . | MMP-2 . | ||
|---|---|---|---|---|
| . | A . | T . | A . | T . |
| Control | 36 ± 9 | 145 ± 23 | 22 ± 10 | 110 ± 31 |
| AcSDKP, 10−9M | 67 ± 9* | 164 ± 14 | 28 ± 6 | 120 ± 6 |
| AcSDKP, 10−11M | 50 ± 7† | 143 ± 9 | 21 ± 1 | 122 ± 7 |
EA.hy926 cells were grown for 24 hours in serum-free DMEM in the presence or absence (control) of AcSDKP. Active (A) or total (T: pro + active) secreted forms of MMP-1 and MMP-2 were quantified by specific ELISA in EA.hy926 cell–conditioned media and calculated in ng/106 cells. Results are expressed as the mean ± SEM of 6 independent experiments.
P < .01.
P < .05.
Angiogenic effect of AcSDKP in CAM assay
The chicken embryo CAM was used as a suitable model to investigate the ability of AcSDKP to modulate angiogenesis in vivo under physiologic conditions. A range of doses between 0.002 and 200 pmol/cm2 AcSDKP was assayed on the CAM at day 9 of embryo development. As shown in Figure 4, AcSDKP induced angiogenesis in a dose-dependent manner, reaching the maximal stimulatory effect at 200 pmol/cm2. The stereoisomer of the tetrapeptide, AcSDDKP, assayed at 2 pmol/cm2, had no effect on CAM vascular density. HARP, a potent inductor of angiogenesis in the CAM and assayed at 100 pmol/cm2, exhibited similar stimulatory potency to AcSDKP at 200 pmol/cm2.
AcSDKP promotes an angiogenic response in the chicken embryo CAM.
AcSDKP, AcSDDKP, and HARP were diluted in 20 μL distilled water and applied to a 1-cm2 area of the CAM on the ninth day of embryo development. The vascular density was estimated 48 hours later using image analysis software. Distilled water, which was used as a solvent, served as an excipient control. AcSDDKP, a stereoisomer of AcSDKP, was used as a negative peptide control and HARP as a positive control. Data are the mean ± SEM of 3 independent experiments each using 10 to 20 embryonated eggs per data point. **P < .01; ***P < .001.
AcSDKP promotes an angiogenic response in the chicken embryo CAM.
AcSDKP, AcSDDKP, and HARP were diluted in 20 μL distilled water and applied to a 1-cm2 area of the CAM on the ninth day of embryo development. The vascular density was estimated 48 hours later using image analysis software. Distilled water, which was used as a solvent, served as an excipient control. AcSDDKP, a stereoisomer of AcSDKP, was used as a negative peptide control and HARP as a positive control. Data are the mean ± SEM of 3 independent experiments each using 10 to 20 embryonated eggs per data point. **P < .01; ***P < .001.
Angiogenic effect of AcSDKP in the in vivo Matrigel plug assay
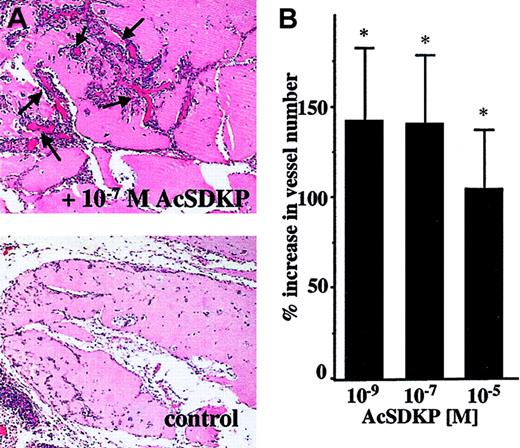
To further assess the angiogenic properties of AcSDKP in vivo, we evaluated blood vessel invasion into Matrigel plugs implanted subcutaneously in rats. As shown in Figure5, AcSDKP significantly stimulates the vascular invasion of Matrigel plugs removed 7 days after implantation. Histologic analysis of Matrigel sections (Figure 5A) identifies new blood vessel development in the AcSDKP-containing plug (upper panel) as compared to the control plug (lower panel). The quantitative evaluation of vessels present in the border region of plugs indicates that AcSDKP induces a significant angiogenic response when used at concentrations of 10−5 M, 10−7 M, and 10−9 M (Figure 5B).
AcSDKP-induced angiogenesis in Matrigel plugs.
Rats were injected subcutaneously with 0.5 mL Matrigel containing saline or AcSDKP. Matrigel plugs were removed after 7 days and processed for histology as described in “Materials and methods.” (A) A representative image of Matrigel plug sections containing saline or 10−7 M AcSDKP (original magnification × 10); arrows indicate newly formed vessels. (B) Vessel numbers were scored in 10 random fields (31.4 mm2 total) localized on the border of plugs. Data are the mean ± SEM of 3 individual experiments including 4 animals per condition in each experiment.
AcSDKP-induced angiogenesis in Matrigel plugs.
Rats were injected subcutaneously with 0.5 mL Matrigel containing saline or AcSDKP. Matrigel plugs were removed after 7 days and processed for histology as described in “Materials and methods.” (A) A representative image of Matrigel plug sections containing saline or 10−7 M AcSDKP (original magnification × 10); arrows indicate newly formed vessels. (B) Vessel numbers were scored in 10 random fields (31.4 mm2 total) localized on the border of plugs. Data are the mean ± SEM of 3 individual experiments including 4 animals per condition in each experiment.
AcSDKP increases vascular density in abdominal muscle of rat
Skeletal muscle is one of the most plastic tissues in the body. To assess angiogenic effect of AcSDKP on the perfusion of abdominal muscles, the tetrapeptide was administrated at 5 μg/kg/injection delivered twice a day for 5 consecutive days and the vascular pattern visualized by India ink angiography. After 7 or 28 days, comparative analysis of the vascular density in the left (treated with AcSDKP) versus the right (control) side of abdominal muscle showed a greater diffusion of the dye in the AcSDKP-injected muscles than that in the contralateral muscles (Figure 6). Effects of the injections of AcSDKP were limited to the region of administration and there was no angioma-like structure or inflammatory infiltration observed in either side. Furthermore, the effects on normal vasculature persisted for up to day 28.
Arteriography of rat ventrolateral abdominal wall performed 7 and 28 days after AcSDKP injection.
AcSDKP was injected into the abdominal wall of rats. Control injections were made with sterile saline. Animals were analyzed at day 7 and day 28 following first day of treatment. Bilateral angiography was obtained using India ink injection into the terminal aorta as described in “Materials and methods.” The markings on the ruler represent cm.
Arteriography of rat ventrolateral abdominal wall performed 7 and 28 days after AcSDKP injection.
AcSDKP was injected into the abdominal wall of rats. Control injections were made with sterile saline. Animals were analyzed at day 7 and day 28 following first day of treatment. Bilateral angiography was obtained using India ink injection into the terminal aorta as described in “Materials and methods.” The markings on the ruler represent cm.
Discussion
This work provides evidence for the angiogenic properties of the tetrapeptide AcSDKP, which belongs to the family of negative physiologic regulators of hematopoiesis. We have demonstrated the ability of this peptide to induce functional angiogenesis in vitro and in vivo. As previously reported, AcSDKP is present in plasma at nanomolar concentrations and has a wide tissue distribution but is most abundant in hematopoietic organs such as the thymus, spleen, and bone marrow.37,38 Measurable tetrapeptide concentrations have also been detected in the intestine, heart, stomach, and lung, which are highly vascular organs.38 Using a highly specific and sensitive EIA, we have shown that the EA.hy926 human endothelial cell line produces AcSDKP and releases it at nanomolar concentrations. The intracellular concentration of AcSDKP increases at the beginning of culture and decreases progressively as the cells approach confluence. These data are consistent with the recently reported presence of AcSDKP in extracts of bone marrow endothelial cells39,40 and suggest that AcSDKP produced constitutively by the endothelium may act in an autocrine manner to induce angiogenesis. Here we report that AcSDKP indeed acts in vitro as a positive regulator of endothelial cell motility and differentiation, 2 essential components for new vessel formation. Maximal chemotactic activity was obtained at 10−11 M to 10−9 M AcSDKP and was similar to that observed for bFGF. The ability of endothelial cells to respond to the same concentrations of AcSDKP was further confirmed by the results of morphogenic assay representative of both migration and differentiation of endothelial cells. The key events regulating cell motility are the polymerization of actin and the formation of actin stress fibers and focal adhesion.41However, AcSDKP does not affect actin assembly at concentrations up to 50 μM and does not compete with Tβ4 for binding to G-actin42; therefore, the observed enhancement of cell migration cannot be mediated by the effect of AcSDKP on actin assembly. Here we report that one of the cellular events induced by AcSDKP is the production of MMP-1. MMPs are thought to play a crucial role in endothelial cell migration and matrix remodeling during angiogenesis.43,44 MMPs are generally secreted by endothelium as zymogens, which are inactive or latent proforms of the enzymes that are next subsequently cleaved to their active forms. Our studies revealed that AcSDKP directly enhances the conversion of the proform of MMP-1 (collagenase I) to its active form without any effect on the total content of this enzyme. Indeed, the up-regulation of MMP-1 following stimulation of endothelial cells by bFGF and vascular endothelial growth factor (VEGF)45,46 was shown to be an absolute requirement for angiogenesis in vitro.47 All these findings indicate that AcSDKP is able to stimulate the early invasive phases of the angiogenic process that leads to endothelial sprouting, characterized by an increase in cell migration and matrix degradation. The enhanced ability of endothelial cells cultured with AcSDKP in Matrigel to form capillary-like structures indicates that this tetrapeptide is also able to stimulate the late differentiation phase required for the formation of hollow vascular structures. Interestingly, the stimulation achieved by AcSDKP at 10−11M in cell assays was similar to that observed for bFGF, a potent promoter of neovascularization, tested at 5 × 10−11 M (1 ng/mL).
In view of the findings obtained in vitro with cultured endothelial cells, it was important to extend the study with AcSDKP into in vivo models. The angiogenic potential of AcSDKP was clearly evident in vivo in the CAM assay. The intensity of the vasoproliferative response defined as vascular index was similar to that observed for HARP, a potent inductor of neovascularization.36 Subsequent work has demonstrated the ability of AcSDKP to induce the recruitment of new vessels into a Matrigel plug implanted in animals at nanomolar concentrations. One important event in endothelial cell invasion is the interaction of endothelial cells with the protein components of the extracellular matrix such as fibronectin and vitronectin.48 Preliminary data have shown the increased adherence of EA.hy926 cells on fibronectin in the presence of AcSDKP (data not shown). These findings are consistent with results obtained using the Matrigel plug assay.
Taking into account the in vivo angiogenic potency of AcSDKP demonstrated in 2 widely used assays for studying angiogenesis as well as the ability of AcSDKP to stimulate neovascularization in normal mammalian muscle, it is possible to hypothesize that the administration of this peptide may improve the insufficient vascularization in the animal model. Indeed, pharmacologic stimulation of angiogenesis is currently under investigation as an important adjunct to conventional therapeutic strategies for the treatment of ischemia, wound healing, and tissue transplantation.49
In vivo, AcSDKP certainly exhibits its angiogenic activity on bone marrow endothelium providing a possible mechanism for the enhancement of stem cell engraftment observed following AcSDKP administration at the time of transplantation. It has been shown that AcSDKP significantly increases the survival of lethally irradiated mice that receive transplants of the unfractionated bone marrow cells. This effect was mediated by an increased homing of hematopoietic precursors in the bone marrow as early as 1 day after transplantation.50 However, another report suggests that AcSDKP may improve the engraftment of ex vivo expanded hematopoietic stem cells through a mechanism unrelated to its activity as a stem cell cycle inhibitor.51 Given the results presented in this report, we hypothesize that AcSDKP mediates homing of hematopoietic stem cells from the peripheral blood to the bone marrow via its activity on endothelial cells.
Bone marrow endothelial cells are intimately involved not only in the homing of hematopoietic progenitor cells to the bone marrow but also in the regulation of their proliferation and differentiation.52 53 The mechanism of inhibitory action of AcSDKP toward hematopoietic stem cell proliferation, however, is far from clear. Nevertheless, evidence suggests that AcSDKP could exert its effect on hematopoietic cells indirectly through its action on accessory cells. The present findings may allow speculation that the interaction of AcSDKP with endothelial cells may regulate putative secondary signals leading directly to the inhibition of primitive hematopoietic cells progression into S phase. Putative regulation may have an impact on endothelial adhesion molecules or the production of specific cytokines.
Angiogenesis is an important process in a variety of pathologic disorders, including tumor growth and metastasis.54 It has long been thought that leukemia and other hematologic malignancies differ from solid tumors in that they do not stimulate or require angiogenesis. However, it is now clear that a large number of hematologic disorders including leukemia, multiple myeloma, polycythemia vera, and myelofibrosis are angiogenesis dependent and reveal significantly increased neovascularity.55,56 It has been found that the microvessel density in bone marrow is tightly correlated with the progression of all these diseases and that the level of intracellular VEGF and FGF can be considered as a predictor of outcome in patients with myeloproliferative diseases.57,58Interestingly, it was observed that the serum concentrations of AcSDKP are frequently higher in adult patients with hematologic malignancies than in healthy subjects.59 These results are consistent with the previous findings that the up-expression of Tβ4, the potential precursor of AcSDKP, occurs in the neoplastic tissues.60 61 Given our present findings and increasing evidence relating angiogenesis and hematoproliferative diseases, it is possible that AcSDKP may contribute not only to leukemic but also to solid tumor angiogenesis.
Taken together, these reports provide the first evidence that AcSDKP has angiogenic potential in addition to its role as an inhibitor of primitive hematopoietic cell proliferation. Moreover, the data suggest that AcSDKP may have therapeutic potential for the clinical treatment of deficient angiogenesis-related diseases. However, a detailed analysis of the role of AcSDKP in maintaining normal vascularization needs to be undertaken. Furthermore, the reported in vivo efficacy of AcSDKP to promote the growth of new vessels suggests that it may be a potential target for therapeutic antiangiogenic strategies targeting mainly tumor dormancy or stabilization. The molecular mechanisms responsible for the angiogenic activity of AcSDKP are currently under investigation. Whatever the modes of action of AcSDKP, it is clear that it exhibits a number of interesting biologic activities that are possibly a consequence of molecular cross-talk due to the redundancy within the numerous angiogenic and hematopoietic pathways supporting these processes. In fact, the angiogenic property of AcSDKP may be pivotal in controlling both hematopoiesis and bone marrow engraftment.
The authors thank Dr Cora J. Edgell for generously supplying EA.hy926 cells. We also thank Dr Josette Badet and Dr José Courty for their helpful advice during these studies and Dr Andrew Riches for his constant interest in our work. The authors wish to thank Dr André Balaton for his expertise with the Matrigel plug assay and Widad Farid for her expert technical assistance. We are extremely grateful to Dr Simon Robinson for critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-07-2315.
Supported by a grant funded by Institut de Chimie des Substances Naturelles, CNRS (M.K.).
J.-M.L. and F.L. contributed equally to this work and share first authorship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joanna Wdzieczak-Bakala, ICSN, CNRS, 91198 Gif-sur-Yvette, France; e-mail:johanna.bakala@icsn.cnrs-gif.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal