The incidence of mold infections in patients with hematologic malignancies continues to increase despite the widespread use of air filtration systems, suggesting the presence of other hospital sources for these molds. Water sources are known to harbor pathogenic molds. We examined samples from water, water surfaces, air, and other environment sources from a bone marrow transplantation unit with optimal air precautions. Molds (Aspergillus species, others) were recovered in 70% of 398 water samples, in 22% of 1311 swabs from plumbing structures and environmental surfaces, and in 83% of 274 indoor air samples. Microscopic examination of the water plumbing lines revealed hyphal forms compatible with molds. Four findings suggest that indoor airborne molds were aerosolized from the water: (1) higher mean airborne concentrations of molds in bathrooms (16.1 colony-forming units [CFU]/m3) than in patient rooms (7 CFU/m3) and hallways (8.6 CFU/m3;P = .00005); (2) a strong type and rank correlation between molds isolated from hospital water and those recovered from indoor hospital; (3) lack of seasonal correlation between the airborne mold concentration in outdoor and indoor air; and (4) molecular relatedness between a clinical strain and a water-related strain (previously reported). Hospital water distribution systems may serve as a potential indoor reservoir of Aspergillus and other molds leading to aerosolization of fungal spores and potential exposure for patients.
Introduction
Nosocomial opportunistic mold infections can be life-threatening in immunocompromised patients, particularly those with hematologic malignancies. These molds are thought to arise from contaminated outdoor air that infiltrates hospital ventilation systems.1,2 Prevention of these infections is critical and has relied on air handling systems such as high-efficiency particulate air (HEPA) filters and laminar airflow (LAF). However, the incidence of mold infections continues to rise despite the widespread use of these air filtration systems,3 suggesting that other hospital sources for molds may exist. We have previously shown thatFusarium species colonize the water system of a hospital and causes nosocomial fusariosis.4 Water systems worldwide have also been shown to be colonized with pathogenic molds.5-7 This 3-year prospective surveillance study of a hospital caring for immunocompromised patients demonstrates that opportunistic molds may colonize hospital water distribution systems and thus lead to spore aerosolization in patient care areas and patient exposure.
Materials and methods
The hospital
The hospital is located in Little Rock, AR, and has 2 bone marrow transplantation (BMT) units. One unit (A) is housed in a newly constructed patient care tower (built in 1997), whereas the second unit (B) is in the old hospital building (built in 1955). Both units are equipped with central HEPA filtration. Unit A also has 3 LAF rooms. All HEPA and LAF filters are monitored and maintained per industry standards.
The hospital water is supplied by the Little Rock Municipal Water Works and meets water industry standards.8 The municipal water enters the hospital from 2 separate substations. One serves the new patient care tower (unit A), and the other serves the old hospital (unit B). After entry into the new building, cold water is pumped immediately to all patient wards. Cold water is also distributed to 2 instantaneous water heaters and then sent to all floors. After entry into the old hospital building, cold water is pumped to 2 large water storage tanks housed on the hospital roof and then gravity-fed to unit B. Cold water is also sent to 4 large heating tanks and pumped to all floors of the old building.
Environmental sampling
Environmental sampling was performed prospectively during a 3-year period (1997-2000). Samples were obtained according to room availability. A total of 416 water samples (1 L each) was collected from municipal mains, cold- and hot-water storage tanks, and showers and sinks in patient rooms. All water samples were collected in sterile polystyrene bottles containing 0.8 mL 3% sodium thiosulfate and passed through sterile 0.45-μm filters (Millipore, Bedford, MA) using a filtration apparatus (Millipore). Using sterile forceps, the filters were placed directly on Sabouraud dextrose agar plates.
A total of 1311 swab cultures from the water distribution system and environmental surfaces was obtained using sterile Culturette swabs (Becton Dickinson, Franklin Lakes, NJ). Sites that were sampled included the inner walls of storage tanks and plumbing lines of shower heads and sinks, sink and shower drains, surfaces of sinks and showers, and internal and external components of disassembled shower heads and faucet aerators. Other surfaces that were swabbed included door and windowsills, floors, walls, HEPA filter surfaces, stethoscope pads, thermometers, curtains, light fixtures, and toilet seats and bowls. Plumbing lines and drains were swabbed by ringing the inner surface of the line once. Flat surfaces were swabbed over an area of approximately 25 cm2.
A total of 283 air samples was collected using a 6-stage Andersen Bioaerosol Sampler (Andersen Instruments, Atlanta, GA) as previously described.9 Air samples were recovered from outdoor air; patient shower facilities, bathrooms, and rooms; and adjoining hallways of HEPA-filtered and LAF rooms.
All samples (water, swabs, and air) were placed on Sabouraud dextrose agar with chloramphenicol and gentamicin, incubated at 30°C, and checked daily for growth for at least 28 days. All fungi were identified according to established methods.10 Colony counts were enumerated as colony-forming units per liter (CFU/L) for water and CFU/m3 for air. 9
Ten swabs of plumbing structures and lines were mounted on slides and stained with lactophenol cotton blue. The slides were then examined by gross microscopy. The free chlorine levels of municipal, cold-water tank, and cold end-use product at patient taps were tested using test strips (Daigger, Lincolnshire, IL).
Statistical analysis
The number of positive samples from each source was compared by 2 × 2 table analysis, with χ2 statistics or 2-tailed Fisher exact test depending on the number of cases. The Mann-WhitneyU test was used for 2-group comparison between independent concentrations of molds from each source. For comparison among more than 2 groups, the Kruskal-Wallis analysis of variance test was used.
Results
Water samples
Molds were recovered from the municipal water, hospital water storage tanks, and hospital water from patient care areas (total of 416 water samples; Table 1). The mean levels of free chlorine in municipal water, cold-water storage tanks, and cold tap water were within standard guidelines at 0.2, 0.1 to 0.2, and 0.05 ppm, respectively (data not shown).
Detection of molds in hospital water using water-filtration sampling methods
| Source of water . | Total positive sites/total tested sites (%) . | Molds recovered* . | Positive sites by mold . | Mean (range) concentration, CFU/L† . |
|---|---|---|---|---|
| Municipal water | 12/18 (67) | Penicillium | 6 | 7.4 (1-20) |
| Aspergillus | 6 | 1.9 (1-4) | ||
| Chaetomium | 2 | 1 (1-1) | ||
| Chrysonilia | 2 | 1 (1-1) | ||
| Water tanks | 33/40 (82) | Aspergillus | 30 | 5.2 (1-24) |
| Penicillium | 17 | 7.1 (1-20) | ||
| Alternaria | 10 | 2.7 (1-10) | ||
| Trichoderma | 8 | 2.2 (1-5) | ||
| Fusarium | 8 | 2.6 (1-9) | ||
| Paecilomyces | 7 | 9.9 (1-30) | ||
| Mucor | 4 | 1.8 (1-4) | ||
| Acremonium | 4 | 2.3 (1-5) | ||
| Chrysonilia | 3 | 2.7 (1-4) | ||
| Sinks | 104/162 (64) | Penicillium | 71 | 5.5 (1-20) |
| Aspergillus | 30 | 2.3 (1-20) | ||
| Showers | 144/196 (73) | Penicillium | 86 | 7.4 (1-20) |
| Aspergillus | 46 | 2.1 (1-20) | ||
| Paecilomyces | 27 | 10.3 (1-23) |
| Source of water . | Total positive sites/total tested sites (%) . | Molds recovered* . | Positive sites by mold . | Mean (range) concentration, CFU/L† . |
|---|---|---|---|---|
| Municipal water | 12/18 (67) | Penicillium | 6 | 7.4 (1-20) |
| Aspergillus | 6 | 1.9 (1-4) | ||
| Chaetomium | 2 | 1 (1-1) | ||
| Chrysonilia | 2 | 1 (1-1) | ||
| Water tanks | 33/40 (82) | Aspergillus | 30 | 5.2 (1-24) |
| Penicillium | 17 | 7.1 (1-20) | ||
| Alternaria | 10 | 2.7 (1-10) | ||
| Trichoderma | 8 | 2.2 (1-5) | ||
| Fusarium | 8 | 2.6 (1-9) | ||
| Paecilomyces | 7 | 9.9 (1-30) | ||
| Mucor | 4 | 1.8 (1-4) | ||
| Acremonium | 4 | 2.3 (1-5) | ||
| Chrysonilia | 3 | 2.7 (1-4) | ||
| Sinks | 104/162 (64) | Penicillium | 71 | 5.5 (1-20) |
| Aspergillus | 30 | 2.3 (1-20) | ||
| Showers | 144/196 (73) | Penicillium | 86 | 7.4 (1-20) |
| Aspergillus | 46 | 2.1 (1-20) | ||
| Paecilomyces | 27 | 10.3 (1-23) |
Aspergillus species included A niger(77%), A fumigatus (11%), A terreus (9%), andA flavus (3%). All the Paecilomyces species were P lilacinus. Three of 14 Fusarium species could be identified; all 3 were F solani.
Molds shown are those that accounted for ≥ 10% of positive water samples. Other molds recovered include Acremonium, Alternaria, Bipolaris, Chrysonilia, Cladosporium, Chrysosporium, Curvularia, Epicoccum, Mucor, Nigrospora, and Ulocladium species.
Represents the mean concentration of the positive cultures from the specified site.
A total of 109 water samples yielded Aspergillus species. The species of Aspergillus found were all recognized opportunistic pathogens, and included A niger (77%),A fumigatus (11%), A terreus (9%), and A flavus (3%). All the Paecilomyces species found in 44 water samples were P lilacinus. Three of 14Fusarium species recovered from water samples were identified to species level. All 3 were F solani (known pathogens). The mean concentration ± SD of molds was 9-fold higher in the water tanks than in the municipal water: mean 11.4 ± 14.0 versus. 1.3 ± 1.7 CFU/L, respectively (P < .01). With the exception of Chaetomiumspecies, all molds recovered from municipal water were also present in the water tank. Penicillium species andAspergillus species were the 2 most common molds recovered from municipal water and from hospital water. Penicilliumspecies were present in 33%, 42%, and 43% of the samples of municipal water, water storage tanks, and water from patient care areas, respectively, whereas Aspergillus species were recovered in 33%, 55%, and 21%, respectively, of water samples obtained from these same sites.
Samples of the first liter of water from taps yielded a higher rate of molds (190 of 250, 70%, mean 5.2 CFU/L) than sampling the second liter (58 of 108, 54%, mean 4.4 CFU/L; P = < .001).
Microscopic examination of lactophenol cotton blue stains of swabs taken from internal plumbing lines revealed hyphal elements compatible with molds (data not shown).
Surface swabs
Molds were recovered on the interior surfaces of water lines, sink drains, and sink plumbing lines, shower drains, shower head surfaces, shower head plumbing lines, and toilet bowls (total of 1311 swabs; Table 2). A total of 58 surface swab samples yielded Aspergillus species including A niger (50%), A fumigatus (19%), A terreus(14%), A flavus (9%), A nidulans (7%), andA sydowi (1%). All Paecilomyces species recovered from swab samples were P lilacinus. Twelve of 37Fusarium species isolated from 34 swab samples could be identified to species level. Eight (67%) were F solani and 4 (33%) were F oxysporum. Cultures of doors, furniture, and personal objects did not yield molds. All molds that were identified are known to cause infections in patients with cancer. Environmental objects (HEPA filter surfaces, walls, floors, and windowsills) harbored molds less frequently than did hospital water samples (38 of 174, 32% versus 248 of 358, 67%, respectively; P = < .001).
Detection of molds in hospital plumbing and on environmental surfaces using the swab culture sampling method
| Source . | Total positive sites/total tested sites (%) . | Molds recovered* . | Positive sites by molds . |
|---|---|---|---|
| Water tanks, inner walls/draining ports | 13/48 (27) | Alternaria | 7 |
| Bipolaris | 4 | ||
| Penicillium | 4 | ||
| Cladosporium | 3 | ||
| Acremonium | 2 | ||
| Sinks† | 78/334 (23) | Paecilomyces | 38 |
| Acremonium | 23 | ||
| Penicillium | 6 | ||
| Showers‡ | 147/721 (20) | Paecilomyces | 63 |
| Aspergillus | 30 | ||
| Penicillium | 30 | ||
| Fusarium | 25 | ||
| Acremonium | 20 | ||
| Toilets2-153 | 8/34 (23) | Aspergillus | 4 |
| Penicillium | 5 | ||
| Paecilomyces | 1 | ||
| Cladosporium | 1 | ||
| Patient rooms2-154 | 38/174 (22) | Penicillium | 19 |
| Aspergillus | 12 | ||
| Alternaria | 10 | ||
| Cladosporium | 6 |
| Source . | Total positive sites/total tested sites (%) . | Molds recovered* . | Positive sites by molds . |
|---|---|---|---|
| Water tanks, inner walls/draining ports | 13/48 (27) | Alternaria | 7 |
| Bipolaris | 4 | ||
| Penicillium | 4 | ||
| Cladosporium | 3 | ||
| Acremonium | 2 | ||
| Sinks† | 78/334 (23) | Paecilomyces | 38 |
| Acremonium | 23 | ||
| Penicillium | 6 | ||
| Showers‡ | 147/721 (20) | Paecilomyces | 63 |
| Aspergillus | 30 | ||
| Penicillium | 30 | ||
| Fusarium | 25 | ||
| Acremonium | 20 | ||
| Toilets2-153 | 8/34 (23) | Aspergillus | 4 |
| Penicillium | 5 | ||
| Paecilomyces | 1 | ||
| Cladosporium | 1 | ||
| Patient rooms2-154 | 38/174 (22) | Penicillium | 19 |
| Aspergillus | 12 | ||
| Alternaria | 10 | ||
| Cladosporium | 6 |
Aspergillus species recovered in surface swab samples included A niger (50 %), A fumigatus (19 %),A terreus (14 %), A flavus (9 %),A nidulans (7%), and A sydowi (1%). All thePaecilomyces species were P lilacinus. Twelve of 37 Fusarium species could be identified; 8 (67%) wereF solani and 4 (33%) were F oxysporum.
Molds shown are those that accounted for ≥10% of positive swab samples. Other molds recovered include Bipolaris, Chaetomium, Chrysonilia, Curvularia, Epicoccum, Mucor, Nigrospora, Oospora, Phialophora, and Trichoderma species.
Includes drains and drain covers (n = 119), surfaces (n = 88), plumbing and faucet aerators (n = 65), and O-ring seals (n = 62).
Includes drain and drain covers (n = 154), floors (n = 154), walls (n = 176), shower heads (n = 158), and plumbing (n = 79).
Includes seats (n = 16) and bowls (n = 18).
Includes HEPA filter surfaces (n = 17), walls (n = 31), floors (n = 34), windowsills (n = 17), room surfaces (n = 51), objects related to patient care (mattress, thermometer case, stethoscope pad, oxygen nozzle; n = 24).
Molds were more frequently recovered from the plumbing and the environmental samples of the old building (149 of 436, 34% and 89 of 444, 20%, respectively) than those from the new building (29 of 139, 20% and 43 of 328, 13%, respectively; P = .002 and .012, respectively).
Air samples
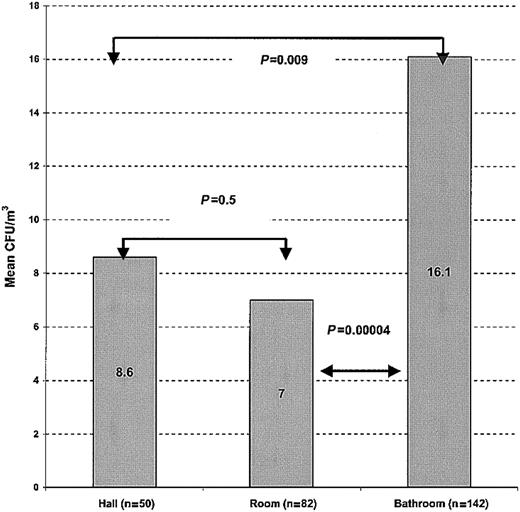
A total of 137 indoor air samples yieldedAspergillus species including A niger (66%),A fumigatus (17%), A nidulans (7%), A terreus (5%), A flavus (3%), A versicolor(1.5%), and A clavatus (0.7%). The great majority of the Paecilomyces species recovered from 42 indoor air samples were P lilacinus (98%) followed by P variotti (2%). Ten of 12 Fusarium species recovered from indoor air samples could be identified to species level. All 10 were F solani. Indoor air samples (n = 274) from bathrooms (n = 142; mean, 16.1; SEM, 1.5), rooms (n = 82; mean, 7; SEM, 1.1), and hallways (n = 50; mean, 8.6; SEM, 2.3) yielded significantly lower concentrations of molds than outdoor samples (n = 9; mean 123; SEM, 26.2 CFU/m3; P = < .001), indicating that the air precautions on these units (sealed windows, HEPA filtration, others) were adequate. The highest mean concentrations of molds in outdoor air were noted during the summer (n = 9 total; 173 CFU/m3) and fall (168 CFU/m3) and the lowest during winter (46 CFU/m3). By contrast the highest concentration of molds in indoor samples was seen during fall (21 CFU/m3) and the lowest during spring (7.1 CFU/m3). A significantly higher mean concentration of airborne molds was present in the bathroom (16.1 CFU m3) than in rooms (7 CFU/m3) or hallways (8.6 CFU/m3; P = 0.00004; Figure1).
Indoor airborne concentrations of molds by site.
A significantly higher concentration of airborne molds was observed in bathrooms (areas of major water use) compared with patient rooms and hallways.
Indoor airborne concentrations of molds by site.
A significantly higher concentration of airborne molds was observed in bathrooms (areas of major water use) compared with patient rooms and hallways.
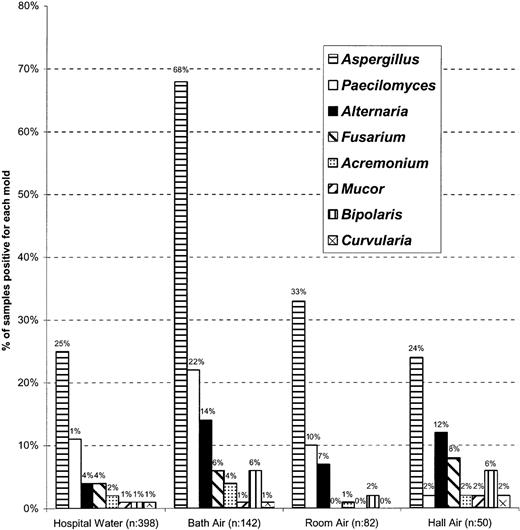
Rank order distribution of molds in hospital water and air samples
The most common molds recovered from hospital water were also the most common ones recovered from air samples. In addition, the distribution of molds by genera and species was comparable in water samples and the bioaerosols from bathrooms, rooms, and hallways (Figure2).
Frequency and distribution of pathogenic molds in hospital water and in air of patients' rooms, bathrooms, and BMT ward hallways.
A similar rank order distribution of molds was observed in water and air at different sites. A significantly higher rate of recovery of airborne molds was noted in bathrooms. Only pathogenic molds were included in this figure. Hospital water includes water samples from storage tanks and taps of patient care areas. Other molds recovered from water and air samples include Acremoniumspecies, Basidiomycota, Bipolarisspecies, Curvularia species, Dreschlera,Fusarium species, and Mucor andPenicillium species.
Frequency and distribution of pathogenic molds in hospital water and in air of patients' rooms, bathrooms, and BMT ward hallways.
A similar rank order distribution of molds was observed in water and air at different sites. A significantly higher rate of recovery of airborne molds was noted in bathrooms. Only pathogenic molds were included in this figure. Hospital water includes water samples from storage tanks and taps of patient care areas. Other molds recovered from water and air samples include Acremoniumspecies, Basidiomycota, Bipolarisspecies, Curvularia species, Dreschlera,Fusarium species, and Mucor andPenicillium species.
Discussion
The results of this 3-year prospective surveillance study that was conducted in a hospital with adequate air filtration precautions and water chlorination show that (1) Aspergillus species and other pathogenic molds inhabit the hospital water distribution system; (2) the same genera and species of molds are recovered from municipal water and hospital water structures; (3) these molds become part of the hospital water system biofilm; (4) a concentration differential of airborne molds exists between areas of water usage such as bathrooms (highest) and other areas such as patient rooms and hallways (lowest); (5) a strong correlation exists between the type and rank orders of molds isolated from hospital water and those recovered from indoor hospital air; and (6) no seasonal correlation in airborne molds exists between the indoor and outdoor air.
These findings suggest that waterborne molds enter the hospital via municipal water and are subsequently integrated in the water distribution system biofilm. The higher recovery of molds in the initial water samples (compared with the subsequent ones) and from the plumbing structures of the older building further support the role of the water biofilm. A higher rate of dislodging of biofilm particles in the initial sample is known to occur with other biofilm-related microbes,11 and the extent of biofilm formation increases with aging of the water structures.12
Taken together, our findings suggest that, in hospitals with adequate air precautions, airborne molds originate from hospital water and not contaminated outside air. Further support for this hypothesis is given by the molecular studies showing relatedness between clinical and water-associated environmental samples of A fumigatus13 This patient with refractory lymphoma died of A fumigatus pneumonia. Isolates from the patient's room shower wall showed the same genotype as the isolate obtained by bronchoscopy, whereas repeated testing of room air failed to yield A fumigatus.
The increasing incidence of aspergillosis reported despite the widespread implementation of air precautions,3 the lack of correlation between the air spore counts of Aspergillusspecies and rate of nosocomial aspergillosis or colonization by aspergilli,14 and the increase in airborne molds in a HEPA-filtered cancer unit in which the source of these molds was ultimately traced to leaking plumbing lines are also suggestive of a water source for these airborne molds.15
In support of possible water-relatedness of at least some mold infections, infections with Aspergillus and other molds have been reported and include pneumonias in near-drowning accidents involving healthy individuals,16-20 nosocomial A niger cutaneous infection in a BMT unit,21disseminated infections by F solani4 andExophiala jeanselmei,22 and endophthalmitis by Acremonium species.23Paecilomyces lilacinus was the fourth most frequently recovered molds from air and water samples in our study. The same pathogen was responsible for an outbreak of serious infections and death in a hematology and BMT unit.24 The organism is phylogenetically related toFusarium species25 and, importantly, tends to shares their resistance to treatment with polyene drugs.
The rank order of the fungal genera recovered is maintained across all sampling areas (water, bath air, room air, and hall air; Figure 2) forAspergillus species. Indeed, Aspergillus species was one of the most frequently isolated organisms in our environmental samples and is also the most common mold infection in this patient population.1 We also frequently recovered other molds such as Penicillium species and Paecilomyces species, organisms that are rarely associated with infections. This lack of correlation between high patient exposure to the latter organisms and the well-known very low rate of infection are best explained by the lower virulence of these organisms compared with Aspergillusspecies.1
The magnitude of the problem of waterborne molds cannot be fully appreciated until prospective studies correlate the concentration of molds in various hospital water systems with the rate of infection in patients cared for at these hospitals and until a genetic identity between the clinical and the environmental isolates is proven. These studies are currently under way.
Our findings that hospital water can be an important source of opportunistic molds have clinically significant implications. An effective and inexpensive approach to prevent patient exposure to waterborne molds in the hospital setting is to provide high-risk patients with sterile (boiled) water for drinking and sterile sponges for bathing (to avoid the aerosolization associated with showering). Such precautions could also be extended to the community, after hospital discharge, as dictated by the net state of immunosuppression of individual patients. Because the recovery of molds in water systems has been reported worldwide in the community and hospital settings as early as in 1982,5,6,26-28 it is likely that our recommendations may be applicable to various communities and hospitals. In addition, we have recently shown that cleaning the floors of patient shower facilities in a BMT unit reduced the mean air concentration ofAspergillus species (from 22 CFU/m3 to 4 CFU/m3; P = .0047) and other airborne pathogenic molds.29 We strongly recommend this approach for patients at high risk for developing serious infections with these fungi.
In conclusion, hospital water distribution systems may be a potential indoor reservoir of Aspergillus species and other molds leading to aerosolization of fungal spores and potential patient exposure.
We thank the University of Arkansas for Medical Sciences Office of Grants and Scientific Publications for their editorial assistance during the preparation of this manuscript and Dr Kieren Marr, from the Fred Hutchinson Cancer Center, Seattle, WA, for her critical review of the manuscript.
Prepublished online as Blood First Edition Paper, December 5, 2002; DOI 10.1182/blood-2002-02-0530.
This work was presented in part at the 42nd Annual Meeting of the American Society of Hematology, San Francisco, CA, December 1-5, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elias J. Anaissie, Professor of Medicine, Clinical Director, Myeloma and Transplantation Research Center, Arkansas Cancer Research Center, University of Arkansas for Medical Sciences 4301 West Markham, Mail Slot 776, Little Rock, AR 72205; e-mail: elias114@aol.com oranaissieeliasj@exchange.uams.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal