Splenic marginal zone lymphoma (SMZL) is a specific low-grade small B-cell lymphoma that is incorporated in the World Health Organization classification. Characteristic features are splenomegaly, moderate lymphocytosis with villous morphology, intrasinusoidal pattern of involvement of various organs, especially bone marrow, and relative indolent course. Tumor progression with increase of blastic forms and aggressive behavior are observed in a minority of patients. Molecular and cytogenetic studies have shown heterogeneous results probably because of the lack of standardized diagnostic criteria. To date, no definitive therapy has been established. Therapeutic options include treatment abstention, splenectomy, splenic irradiation, and chemotherapy.
Introduction
Splenic marginal zone lymphoma (SMZL) is a distinctive and well-characterized B-cell neoplasm that involves the spleen and various organs. Although SMZL is accepted as an entity in the World Health Organization (WHO) classification,1 its histogenesis remains obscure.2
SMZL was originally recognized either after histopathologic examination of surgically removed spleens as SMZL itself3or by means of morphologic and immunophenotypical characterization of circulating neoplastic lymphocytes as splenic lymphoma with villous lymphocytes (SLVLs).4 Now it is quite clear that the 2 entities have the same pathologic basis but different expressions of circulating cells.5,6 After the recognition of a peculiar intrasinusoidal bone marrow infiltration in SMZL with or without villous lymphocytes,7 the diagnosis can even be made with the bone marrow biopsy alone.8,9 This feature is of great help because it allows a definitive diagnosis to be made, avoiding an unnecessary splenectomy. O'Reilly,10 who studied retrospectively a large series of patients with splenomegaly, concluded that in the case of splenomegaly of unknown origin, the invasive procedure of choice for patients with hematologic associations could be a bone marrow biopsy.
For many years, the approaches to SMZL and SLVLs have been different. SMZL has been prevalently diagnosed by pathologists and SLVL by clinicians. Here we integrate the clinical and pathologic aspects of SMZL, along with cytogenetics, molecular biology, special features, and therapeutic options.
Clinical features
Incidence
The real incidence of SMZL has never been exactly calculated, even if it has been estimated as less than 1% of non-Hodgkin lymphoma (NHL).11,12 However, in the series of Berger et al,13 the splenic subtype of marginal zone B-cell lymphoma accounts for 2.7% of all patients with lymphoma treated in their department. This figure seems to be high and should be critically evaluated. SMZL constitutes 8% to 14% of lymphoma in surgically removed spleens involved by lymphoproliferative disorders.14 15 The median age of patients is 68 years (range, 22-79 years) with a male-to-female ratio of 1:1.8.
Clinical presentation and laboratory findings
Almost all patients present moderate-to-massive splenomegaly that can cause discomfort in the left hypochondrium. Hepatomegaly can be sometimes observed, but lymphadenopathy is extremely rare. Nonspecific symptoms relating to moderate anemia, which is reported in up to 64% of cases, are frequently noted. Thrombocytopenia is sometimes seen, but it is severe only in 15% of cases. Mild neutropenia due to a combination of splenic sequestration and bone marrow infiltration is commonly observed, but only 5% of patients present neutropenia below 1 × 109/L.16Absolute lymphocytosis is reported in 75% of patients. Lymphocytosis can ensue in the course of the disease after diagnosis. A frequent rise in lymphocyte count is observed after splenectomy. The presence of a small M band, IgM or IgG, usually less than 30 g/L, can be documented in up to 50% of patients.6
B symptoms such as fever and night sweats are rare. Similarly, alterations in levels of serum albumin, lactic dehydrogenase (LDH), and β2-microglobulin may be seen in variable percentages.
Occasionally diagnosis can be made on splenectomy specimens for traumatic or spontaneous rupture.17
Associated clinical conditions
Autoimmune phenomena, such as primary biliary cirrhosis,18 rheumatoid arthritis,19 immune thrombocytopenia, appearance of lupus anticoagulant,20 and autoimmune hemolytic anemia,21-23 represent the most frequent associated conditions, even though in some cases they could be induced by treatment.24 A combination of warm autoimmune hemolytic anemia and pure red cell aplasia has been reported in a single patient.25
A type II (IgM-IgG) cryoglobulin, detected in the serum, caused leukocytoclastic vasculitis in one patient.26
Clinical evolution
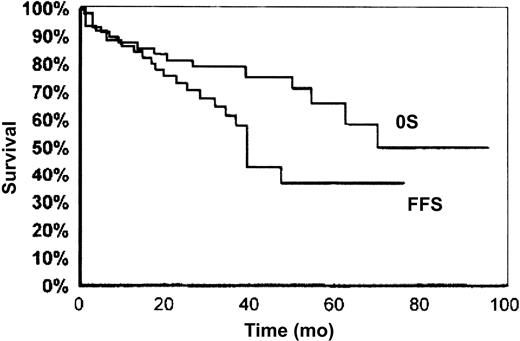
SMZL is universally considered a low-grade lymphoma with an indolent clinical course. Many cases show a protracted uncomplicated evolution, a good response to splenectomy or chemotherapy, or even an unmodified clinical picture in the absence of any kind of treatment. The 5-year overall survival rate ranges from 65% to 78%16,27 (Figure 1). Even in the absence of treatment or complete response, the time to progression exceeds 5 years.27 After therapy, complete remission and good clinical hematologic response with no evidence of active disease were cumulatively observed in 80% of cases in the series of Chacòn et al,27 whereas partial response is observed in 13.3% of cases. Failure to obtain complete remission, involvement of nonhematopoietic sites at diagnosis, high performance status scores, and p53 expression are the 4 factors associated with poorer survival.27 Thieblemont et al28reported a median survival of 10.5 years, which becomes significantly shorter in the presence of M component, elevated β2-microglobulin level, leukocyte count more than 20 000/μL, and lymphocytes more than 9000/μL. Despite its low aggressive course, histologic progression with increased number of blasts has been described in sporadic reports.29-31 In the series of Camacho et al,31 blastic transformation occurred in 13% of cases, which was lower than in follicular lymphoma (25%-60%) and mantle cell lymphoma (11%-39%) and similar to that observed in small lymphocytic lymphoma (1%-10%). Blastic transformation correlated with higher mean proliferative index and could be associated with p53 deletion but seems independent of p16INK4a inactivation.32
Overall survival (OS) and failure-free survival (FFS) in SMZL.
Reprinted with permission from Blood.27Copyright 2002.
Overall survival (OS) and failure-free survival (FFS) in SMZL.
Reprinted with permission from Blood.27Copyright 2002.
Pathologic features
Spleen
Recognition of this kind of lymphoma started with the pathologic studies on surgically removed spleens. Several reports have outlined the pathologic picture.3,17,33-35 The spleen is enlarged, weighing from 270 to 5500 g with a median of 1750 g. The cut surface generally shows multiple, small-to-moderate gray-tan nodules throughout the parenchyma (Figure 2A). A diffuse variant with no nodule formation has been recently described.36
Pathologic features of SMZL.
(A) Splenic cut surface in typical SMZL. Note the widespread micronodular appearance. (B) Histologic picture of spleen in SMZL. Widening of the marginal zone is evident; in initial phase, the differentiation with a reactive marginal expansion can be impossible (hematoxylin and eosin, × 125). (C) Splenic sinuses are characterized by the presence of mature lymphocytes (hematoxylin and eosin, × 500). (D) CD20 positivity in SMZL. Note sinusal involvement (avidin-biotin-peroxidase complex [ABC] method, × 250). (E) Imprint cytology of SMZL. Medium-sized lymphoid cells with clumped nucleus and villous cytoplasmic borders (arrow; Giemsa, × 1000). (F) Bone marrow biopsy in a typical case of SMZL shows peculiar intrasinusoidal infiltration (arrows; × 400). (G) Intrasinusoidal infiltration in bone marrow biopsy (Cd45RB; ABC method, × 500). (H) Rarely, lymphoid cells infiltrate bone marrow in a perisinusoidal pattern (CD20; ABC method, × 300). (I) Ultrastructure of circulating villous lymphocytes.(uranyl acetate-lead citrate, × 2000). (J) CD79a positivity in a hilar splenic lymph node involved by SMZL (ABC method, × 500). (K) Liver involvement in SMZL. Lymphoid infiltration is present in portal tract and in sinusoids (hematoxylin and eosin, × 250). (L) Sinusoidal involvement in liver biopsy is highlighted by immunocytochemistry (CD45 RA; ABC method, × 250).
Pathologic features of SMZL.
(A) Splenic cut surface in typical SMZL. Note the widespread micronodular appearance. (B) Histologic picture of spleen in SMZL. Widening of the marginal zone is evident; in initial phase, the differentiation with a reactive marginal expansion can be impossible (hematoxylin and eosin, × 125). (C) Splenic sinuses are characterized by the presence of mature lymphocytes (hematoxylin and eosin, × 500). (D) CD20 positivity in SMZL. Note sinusal involvement (avidin-biotin-peroxidase complex [ABC] method, × 250). (E) Imprint cytology of SMZL. Medium-sized lymphoid cells with clumped nucleus and villous cytoplasmic borders (arrow; Giemsa, × 1000). (F) Bone marrow biopsy in a typical case of SMZL shows peculiar intrasinusoidal infiltration (arrows; × 400). (G) Intrasinusoidal infiltration in bone marrow biopsy (Cd45RB; ABC method, × 500). (H) Rarely, lymphoid cells infiltrate bone marrow in a perisinusoidal pattern (CD20; ABC method, × 300). (I) Ultrastructure of circulating villous lymphocytes.(uranyl acetate-lead citrate, × 2000). (J) CD79a positivity in a hilar splenic lymph node involved by SMZL (ABC method, × 500). (K) Liver involvement in SMZL. Lymphoid infiltration is present in portal tract and in sinusoids (hematoxylin and eosin, × 250). (L) Sinusoidal involvement in liver biopsy is highlighted by immunocytochemistry (CD45 RA; ABC method, × 250).
Histologically, the splenic architecture can be retained in initial phases. Hyperplastic white pulp shows expansion of marginal zones (Figure 2B), with frequent merging and coalescence. Residual reactive follicles with germinal centers can be occasionally noted. Germinal centers can be frequently involuted. Neoplastic cells extend from the marginal zone to the red pulp with variable involvement. In advanced phases, diffuse obliteration of splenic architecture is made by sheets of neoplastic cells that obscure white and red compartments; in these cases no residual follicular structures can be identified. Involvement of splenic sinuses is typical (Figure 2C-D). The sinuses are filled up with neoplastic cells morphologically identical to the cells seen in the marginal zones. Cytologically, the neoplastic cells are medium sized with roundish or slightly irregular nucleus, clumped chromatin, frequent small nucleolus, and a moderate amount of cytoplasm with distinct borders sometimes of villous appearance (Figure 2E).
Bone marrow
Bone marrow is invariably involved. Isolated and initial reports of cases with no bone marrow involvement can be attributed to subtle lymphoid infiltration, not easily recognized without immunohistochemical studies.7 Different types of infiltration have been described, namely, intrasinusoidal, interstitial, nodular, and even paratrabecular.6-8,13,17,24,33 However, intrasinusoidal infiltration can be considered highly characteristic of SMZL (Figure 2F-G); so far there is no report of intrasinusoidal infiltration in other kinds of low-grade B-cell lymphoma, with the exception of subtle infiltration in a proportion of cases of extranodal marginal lymphoma.37 Sometimes, perisinusoidal infiltration can be seen (Figure 2H). Different patterns of infiltration could be an expression of different phases of the disease; in early phases, the intrasinusoidal pattern prevails, whereas in advanced ones it tends to diminish and nodular formations tend to increase.38
Peripheral blood
Peripheral blood involvement is reported at different percentages. The previous provisional nomenclature of SMZL with or without villous lymphocytes exemplifies at best this variability. The amount of circulating cells can be so small that it cannot be detected. In obvious peripheral blood involvement, numerous mature B lymphocytes with pale cytoplasm, irregular cytoplasmic borders, and villous projections can easily be recognized (Figure 2I). Larger cells with prominent nucleoli can be seen in the aggressive variant.29
Lymph nodes
Involvement of hilar splenic lymph nodes is commonly observed in SMZL39 (Figure 2J). Peripheral lymph nodes can be involved as well, but to a lesser extent. Complete or partial effacement of lymph node architecture is made by a nodular lymphoid infiltrate. Neoplastic nodules can contain a central reactive follicular center. Sinuses are generally spared or can show variable degrees of dilation. The characteristic sinusoidal involvement seen in other organs has not been described in lymph nodes. Different expression of adhesion molecules in the various lymphoid compartments might explain, at least in part, this phenomenon. Extensive lymph node involvement can rarely produce diffuse replacement. Scattered blasts with nucleoli can sometimes be present, especially at the periphery of the nodules. A primary nodal counterpart of SMZL, occurring in the absence of splenomegaly, has been hypothesized and shows a similar histologic picture to that observed in hilar splenic lymph nodes of SMZL.40
Liver
Few data are available on liver involvement in SMZL.3,17,21,35,41 The liver is involved in almost 90% of cases prevalently showing nodular infiltration of portal tracts. Lobular invasion is reported to a lesser extent.21However, no special reference is made on immunohistochemical studies. Liver biopsies in our patients show an invariable sinusoidal infiltration together with lymphoid nodules in portal tracts (V.F., personal data, 2002; Figure 2K-L).
Other organs
Rarely are nonhematolymphoid organs massively involved, at least at presentation. Involvement of nonhematopoietic sites is reported in 6.6% of cases in a recent series.27 Cutaneous involvement has been reported in 2 patients showing dermal tumoral infiltration located around cutaneous appendages and blood vessels with some degree of epidermotropism.42 Pleura localization is seen in 5% of cases in a large series.13 In a single patient of ours we found a massive perirectal localization of soft tissue. Sporadic soft-tissue involvement in SLVLs has been previously described by Orero et al43 in the thoracic region and by Yamazaki et al44 in perirenal area. Meningeal involvement has also been reported and can induce altered mental status and seizures.44 45
Immunophenotype
The B-cell nature of tumor cells in SMZL is well known. Table1 shows the immunophenotypic profile of SMZL. Positivity for CD20, CD45RA, CD45RB, CD79a, PAX5/BSAP, IgM, and bcl2 and negativity for CD43, CD23, CD10, bcl6, and cyclin D1 are constantly observed. Occasional positivity for CD5 has been reported in a minority of patients,16,60 but it should be critically analyzed. T-cell antigens are always negative. DBA44 gives variable staining; weak positivity for acid phosphatase isoenzyme 5 (tartrate-resistant) is reported in a proportion of cases.54 SMZL shares with nodal and extranodal marginal lymphomas the absence of telomerase activity, which is conversely highly expressed in other kinds of low-grade B-cell neoplasms.61 High, low, or no expression of p27 has also been reported.55,56 62
Immunohistochemical reactivity of SMZL
| Antibody . | Result . | References . |
|---|---|---|
| CD20 | + | 3, 7, 17, 33, 34, 46 |
| CD3 | − | 7, 17, 33–35 |
| CD43 | − | 7, 17, 34, 35, 46, 47 |
| CD23 | − | 3, 34, 35, 48 |
| CD5 | − | 3, 33–35, 48 |
| CD10 | − | 3, 34, 35, 48, 49 |
| CD34 | − | 7 |
| CD38 | − | 34, 35 |
| DBA44 (CD72) | −/+ | 2, 17, 34, 46, 50 |
| Light chains | + | 3, 7, 17, 33–35, 46 |
| MIB1 | Low | 7, 33–35 |
| Cyclin D1 | − | 34, 51–53 |
| P53 | − | 7, 33, 34 |
| Bcl2 | + | 7, 17, 33–35 |
| Bcl6 | − | 34, 49 |
| CD45RO | − | 7, 17, 33, 35 |
| CD21 | −/+ | 3, 7, 17, 35 |
| CD35 | +/− | 3, 17, 35 |
| CD45 | + | 7, 17, 35, 46 |
| CD45RA | + | 7, 46 |
| CD68 | − | 33, 35, 46 |
| CDw75 | −/+ | 7, 17, 33, 46 |
| CD74 | + | 7, 46 |
| Ln3 | + | 7, 46 |
| Kib3 | −/+ | 3, 33, 46 |
| CD19 | + | 35 |
| CD22 | + | 35 |
| CD11c | − | 35 |
| CD14 | − | 35 |
| CD25 | − | 35 |
| TRAP | −/+ | 35, 54 |
| P27 | −/+ | 55, 56 |
| CXCR3 | + | 57 |
| IRTA-1 | + | 58 |
| BSAP | + | 2 |
| IRF4 | − | 2 |
| E2F-1 | − | 59 |
| Antibody . | Result . | References . |
|---|---|---|
| CD20 | + | 3, 7, 17, 33, 34, 46 |
| CD3 | − | 7, 17, 33–35 |
| CD43 | − | 7, 17, 34, 35, 46, 47 |
| CD23 | − | 3, 34, 35, 48 |
| CD5 | − | 3, 33–35, 48 |
| CD10 | − | 3, 34, 35, 48, 49 |
| CD34 | − | 7 |
| CD38 | − | 34, 35 |
| DBA44 (CD72) | −/+ | 2, 17, 34, 46, 50 |
| Light chains | + | 3, 7, 17, 33–35, 46 |
| MIB1 | Low | 7, 33–35 |
| Cyclin D1 | − | 34, 51–53 |
| P53 | − | 7, 33, 34 |
| Bcl2 | + | 7, 17, 33–35 |
| Bcl6 | − | 34, 49 |
| CD45RO | − | 7, 17, 33, 35 |
| CD21 | −/+ | 3, 7, 17, 35 |
| CD35 | +/− | 3, 17, 35 |
| CD45 | + | 7, 17, 35, 46 |
| CD45RA | + | 7, 46 |
| CD68 | − | 33, 35, 46 |
| CDw75 | −/+ | 7, 17, 33, 46 |
| CD74 | + | 7, 46 |
| Ln3 | + | 7, 46 |
| Kib3 | −/+ | 3, 33, 46 |
| CD19 | + | 35 |
| CD22 | + | 35 |
| CD11c | − | 35 |
| CD14 | − | 35 |
| CD25 | − | 35 |
| TRAP | −/+ | 35, 54 |
| P27 | −/+ | 55, 56 |
| CXCR3 | + | 57 |
| IRTA-1 | + | 58 |
| BSAP | + | 2 |
| IRF4 | − | 2 |
| E2F-1 | − | 59 |
Ultrastructure
Ultrastructural studies have been rarely performed. Hammer et al17 studied 11 of 14 cases of their series by electron microscopy. Neoplastic marginal zone cells have a moderately developed curvilinear rough endoplasmic reticulum, approaching that seen in plasmacytoid cells. The cytoplasmic ribosomes are either clustered or scattered. Nuclei generally show more evident and peripherally located nucleoli than in normal marginal zone cells. Cell-to-cell contact is characterized by surface interdigitations, focally prominent.
Differential diagnosis
Among low-grade lymphoid neoplasias, various disorders can enter into differential diagnosis of SMZL. In most cases, a definitive diagnostic judgment can be reached after histologic examination along with immunohistochemical study in conjunction with clinical data. Only rarely are molecular biology and cytogenetics or ultrastructure helpful in distinguishing the various entities.
Hairy cell leukemia
Although circulating cells and clinical setting are similar in hairy cell leukemia (HCL) and SMZL, many distinguishing features make the differential diagnosis quite simple. In the bone marrow, hairy cells produce patchy infiltration with progressive replacement of normal hematopoietic series and low cellular density. Neoplastic infiltration is characteristically intermingled with extravasated red cells. The reticulin fiber content is almost invariably increased and accounts for the high frequency of “dry taps.” In the spleen, HCL diffusely involves the red pulp, whereas the white pulp is atrophied. Cytochemically, hairy cells are diffusely and strongly positive for tartrate-resistant acid phosphatase (TRAP). The immunophenotype profile of HCL is similar to that of SMZL. However, HCL differs for CD25 and CD103 positivity. Ultrastructural analysis shows characteristic ribosome-lamella complex in hairy cell cytoplasm.
B-CLL
Morphologically, B-cell chronic lymphocytic leukemia (B-CLL) is composed of small mature lymphoid cells, with high nuclear-to-cytoplasm ratio, scant cytoplasm, and round nuclei with highly condensed chromatin and inconspicuous nucleolus. Admixed with these cells may be prolymphocytes and paraimmunoblasts, characterized by larger size and prominent nucleoli. In the bone marrow the pattern of infiltration can be interstitial, nodular, or diffuse, but never intrasinusoidal. Immunohistochemically, B-CLL differs from SMZL for a lower expression of CD20 and positivity for CD23.
Mantle cell lymphoma
Mantle cell lymphoma (MCL) is clinically different from SMZL due to the high frequency of peripheral lymphadenopathy, but some example of massive splenomegaly with bone marrow involvement without prominent lymphadenopathy can be observed. Neoplastic cells are small to medium sized with irregular nuclei, morphologically resembling centrocytes/cleaved follicular center cells, but with less irregular nuclear contours. The bone marrow infiltration can be interstitial, diffuse, or nodular; an intrasinusoidal component has never been observed. MCL is characteristically CD5+, CD43+, and cyclin D1+.
Cytogenetics and molecular biology
In contrast to the well-established chromosomal changes associated with other B-cell NHLs, few genetic alterations have been reported in association with SMZL and no consistent or unique abnormalities have been so far documented. IgVH mutations have been described and are consistent with their postfollicular marginal zone B-cell origin,51 whereas the occurrence of ongoing mutations could be acquired in the splenic environment.63Recently, the study of matched splenic and blood neoplastic cells in 4 patients with SMZL showed identical VH sequences between cells from different tissues; 3 patients showed an unmutated germ line configuration, whereas the fourth one, who had developed a clonally related diffuse large B-cell lymphoma (DLBCL) of the chest wall, showed a significant somatic mutation of the subclone with intraclonal heterogeneity. This finding stresses the concept that an environmental factor can initiate in vivo somatic mutation, thus influencing tumor behavior and, possibly, disease progression.64 Rearrangement of bcl-6 was identified in some marginal zone lymphomas (MZLs),65 whereas another study did not confirm this finding.66 A molecular heterogeneity of SMZL has been therefore suggested, thus supporting the hypothesis that besides the proportion of cases derived from marginal zone cells, showing bcl-6 hypermutation, another significant proportion of cases could originate from an unmutated naive precursor possibly located in the mantle zone.67 The t(11;14), typical of MCL, was reported in some cases of SMZL leading to 2 possible explanations: (1) misdiagnosis because review of lymph node histology was not performed, and (2) a different bcl-1 breakpoint in SLVLs.68
Abnormalities of chromosome 14, at band q32.33 harboring the immunoglobulin heavy chain (IgH) gene, are found throughout among NHLs with different morphologic and clinical manifestations.69 The chromosomal abnormalities involving chromosome 14 more frequently encountered in patients with SLVLs were translocation t(6;14)70,71 and microdeletion of 13q14.72 The t(9;14)(p13;q32) translocation involves the IgH 14q32 in a head-to-head configuration to thePAX5 gene on chromosome 9p13. PAX5 normally codes for the transcription factor BSAP and is expressed from the earliest B-cell lymphopoiesis stages up to the mature B-cell stage with subsequent down-regulation during terminal plasma cell differentiation. The finding of t(9;14)(p13;q32) translocation in B-cell NHLs with plasmacytoid differentiation suggests that deregulation of PAX5 interferes with the cell cycle regulation of mature B cells and, by preventing them to enter the quiescent state, may contribute to the lymphomagenesis. This finding was reported in cases of SMZLs, whose origin is postulated to derive from transformed memory B cells of the marginal zone with potential of differentiation into plasma cells. The t(9;14)(p13;q32) translocation corresponds to a gain-of-function mutation of the humanPAX5 gene with coding of a wild-type of BSAP protein.9
Structural abnormalities of 7q (7q32 deletions and 7q22 translocation), commonly reported in myeloid malignancies, were found to occur in 3.6% of patients with chronic lymphoproliferative disorders, mainly in patients with SLVLs and HCL variant (26%).73 Moreover, alterations of chromosome 7q, mainly allelic loss, are frequently observed in splenic lymphomas74-77 with subsequent dysregulation of cyclin-dependent kinase 6 (CDK6) gene, possibly playing a role in the pathogenesis of SMZL and SLVLs.78 The occurrence of 2 cytogenetic subtypes, characterized, respectively, from gain of 3q and loss of 7q, was suggested.79 Recently, by means of analysis of the IgVH somatic mutations in SMZL, a significant group (49%) of unmutated cases with frequent 7q− and adverse clinical course was reported,80 suggesting that 7q deletion may play an alternative role in the inactivation of p53 pathway for tumor progression.31
The relationship between SMZL and MZLs arising at other sites, particularly those of mucosa-associated lymphoid tissue (MALT) type, has also been discussed. The t(11;18) (q21;q21), which represents the most frequent structural chromosomal alteration in extranodal low-grade MALT lymphomas, leads to a fusion between the apoptosis inhibitor-2 (API2) gene, on the chromosome 11 and the MALT lymphoma-associated translocation (MLT) gene on chromosome 18, thus prompting speculation about the critical role of abrogation of apoptosis in the development of these lymphomas.81,82Despite the common pathogenesis postulated,83 no case of SMZL shows API2-MLT fusion, thus favoring the hypothesis of separate lymphoma entities.84-86
Similarly, the search in cases of SMZL for microsatellite instability, described in association with p53 mutation in patients with MZL of MALT type, gave negative results.87
This concept was further strengthened by the finding of different genetic alterations in MZLs arising at different sites, suggesting that fundamental differences exist between the subtypes of MZLs.88
Trisomy 3 represents the most frequent cytogenetic abnormalities reported in MZLs, occurring in a high proportion of extranodal, nodal, and splenic MZLs89,90 and SLVLs.91 In contrast with this finding, another study reported a high frequency of trisomy 3 in extranodal and nodal MZLs but not in splenic ones. This fact strengthens the idea that these different subtypes are genetically distinct.92
In some studies, the finding of cytogenetic alterations was put into relationship with clinical course and prognosis. Translocations t(2;8)(p12;q24) and t(14;18)(q32;q21) were reported in a case of an aggressive variant of SMZL.93 Complex chromosome defects including 6q−, 11q−, +12, and 17p were found to be usually associated with switching into high-grade histology94 and, among several genomic imbalances encountered, gains, involving more frequently 3q, 5q, 12q, 20q, 9q, and 4q were more frequent than losses; the latter, mainly involving 7q and 17p, were associated with shorter survival.95 The 17p losses, which occur frequently in human cancers96 including several histotypes of NHLs,97 usually involve the region encompassing the p53 tumor suppressor gene locus, 17p13.1. The occurrence of p53 abnormalities in cases of SLVL has been very infrequently reported and seems to be associated with a more aggressive disease and poor prognosis.98 99
Other studies, dealing with SMZL or SLVLs and cytogenetics, reported the expression of TCL1 oncogene, activated by recurrent reciprocal translocations at chromosome 14q32100 or the presence of a point mutation in the mtDNA tRNA methionine gene.101
In a recent paper, Bahler et al102 postulated the existence of 2 different groups of SMZL, the first carrying unmutated VH genes and showing IgD expression consistent with a naı̈ve B-cell origin and the other with mutated VH genes and IgD−, consistent with a memory B-cell origin.
Special aspects
SMZL in animal models
Some mouse lymphomas have strong histologic similarities with human SMZL.103 SMZLs have been found to occur spontaneously at a high frequency in particular strains of mice.104,105 In transgenic mice, in which a transcription factor derived from human lymphoblastic leukemia was placed in the DNA of their lymphocytes, a lymphoid expansion of splenic marginal zones was evident before the development of diffuse B-cell lymphoma.106 Clonal integration of murine leukemia virus is able to develop B-cell lymphoma in the splenic marginal zone.107 Homozygous p53-deficient mice develop SMZL, assessed with morphologic, immunohistochemical, flow cytometry, and immunoglobulin heavy-chain rearrangement techniques.108However, the mechanism of lymphoma development in animal models remains undetermined.107 SMZL has been included in the Bethesda proposals for classification of lymphoid neoplasms in mice.109
SMZL and infectious agents
A high prevalence of a chronic liver disease, usually chronic hepatitis or cirrhosis, was described in Japan by Murakami et al110 in 1988 in a review of primary splenic lymphoma. Hepatitis C virus (HCV) has been observed in 36% of cases of primary splenic lymphoma.111 Satoh et al112hypothesized a role of HCV infection in the development of primary splenic lymphoma after the description of a HCV+ patient whose spleen was grossly and histologically indistinguishable from SMZL. Other descriptions of SMZL in patients with HCV infection make this association more than casual.113-115 In nodal marginal lymphoma, HCV+ specimens harbor different VH somatic mutation compared to that of HCV− ones, suggesting a role for a HCV antigen epitope in the B-cell selection.116 The pathogenetic mechanism underlying the development of SMZL during HCV infection remains unexplained. HCV might provide the initial antigenic stimulus for B-cell clonal expansion, as part of the multistep progression toward lymphomagenesis.
Abruzzo et al117 recently described data on a patient with SMZL, treated with fludarabine, who subsequently developed an Epstein-Barr virus (EBV)–positive B-cell lymphoproliferative disorder. Raised EBV antibody levels without a concomitant increase in the detection of viral genomes in the peripheral blood has been demonstrated in tropical SLVL.118
A relationship between SLVL and malaria has been hypothesized.119 In West Africa, SLVL and hyperreactive malarial splenomegaly are demographically, clinically, and immunologically indistinguishable.120 Polymerase chain reaction (PCR) analysis of immunoglobulin genes can be useful in distinguishing the 2 conditions.121 Although the etiopathologic link is not yet fully understood, it is possible that an altered immune response to repeated malaria infections can stimulate or select a proliferating pool of naive B cells, altering their growth and apoptosis.122
Adhesion molecules
The presence of its circulating counterpart, previously classified as SLVL, indicates that homing and circulation of neoplastic cells must be under the influence of adhesion molecule action.
The migration and localization of lymphocytes from the blood into the splenic white pulp is influenced by adhesive interactions, as evidenced by the expression of adhesion molecules on sinus lining cells in the marginal zone of the spleen.123 It is now clear that the recruitment and retention of both T and B lymphocytes are based on selective interactions involving several adhesion molecules like human mucosal addressin cell adhesion molecule 1 (MAdCAM-1),124whose expression is well documented in endothelial cells at mucosal sites such as mesenteric lymph nodes, lamina propria of small and large intestine and lactating mammary gland, and its exclusive integrin receptor α4β7.125 Moreover, splenic white pulp B cells in marginal zones have been shown to be diffusely positive for L (leukocyte)–selectin.126Similarly, the first step in the homing of hematopoietic progenitor cells from the peripheral blood to the bone marrow is regulated by selective mechanisms controlling their adhesion to the bone marrow endothelium.127,128 This step is then followed by their migration through the endothelial cell barrier and adhesion to stromal cells and extracellular matrix.129 In this process of adhesion, molecules such as E (endothelium)–selectin, vascular cell adhesion molecule 1 (VCAM-1), intracellular adhesion molecule 1 (ICAM-1), and the cell surface receptor for hyaluronic acid CD44 have been shown to play a role.130
SLVL shows a low expression of L-selectin that represents a discordant feature if compared to the fact that all white pulp B cells, including marginal zone ones, are L-selectin positive.126 Moreover, in HCL, a pathologic condition closely related to SLVL, adhesion molecules have an important role in the interactions between hairy cells and endothelium/accessory cells in the red pulp of the spleen.131 The expression of a series of adhesion receptors (L-selectin, several integrins, ICAM-1, and CD44) investigated by means of flow cytometry in cases of both B-cell leukemic disorders and related lymphomas showed a reverse pattern of expression, namely, high expression of L-selectin and low expression of integrin molecules (leukocyte function associated antigen 1 [LFA-1], very late activation antigen 4 [VLA-4], ICAM-1) in CLL and low L-selectin positivity and high integrin expression in non-CLL disorders, including SMZL.132
Treatment
To date there is no definitive standard treatment for SMZL. About two thirds of patients are asymptomatic at diagnosis and as many as one third will never require therapy. Both the selection and the timing of therapy are basically established on an empirical ground because of the indolent natural history of SMZL, the lack of prospective therapeutic trials, and the absence of response criteria and prognostic scores. There are 4 main types of initial treatment strategies: (1) no treatment, (2) splenic irradiation, (3) chemotherapy, and (4) splenectomy. In patients who do not undergo splenectomy the diagnosis is made with morphologic and immunophenotypical analysis of peripheral blood and bone marrow.
No treatment
Treatment abstention should be considered in patients with favorable prognostic factors. Patients with mild lymphocytosis and no cytopenia are in the eligible condition for the “wait and see” policy.6,13 In untreated patients the 5-year overall survival rate is 88%.16 Ten of 14 untreated patients of the series of Mulligan et al133 remained alive between 1 and 6 years from diagnosis. Patients with stable disease have been monitored for up to 15 years, without requiring any treatment.6
Splenic irradiation
Splenic irradiation has been used in a limited series of patients. Three of 7 patients in the Mulligan series benefited from splenic irradiation.133 Among the 7 patients treated by Troussard et al16 with 6 to 8 Gy with 1 or 2 fractions over 2 weeks, 3 had a relapse; none of the patients receiving only irradiation died. El Weshi et al134 reported that even low-dose radiotherapy (4 Gy) may be effective, producing a dramatic reduction in circulating villous lymphocytes, regression of splenomegaly, and improvement of cytopenias. Radiotherapy seems to be a reasonable and effective treatment option in SLVL when splenectomy is contraindicated and/or when pancytopenia is present and likely to give rise to excessive toxicity when chemotherapy is administered.
Chemotherapy
Many drugs with different schemes have been administered. There is no univocal convergence when and how to use chemotherapy. Chemotherapy is generally used as first-line treatment in patients with more advanced disease. The role of alkylating agents such as chlorambucil or cyclophosphamide is marginal. Few patients benefit when these are used as first-line therapy.6 However, in cases of disease progression, especially after splenectomy, alkylating agents may achieve good response, but seldom complete remission. Mean duration of response in patients treated with alkylating agents alone or in combination with other drugs is 6 months, whereas the 5-year overall survival rate is 64%.16 The use of purine analogues is more promising, but so far it has been tested in relatively few patients. Complete or partial hematologic remission has been achieved with 2-deoxycoformycin.135,136 Good responses with 2-chlorodeoxyadenosine have been also reported,137 whereas in other cases only partial responses with high frequency of relapses were obtained.138 Some complete remissions have been achieved with fludarabine as first- or second-line therapy.139-141
Splenectomy
There is a general consensus, as indicated by the 2 largest retrospective studies, in considering splenectomy the best first-line therapy.16,133 A huge symptomatic splenomegaly or a severe cytopenia or both are the main indications to perform splenectomy. Long and sustained improvement of cytopenia and relief of abdominal discomfort have been achieved with splenectomy alone. Moreover, in retrospective studies, splenectomized patients have a significantly better overall survival than those treated with chemotherapy.16 However, these data should be interpreted cautiously because this could be, at least partially, an expression of a selection bias, because patients with more aggressive disease are more likely to be treated with chemotherapy. Furthermore, it is intuitive that splenectomy alone cannot reduce extrasplenic lymphomatous infiltrations. Our group has reported a change in the bone marrow infiltration with increase in tumor burden after splenectomy.38 In particular, we demonstrated a modification from intrasinusoidal to nodular of the infiltrates in the bone marrow after splenectomy in most patients with SMZL. Conversely, in those patients who did not undergo splenectomy, bone marrow intrasinusoidal infiltration remained stable. Therefore, splenectomy seems to induce important changes in bone marrow infiltration, probably through the lack of microenvironmental homing factors on circulating B cells.
Other modalities
Additional treatment modalities such as single-agent rituximab are under investigation.142 Radiolabeled anti-CD20 monoclonal antibodies are currently in development for the treatment of low-grade B-cell NHLs.143 These include the iodine-labeled (tositumomab) and yttrium-labeled anti-CD20 (ibritumomab). Such approaches possess potential as future therapeutic agents but await investigations and clinical trials.
The unexpected regression of SLVL in a HCV patient who was treated with interferon-alfa for symptomatic type II cryoglobulinemia prompted Hermine et al144 to evaluate the effect of interferon-alfa in 8 additional patients. Seven of 9 HCV+ patients with SLVL had a complete hematologic remission after the loss of detectable HCV-RNA, whereas none of HCV− patients with SLVL had hematologic response to the treatment with interferon. However, the rearrangement of the monoclonal immunoglobulin gene persisted in the blood of the HCV+ patients even after a complete hematologic response had been achieved. In the same study, Hermine et al144 showed that a complete hematologic response occurred after the addition of ribavirin treatment in patients who had a relapse or a partial response to interferon.
The authors thank Dr M. Salvato for immunohistochemical stainings and Dr E. Barresi for the electron microscopy study.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-07-2216.
Supported by Ministero dell'Istruzione, dell'Universitàe della Ricerca (MIUR), Rome, Italy.
References
Author notes
Vito Franco, Istituto di Anatomia e Istologia Patologica- Policlinico, Via del Vespro 129 90127 Palermo, Italy; e-mail: vfranco@unipa.it. © 2003 by The American Society of Hematology

![Fig. 2. Pathologic features of SMZL. / (A) Splenic cut surface in typical SMZL. Note the widespread micronodular appearance. (B) Histologic picture of spleen in SMZL. Widening of the marginal zone is evident; in initial phase, the differentiation with a reactive marginal expansion can be impossible (hematoxylin and eosin, × 125). (C) Splenic sinuses are characterized by the presence of mature lymphocytes (hematoxylin and eosin, × 500). (D) CD20 positivity in SMZL. Note sinusal involvement (avidin-biotin-peroxidase complex [ABC] method, × 250). (E) Imprint cytology of SMZL. Medium-sized lymphoid cells with clumped nucleus and villous cytoplasmic borders (arrow; Giemsa, × 1000). (F) Bone marrow biopsy in a typical case of SMZL shows peculiar intrasinusoidal infiltration (arrows; × 400). (G) Intrasinusoidal infiltration in bone marrow biopsy (Cd45RB; ABC method, × 500). (H) Rarely, lymphoid cells infiltrate bone marrow in a perisinusoidal pattern (CD20; ABC method, × 300). (I) Ultrastructure of circulating villous lymphocytes.(uranyl acetate-lead citrate, × 2000). (J) CD79a positivity in a hilar splenic lymph node involved by SMZL (ABC method, × 500). (K) Liver involvement in SMZL. Lymphoid infiltration is present in portal tract and in sinusoids (hematoxylin and eosin, × 250). (L) Sinusoidal involvement in liver biopsy is highlighted by immunocytochemistry (CD45 RA; ABC method, × 250).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-07-2216/3/m_h80734046002.jpeg?Expires=1770927191&Signature=0yPsVj0kctFjlYi5mn5QgWtYvB33Ch2FIIKFWXbg6Qxr2qwIxlsqcdWfhd6r3NLIEgVTqM6MIP-Lxqm6-RPsBN1LAQUdLJ21gObsbS-lCr2rK5EhkD6gy22FnFZu5r6sY0b~-WCWPFUQttYRSK-JkyfC03LELdC3VYQAqNkzL-9jFDZ8NrtvOttNQGdNnOxx-NZmb~YjtKJY357VkD6~R9LfZAUQJSLh2IQNW1Q-gHqfWlEsmj1HCZQ-Upkbj9KGMM~lbfKc-MVnldViL6~jZf2s94vKZP6AWCNZ0CZtOdVbFop-bpktCHSW-IXcxEjleBynEGFi3QL8xLJxddgHEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal