Myeloma bone disease is due to interactions of myeloma cells with the bone marrow microenvironment, and is associated with pathologic fractures, neurologic symptoms and hypercalcemia. Adjacent to myeloma cells, the formation and activation of osteoclasts is increased, which results in enhanced bone resorption. The recent characterization of the essential cytokine of osteoclast cell biology, receptor activator of NF-κB ligand (RANKL) and its antagonist osteoprotegerin (OPG), have led to a detailed molecular and cellular understanding of myeloma bone disease. Myeloma cells induce RANKL expression in bone marrow stromal cells, and direct RANKL expression by myeloma cells may contribute to enhanced osteoclastogenesis in the bone microenvironment in myeloma bone disease. Furthermore, myeloma cells inhibit production and induce degradation of OPG. These effects result in an increased RANKL-to-OPG ratio that favors the formation and activation of osteoclasts. Patients with myeloma bone disease have inappropriately low serum and bone marrow levels of OPG. Specific blockade of RANKL prevented the skeletal complications in various animal models of myeloma, and suppressed bone resorption in a preliminary study of patients with myeloma bone disease.
Osteoclasts as effector cells in skeletal malignancies
Skeletal complications represent frequent and significant events in patients with multiple myeloma, and include osteolytic lesions, pathologic fractures, neurologic symptoms (pain, paralysis), and profound hypercalcemia.1,2 At the cellular level, these complications are due to an excessive growth of malignant myeloma cells within the bone marrow microenvironment and their interactions with osteoblastic and osteoclastic lineage cells.1,3,4 A consistent histologic finding in myeloma bone disease is enhanced and uncontrolled osteoclastic bone resorption adjacent to areas of plasma cell infiltrates.2 Moreover, antiresorptive drugs that inhibit osteoclastic functions such as bisphosphonates are successfully used in patients with myeloma bone disease, indicating that osteoclasts are essential mediators in the pathogenesis of myeloma bone disease.5
In the past 5 years, an essential cytokine system for osteoclast biology has been characterized.6,7 This system consists of a ligand, receptor activator of NF-κB ligand (RANKL),8,9a cellular receptor, RANK,8,10 and a soluble decoy receptor, osteoprotegerin (OPG).11 While RANKL stimulates several aspects of osteoclast function, thus enhancing bone resorption, OPG blocks RANKL, and prevents bone resorption.9,12Abnormalities of this system have been implicated in the pathogenesis of various skeletal diseases characterized by enhanced osteoclastic activity and increased bone resorption, including osteolytic metastasis and tumor- associated hypercalcemia.13
RANKL and OPG in bone cell biology
Osteoclasts are derived from macrophagic/monocytic lineage cells and represent differentiated, multinucleated cells specialized in resorbing bone.6,7 Recently, the essential cytokines of osteoclast biology have been identified and extensively characterized. Osteoclastic lineage cells express RANK, a member of the tumor necrosis factor receptor superfamily.8,10 Following activation of RANK by its ligand, RANKL, differentiation, proliferation, and survival of preosteoclast is enhanced, osteoclastic fusion and activation is promoted, and osteoclastic apoptosis is suppressed, resulting in a marked increase of the number and activity of osteoclasts.9 12
RANKL is mainly produced by osteoblastic lineage cells,14immune cells,8,15 and some cancer cells.16,17This provides the cellular and molecular basis for osteoblast-osteoclast cross-talks, which are crucial for an orderly sequence of bone resorption and formation during bone remodeling.14 However, RANKL production by immune and cancer cells also forms the basis of skeletal complications of inflammatory and malignant diseases, because activated T cells and cancer cells are able to directly activate RANK on osteoclasts by virtue of expressing RANKL.4,7 The potent stimulatory effects of RANKL on RANK are counteracted by a safeguard mechanism. Many cell types—in the bone marrow microenvironment, mainly osteoblastic lineage cells—secrete OPG, which acts as a decoy receptor and blocks RANKL, thus preventing RANK activation.11
Malignant tumors capable of forming skeletal metastases or causing hypercalcemia utilize the cellular machinery (osteoclasts) and molecular pathways (RANKL/RANK/OPG) of normal bone cell biology.3,4 Focally or systemically enhanced osteoclastic activation results in tumor-associated hypercalcemia, osteolysis, pathologic fractures, and severe pain. Such RANKL-mediated mechanisms have been described for a variety of osteotropic malignancies, including breast cancer,18,19 prostate cancer,20,21 squamous cell carcinoma,16 adult T-cell leukemia,17 Hodgkin disease,22 and neuroblastoma.23
RANKL and OPG production in myeloma bone disease
Myeloma cells increase RANKL expression within the bone microenvironment
There are several distinct mechanisms whereby myeloma cells increase the expression of RANKL within the bone microenvironment. Bone marrow plasma cells derived from patients with multiple myeloma revealed high positive RANKL immunoreactivity as compared to healthy controls, and among patients with multiple myeloma RANKL immunoreactivity on plasma cells was positively correlated with the presence of osteolytic lesions.24 However, there is controversy as to whether myeloma cells directly express RANKL. While several studies reported RANKL expression by myeloma cells using either human primary myeloma cells from patients,24-26 human myeloma cell lines,27 or the murine myeloma cell line 5T2MM,28 other studies could not detect RANKL expression in human myeloma cell lines or primary myeloma cells.29-31
Despite this open question, several studies have unambiguously demonstrated that myeloma cells enhance RANKL expression by bone marrow–residing stromal cells through direct cell-to-cell contact.29-31 RANKL induction by stromal cells was present in patients with multiple myeloma but not in patients with monoclonal gammopathy of undetermined significance (MGUS),29,31indicating a specific threshold effect. In addition, human myeloma cell lines and primary myeloma cells have also been demonstrated to up-regulate RANKL production by activated T cells, although the precise role of this interaction in the pathogenesis of myeloma bone disease remains unclear.32
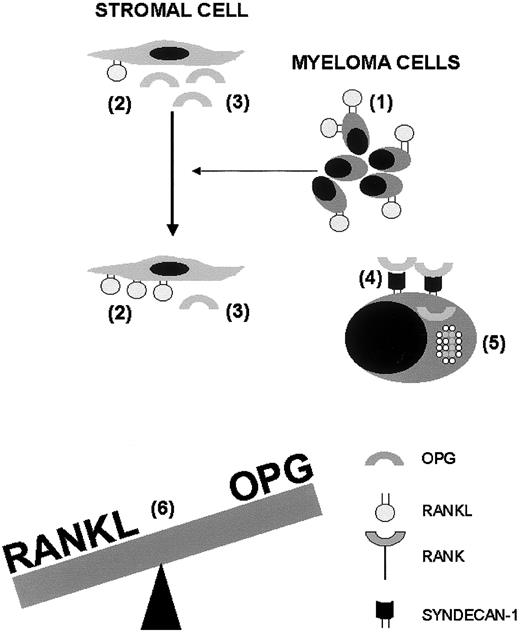
Increased expression of RANKL by bone marrow stromal cells was associated with enhanced osteoclastogenesis, and this effect could be prevented by RANK-Fc, a specific inhibitor of RANKL.29Taken together, enhancement of marrow stromal (and possibly T cell) expression of RANKL by myeloma cells and direct RANKL expression by myeloma cells contribute to enhanced osteoclastogenesis in the bone microenvironment in myeloma bone disease (Figure1).
Interactions of the RANKL-OPG system with myeloma cells, bone marrow stromal cells, and osteoclasts in the pathogenesis of myeloma bone disease.
Myeloma cells express RANKL (1) and cause bone marrow–residing stromal cells to overexpress RANKL (2). In addition, myeloma cells inhibit OPG production by stromal cells (3). Syndecan-1 is expressed on the surface of myeloma cells and binds the heparin-binding domain of OPG (4), thus facilitating internalisation and lysosomal degradation of OPG (5). The physiologic balance between RANKL and OPG is tilted by these combined effects (6), and the ensuing enhanced RANKL-to-OPG ratio promotes osteoclast formation and activation, which is responsible for osteolysis, hypercalcemia, fractures, and pain. OPG indicates osteoprotegerin; RANKL, receptor activator of NF-κB ligand; RANK, receptor activator of NF-κB.
Interactions of the RANKL-OPG system with myeloma cells, bone marrow stromal cells, and osteoclasts in the pathogenesis of myeloma bone disease.
Myeloma cells express RANKL (1) and cause bone marrow–residing stromal cells to overexpress RANKL (2). In addition, myeloma cells inhibit OPG production by stromal cells (3). Syndecan-1 is expressed on the surface of myeloma cells and binds the heparin-binding domain of OPG (4), thus facilitating internalisation and lysosomal degradation of OPG (5). The physiologic balance between RANKL and OPG is tilted by these combined effects (6), and the ensuing enhanced RANKL-to-OPG ratio promotes osteoclast formation and activation, which is responsible for osteolysis, hypercalcemia, fractures, and pain. OPG indicates osteoprotegerin; RANKL, receptor activator of NF-κB ligand; RANK, receptor activator of NF-κB.
Myeloma cells decrease OPG availability in the bone microenvironment
In contrast to many other tissues and cell types,11OPG mRNA expression or protein secretion was undetectable in myeloma cells and cell lines assessed (O.S. and L.H., unpublished observation, May 2002).30 In addition, myeloma cells use several mechanisms to inhibit OPG production or availability within the bone microenvironment. Cell-to-cell contact of myeloma cells with bone marrow stromal cells and osteoblasts inhibited OPG mRNA levels and protein secretion by stromal cells, as evident from coculture models (Figure 1).29 30
Furthermore, syndecan-1 (CD 138), a transmembrane proteoglycan with heparan sulfates that is expressed by myeloma cells, has been hypothesized to bind and sequestrate OPG through interaction with the heparin-binding domain of the OPG protein. A recent study provided details of these mechanisms.33 OPG binding to syndecan-1 of myeloma cells was dependent on the presence of heparan sulfates, and did not occur in syndecan-1 lacking heparan sulfates or in the presence of heparin.33 Following binding to syndecan-1, OPG was internalized and degraded within the lysosomal compartment of myeloma cells with a kinetic of 1 ng/h per 106 cells (Figure1).33 This posttranslational mechanism may contribute to low local and systemic OPG levels in patients with multiple myeloma.33-35
In summary, inhibition of OPG gene expression and protein production and posttranslational degradation of OPG by myeloma cells combined with the stimulatory effects of myeloma cells on RANKL expression in the bone microenvironment markedly enhances the RANKL-to-OPG ratio within affected bone areas, thus favoring osteoclast differentiation and activation, and enhancing bone resorption (Figure 1).
Effects of commonly used drugs on RANKL and OPG production
Several drugs that are commonly used in patients with multiple myeloma may adversely affect the RANKL-OPG system.13 In vitro, glucocorticoids have been demonstrated to concurrently up-regulate RANKL mRNA levels and to suppress OPG mRNA levels and protein concentrations in human osteoblasts.36 A similar pattern of RANKL and OPG regulation has been reported in human bone marrow stromal cells for immunosuppressants (cyclosporine A, rapamycin, tacrolimus) that may be used following allogeneic stem cell transplantation.37 By contrast, the bisphosphonates pamidronate and zoledronic acid have been shown to up-regulate OPG mRNA levels and protein secretion by human osteoblastic cells.38
RANKL and OPG serum levels in patients with myeloma bone disease
Sensitive assay systems now allow measurement of the soluble form of RANKL (sRANKL) and OPG in health and disease.39 While data on sRANKL serum levels in bone diseases are limited, several studies have reported alterations of OPG serum levels in metabolic bone diseases. Some limitations need to be considered when interpreting such data, including (1) that OPG is produced by various skeletal and extra-skeletal tissues, (2) that there is no bone-specific fraction of OPG (in contrast to other skeletal makers such as alkaline phosphatase), and (3) that most OPG assays measure both free and sRANKL-bound OPG and do not distinguish between these 2 fractions.39 Despite these limitations, Brown et al40 and Jung et al41 have unambiguously shown that OPG serum levels are significantly higher in men with prostate cancer and osseous metastases compared to local prostate cancer or benign prostate diseases.
Three studies have recently evaluated the role of OPG serum levels in myeloma bone disease.33-35 In the first study, OPG serum levels of 225 patients with myeloma were compared with those of 40 healthy age- and sex-matched controls. Patients with myeloma were found to have OPG serum levels that were 18% lower than those of controls.34 Of note, OPG serum levels of patients with multiple myeloma were inversely correlated with the number of radiographic osteolytic lesions and World Health Organization (WHO) performance status, and were positively correlated with the carboxy-terminal propeptide of type I collagen, a biochemical marker of bone turnover.34 These findings were in part confirmed by Lipton et al,35 who assessed OPG serum levels of 112 healthy controls and 111 patients with various hematologic malignancies. OPG serum levels were 29% lower in patients with multiple myeloma (n = 34) as compared to healthy controls, but 71% and 41% higher in patients with Hodgkin disease and non-Hodgkin lymphoma, respectively.35 A recent study by Standal et al33 analyzed local OPG concentrations in plasma samples obtained from bone marrow aspirates of 33 patients with multiple myeloma and 27 healthy controls. In this study, OPG protein concentrations within the bone marrow microenvironment were 27% lower in patients with myeloma as compared with healthy controls.33 Of note, OPG concentrations were 2-fold higher in bone marrow plasma compared to serum and were found to be positively correlated with each other.33
RANKL as a potential target in the treatment of myeloma bone disease
Effects of RANKL blockade in animal models of myeloma bone disease
Systemic RANKL blockade using OPG, OPG-Fc fusion protein, or inhibitory RANK antibodies has been successfully used to treat osteolytic metastases,23,42-44 humoral hypercalcemia,45-47 and tumor-associated bone pain43 48 in various animal models of nonmyeloma malignancies.
The first therapeutic study on RANKL blockade in an animal model of myeloma bone disease was performed by Pearse et al29 using the severe combined immunodeficiency (SCID) ARH-77 xenograft model in which the human myeloma cell line (ARH-77) was injected into mice. Compared to controls, SCID ARH-77 mice receiving intravenous injections of RANK-Fc, a fusion protein of the murine RANK with the human IgG region (200 μg, 3 times per week), displayed markedly reduced bone resorption markers and absence of radiographic evidence of skeletal destruction after 6 weeks.29 After 7 weeks of treatment, 80% of control animals, but none of the treated animals had hind limb paralysis. In a second xenograft model, in which primary human bone marrow cells from a patient with myeloma bone disease were injected into mice (SCID-hu-MM), treatment with RANK-Fc (200 μg, 3 times per week) prevented resorption of xenografts, and resulted in a markedly lower number of osteoclasts in affected lesions as compared to controls receiving negative controls.29 Another study by the same group49 evaluated the effects of bisphosphonates and RANK-Fc on myeloma tumor burden and osteoclast formation in the SCID-hu-MM model. Injections of zoledronic acid (0.1 mg/kg once per week, starting 3 weeks after injection of tumor cells) or RANK-Fc (200 μg, 3 times per week, starting 5 weeks after injection of tumor cells) resulted in a similar, sustained suppression of paraprotein levels by more than 80% and inhibition of osteoclast numbers by more than 50%.49
In a second study, Croucher et al28 used the 5T2MM model in which murine 5T2MM myeloma cells were injected into syngeneic mice. While mice receiving the vehicle control developed extensive osteolytic lesions due to enhanced osteoclastic bone resorption, mice intravenously treated with OPG-Fc, a fusion protein of the human OPG with the human IgG region (30 mg/kg, 3 times per week), displayed only 6% and 13% of the numbers of osteolytic lesions in their tibiae and femora, respectively. Moreover, treatment with OPG-Fc not only prevented bone loss following 5T2MM injection, but increased bone mineral density and resulted in a complete absence of osteoclasts,28 which is most likely due to the relatively high OPG dose and consistent with OPG effects in healthy rodents.11
In a different approach, Doran et al50 recently reported the effects of ex vivo gene transfer of the OPG-Fc gene using a lentiviral vector in the SCID ARH-77 xenograft model. Compared to SCID ARH-77 mice treated with the empty vector, mice carrying OPG-Fc-expressing tumors had a lower incidence of complete paraplegia (39% vs 84%), osteolytic lesions (17% vs 78%), and a longer survival (37 days vs 32 days).50
Effects of RANKL blockade in humans
Skeletal effects of RANKL blockade have been evaluated in 52 postmenopausal women with enhanced bone turnover who received a single subcutaneous injection of the OPG-Fc fusion protein (3 mg/kg).51 In this study, biochemical markers of bone turnover rapidly decreased by 30-80%. More recently, a similar approach has been used in patients with myeloma bone disease.52 In this controlled double-blind dose escalation study, patients received either OPG-Fc (0.1, 0.3, 1.0, or 3.0 mg/kg administered subcutaneously; n = 20) or pamidronate (90 mg administered intravenously; n = 6) and were followed for 57 days. Patients receiving 1 mg/kg of OPG-Fc displayed a rapid, sustained decrease of the biochemical marker of bone resorption, N-telopeptide of collagen, of more than 50% after 8 and 29 days following initiation of treatment which was similar to the pamidronate group.52Except for transient asymptomatic hypocalcemia, the treatment was well tolerated and without adverse effects. Although long-term effects of such intervention on tumor burden, bone mass, number of osteolytic lesions, and patient survival have not been assessed, these preliminary data provide proof-of-principle that RANKL blockade may be feasible and effective in human myeloma bone disease. However, future studies need to address the undesired possibility that OPG may also bind tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) in vivo, as suggested by in vitro studies.53
Role of other cytokines in myeloma bone disease
In addition to RANKL and OPG, a variety of chemokines and cytokines has been implicated in the pathogenesis of myeloma bone disease, including macrophage inflammatory protein (MIP)-1α and MIP-1β,54-56 interleukin (IL)-1β,57,58 IL-6,59 and hepatocyte growth factor (HGF).60 Some of these factors such as IL-1β and IL-657-59 may use RANKL-dependent and -independent pathways to stimulate osteoclasts, and have been shown to up-regulate RANKL expression by marrow stromal cells.13 Others, including MIP-1 act independently of RANKL,54-56indicating a high degree of redundancy of myeloma cells to induce osteoclastic bone resorption. Among the factors listed above, few are elevated in most patients with myeloma bone disease, are correlated with disease activity, and associated with enhanced osteoclastogenesis.4 At present, MIP-1α and MIP-1β—along with RANKL—best fulfill the criteria of the putative osteoclast-activating factors (OAFs) in myeloma bone disease.
Summary and integration of mechanisms
RANKL and OPG play an essential role for osteoclast formation and activation, and various bone tumors use this cytokine system to trigger osteoclastic bone resorption. While RANKL stimulates osteoclast functions through binding to its osteoclastic receptor RANK, OPG acts as a decoy receptor that blocks RANKL. Myeloma cells express RANKL, and cause bone-marrow stromal cells to overexpress RANKL (Figure1). Concurrently, myeloma cells inhibit OPG secretion by stromal cells through cell-to-cell contact and inactivate OPG through expression of syndecan-1, which binds the heparin-binding domain of OPG, and mediates its internalization and lysosomal degradation (Figure 1). The ensuing increased RANKL-to-OPG adjacent to myeloma cells promotes osteoclast formation and activation. Enhanced osteoclastic bone resorption releases various cytokines and growth factors from the extracellular matrix of bone that further stimulate myeloma cell proliferation, thus initiating and maintaining a vicious circle between osteoclasts and myeloma cells. This concept provides the rationale that strategies that reduce the RANKL-to-OPG ratio may suppress bone resorption and myeloma cell burden alike.
Compared with healthy subjects or patients with other tumors, patients with myeloma bone disease have lower OPG levels in serum and within the bone microenvironment, and low OPG serum levels were inversely correlated with the severity of the disease. In animal models of myeloma bone disease, RANKL blockade by exogenous administration of RANK or OPG fusion proteins or gene transfer reduced the number of osteoclasts and osteolytic lesions, levels of bone resorption markers and monoclonal protein, and the incidence of complications such as paraplegia and prolonged survival. Preliminary data in human myeloma bone disease indicated profound antiresorptive effects of OPG administration as evident from biochemical markers of bone turnover, indicating that RANKL blockade may be a future therapeutic option for patients suffering from myeloma bone disease.
Prepublished online as Blood First Edition Paper, November 7, 2002; DOI 10.1182/blood-2002-09-2684.
Supported by grants from the Alfred und Ursula Kulemann Foundation, Marburg, Germany, and the Deutsche Krebshilfe (10-1697-Ho1), Bonn, Germany (L.C.H.).
References
Author notes
Lorenz Christian Hofbauer, Division of Gastroenterology, Endocrinology and Metabolism, Department of Medicine, Philipps University, Baldingerstrasse, D-35033 Marburg, Germany; e-mail: hofbauer@post.med.uni-marburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal