Mouse long-term hematopoietic reconstituting cells exist in the c-Kit+Sca-1+Lin− (KSL) cell population; among them, CD34low/− cells represent the most highly purified population of hematopoietic stem cells in the adult bone marrow. Here, we demonstrate that retrovirus-mediated transduction of CD34low/−c-Kit+Sca-1+Lin−(34−KSL) cells with the HES-1 gene, which encodes a basic helix-loop-helix transcription factor functioning downstream of the Notch receptor, and is a key molecule for the growth phase of neural stem cells in the embryo, preserves the long-term reconstituting activity of these cells in vitro. We also show that cells derived from the HES-1–transduced 34−KSL population produce progenies characterized by negative Hoechst dye staining, which defines the side population, and by CD34low/− profile in the bone marrow KSL population in each recipient mouse at ratios 3.5- and 7.8-fold those produced by nontransduced 34−KSL-derived competitor cells. We conclude that HES-1 preserves the long-term reconstituting hematopoietic activity of 34−KSL stem cells ex vivo. Up-regulation of HES-1 protein in the 34−KSL population before unnecessary cell division, that is, without retrovirus transduction, may represent a potent approach to absolute expansion of hematopoietic stem cells.
Introduction
Hematopoietic stem cells (HSCs) are generated during ontogeny and supply all mature hematopoietic lineages throughout life with their self-renewal and multilineage differentiation capacity.1 Efforts have been made to expand HSCs ex vivo without loss of their original potency. Long-term reconstitution capacity of mouse and human HSCs is maintained for up to 2 to 3 weeks by coculture with certain stromal cells.2-4 For expansion of HSCs without stromal cells, various combinations of cytokines that are active for immature hematopoietic progenitors have been surveyed.5-9 Of interest are approaches using Notch signaling, since it has been shown to inhibit differentiation of diverse types of cells in vertebrates.10-14 Notch signals are mediated by interactions between Notch receptors and their membrane-anchored ligands expressed in adjacent cells.15In the hematopoietic compartment, Notch receptors and ligands are expressed in hematopoietic progenitors and certain stromal cells, respectively.16-19 It was recently reported that the Notch ligand Jagged-1 maintained the severe combined immunodeficiency (scid)–repopulating activity of human cord blood–derived CD34+CD38− cells in vitro significantly longer than the control.20 Further evidence implying the potential usefulness of Notch signaling in HSC expansion comes from the establishment of a line of cytokine-dependent cells which differentiate into myeloid and lymphoid lineages in vivo when transplanted into syngeneic mice, by retroviral transduction of stem cell–enriched bone marrow cells with an activated form of Notch-1.21
In these previous investigations, however, it was not certain whether HSC expansion was achieved without loss of the original biologic phenotype, partly because unpurified cell populations were used as the starting materials. Mouse HSCs are enriched in the c-Kit+Sca-1+Lin− cell population (KSL). Further enrichment, in steady-state mouse bone marrow, showed that the highest purification was obtained with the CD34low/− population (34−KSL). In fact, a single 34−KSL cell was able to repopulate all hematopoietic lineages.22 Tracking of 34−KSL, therefore, after culturing in vitro and growing in recipient mice, may provide a better answer to the issue of HSC expansion.
Here, we used retrovirus-mediated transduction of 34−KSL with the HES-1 (hairy enhancer of split-1) gene.23HES-1 is known to code for a basic helix-loop-helix transcription factor functioning downstream of the Notch receptor,24-27 and together with HES-5, is a key molecule for the growth phase of neural stem cells in the developing mouse.28 Although it has also been suggested that HES-1 plays an important role in the development of perinatal T cells19 and myocytes,29 virtually no information is available about whether HES-1 plays a significant role in hematopoietic stem cell expansion. We demonstrate here that the introduction of HES-1 into 34−KSL significantly preserves the long-term reconstituting activity of these cells during culture. Moreover, the ratios of the Hoechst dye-staining–defined side population (SP)30-32 and CD34low/− cells in HES-1+ KSL are significantly higher than those in competitor-derived HES-1−KSL in the bone marrow of each recipient mouse. Given that retroviral transduction in vitro inevitably requires cell division, which typically reduces long-term reconstituting potency, these observations indicate that HES-1 is a highly potent molecule for the ex vivo expansion of the most primitive hematopoietic stem cells.
Materials and methods
Mice
C57BL/6 (B6-Ly5.2) mice were purchased from SLC (Tokyo, Japan). Mice congenic for Ly5 locus (B6-Ly5.1) were bred and maintained at the University of Tsukuba Animal Research Center (Tsukuba, Japan).
Antibodies and cytokines
The following materials were purchased from PharMingen (San Diego, CA): both biotinylated and unmodified sets of rat IgG2b anti–lineage markers Gr-1 (RB6-8C5), B220 (RA3-6B2), CD4 (GK1.5), CD8 (53-6.7), Mac1 (M1/70), and Ter119 (TER119); fluorescence-labeled antibodies phycoerythrin (PE)–Gr-1, PE-Mac1, allophycocyanin (APC)–B220, APC-Thy1.2, PE-Ly5.1 (A20), fluorescein isothiocyanate (FITC)–Ly5.1, FITC-Ly5.2 (104), PE-Sca-1 (D7), APC-c-Kit (2B8), and FITC–antimurine CD34 (RAM34); biotinylated antimurine CD34; and peridinin chlorophyll protein (PerCP)–Cy5.5-streptavidin. Energy-coupled dye (ECD)–streptavidin was from Beckman Coulter (Fullerton, CA). All cytokines were formulated at Kirin Brewery Research Laboratory (Takasaki, Japan), except Flt3 ligand (FL), which was purchased from Genzyme (Boston, MA).
Stem cell purification
Bone marrow cells were obtained from 8- to 12-week-old mice and fetal liver cells from E14 embryo (B6-Ly5.1). Adult bone marrow– and fetal liver–derived KSL (B-KSL and L-KSL, respectively) and 34−KSL were sorted in accordance with a previously described protocol.22 Briefly, lineage depletion from low-density cells isolated on Histpaque (Sigma, St Louis, MO) was performed with biotinylated rat IgG2b anti–lineage markers Gr-1, B220, CD4, CD8, Mac1, and Ter119, and streptavidin-conjugated magnetic beads (Biomag Binding Streptavidin; Polysciences, Warrington, PA). These cells were stained with ECD-streptavidin, PE-Sca-1, APC-c-Kit, and FITC–antimurine CD34, and analyzed and sorted with a FACS Vantage (Becton Dickinson, Franklin Lakes, NJ). The sorted cells were used for virus infection and cultivation as described below.
Retrovirus production and infection
A cDNA fragment for mouse HES-133 was subcloned into a retrovirus vector, pMY/IRES-EGFP (a gift from T. Kitamura, IMSUT, Tokyo, Japan). The resulting pMY/HES-1-IRES-EGFP and pMY/IRES-EGFP were transfected into ψMP34 cells34(a gift from Wakunaga Pharmaceuticals, Hiroshima, Japan; the resulting viruses were defined as HES-1IGv and GFPv, respectively), which were single cell–sorted for enhanced green fluorescence protein (GFP) with the FACS Vantage. Clones giving the highest infection efficiency, namely 4.5 × 108/mL for NIH/3T3 in both HES-1IGv and GFPv, were used for the rest of the experiments.
Either of the above-sorted KSL or 34−KSL was deposited into a single well of a 24-well dish coated with a fragment of RetroNectin (Takara, Shiga, Japan), at 1 × 104 to 5 × 104 per well, and cultured in a 1:2 mixture of the supernatant of the virus-producing ψMP34 clones and serum-free StemPro34 medium (Invitrogen, San Diego, CA; final serum concentration, 3.3%) containing 50 ng/mL mouse stem cell factor (SCF), 20 ng/mL mouse thrombopoietin (TPO), and 20 ng/mL human FL (for KSL) or 100 ng/mL SCF and 30 ng/mL TPO (for 34−KSL). After 24 hours, the culture medium was removed and the same medium containing freshly prepared supernatant of ψMP34 was furnished for an additional 24 hours.
RT-PCR analysis
Total RNA was isolated using RNeasy (QIAGEN, Hilden, Germany) from 1.5 × 104 to 2.5 × 104 of GFP+-sorted cells after culture, and used for semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR). Primer pairs were as follows: glyceraldehyde phosphate dehydrogenase (GAPDH), 5′-GCATTGTGGAAGGGCTCATG-3′ and 5′-TTGCTGTTGAAGTCGCAGGAG-3′; HES-1, 5′-CGGCATTCCAAGCTAGAGAAGG-3′ and 5′-GGTAGGTCATGGCGTTGATCTG-3′.
Colony assay
GFP+ KSL-derived cells were sorted at the end of the 48-hour infection period. Soon after this and after a further 3 days of culture in the presence of SCF, TPO, and FL, the cells were subjected to a colony assay using methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada).
Noncompetitive and competitive long-term reconstitution assay
For long-term reconstitution assay (LTRA) using the KSL-derived cells, Ly5.2 mice were exposed to 7.5 Gy (defined as “sublethal dose”) irradiation before injection of 1000 KSL-derived GFP+-sorted cells (Ly5.1) into the tail vein. At each time point, chimerism of GFP+ (Ly5.1) and GFP−(Ly5.2) cells in the blood of recipients was analyzed. For competitive LTRA using the 34−KSL-derived cells, Ly5.2 mice were exposed to 9.5 Gy (“lethal dose”) irradiation and injected with 1000 pMY/HES-1-IRES-EGFP– or pMY/IRES-EGFP–transduced 34−KSL-derived GFP+-sorted cells (Ly5.1) together with the same number of nontransduced 34−KSL-derived cells (Ly5.1) that were cultured for 2 days in the same manner except for the absence of the virus. At each time point, chimerism of GFP+Ly5.1+ and GFP− Ly5.1+ cells in the blood of recipients was analyzed. Decrease of Ly5.2+ (GFP−) cells was simultaneously confirmed.
Identification of SP and CD34low/− cells in the recipient bone marrow KSL cells
Analysis of SP in the competitive LTRA recipient's bone marrow KSL population was performed as previously described30 31with Hoechst 33 342 (Sigma).
For analysis of CD34low/− cells in the competitive LTRA recipient's bone marrow KSL population, a staining strategy different from the usual strategy that is described above was used because GFP occupied the FITC wave length. Briefly, lineage depletion was executed by the same set of, but unmodified series of, lineage marker antibodies used for the initial cell sorting, and anti–rat IgG beads (Dynabeads M-450; Dynal, Oslo, Norway). The lineage-depleted cells were stained with PE-Sca-1 (D7), APC-c-Kit (2B8), and biotinylated antimurine CD34 plus PerCP-Cy5.5-streptavidine, after confirmation of lineage depletion with a portion of the cells.
Results
Retrovirus containing HES-1 preserves immature progenitors in bone marrow– and fetal liver–derived KSL
We placed HES-1 cDNA in the retroviral vector pMY/IRES-EGFP, which drives expression of a cDNA of interest and of GFP as a marker from a single bicistronic mRNA (Figure1A).35 36 The infection efficiencies of the resulting HES-1IGv and GFPv in B-KSL and L-KSL (Figure 1B) after 48-hour culture were 20% to 75% and 40% to 90%, respectively, in the presence of SCF, TPO, and FL (Figure 1C).
Retroviral construct, stem cells, and transduction.
(A) Schematic representation of the retroviral vector, pMY/HES-1IG, encoding HES-1 linked by internal ribosome entry site (IRES) to a cDNA encoding EGFP. (B) Flow cytometric analysis of murine hematopoietic stem cells in adult bone marrow and fetal liver. Staining profile of Sca-1 versus c-Kit on lineage-negative populations in bone marrow (left) and fetal liver (right) cells. (C) Flow cytometric analysis for GFP in infected c-Kit+ Sca-1+ Lin−(KSL) cells of bone marrow (top) and fetal liver (bottom). Efficiency of infection of these populations is indicated.
Retroviral construct, stem cells, and transduction.
(A) Schematic representation of the retroviral vector, pMY/HES-1IG, encoding HES-1 linked by internal ribosome entry site (IRES) to a cDNA encoding EGFP. (B) Flow cytometric analysis of murine hematopoietic stem cells in adult bone marrow and fetal liver. Staining profile of Sca-1 versus c-Kit on lineage-negative populations in bone marrow (left) and fetal liver (right) cells. (C) Flow cytometric analysis for GFP in infected c-Kit+ Sca-1+ Lin−(KSL) cells of bone marrow (top) and fetal liver (bottom). Efficiency of infection of these populations is indicated.
Next, the sorted GFP+ cells were subjected to the colony-forming assay before and after an additional 3-day culture in the presence of SCF, TPO, and FL. Results showed that the numbers of mature progenitor-derived colonies such as granulocyte colonies and erythroid colony-forming unit–derived colonies were similar between the HES-1Igv–transduced and GFPv-transduced B-KSL–derived cells. However, the number of high-proliferative-potential-mix (HPP-mix)–derived colonies was greater in the HES-1Igv–transduced than in the GFPv-transduced B-KSL–derived cells, particularly when the assay was performed after an additional 3-day culture. The HES-1Igv–transduced L-KSL–derived cells also formed a significantly higher number of HPP-mix–derived colonies than the GFPv-transduced L-KSL–derived cells (Figure 2A). We then transplanted 1000 of the GFP+ B-KSL– and L-KSL–derived cells sorted soon after the 48-hour infection period to sublethally irradiated recipient mice. In both B-KSL– and L-KSL–derived cell transplant recipients, donor cell chimerism was initially established but rapidly decreased within 2 months after transplantation in the control GFPv-transduced group, whereas high levels of chimerism were maintained for up to 6 months after transplantation in the HES-1Igv–transduced group in both myeloid and lymphoid lineages (Figure 2B). Immunophenotyping of the bone marrow, spleen, and thymus cells at 3 months after transplantation confirmed that donor chimerism in these tissues was similar to that in the blood cells (data not shown).
Relative preservation of KSL phenotype by constitutive expression of HES-1.
(A) Colony-forming ability of transduced cells was examined soon after sorting GFP+ cells and after an additional 3-day culture. HPP-mix; colonies larger than 1.5 mm in diameter and consisting of at least granulocyte/macrophage and erythroid clusters. The number of HPP-mix–derived colonies in 1000 cells (culture day 2; sort day 0) or 2000 cells (culture day 5; sort day 3) is shown. Data show the mean ± SD of triplicates from 2 independent experiments. (B) Long-term repopulation ability of selected transduced cells was assessed by transplantation to sublethally irradiated recipients. Donor (GFP+Ly5.1+)–derived cells were detected in peripheral blood of recipient mice (Ly5.2). The percentage of donor-derived cells within myeloid (Mac-1+ and Gr-1+) and lymphoid (Thy1.2+ and B220+) cells was measured at various time points after transplantation. Results for HES-1IGv and GFPv are shown as the mean ± SD of 12 and 9 measurements, respectively, from 3 independent experiments (2-6 animals per experiment).
Relative preservation of KSL phenotype by constitutive expression of HES-1.
(A) Colony-forming ability of transduced cells was examined soon after sorting GFP+ cells and after an additional 3-day culture. HPP-mix; colonies larger than 1.5 mm in diameter and consisting of at least granulocyte/macrophage and erythroid clusters. The number of HPP-mix–derived colonies in 1000 cells (culture day 2; sort day 0) or 2000 cells (culture day 5; sort day 3) is shown. Data show the mean ± SD of triplicates from 2 independent experiments. (B) Long-term repopulation ability of selected transduced cells was assessed by transplantation to sublethally irradiated recipients. Donor (GFP+Ly5.1+)–derived cells were detected in peripheral blood of recipient mice (Ly5.2). The percentage of donor-derived cells within myeloid (Mac-1+ and Gr-1+) and lymphoid (Thy1.2+ and B220+) cells was measured at various time points after transplantation. Results for HES-1IGv and GFPv are shown as the mean ± SD of 12 and 9 measurements, respectively, from 3 independent experiments (2-6 animals per experiment).
We next examined the ratios of KSL populations in the recipient bone marrow. Recipient-derived (GFP−) and donor-derived (GFP+) bone marrow cells contained similar ratios (0.117% ± 0.054% and 0.092% ± 0.021%, respectively) of KSL in the GFPv-transduced cell transplant group. In contrast, an approximately 5-fold higher ratio (0.577% ± 0.109%) of donor-derived bone marrow cells showed the KSL phenotype in the HES-1Igv–transduced cell transplant group (Figure3A). In GFP+ KSL sorted from the recipient bone marrow of the GFPv and HES-1IGv groups, RT-PCR confirmed a lower level of HES-1 mRNA expression in KSL from the GFPv-transduced group and a higher level in KSL from the HES-1Igv–transduced group (Figure 3B).
Analysis of bone marrow cells in recipients at 3 months after transplantation.
(A) Flow cytometric analysis for KSL in recipient mice. Respective gates for GFP− (recipient-derived, left) and GFP+ (donor-derived: HES-1Igv–transduced, middle; GFPv-transduced, right) cells of the lineage-negative population were analyzed for c-Kit and Sca-1 staining. (B) Expression ofHES-1 mRNA in reconstituted cells. The amount of templates for RT-PCR was normalized to give equivalent signals for GAPDH. RT− indicates PCR performed without reverse transcriptase reaction.
Analysis of bone marrow cells in recipients at 3 months after transplantation.
(A) Flow cytometric analysis for KSL in recipient mice. Respective gates for GFP− (recipient-derived, left) and GFP+ (donor-derived: HES-1Igv–transduced, middle; GFPv-transduced, right) cells of the lineage-negative population were analyzed for c-Kit and Sca-1 staining. (B) Expression ofHES-1 mRNA in reconstituted cells. The amount of templates for RT-PCR was normalized to give equivalent signals for GAPDH. RT− indicates PCR performed without reverse transcriptase reaction.
HES-1 maintains stem cell activity of 34−KSL in vitro
The findings above indicated that HES-1 decreases the rate of loss of immaturity and preserves self-renewability of KSL in vitro. This encouraged us to examine whether HES-1 could maintain 34−KSL cells, the most highly purified HSCs, which have never been shown to be expanded or even maintained in vitro. Although FL has often been used for retrovirus gene transfer directed to HSCs because infection efficiency is generally very low without it, it has been reported that the use of FL is associated with the loss of self-renewal capacity of KSL cells.37 38 Therefore, we took advantage of omitting FL and used only SCF and TPO for HES-1IGv and GFPv infection of 34−KSL cells (Figure4A). Both viruses gave an infection efficiency of no less than 20% (Figure 4B). For a competitive LTRA, we also cultured 34−KSL for 48 hours in the same conditions as those for the virus infection, except for the absence of virus. At the end of the 48-hour culture period, most cells lost the 34−KSL phenotype in any virus-transduced and virus-nontransduced group, as anticipated (data not shown). Even KSL cells did not represent a major population in any groups. Moreover, although the nontransduced 34−KSL-derived cells kept the KSL phenotype at 16.7% to 24.9%, GFPv-transduced 34−KSL-derived cells kept the KSL phenotype at as low as 8.5% to 13.2%, probably reflecting the fact that only unquiescent and thus differentiation-prone cells are susceptible to retrovirus infection. In contrast, the ratio of KSL in the HES-1Igv–transduced 34−KSL-derived cells was 18.6% to 24.7%, which was as high as that in the nontransduced 34−KSL-derived cells (Figure 4C).
Competitive repopulation assay using 34−KSL-derived cells.
(A) Staining profile of Sca-1 versus c-Kit in lineage-depleted and CD34−/low–gated populations in bone marrow. (B) Flow cytometric analysis for GFP in infected 34−KSL-derived cells. Effieciency of infection at the end of the 2-day infection period is indicated. (C) Maintenance of the KSL phenotype in 34−KSL-derived cells by HES-1. Left, nontransduced 34−KSL-derived cells; middle, HES-1Igv–transduced 34−KSL-derived cells; right, GFPv-transduced 34−KSL-derived cells. (D) Chimerism of GFP+Ly5.1+ cells in the total Ly5.1+ cells in the blood of recipients. Results for HES-1IGv and GFPv are shown as the mean ± SD of 12 and 8 measurements, respectively, from 3 independent experiments (2 to 6 animals per experiment).
Competitive repopulation assay using 34−KSL-derived cells.
(A) Staining profile of Sca-1 versus c-Kit in lineage-depleted and CD34−/low–gated populations in bone marrow. (B) Flow cytometric analysis for GFP in infected 34−KSL-derived cells. Effieciency of infection at the end of the 2-day infection period is indicated. (C) Maintenance of the KSL phenotype in 34−KSL-derived cells by HES-1. Left, nontransduced 34−KSL-derived cells; middle, HES-1Igv–transduced 34−KSL-derived cells; right, GFPv-transduced 34−KSL-derived cells. (D) Chimerism of GFP+Ly5.1+ cells in the total Ly5.1+ cells in the blood of recipients. Results for HES-1IGv and GFPv are shown as the mean ± SD of 12 and 8 measurements, respectively, from 3 independent experiments (2 to 6 animals per experiment).
To better evaluate the effect of HES-1 on 34−KSL cells, we performed a competitive LTRA. We transplanted 1000 transduced (GFP+-selected, Ly5.1+) and the same number of nontransduced 34−KSL-derived cells (GFP−Ly5.1+) to lethally irradiated recipient mice (Ly5.2+), and assessed chimerism of the GFP+Ly5.1+ (tester) and GFP−Ly5.1+ (competitor) populations in myeloid and lymphoid lineages in the blood at several time points. At all time points examined, the ratios of the tester-derived cells in the total donor-derived cells, that is, the percent of GFP+ cells among the Ly5.1+ cells, were more than 40% and more than 35% in the myeloid and lymphoid lineages, respectively, in the HES-1Igv–transduced group. In the GFPv-transduced group, in contrast, chimerism of the tester-derived cells in the total donor-derived cells was less than 15% in both lineages at 2 months after the transplantation (Figure 4D). We also assessed GFP−Ly5.2+ (recipient-derived) cells at each time point and confirmed that this population decreased to a negligible level. These results indicated that stem cell activity of the HES-1Igv–transduced, but not GFPv-transduced, 34−KSL-derived cells was almost equivalent to that of the nontransduced 34−KSL-derived cells.
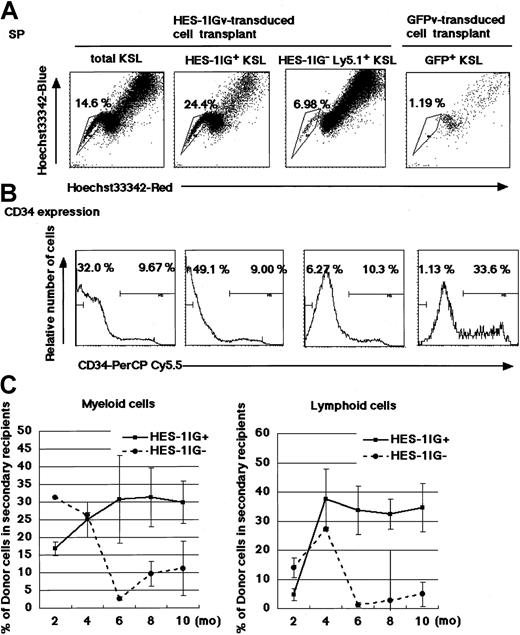
The HES-1+ KSL population contains higher ratios of SP and 34low/− cells in the recipient bone marrow and shows higher reconstituting activity
We compared the SP phenotype and CD34 expression profile of the virus-transduced and nontransduced recipient bone marrow KSL in each competitive LTRA recipient mouse. At 3 months after the transplantation, ratios of SP and CD34low/− cells in total KSL were 14.6% and 32.0% in the recipient of the HES-1Igv–transduced 34−KSL-derived cell transplant (Figure 5A-B), and 10.7% and 27.3% in the recipient of the GFPv-transduced 34−KSL-derived cell transplant (data not shown), respectively. We then separated total KSL into GFP− (mostly virus-nontransduced donor [competitor]–derived) and GFP+ (tester-derived) fractions. Remarkably, ratios of SP and CD34low/− cells in the GFP+ KSL population were 3.5- and 8-fold higher than those in the GFP− KSL population from the HES-1Igv–transduced cell transplant recipient. In contrast, ratios of SP and CD34low/− cells in the GFP+ KSL population were much lower than those in the GFP− KSL population from the GFPv-transduced cell transplant recipient (Figure 5A-B). This indicated that HES-1IGv transduction preserved the KSL cells characterized by the SP and CD34low/− profile, suggesting that HES-1 stores the most immature HSCs without preventing them from supplying mature blood cells.
To further investigate whether the greater ratios of SP and CD34low/− cells in the HES-1+ than in the HES-1−KSL population truly reflect the greater reconstitution activity of HES-1+ than that of HES-1−KSL cells, GFP+ and GFP−KSL cells were sorted from the bone marrow of HES-1Igv–transduced cell transplant recipients 3 months after the primary transplantation, and 1000 cells from either KSL population were transplanted to lethally irradiated secondary recipient mice (Ly5.2+). At 6 months and 10 months after the secondary transplantation, donor chimerism in the blood cells of the secondary recipient was approximately 30% to 40% in the GFP+ KSL transplant group and less than 10% in the GFP− KSL transplant group, both in the myeloid and lymphoid lineages (Figure 5C). Although it is more than 1 year since the primary transplantation, no leukemias or lymphomas have developed in any primary or secondary recipient mice (data not shown).
Identification of SP and CD34low/− cells in recipient bone marrow KSL cells.
(A) Hoechst dye efflux in various gates of KSL prepared from bone marrow of competitive LTRA recipients. Total KSL, GFP+Ly5.1+ KSL, and GFP−Ly5.1+ KSL from a representative recipient of HES-1Igv–transduced tester cells and competitor cells, and GFP+Ly5.1+ KSL from a representative recipient of GFPv-transduced tester cells and competitor cells were analyzed for staining for Hoechst 33342Blue and Hoechst 33342Red. (B) CD34 expression profile in various gates of KSL prepared from recipients of competitive LTRA. The same gates as panel A were used. To stain CD34 without FITC, PerCP-Cy5.5–labeled mouse CD34 antibody was used. This makes the fluorescence activated cell sorting (FACS) pattern different from that used for CD34−KSL sorting. (C) Evaluation of stem cell activity on secondary transplantation. At each time point, chimerism of donor-derived cells in recipient blood was plotted. Plots are shown as the mean ± SD of 4 animals.
Identification of SP and CD34low/− cells in recipient bone marrow KSL cells.
(A) Hoechst dye efflux in various gates of KSL prepared from bone marrow of competitive LTRA recipients. Total KSL, GFP+Ly5.1+ KSL, and GFP−Ly5.1+ KSL from a representative recipient of HES-1Igv–transduced tester cells and competitor cells, and GFP+Ly5.1+ KSL from a representative recipient of GFPv-transduced tester cells and competitor cells were analyzed for staining for Hoechst 33342Blue and Hoechst 33342Red. (B) CD34 expression profile in various gates of KSL prepared from recipients of competitive LTRA. The same gates as panel A were used. To stain CD34 without FITC, PerCP-Cy5.5–labeled mouse CD34 antibody was used. This makes the fluorescence activated cell sorting (FACS) pattern different from that used for CD34−KSL sorting. (C) Evaluation of stem cell activity on secondary transplantation. At each time point, chimerism of donor-derived cells in recipient blood was plotted. Plots are shown as the mean ± SD of 4 animals.
Discussion
In the present study, we demonstrate that retroviral transduction of KSL and 34−KSL with the HES-1 gene results in preservation of the stem cell activity of these cells in vitro. Moreover, transduction of 34−KSL with HES-1results in a striking accumulation of SP and CD34low/−cells among the KSL population in the recipient bone marrow, while maintaining production of differentiated blood cells.
Many investigators have introduced a gene of interest into bone marrow and fetal liver cells by a retroviral gene transfer method and transplanted the transduced cells to recipient animals.39,40 These studies have described a number of interesting phenotypes in the blood compartment of recipients, such as those showing the development of leukemia and an increase or decrease in hematopoiesis in a certain lineage, and aided understanding of the roles of introduced genes in HSCs. However, with rare exceptions, the use of highly purified HSCs for virus transduction is rare and it has therefore been unclear which cells are transduced. Strictly, none of the previous studies have directly proved the expansion or maintenance of virus-transduced HSCs. A few reports22,41 have shown multilineage hematolymphopoietic reconstitution in the recipient mice with a sorted single HSC characterized by Lin−, Sca-1+, c-Kit+, and CD34low/−. Although 34−KSL cells do not represent a homogenous population, they are the most highly purified HSCs, at least in steady-state adult bone marrow. The use of this population as a target of gene transduction and transplantation to recipient mice greatly facilitates study of the function of the gene of interest and the technology of ex vivo HSC expansion. Normally, culturing purified HSCs for only 48 hours without stromal cells significantly reduces their stem cell activity. Moreover, retrovirus transduction requires cell division, which in general indicates a further decrease in stem cell activity.37 Given these factors, the decreased ability of GFPv-transduced 34−KSL-derived cells to retain the immature phenotype in vitro and decreased engraftment capacity in mice receiving these cells compared with the nontransduced 34−KSL-derived cells (Figure 4C-D) is reasonable. In clear contrast, these capacities in HES-1Igv–transduced 34−KSL-derived cells are at least the same as those of nontransduced 34−KSL-derived cells (Figure 4C-D), indicating that HES-1 introduction in 34−KSL cells strongly preserves the original biologic phenotype of these cells in vitro.
HES-1 is a basic helix-loop-helix transcription factor, which functions as a negative regulator for cell differentiation in various systems, such as neurogenesis,24,42,43myogenesis,25,29 and hair cell formation,44although it also induces cell differentiation in selected contexts, such as in glial cell differentiation.33 Down-regulation and up-regulation of HES-1, together with HES-5, in neuronal stem cells in developing mice indicate that this gene functions as a positive regulator of neuronal stem cell self-renewal.28 Our observation suggests that HES-1 has a similar effect on hematopoietic stem cells.
In addition to its strong ex vivo maintenance capacity for 34−KSL, we showed that HES-1 dramatically accumulates SP and CD34low/− cells in the recipient bone marrow KSL cells. The ability to escape Hoechst dye staining is considered to represent an important feature common to stem cells of various lineages.32 In the hematopoietic compartment, it has been shown that long-term reconstituting cells exist in SP and that SP and 34−KSL partially overlap.45 We indeed show that the HES-1+ KSL population that contains a greater ratio of SP and CD34low/− cells has a reconstitution capacity greater than the HES-1−KSL population (Figure5C). This implies that HES-1 confers an increased self-renewal capacity to the most primitive HSCs in vivo, and we propose that HES-1 is a positive regulator of the expansion of HSCs without exhausting their stem cell activity. The mechanism by which HSC-containing populations such as SP and 34−KSL accumulate while normal differentiation and supply of blood cells are maintained remains to be elucidated.
Recently, phenotypes of the recipients who received transplants of lineage-negative bone marrow cells introduced with HES-1 and HES-5, as well as active Notch-1, were reported.46 The phenotype ofHES-1–transduced lineage-negative cell transplant recipients described in that report was similar to ours in that the recipients do not develop T-cell leukemia, in contrast to the fact that T-cell leukemia develops in active Notch-1–transduced lineage-negative cell transplant recipients.46 47 The finding in the previous report that the recipients had a greater number of clonogenic cells in bone marrow could be similar to our finding that immature clonogenic cells were maintained in vitro or that the recipients had a greater number of stem cells such as SP cells in bone marrow. However, there is a significant difference in the development of lymphoid cells in the recipients. In contrast to the results described in the previous report, we did not find any distortion in the lymphoid compartment of the HES-1–transduced HSC transplant recipients. This difference could be attributed to the difference in the experimental system. We used highly purified HSCs for retroviral gene transfer, implying that we transplanted much fewer numbers ofHES-1–transduced committed progenitor cells. If less-purified bone marrow cells are used for retroviral transduction, it might be the case that the gene of interest is preferentially introduced into the committed progenitors such as T-cell progenitors, rather than into stem cells, and that in such cases the phenotype derived from progenitors rather than stem cells is prominent.
Interestingly, HES-1 is a direct target of a transcriptional activator complex comprising RBPJ and activated Notch in neurogenic, myogenic, and lymphoid cells. Further, a growing body of evidence shows that activation of Notch signaling results in prevention of differentiation. We therefore expect that if ligands for Notch can efficiently up-regulate HES-1 expression in 34−KSL, they may serve as potential tools for ex vivo HSC expansion, particularly given that ligand-induced up-regulation of HES-1 does not require retrovirus transduction, an experimental system used in this work which is nevertheless disadvantageous to the maintenance of stem cell activity because of requirement of cell division. Indeed, others have already investigated the possibility of Notch ligands for this purpose.17,18 20 Although these efforts have yet to show clinical impact, our data strongly support their value and the potential for further methodologic development.
In the present study we show using several independent experimental designs that purified HSCs can be maintained ex vivo by HES-1 up-regulation. With technical improvement, the use of Notch ligands or other methods to up-regulate HES-1 carries strong promise for future clinical use.
We thank Y. Koyama of Becton Dickinson Hongo Laboratory for assistance in SP analysis. We also thank T. Kitamura for the pMY/IRES-EGFP retrovirus vector, T. Yoshimatsu for the ψMP34 cells, and G. Harris for review of the manuscript.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-07-2051.
Supported in part by grants-in-aid from the Ministries of Education, Culture, Sports and Technology, and Health, Labour and Welfare of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hisamaru Hirai, University of Tokyo, Graduate School of Medicine, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan; e-mail: hhirai-tky@umin.ac.jp.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal