Coculture with stromal cells tends to maintain normal hematopoietic progenitors and their leukemic counterparts in an undifferentiated, proliferative state. An example of this effect is seen with megakaryocytic differentiation, wherein stromal contact renders many cell types refractory to potent induction stimuli. This inhibitory effect of stroma on megakaryocytic differentiation correlates with a blockade within hematopoietic cells of protein kinase C-ε (PKC-ε) up-regulation and of extracellular signal-regulated kinase/mitogen-activated protein (ERK/MAP) kinase activation, both of which have been implicated in promoting megakaryocytic differentiation. In this study K562ΔRafER.5 cells, expressing an estradiol-responsive mutant of the protein kinase Raf-1, were used to determine the relevance and stage of ERK/MAPK pathway blockade by stromal contact. Activation of ΔRafER by estradiol overrode stromal blockade of megakaryocytic differentiation, implicating the proximal stage of the ERK/MAPK pathway as a relevant control point. Because stromal contact blocked delayed but not early ERK activation, the small guanosine triphosphatase (GTPase) Rap1 was considered as a candidate inhibitory target. Activation assays confirmed that Rap1 underwent sustained activation as a result of megakaryocytic induction, as previously described. As with ERK activation, stromal contact selectively blocked delayed but not early Rap1 activation, having no effect on Ras activation. Enforced expression of either wild-type Rap1 or the GTPase (GAP) resistant mutant Rap1 V12 failed to override stromal inhibition, suggesting that the inhibitory mechanism does not involve GAP up-regulation but rather may target upstream guanine nucleotide exchange factor (GEF) complexes. Accordingly, coimmunoprecipitation demonstrated stromally induced alterations in a protein complex associated with c-Cbl, a scaffolding factor for Rap1-GEF complexes.
Introduction
Within the bone marrow microenvironment, stromal cells provide critical extrinsic cues to hematopoietic progenitor cells, influencing such fundamental processes as survival, proliferation, and differentiation. The influence of stromal cells on normal and neoplastic hematopoiesis has been reflected by several in vitro culture systems, in which stromal contact supports (a)long-term culture of multipotent progenitors,1(b) maintenance of normal B-cell progenitors,2and (c) leukemic cell growth and survival.3 The mechanisms through which stromal cells influence hematopoiesis include paracrine secretion of soluble factors, production of extracellular matrix, and direct receptor-counterreceptor engagements.4An important component of the stromal influence in these culture systems, and perhaps also in vivo, lies in the ability to block hematopoietic differentiation.5
An inhibitory effect of stromal cells on in vitro megakaryopoiesis occurs both with primary human CD34+ cells and erythroleukemic cell line model systems.6,7 The stromal inhibition of megakaryocyte production from CD34+ cells clearly requires direct cell-cell contact.8 This inhibitory effect may partly account for the impaired megakaryocytic engraftment frequently seen after bone marrow transplantation. For example, whereas CD34+ cells alone in vitro undergo efficient megakaryocytic differentiation in response to thrombopoietin (TPO), CD34+ cells infused into an irradiated recipient show minimal evidence of megakaryocytic induction in response to pharmacologic TPO doses.9
Stromal inhibition of hematopoietic differentiation most likely occurs due to alterations in signal transduction and transcriptional regulatory pathways within progenitor cells. In the case of megakaryocytic differentiation induction, stromal contact can block both up-regulation of protein kinase C-ε (PKC-ε) and sustained activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) pathway.7 PKC-ε has previously been implicated in the programming of megakaryocytic lineage commitment, possibly through functional interaction with the GATA-1 erythromegakaryocytic zinc finger transcription factor.10 However, enforced expression of PKC-ε only weakly promotes megakaryocytic differentiation and does not suffice to override stromal inhibition of differentiation.7 Another possibility is that the stromal inhibition of ERK/MAPK signaling may be responsible for blocking megakaryocytic differentiation. Supporting this conclusion, an array of studies using a variety of experimental systems, including primary human CD34+ progenitors, have demonstrated a requirement for ERK/MAPK signaling in megakaryocytic differentiation.11-15 In some of these experimental systems, ERK/MAPK signaling actually appears to be sufficient to trigger megakaryocytic differentiation.12 15
A critical aspect of ERK/MAPK signaling in promoting megakaryocytic differentiation is sustained activation.11,13,16Interruption of sustained ERK/MAPK activation, even after 24 hours, completely aborts megakaryocytic lineage commitment.11 In 2 different model systems, the differentiation-promoting component of TPO receptor signaling correlates with prolonged activation of the ERK/MAPK pathway.13,16 As with nerve growth factor (NGF)–induced neuronal differentiation, the sustained phase of TPO-induced ERK/MAPK activation occurs via the Ras-like guanosine triphosphatase (GTPase) Rap1.17
In the current study, experiments addressed both the significance and the mechanism of stromal inhibition of sustained ERK/MAPK activation. Using an epistasis approach in the K562 system, it was found that the ERK/MAPK pathway is indeed the relevant target of stromal inhibition of megakaryocytic differentiation. Specifically, stromal contact caused a decay in late-phase ERK/MAPK signaling through delayed down-regulation of Rap1 activation, an effect most likely achieved through inhibition of guanine nucleotide exchange factor (GEF) activity. Similarly in HEL cells, stromal inhibition of megakaryocytic differentiation correlated with inhibition of Rap1 activation. Coimmunoprecipitation studies showed that stromal contact caused alterations in a protein complex associated with c-Cbl, a scaffolding factor involved in Rap1 activation.18 Therefore, Rap1 may represent a critical control point in the stromal inhibition of megakaryocytic differentiation.
Materials and methods
Cell culture and transfection
The bone marrow stromal cell line HESS-5 was provided by Dr Takashi Tsuji from the Pharmaceutical Frontier Research Laboratory (Yokohama, Japan) and was maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). HESS-5 cells were used because of their superlative ability to support a variety of primary hematopoietic functions, including Dexter long-term cultures, Whitlock-Witte long-term cultures, cobblestone formation, and adhesion of lin− cells.19 In addition, HESS-5 cells promote serum-free proliferation of human leukemic cells.20 The human erythroleukemia cell lines K562 and HEL were grown in RPMI 1640 with 10% FBS. K562ΔRafER.5, a stable transfectant expressing the ΔRafER estradiol-responsive Raf-1 mutant, was kindly provided by Dr Dan Rosson (Lankenau Medical Research Center, Wynnewood, PA) and maintained in phenol red–free RPMI 1640 with 10% FBS and 1 mg/mL G418.21 Experiments using K562, K562ΔRafER.5, and HEL cells employed midlog phase cells at a density of 0.5 × 106 to 1.0 × 106/mL. In general, megakaryocytic induction of either K562 or HEL cells was accomplished with 25 nM 12-O-tetradecanoyl phorbol 13-acetate (TPA) (Sigma, St Louis, MO) for 48 hours. Human primary 34+ stem cells (Poietic Technologies, Gaithersburg, MD) purified from the peripheral blood of granulocyte colony-stimulating factor (G-CSF)–treated healthy adult donors were grown in StemPro 34-SFM serum free medium (Gibco, Carlsbad, CA) supplemented with cytokines (R & D Systems, Minneapolis, MN). Cytokine supplements consisted of stem cell factor (SCF) at 25 ng/mL plus interleukin-3 (IL-3) at 10 ng/mL (myeloid progenitor expansion) or SCF at 25 ng/mL plus thrombopoietin (TPO) at 40 ng/mL (megakaryocyte development). Ingenol 3,20-dibenzoate (IDB) at 25 nM was used to enhance megakaryocyte induction of CD34+ stem cells. Human primary bone marrow stromal cells obtained with informed consent from patients undergoing spinal fusion surgery (Department of Orthopedics, Case Western Reserve University and University Hospitals Research Institute) were isolated and expanded as previously described.22 Experiments with the human CD34+cells were approved by the Human Investigation Committee of the University of Virginia. The use of the human primary bone marrow stromal cells was approved by the Case Western Reserve University institutional review board; informed consent was provided according to the Declaration of Helsinki.
Transfections of K562 cells were carried out using the X-tremeGENE Q2 Transfection Reagent as recommended by the manufacturer (Roche). For transient transfections, cells were subjected to indicated treatments about 18 hours after initiating transfection. For stable transfection, cells were subjected to 1 mg/mL G418 selection followed by limiting dilution cloning, with selection of stably YFP+ clones using an inverted epifluorescent/phase contrast microscope.
Plasmids
pGEX-RalGDS(RBD) encoding a glutathione-S-transferase (GST) fusion with the 98–amino acid Rap/Ras binding domain of RalGDS was kindly provided by Dr Judy Meinkoth (Department of Pharmacology, University of Pennsylvania School of Medicine).23Mammalian expression vectors for Rap1 wild type and V12, pMT-Ha-rap1A and pMT-rap V12-HA, were a generous gift from Dr Johannes Bos (Department of Physiological Chemistry, Utrecht University, The Netherlands).24 Expression constructs for GFP-Rap1 fusions were made by subcloning polymerase chain reaction (PCR)–amplified Rap1 wild-type and V12 coding regions intoXhoI-EcoRI sites of pEYFP-C1 (Clontech); the 3′ primers in the PCR amplification introduced in-frame carboxy-terminal FLAG tag sequence.
Immunoblot assays
For whole cell lysates, equivalent numbers of cells for each sample were washed with cold phosphate-buffered saline (PBS) and lysed in 100 μL 1 × sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer per 106cells. Samples were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were preblocked and probed with primary antibodies per the manufacturer's instructions, followed by the appropriate horseradish peroxidase–conjugated secondary antibody (Pierce, Rockford, IL). Signal detection used enhanced chemiluminescence. Rabbit polyclonal anti-gpIIb was a generous gift from Dr Dan Rosson (Lankenau Medical Research Center, Wynnewood, PA).21 Rabbit polyclonal antiphospho-ERK was purchased from Promega (Madison, WI). Rabbit polyclonal antiphospho-Akt (Ser473) was purchased from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal anti-Rap1a was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-Ras (clone RAS10) was purchased from Upstate Biotechnology (Lake Placid, NY). Mouse monoclonal antitubulin was purchased from Sigma. Mouse monoclonal anti-Crk was purchased from BD Transduction Laboratories (San Diego, CA). Mouse monoclonal antiphosphotyrosine antibodies 4G10 and ptyr-100 were purchased, respectively, from Upstate Biotechnology and Cell Signaling Technology. Rabbit anti-C3G and anti-FRS2 were purchased from Santa Cruz Biotechnology. Quantitative scanning densitometry with normalization for lane loading (tubulin signal) was performed using a Molecular Dynamics (Piscataway, NJ) Personal Densitometer with ImageQuant 5.0 software.
Rap1 and Ras activation assays
GTP-bound forms of Rap1 and Ras were detected as described by Tsyganova et al with minor modifications.23 In brief, 3 × 106 cells were resuspended in 300 μL ice-cold radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [tris(hydroxymethyl)aminomethane] HCl, pH 8.0; 150 mM NaCl; 0.5% sodium deoxycholate; 1% Nonidet P-40 [NP-40]; 0.1% SDS; 10 mM NaF; 2 mM Na3VO4) with protease inhibitors (1 tablet per 10 mL BMB-complete, EDTA [ethylenediaminetetraacetic acid]–free; Boehringer-Mannheim Biochemicals, Mannheim, Germany). Cellular extracts were incubated with glutathione agarose beads preloaded with bacterially expressed GST-RalGDS(RBD) for 1 hour at 4°C. After thorough washing, beads were subjected to elution by boiling 5 minutes in 1 × SDS-PAGE loading buffer. Eluates were then subjected to immunoblotting with antibodies to Rap1 and Ras.
Phalloidin staining of actin cytoskeleton
K562-EYFP-Rap1 stable transfectants cultured on sterile glass coverslips 48 hours with or without 25 nM TPA were washed with ice-cold PBS and fixed with 4% paraformaldehyde/PBS for 20 minutes at room temperature. Cells were then permeabilized with 0.2% Triton X-100 in PBS with 20% normal goat serum (NGS) for 30 minutes at room temperature followed by blocking with 20% NGS/PBS another 30 minutes at room temperature. Cells were stained with rhodamine-phalloidin (Molecular Probes, Eugene, OR) at a 1:500 dilution in 5% NGS/PBS for 1 hour at room temperature. After washing and mounting, dual-color confocal laser scanning microscopy of cells was carried out on a Zeiss LSM 5 Pascal (Jena, Germany) with Zeiss LSM analysis software.
Coimmunoprecipitation
A total of 1 × 107 cells were washed and resuspended in 500 μL ice-cold extraction buffer (50 mM Tris HCl, pH 7.6; 150 mM NaCl; 1.0% NP-40; 1 mM EDTA; 1 mM NaVO4; 1 mM NaF) with protease inhibitors (1 tablet per 10 mL BMB-complete, EDTA-free; Roche). After a 20-minute incubation on ice with intermittent tapping, debris was pelleted at 10 000 rpm, 10 minutes at 4°C. Supernatants were adjusted to 10% glycerol and stored at −20°C. For c-Cbl immunoprecipitation, 4 μg rabbit anti–c-Cbl (sc-170; Santa Cruz Biotechnology) was added to 500 μL of thawed, clarified extracts followed by incubation on a rocker 3 hours at 4°C. Immune complexes captured on protein G beads (Ultralink, Pierce) were subjected to washing followed by elution in Laemmli sample buffer.
Flow cytometry
Staining of cells for surface CD41a and glycophorin A (GPA) used the phycoerythrin (PE)–conjugated antibodies HIP8-PE and GA-R 2-PE, respectively (PharMingen, San Diego, CA). Phycoerythrin-conjugated isotype-matched antibody controls were used to establish thresholds for specific staining. Flow cytometric analyses were conducted on either a FACSCalibur (Figures 1, 3, and 6) or FACScan (Figure 7) system using CellQuest software (Becton Dickinson, San Jose, CA).
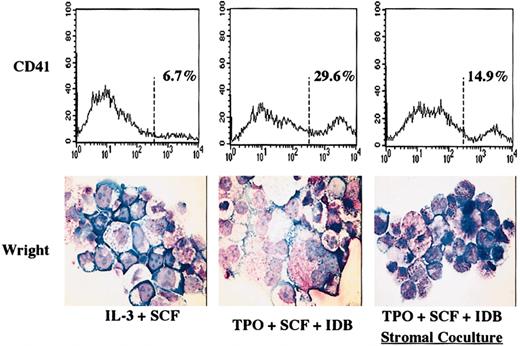
Stromal cell exposure inhibits megakaryocytic differentiation in primary human stem cells.
Primary human hematopoietic stem cells were cultured 7 days with either IL-3 plus SCF to expand myeloid progenitors or with TPO plus SCF plus IDB to induce megakaryocytic differentiation. Cells were either cultured alone or on a monolayer of primary human bone marrow stromal cells. Cells treated as indicated were stained with PE–anti-CD41a (HIP8-PE) or isotype-matched control antibodies and analyzed by single-color flow cytometry. Log fluorescence intensity is indicated on the x-axis. Original magnification, × 500.
Stromal cell exposure inhibits megakaryocytic differentiation in primary human stem cells.
Primary human hematopoietic stem cells were cultured 7 days with either IL-3 plus SCF to expand myeloid progenitors or with TPO plus SCF plus IDB to induce megakaryocytic differentiation. Cells were either cultured alone or on a monolayer of primary human bone marrow stromal cells. Cells treated as indicated were stained with PE–anti-CD41a (HIP8-PE) or isotype-matched control antibodies and analyzed by single-color flow cytometry. Log fluorescence intensity is indicated on the x-axis. Original magnification, × 500.
Results
Stromal blockade of megakaryopoiesis in primary cultures
We have previously demonstrated with cell lines that stromal contact potently blocks megakaryocytic differentiation induction.7 To confirm these results with primary human cells, purified adult CD34+ cells were cultured under conditions that strongly promote megakaryocytic differentiation, in the absence or presence of primary human bone marrow stromal cells. We analyzed the earliest stages of megakaryocytic differentiation (day 7 of cultures) in an attempt to focus on lineage commitment as opposed to subsequent lineage expansion. As shown in Figure1, control CD34+ cells grown alone in myeloid expansion medium (containing IL-3 and SCF) showed minimal evidence of megakaryocytic differentiation, consisting predominantly of CD41− cells with blastic or granulocytic morphology. CD34+ cells grown alone in megakaryocytic medium (containing TPO, SCF, and IDB) displayed significant megakaryocytic differentiation at 7 days, with about 30% of cells expressing bright CD41a (glycoprotein IIb-IIIa complex) and frequent cells displaying characteristic megakaryocytic morphology on Wright stain. In contrast, CD34+ cells cocultured with bone marrow stroma in megakaryocytic medium manifested a clear decrease in megakaryocytic differentiation, with only about 15% of cells expressing bright CD41a on flow cytometry and only rare cells displaying megakaryocytic morphology. The final yield of cells in megakaryocytic medium did not differ according to the absence or presence of stroma (3.8 × 106 cells in the absence of stroma and 4.2 × 106 cells in the presence of stroma). Therefore, both relative and absolute numbers of megakaryocytes were diminished with stromal coculture. These results confirm previous observations that stromal inhibiton of megakaryocytic differentiation may occur with primary hematopoietic progenitors as well as with cell lines.6
Enforced Raf-1 activation overrides stromal inhibition
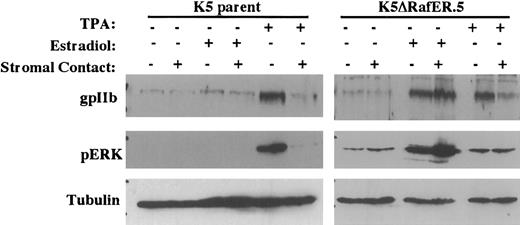
We have previously shown that stromal inhibition of megakaryocytic differentiation correlates with blockade of ERK/MAPK activation.7 To determine the significance of this observation, we tested the effects of stromal contact on K562ΔRafER.5 cells, which contain an estradiol-responsive mutant of the kinase Raf-1. As illustrated in Figure 2, K562 parental cells lacked responsiveness to estradiol as assessed by ERK phosphorylation and up-regulation of glycoprotein IIb (gpIIb). Treatment of the parental cells with TPA, as expected, caused up-regulation of gpIIb and ERK phosphorylation, both of which were completely inhibited by stromal contact. K562ΔRafER.5 cells, on the other hand, responded to estradiol treatment with potent activation of ERK phosphorylation accompanied by up-regulation of gpIIb. Notably, stromal contact failed to inhibit either ERK phosphorylation or gpIIb up-regulation in estradiol-treated K562ΔRafER.5 cells. In contrast, TPA-induced gpIIb up-regulation in K562ΔRafER.5 cells was completely blocked by stromal contact, indicating that these cells retained the ability to respond to stromal inhibition. Interestingly, scanning densitometry with tubulin normalization showed that estradiol treatment of K562ΔRafER.5 cells consistently activated ERK phosphorylation to a greater degree (without stroma: 24-fold; with stroma: 17-fold) than did TPA treatment (without stroma: 4.5-fold; with stroma: 1.5-fold). A possible explanation is that the ΔRafER mutant protein in the absence of estradiol might partially block the action of TPA.
Enforced, selective activation of the ERK/MAPK pathway overrides stromal inhibition of megakaryocytic differentiation (indicated by gpIIb up-regulation).
Cells analyzed consisted of either K562 parent cells or K5ΔRafER.5 cells engineered to express the estrogen-responsive Raf-1 kinase mutant ΔRafER. Cells were treated for 48 hours as indicated with either 25 nM TPA or 1 μM estradiol in the presence or absence of HESS-5 stromal cell monolayers. Whole cell lysates from the indicated samples were subjected to immunoblot analysis with the indicated antibodies. Membranes were stripped and reprobed to correlate gpIIb, phospho-ERK, and tubulin expression within each sample.
Enforced, selective activation of the ERK/MAPK pathway overrides stromal inhibition of megakaryocytic differentiation (indicated by gpIIb up-regulation).
Cells analyzed consisted of either K562 parent cells or K5ΔRafER.5 cells engineered to express the estrogen-responsive Raf-1 kinase mutant ΔRafER. Cells were treated for 48 hours as indicated with either 25 nM TPA or 1 μM estradiol in the presence or absence of HESS-5 stromal cell monolayers. Whole cell lysates from the indicated samples were subjected to immunoblot analysis with the indicated antibodies. Membranes were stripped and reprobed to correlate gpIIb, phospho-ERK, and tubulin expression within each sample.
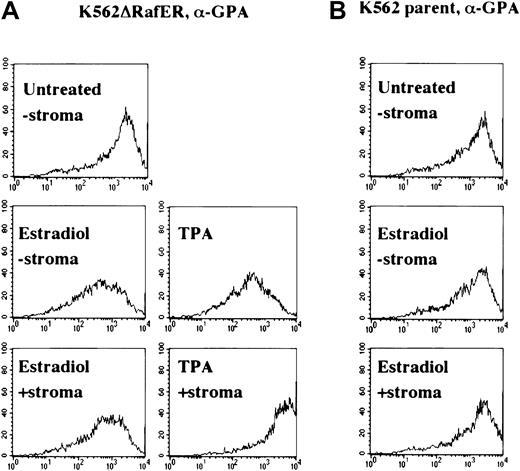
Another parameter associated with megakaryocytic differentiation consists of down-regulation of glycophorin A (GPA), an erythroid marker.10 To confirm the results obtained with gpIIb up-regulation in Figure 2, K562ΔRafER.5 cells with or without stroma were analyzed for GPA down-regulation in response to estradiol or TPA. Figure 3A shows that K562ΔRafER.5 cells treated with estradiol down-regulated GPA equally well in absence or presence of stromal cells. In contrast, GPA down-regulation associated with TPA treatment was completely blocked by stromal contact. As expected, K562 parental cells showed no response to estradiol treatment (Figure 3B). The results in Figures 2 and 3 both demonstrate that stromal inhibition of megakaryocytic differentiation in K562 cells results from blockade of the ERK/MAPK pathway at a point concident with or upstream of Raf kinases.
Enforced, selective activation of the ERK/MAPK pathway overrides stromal inhibition of megakaryocytic differentiation (indicated by glycophorin A down-regulation).
As in Figure 2, the cells analyzed consisted of either K562 parent cells (B) or K5ΔRafER.5 cells (A) engineered to express the estrogen-responsive Raf-1 kinase mutant ΔRafER. Cells were treated for 48 hours as indicated with either 25 nM TPA or 1 μM estradiol in the presence or absence of HESS-5 stromal cell monolayers. Cells treated as indicated were stained with PE–anti-GPA (GA-R 2-PE) or isotype-matched control antibodies and analyzed by single-color flow cytometry. Log fluorescence intensity is indicated on the x-axis.
Enforced, selective activation of the ERK/MAPK pathway overrides stromal inhibition of megakaryocytic differentiation (indicated by glycophorin A down-regulation).
As in Figure 2, the cells analyzed consisted of either K562 parent cells (B) or K5ΔRafER.5 cells (A) engineered to express the estrogen-responsive Raf-1 kinase mutant ΔRafER. Cells were treated for 48 hours as indicated with either 25 nM TPA or 1 μM estradiol in the presence or absence of HESS-5 stromal cell monolayers. Cells treated as indicated were stained with PE–anti-GPA (GA-R 2-PE) or isotype-matched control antibodies and analyzed by single-color flow cytometry. Log fluorescence intensity is indicated on the x-axis.
Stromal contact blocks late-phase but not early-phase ERK/MAPK activation
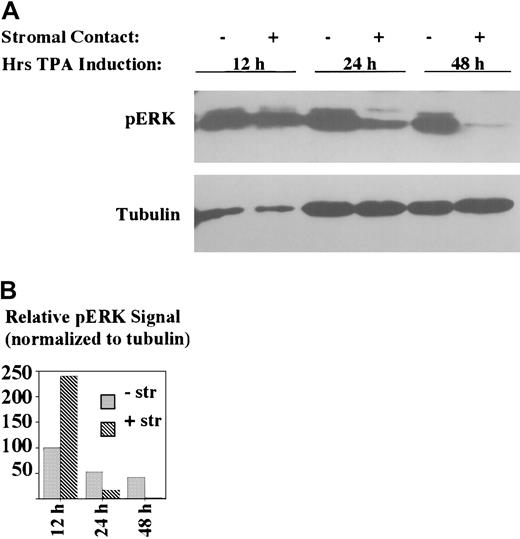
Analysis of the kinetics of stromal inhibition of ERK/MAPK activation indicated a selective effect on later stages (Figure4). In particular, stromal contact had no inhibitory effect on the level of ERK/MAPK phosphorylation at 12 hours of treatment. In fact, scanning densitometry indicated an about 2-fold enhancement of ERK activation in stromal cocultures at 12 hours as compared with nonstromal cultures. However, by 24 hours, stromal contact correlated with an about 15-fold decline in ERK/MAPK phosphorylation, and by 48 hours there was almost a complete loss of ERK/MAPK phosphorylation associated with stromal coculture. In the absence of stromal contact, ERK/MAPK phosphorylation declined by about 2-fold from 12 to 24 hours with no further decline at 48 hours. Thus, stromal contact permitted early activation of ERK/MAPK but prevented the sustained activation known to be critical for megakaryocytic differentiation.11,13 16
Stromal contact selectively blocks late-phase and not early-phase ERK/MAPK activation.
K562 cells were treated with TPA with or without HESS-5 stromal cell contact for the indicated durations. The adjacent graph shows the relative phospho-ERK signal intensities, as determined by scanning densitometry, normalized according to tubulin signal. -str indicates no stromal cells present; +str, coculture with stromal cells. Whole cell lysates of indicated samples were subjected to immunoblotting with antibodies specific for phospho-ERK.
Stromal contact selectively blocks late-phase and not early-phase ERK/MAPK activation.
K562 cells were treated with TPA with or without HESS-5 stromal cell contact for the indicated durations. The adjacent graph shows the relative phospho-ERK signal intensities, as determined by scanning densitometry, normalized according to tubulin signal. -str indicates no stromal cells present; +str, coculture with stromal cells. Whole cell lysates of indicated samples were subjected to immunoblotting with antibodies specific for phospho-ERK.
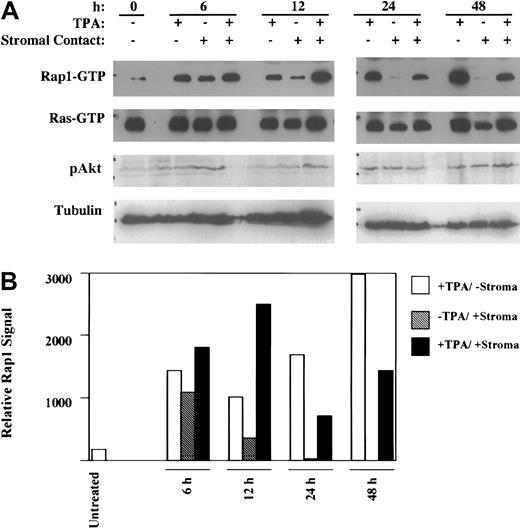
Stromal contact selectively blocks late-phase Rap1 signaling
The recent implication of Rap1 in sustained ERK/MAPK activation17 25 prompted us to examine its involvement in stromal inhibition of megakaryocytic differentiation. To this end, the kinetic profiles of Rap1 and Ras activation were determined in TPA-induced K562 cells in the absence or presence of stromal contact (Figure 5). Notably, the Rap1 activation observed at earlier time points (ie, 6 hours and 12 hours) showed no inhibition by stromal contact. However, at the later time points of 24 and 48 hours of induction, there was clear indication of stromal inhibition of Rap1 activation, as confirmed by scanning densitometry (Figure 5B). This kinetic profile paralleled that observed with ERK/MAPK phosphorylation, with stromal inhibition manifesting at 24 hours but not at 12 hours (Figure 4). Ras activation, in contrast, showed essentially no change as a consequence of TPA induction and no evidence of inhibition by stromal contact. Furthermore, phospho-Akt levels remained essentially unchanged as a result of stromal contact. These results suggested that stromal inhibitory effects were specific for Rap1 signaling via the ERK/MAPK pathway.
Stromal contact selectively blocks late-phase Rap1 activation.
(A) K562 cells cultured with or without TPA and with or without HESS-5 stromal cell contact for the indicated durations were analyzed for Rap1 and Ras activation as well as for Akt activation. (B) Graph showing relative Rap1 activation was derived by scanning densitometry of the Rap1 signals in the immunoblot in panel A. The results shown are representative of experiments performed 3 times. Rap1 and Ras activation were assayed by pulldown using GST-RalGDS(RBD) followed by immunoblot detection. Akt activation was detected by probing immunoblots of whole cell lysates with antiphospho-Akt (Ser473).
Stromal contact selectively blocks late-phase Rap1 activation.
(A) K562 cells cultured with or without TPA and with or without HESS-5 stromal cell contact for the indicated durations were analyzed for Rap1 and Ras activation as well as for Akt activation. (B) Graph showing relative Rap1 activation was derived by scanning densitometry of the Rap1 signals in the immunoblot in panel A. The results shown are representative of experiments performed 3 times. Rap1 and Ras activation were assayed by pulldown using GST-RalGDS(RBD) followed by immunoblot detection. Akt activation was detected by probing immunoblots of whole cell lysates with antiphospho-Akt (Ser473).
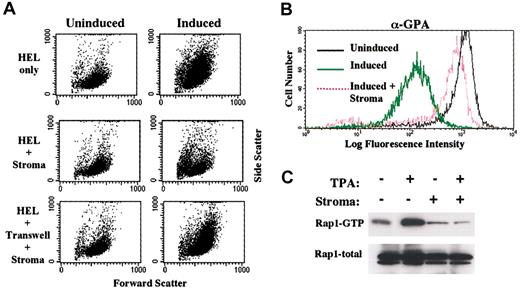
To extend these observations to a different model system, the effects of stromal contact were examined on the megakaryocytic induction of HEL cells. As shown in Figure 6A, stromal contact blocked the morphologic changes (flow cytometric forward and side light scatter) associated with megakaryocytic induction. As has been observed for CD34+ cells,6 transwell separation of stroma and HEL cells eliminated the inhibition, reinforcing the importance of direct stromal contact. Stromal contact also blocked the down-regulation of GPA observed in HEL cells undergoing megakaryocytic differentiation (Figure 6B). Importantly, as with K562 cells, Rap1 activation in induced HEL cells showed complete inhibition occurring as a consequence of stromal contact (Figure 6C). Ras activation was unevaluable in the HEL cells due to undetectable protein levels.
Stromal inhibition of megakaryocytic induction in HEL cells also correlates with blockade in Rap1 activation.
(A) Coculture of HEL cells with HESS-5 stromal cells blocked the morphologic changes associated with megakaryocytic induction. Morphologic changes are reflected by forward versus right-angle light scatter characteristics on flow cytometry. (B) Coculture of HEL cells with stromal cells blocked surface antigen changes associated with megakaryocytic induction. Shown on the x-axis is mean fluorescent intensity of cells stained with PE-conjugated antibody to GPA. HEL cells either untreated or induced for 48 hours were analyzed by flow cytometry for forward versus side scatter and for glycophorin A expression (GPA). (C) Coculture of HEL cells with stroma blocked Rap1 activation associated with megakaryocytic induction. HEL cells either untreated or induced for 48 hours were analyzed for Rap1 activation as described in “Materials and methods.” In addition, whole cell lysates were immunoblotted for total Rap1 levels.
Stromal inhibition of megakaryocytic induction in HEL cells also correlates with blockade in Rap1 activation.
(A) Coculture of HEL cells with HESS-5 stromal cells blocked the morphologic changes associated with megakaryocytic induction. Morphologic changes are reflected by forward versus right-angle light scatter characteristics on flow cytometry. (B) Coculture of HEL cells with stromal cells blocked surface antigen changes associated with megakaryocytic induction. Shown on the x-axis is mean fluorescent intensity of cells stained with PE-conjugated antibody to GPA. HEL cells either untreated or induced for 48 hours were analyzed by flow cytometry for forward versus side scatter and for glycophorin A expression (GPA). (C) Coculture of HEL cells with stroma blocked Rap1 activation associated with megakaryocytic induction. HEL cells either untreated or induced for 48 hours were analyzed for Rap1 activation as described in “Materials and methods.” In addition, whole cell lysates were immunoblotted for total Rap1 levels.
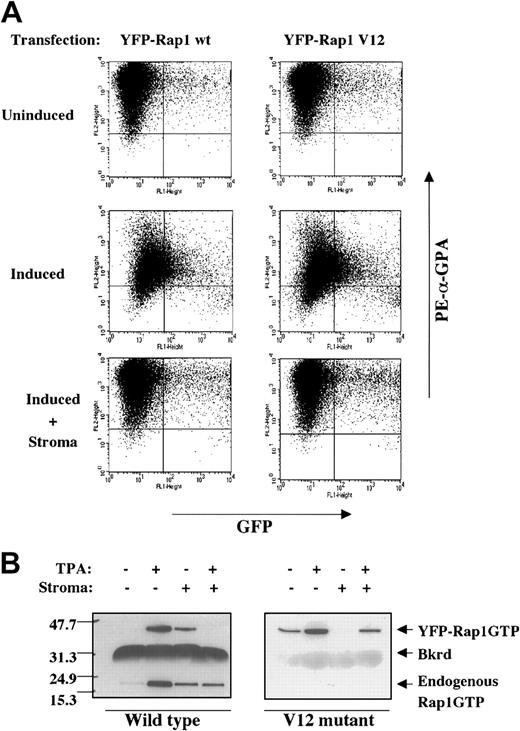
Stromal inhibition of the Rap1 V12 mutant
Inactivation of Rap1 may occur through 2 different mechanisms:(a) up-regulation of a Rap1 GTPase-activating protein (Rap-GAP) or (b) inhibition of the function of guanine nucleotide exchange factors (GEFs). To distinguish between these 2 mechanisms, K562 cells were transfected with expression vectors for YFP fusion proteins of wild-type Rap1 and the Rap1 V12 mutant. The V12 mutant of Rap1 is insensitive to Rap-GAP activity26 and therefore should override any stromal inhibition due to up-regulation of Rap-GAP proteins. Two-color flow cytometric analysis monitored down-regulation GPA in YFP+ cells, thus providing a readout of the degree of megakaryocytic differentiation as a function of the level of fusion protein expression. As shown in Figure7A, cells expressing high levels of either YFP-Rap1 wild type or YFP-Rap1 V12 showed minimal spontaneous GPA down-regulation. With TPA treatment, both transfectants showed similar GPA down-regulation, with direct correlation between the degrees of YFP fusion expression and the extent of GPA down-regulation. Notably, stromal contact blocked down-regulation of GPA in both YFP-Rap1 wild-type and YFP-Rap1 V12 transfectants regardless of the degree of YFP fusion protein expression.
The Rap1 V12 mutant fails to override stromal inhibition.
(A) Enforced expression in K562 cells of neither Rap1 wild type (wt) nor Rap1 V12 could reverse stromal inhibition of GPA down-regulation. Expression of the indicated YFP-Rap1 fusions, a function of GFP fluorescence, was correlated with GPA expression, a function of PE/red fluorescence. K562 cells transiently transfected with expression vectors for the indicated YFP fusion proteins were induced with or without HESS-5 stromal cells for 48 hours prior to staining with PE-conjugated antibody to GPA. (B) Stromal contact blocked activation of both YFP-Rap1 wt and YFP-Rap1 V12. Similar results were obtained with multiple independently isolated stable transfectants (not shown). Stable transfectants of K562 expressing the indicated YFP fusion proteins were induced with or without stromal cells for 48 hours prior to analysis of Rap1 activation as described in “Materials and methods.” The positions of the YFP-Rap1 fusions, endogenous Rap1, and a background band (Bkrd) are indicated.
The Rap1 V12 mutant fails to override stromal inhibition.
(A) Enforced expression in K562 cells of neither Rap1 wild type (wt) nor Rap1 V12 could reverse stromal inhibition of GPA down-regulation. Expression of the indicated YFP-Rap1 fusions, a function of GFP fluorescence, was correlated with GPA expression, a function of PE/red fluorescence. K562 cells transiently transfected with expression vectors for the indicated YFP fusion proteins were induced with or without HESS-5 stromal cells for 48 hours prior to staining with PE-conjugated antibody to GPA. (B) Stromal contact blocked activation of both YFP-Rap1 wt and YFP-Rap1 V12. Similar results were obtained with multiple independently isolated stable transfectants (not shown). Stable transfectants of K562 expressing the indicated YFP fusion proteins were induced with or without stromal cells for 48 hours prior to analysis of Rap1 activation as described in “Materials and methods.” The positions of the YFP-Rap1 fusions, endogenous Rap1, and a background band (Bkrd) are indicated.
To confirm these findings at a biochemical level, cells stably expressing either YFP-Rap1 wild type or YFP-Rap1 V12 were analyzed for Rap1 activation (Figure 7B). The activation status of the YFP fusion could be distinguished from that of the endogenous Rap1 protein by their difference in migration on SDS-PAGE (about 46 kDa vs 21 kDa, respectively). As shown in Figure 7B, YFP-Rap1 wild type mirrored endogenous Rap1 in its activation by TPA and inhibition by stromal contact. Interestingly, cells expressing YFP-Rap1 V12 markedly down-regulated endogenous Rap1 activation, due to an unconfirmed mechanism possibly involving GAP up-regulation.27Nevertheless, the YFP-Rap1 V12 fusion itself manifested activation in response to TPA and retained sensitivity to stromal inhibition of activation. Therefore, at both phenotypic and biochemical levels of analysis, stromal contact maintained the ability to block activation of the V12 mutant of Rap1.
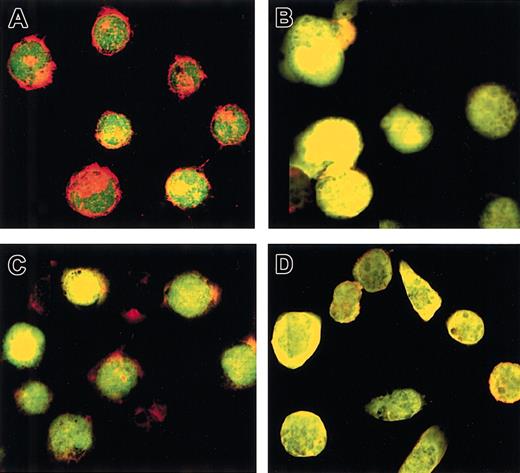
To confirm that the Rap1 V12 mutant displayed constitutive biologic activity in vivo, we examined the status of the actin cytoskeleton in cells stably expressing wild-type or V12 Rap1 fused to YFP. Recent data have suggested a role for Rap1 in remodeling of the actin cytoskeleton.28 As shown in Figure 8A-B, K562 cells expressing YFP-Rap1 wild type (wt) (green) possessed well-defined cortical actin filaments (red), which underwent elimination during 48 hours of TPA treatment. Notably, cells expressing YFP-Rap1 V12 showed loss of cortical actin both in the untreated and TPA treated states (Figure 8C-D). Therefore, YFP-Rap1 V12 did manifest constitutive in vivo signaling, resulting in remodeling of the cellular actin cytoskeleton.
Stromal effects on signaling complexes upstream of Rap1
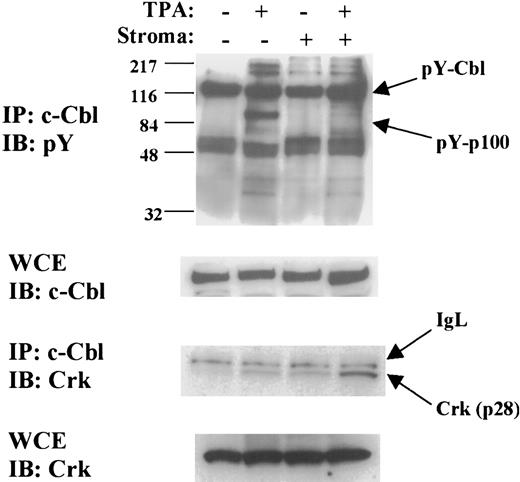
The ability of stromal contact to block the activation of a Rap1 V12 mutant (Figure 7) suggested a mechanism targeting upstream activators of Rap1. At least 9 different Rap1 GEFs, capable of direct Rap1 activation in response to a variety of signals, have been characterized.29 The best-characterized Rap1 GEF, C3G, functions in a complex with the Crk small adaptor molecule.18 We were unable to identify C3G or Crk associated with Rap1-FLAG in large-scale immunoprecipitations performed on stably transfected K562 cells with or without TPA and with or without stroma (not shown); these results raised the possibility that one of the many alternative GEF complexes might regulate Rap1 activation in our system. In an attempt to identify relevant factors, we examined tyrosine-phosphorylated proteins associated with the scaffolding factor c-Cbl. The rationale for this approach was that c-Cbl is known to function as a scaffolding factor for Rap1 activation complexes18 and that components of Rap1 activation complexes often undergo inducible tyrosine phosphorylation.30 In the results shown in Figure9, an about 100 kDa tyrosine-phosphorylated protein, pY-p100, was found to associate with c-Cbl upon TPA treatment of K562 cells. Significantly, in the presence of stromal contact, pY-p100 failed to associate with c-Cbl upon TPA treatment. The levels of tyrosine-phosphorylated and total c-Cbl were not significantly affected by any of the treatments. Crk also showed TPA-inducible association with c-Cbl, but stromal contact enhanced the association of Crk with c-Cbl, possibly providing an explanation for the initial activation of Rap1 caused by stromal contact (Figure 5). In summary, stromal contact prevented the TPA-induced recruitment to c-Cbl of pY-p100, a tyrosine-phosphorylated approximate 100 kDa protein, but enhanced the recruitment of Crk.
The Rap1 V12 mutant induces remodeling of cortical actin.
(A) Untreated K562 cells expressing YFP-Rap1 wt displayed cortical actin, which did not colocalize significantly with Rap1. (B) TPA-treated K562 cells expressing YFP-Rap1 wt show loss of cortical actin with apparent colocalization of actin and Rap1. (C) Untreated K562 cells expressing YFP-Rap1 V12 show loss of cortical actin and apparent colocalization of actin and Rap1. (D) TPA-treated K562 cells expressing YFP-Rap1 V12 show absent cortical actin with apparent colocalization of actin and Rap1. These findings were similar in 2 different experiments using 2 independently isolated K562-YFP-Rap1 V12 clones. K562 stable transfectants with or without TPA were grown 48 hours on glass coverslips, followed by fixation and staining for actin with rhodamine-conjugated phalloidin. YFP fusions and rhodamine-phalloidin were detected simultaneously by 2-color confocal imaging at a total magnification × 520.
The Rap1 V12 mutant induces remodeling of cortical actin.
(A) Untreated K562 cells expressing YFP-Rap1 wt displayed cortical actin, which did not colocalize significantly with Rap1. (B) TPA-treated K562 cells expressing YFP-Rap1 wt show loss of cortical actin with apparent colocalization of actin and Rap1. (C) Untreated K562 cells expressing YFP-Rap1 V12 show loss of cortical actin and apparent colocalization of actin and Rap1. (D) TPA-treated K562 cells expressing YFP-Rap1 V12 show absent cortical actin with apparent colocalization of actin and Rap1. These findings were similar in 2 different experiments using 2 independently isolated K562-YFP-Rap1 V12 clones. K562 stable transfectants with or without TPA were grown 48 hours on glass coverslips, followed by fixation and staining for actin with rhodamine-conjugated phalloidin. YFP fusions and rhodamine-phalloidin were detected simultaneously by 2-color confocal imaging at a total magnification × 520.
Stromal contact prevents TPA-induced recruitment of pY-p100 to c-Cbl but enhances recruitment of Crk to c-Cbl.
Immunoprecipitation of c-Cbl followed by immunoblotting for phosphotyrosine showed TPA-induced recruitment of an about 100 kDa tyrosine-phosphorylated protein (indicated by arrow as pY-p100). Stromal contact completely blocked TPA-induced recruitment of pY-p100. Levels of tyrosine-phosphorylated c-Cbl (arrow: pY-Cbl) did not vary significantly with any of the treatments. Reprobing for Crk showed that stromal contact, by contrast, enhanced recruitment of Crk (p28) to c-Cbl. IgL indicates immunoglobulin light chain. Immunoblotting of the whole cell extracts (WCE) showed no significant variation in total levels of c-Cbl or Crk (p28) in any of the treatments. The results shown are representative of experiments performed 3 times. K562 cells with or without TPA for 48 hours and with or without stromal contact were subjected to whole cell extraction as described in “Materials and methods.” Whole cell extracts were then used both for direct immunoblotting and for immunoprecipitation of c-Cbl followed by immunoblotting.
Stromal contact prevents TPA-induced recruitment of pY-p100 to c-Cbl but enhances recruitment of Crk to c-Cbl.
Immunoprecipitation of c-Cbl followed by immunoblotting for phosphotyrosine showed TPA-induced recruitment of an about 100 kDa tyrosine-phosphorylated protein (indicated by arrow as pY-p100). Stromal contact completely blocked TPA-induced recruitment of pY-p100. Levels of tyrosine-phosphorylated c-Cbl (arrow: pY-Cbl) did not vary significantly with any of the treatments. Reprobing for Crk showed that stromal contact, by contrast, enhanced recruitment of Crk (p28) to c-Cbl. IgL indicates immunoglobulin light chain. Immunoblotting of the whole cell extracts (WCE) showed no significant variation in total levels of c-Cbl or Crk (p28) in any of the treatments. The results shown are representative of experiments performed 3 times. K562 cells with or without TPA for 48 hours and with or without stromal contact were subjected to whole cell extraction as described in “Materials and methods.” Whole cell extracts were then used both for direct immunoblotting and for immunoprecipitation of c-Cbl followed by immunoblotting.
Discussion
Stromal inhibition of cellular differentiation most likely provides a homeostatic mechanism for maintenance of hematopoietic progenitor cell pools, which might otherwise undergo rapid depletion at the expense of differentiation. At the other extreme, however, stromal cells may contribute to defective marrow function through interference with normal differentiation, both in the case of leukemic cell expansion as well as with defective megakaryocytic engraftment after bone marrow transplantation. The finding that purified CD34+ cells show superior megakaryopoiesis ex vivo than when injected into myeloablated hosts9 suggests that the bone marrow microenvironment under some circumstances—for example, vast stromal cell excess—may impair normal maturation. In support of this hypothesis, we and others have demonstrated that megakaryocytic differentiation of primary human CD34+ progenitor cells is inhibited by freshly isolated human bone marrow stroma.6 8Understanding the mechanisms involved in stromal inhibition of megakaryocytic differentiation may assist in the design of future strategies to reconstitute megakaryopoiesis after myeloablation. These mechanisms may also elucidate the biochemical function of marrow stroma in normal and leukemic progenitor cell expansion.
The current data demonstrate that stromal contact may block megakaryocytic differentiation through interference with sustained ERK/MAPK activation. Several previous studies using a variety of experimental systems have reinforced the central role of sustained ERK/MAPK activation in the programming of megakaryocytic lineage commitment.11,13,16 More recent experiments have implicated Rap1, rather than Ras proteins, as the upstream driver of sustained, late-phase ERK activation, both in neuronal and in megakaryocytic differentiation.17 25 In support of these findings, our results showed that activation of Rap1, but not Ras, is targeted for inhibition by stromal contact. Furthermore, the kinetics of stromal inhibition of Rap1 activation closely paralleled the kinetics of stromal inhibition of ERK/MAPK activation (Figures 4-5). Therefore, Rap1 most likely represents a critical target in the stromal inhibition of megakaryocytic differentiation. One major caveat is that the current results were obtained with leukemic cell lines induced with a pharmacologic agent (phorbol ester) to undergo incomplete megakaryocytic differentiation. Further verification of the importance of Rap1 in regulation of megakaryopoiesis will rely on additional model systems including primary human hematopoietic progenitor cell cultures, human and murine embryonic stem cell hematopoiesis, and genetically manipulated mice.
The mechanism of stromal inhibition of Rap1 activation appears to involve destabilization of an upstream activation complex. Such a mechanism is suggested both by the susceptiblity of the Rap1 V12 mutant to stromal inhibition (Figure 7) and by the delayed kinetics of Rap1 inactivation (Figure 5). Stromal contact initially appears to be associated with transient Rap1 activation (Figure 5), possibly related to Crk recruitment to c-Cbl complexes (Figure 9); previous work has correlated Crk recruitment to Cbl with activation of Rap1a by forskolin.18 However, stromal contact caused eventual decay in TPA-induced Rap1 activation between 12 hours and 24 hours of treatment. The concept of stable versus unstable Rap1 upstream activation complexes has found support in previous experiments in the PC12 system of neuronal differentiation, in which treatment of cells with EGF led to labile signaling complexes composed of EGF-R, Crk, and C3G.31 Our data show that stromal contact altered the composition of a c-Cbl–associated complex, preventing TPA-induced recruitment of a 100 kDa tyrosine-phosphorylated protein, pY-p100, and enhancing recruitment of Crk (Figure 9). Because c-Cbl serves as a critical scaffolding factor for Rap1 upstream activation complexes,18 it is reasonable to postulate that the stromally induced alterations in the c-Cbl complex may be associated with loss of capacity for stable Rap1 activation. However, the precise significance of pY-p100 and Crk recruitment to Cbl remains to be determined. The identity of pY-p100 and its function (direct Rap1 GEF versus regulator of Rap1 GEF activity) are currently under investigation.
Cell membrane molecules obviously must participate in transmitting the stromal signal to the hematopoietic target cells. A candidate interaction consists of ephrin (Eph) ligand binding to Eph receptor tyrosine kinases, signaling from which can inhibit the Ras/MAPK pathway.32,33 Arguing against a role for Eph receptors in stromal inhibition of megakaryocyte differentiation are(a) their rapid kinetics of ERK/MAPK inhibition,(b) their ability to inhibit Ras, and (c) their inability to inhibit V12 mutants.32,33 Aside from the Eph kinases, a broad array of other tyrosine kinases and phosphatases regulate megakaryocytic development and might participate in the stromal inhibitory mechanism.34 Another appealing candidate consists of the Sprouty (Spry) signaling pathway, which can potently inhibit ERK/MAPK activation and thereby block neuronal differentiation of PC12 cells.35 However, in contrast to the stromal mechanism, both Spry1 and Spry2 can prevent early-phase ERK/MAPK activation and are able to inhibit Ras activation.35 Nevertheless, Spry function may be influenced by cellular context and by the array of Spry isoforms being expressed. Future experiments will address potential upstream factors, such as Spry proteins, mediating stromal inhibition of Rap1 activation.
We thank Drs Dan Rosson and Takashi Tsuji for generous provision of cell lines, Dr Judy Meinkoth for helpful advice and reagents in the analysis of Rap1 activation, Dr Johannes Bos and Miranda van Triest for kindly providing Rap1 mammalian expression vectors, and Drs Joe Sung and Shu Man Fu for invaluable assistance with and access to the FACScan. We also thank Dr Donna Webb for advice and reagents for actin staining and the laboratory of Dr Kodi Ravichandran for advice and reagents for c-Cbl immunoprecipitation.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-04-1278.
Supported by grants RO1 CA93735 of the National Cancer Institute of the National Institutes of Health (A.N.G.), K08 HL04017 of the National Heart, Lung, and Blood Institute of the National Institutes of Health (F.K.R.), and the Eugene and Mary B. Meyer Center for Advanced Transfusion Practices and Blood Research (F.K.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Adam Goldfarb, Department of Pathology, University of Virginia, PO Box 800904, Charlottesville, VA 22908; e-mail: ang3x@virginia.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal