Leukocyte adhesion deficiency type 2 (LADII) is characterized by defective selectin ligand formation, recurrent infection, and mental retardation. This rare syndrome has only been described in 2 kindreds of Middle Eastern descent who have differentially responded to exogenous fucose treatment. The molecular defect was recently ascribed to single and distinct missense mutations in a putative Golgi guanosine diphosphate (GDP)–fucose transporter. Here, we describe a patient of Brazilian origin with features of LADII. Sequencing of the GDP-fucose transporter revealed a novel single nucleotide deletion producing a shift in the open-reading frame and severe truncation of the polypeptide. Overexpression of the mutant protein in the patient's fibroblasts did not rescue fucosylation, suggesting that the deletion ablated the activity of the transporter. Administration of oral L-fucose to the patient produced molecular and clinical responses, as measured by the appearance of selectin ligands, normalization of neutrophil counts, and prevention of infectious recurrence. The lower neutrophil counts paralleled improved neutrophil interactions with activated endothelium in cremasteric venules of nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice. However, fucose supplementation induced autoimmune neutropenia and the appearance of H antigen on erythrocytes, albeit without evidence of intravascular hemolysis. The robust response to fucose despite a severely truncated transporter suggests alternative means to transport GDP-fucose into the Golgi complex.
Introduction
The interactions of neutrophils with endothelial cells that line the blood vessels are necessary for their migration to sites of inflammation. This dynamic process follows a multistep sequence that is initiated by initial tethering and rolling mediated by P- and E-selectins expressed on activated venules and their glycoconjugated ligands on leukocytes.1-3
Selectin ligands undergo several key posttranslational modifications for selectin binding, including sialylation, fucosylation, and tyrosine or carbohydrate sulfation.4,5 In particular, the addition of fucose to O-linked glycan structures was shown to be critical for selectin recognition because mice deficient in the leukocyte fucosyltransferases IV and VII display leukocyte adhesion defects that parallel those found in selectin-deficient mice.6-8 The importance of fucosylation in selectin-mediated interactions is further evidenced with the description of a rare leukocyte adhesion deficiency (LAD) syndrome characterized by a systemic defect in fucose metabolism.9Patients with LAD syndromes have marked leukocytosis and recurrent infections without pus formation.10 In contrast to LAD type 1 (LADI), in which the function or expression of β2 integrins is defective,11 LAD type 2 (LADII, also termed congenital disorder of glycosylation IIc) is characterized by normal β2 integrin function but altered selectin binding. Neutrophils from these patients are unable to roll on selectins or activated endothelial cells in vitro or on inflamed mesenteric microvessels in vivo, leading to impaired migration to sites of inflammation.12-14 Given that fucose is absent from all glycoproteins, LADII patients have other defects, including the Bombay blood type, and they lack the Lewis blood group.15 Patients also have severe mental retardation, suggesting that fucosylated glycoproteins, glycolipids, or both are important for brain function. All 5 LADII patients described to date are of Middle Eastern descent (4 Arab, 1 Turkish).16 In search for the defective pathway in the fucose metabolism, it was found that when fibroblasts and lymphoblastoid cells derived from a LADII patient were grown in the presence of millimolar concentrations of fucose, cell surface fucosylation could be restored.17Following this observation, Marquardt et al18 orally administered L-fucose to the Turkish LADII patient and observed a remarkable clinical response. Within days of fucose intake, neutrophil counts normalized and functional E- and P-selectin ligands were expressed on myeloid cells. However, the Arab patients have not responded to oral fucose therapy,19 20 suggesting a different molecular basis for their disease.
The gene defective in LADII was recently cloned and was found to encode a putative guanosine diphosphate (GDP)–fucose transporter predicted to span the Golgi membrane 10 times.21 22 Single and distinct missense mutations were identified in Arab and Turkish kindred, thus providing a molecular explanation for the differential response to fucose. In the present report, we describe a new manifestation of LADII in a patient of Brazilian origin and no known Middle Eastern descent. The disease in this patient stems from a novel single nucleotide deletion leading to frameshift and premature arrest in the translation of the GDP-fucose transporter gene. We show that this patient responds well to fucose therapy and that partial restoration of P-selectin ligand function on the patient's neutrophils correlates with an increased ability to roll in cremasteric venules of immunodeficient nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice. We further demonstrate that the induction of fucosylated conjugates leads to the expression of the H antigen on erythrocytes and the production of antineutrophil antibodies.
Patients, materials, and methods
Antibodies, reagents, and fucose administration
Biotinylated Aleuria aurantia lectin (AAL), which recognizes fucose in α1,3- and α1,6-linkages, was purchased from Vector Laboratories (Burlingame, CA), and the rat anti-CLA antibody (HECA 452) was from PharMingen (San Diego, CA). The mouse antihuman PSGL-1 antibody (PSL-275) and P-selectin–immunoglobulin G (IgG) chimera were generously provided by Dr Anjali Kumar (Genetics Institute, Cambridge, MA). Murine E-selectin–IgM chimera was produced by the transfection of COS cells with selectin–IgM DNA vector (kind gift of Dr John Lowe, University of Michigan) using the 2-diethylaminoethanol (DEAE)–dextran method.6 L-fucose for in vitro studies was from Aldrich Chemical (Milwaukee, WI). The patient was treated at the Mount Sinai Medical Center after departmental approval and the issuance of compassionate Investigational New Drug (IND) use from the Food and Drug Administration (no. 62036). L-fucose (Pfanstiehl Laboratories, Waukegan, IL) was administered orally, in 4 or 5 daily divided doses, under close monitoring as described in “Results.”
Mice
An NOD/SCID mouse colony was established from breeding pairs obtained from Jackson Laboratories (Bar Harbor, ME) and was bred in the barrier facility at Mount Sinai School of Medicine. Mice were fed with sterilely irradiated chow and autoclaved water. The Animal Care and Use Committee at the Mount Sinai School of Medicine approved all experimental procedures on animals.
Isolation of neutrophils
Neutrophils were purified from anticoagulated venous blood samples from the LADII patient or from healthy adult volunteers in a Histopaque1119 (1.119 g/mL; Sigma Diagnostics, St Louis, MO) and Ficoll-Paque (1.077 g/mL; Amersham Pharmacia Biotec, Uppsala, Sweden) discontinuous gradient, and they were washed in phosphate-buffered saline (PBS) containing 2 mM EDTA (ethylenediaminetetraacetic acid) and 0.5% bovine serum albumin (BSA) (PEB buffer). For intravital microscopy experiments, purified human neutrophils were fluorescently labeled by incubation with 33 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 30 minutes at 6°C and were washed 3 times in RPMI 1640 before injection into mice through a carotid artery catheter. Human blood samples were obtained in accordance with protocols approved by the Internal Review Board of Mount Sinai School of Medicine.
Antineutrophil antibody and H antigen assays
Antineutrophil antibody activity in the patient's serum was detected by flow cytometry. Purified neutrophils from healthy donors were incubated with a 1:100 dilution of sera obtained from the peripheral blood of healthy donors or the LADII patient, washed, and further incubated with Cy5-conjugated antihuman IgG antibody. After a final wash, cells were analyzed on a FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA) or were subjected to cytospin and prepared for fluorescence microscopy. Granulocyte agglutination (GA) and monoclonal antibody–specific immobilization of neutrophil antigens (MAIGA) assays were also performed in parallel (Neutrophil Serology Laboratory; American Red Cross, St Paul, MN) using a panel of donor neutrophils serologically phenotyped for the NA1, NA2, NB1, NB2, 5A, 5B, 9A, and MART antigens. Neutrophils were incubated with patient serum and monoclonal antibodies specific for these epitopes. GA was graded subjectively, and MAIGA was graded by enzyme-linked immunosorbent assay (ELISA). Titers were measured using doubling dilutions in the GA assays. Red blood cell agglutination with anti-A, anti-B, anti-A/B, and anti-H was examined according to standard blood bank procedures (Mount Sinai Medical Center and New York Blood Center). H antigens on the patient's RBCs were detected with polyclonal anti-H antibodies from human Bombay donors and the α-linked, fucose-specific lectin A aurantia.
Cell culture and generation of the lymphoblastoid cell line
Fibroblasts were obtained through skin biopsy of the LADII patient and were cultured in Dulbecco modified Eagle medium (DMEM; CellGro, Herndon, VA) containing 10% fetal calf serum (FCS; Hyclone, Logan, UT) and antibiotics. For the generation of a lymphoblastoid cell line, low-density mononuclear cells (MNCs) were collected after centrifugation at 250g over Ficoll-Paque of a blood sample from the LADII patient. B-lymphoid cells were transformed and selected by plating MNCs in 10 mL RPMI 1640 plus 10% FCS, also containing 2 μg/mL cyclosporin A (CSA; Novartis Pharmaceutical, East Hanover, NJ), 10 μg/mL phytohemagglutinin (Gibco, Grand Island, NY), and 1 mL Epstein-Barr virus (EBV) supernatant. After 10 days of culture, lymphoid colonies were expanded and selected in RPMI plus 10% FCS containing 2 μg/mL CSA. The transformed LADII-derived lymphoid cell line was further cultured in RMPI plus 10% FCS and antibiotics. The B7 EBV-transformed lymphoblastoid cell line established from a healthy donor (generous gift of Yande Kuang, Mount Sinai School of Medicine, NY) was used as control. All cultures were performed at 37°C and 5% CO2 atmosphere.
Flow cytometry and selectin chimera binding assay
Cells were washed in PEB buffer before incubation with 15 μg/mL biotinylated A aurantia lectin or 10 μg/mL primary antibodies. After washing in PEB buffer, the cells were incubated with a 1:100 dilution of fluorescein isothiocyanate (FITC)–conjugated streptavidin or Cy5-conjugated antirat antibody (both from Jackson Immunoresearch, West Grove, PA). After a final wash, cells were analyzed by flow cytometry. All incubations were performed for 15 minutes at 6°C.
Selectin chimera binding assays were performed as described.23 Briefly, neutrophils were incubated for 15 minutes at 6°C in staining buffer (RPMI containing 5% FCS and 0.05% NaN3) containing a human P-selectin IgG that had been preconjugated to biotinylated Protein A or with the supernatant of COS-7 cells transfected with the murine E-selectin IgM chimera vector. Selectin binding was detected by incubation with an excess of FITC-conjugated streptavidin (1.5 μg) or an FITC-conjugated antihuman IgM antibody (1:50 dilution; Jackson Immunoresearch). Cells were washed in staining buffer and analyzed by flow cytometry. In control samples, the staining was carried out in the presence of 5 mM EDTA.
cDNA cloning and sequencing
mRNA was isolated from the healthy B7 or the LADII-derived DF1 lymphoblastoid cell lines using the Trizol reagent (Life Technologies), and cDNA was prepared using the ThermoScrip reverse transcription–polymerase chain reaction (RT-PCR) system (Invitrogen, Carlsbad, CA) as indicated by the manufacturer. The putative GDP-fucose transporter (GDP-FucTP) cDNA (encompassing the entire coding region) was amplified by PCR using the high-fidelity Platinum Taq polymerase (Invitrogen) and the following primers: forward ORF-F, 5′-GATCCTGCACATGGCGCTGA-3′; reverse ORF-R, 5′-AGTAGCACCCAGTCCACACC-3′. PCR was performed as follows: 94°C for 5 minutes followed by 35 cycles of 94°C for 30 seconds, 68°C for 30 seconds, and 72°C for 1.5 minutes, and a final extension at 72°C for 7 minutes. PCR products were subcloned into the Zero Blunt TOPO TA PCR cloning system (Invitrogen) and finally cloned for expression in the bicistronic pIRES-hrGFP-1a vector (Stratagene). Sequencing of the GDP-FucTP gene was performed using ORF-F and ORF-R as external primers and ORF-2F (5′-TCAACGCCATCTACACCACGA-3′) and ORF-2R (5′-CGTTGTTGTAGAAAGTCA-3′) as internal primers, using an ABI Prism 3700 automatic DNA analyzer (DNA sequencing core facility; Mount Sinai School of Medicine). For sequencing of genomic DNA, total cell DNA was extracted from the DF1 cell line and skin fibroblasts (both of patient's origin) or MNCs from both the mother and the father, and the 2 exons of the GDP-FucTP gene were amplified by PCR using the following primers: exon 1 (forward, 5′-GGGCTGCGGCTTCCTT-3′; reverse, TCCCCATGACCACTCTATCC-3′) and exon 2 (forward, 5′-TCACCCTTCCCCACTCCTCCTCTC-3′; reverse, 5′-GTAGCACCCAGTCCACACCACAGC-3′). PCR was performed as follows: 94°C for 3 minutes followed by 35 cycles of 94°C for 30 seconds, 59.5°C (for exon 1) or 63.3°C (for exon 2) for 30 seconds, and 72°C for 1 minute, and a final extension at 72°C for 7 minutes. The same sets of primers were used for sequencing. Sequencing data were analyzed using the Sequencher Software (Genes Codes, Ann Arbor, MI).
Complementation assays
Skin fibroblasts derived from the LADII patient were transfected by electroporation (960 μF, 280 V) with the pIRES-hrGFP-1a vector (Stratagene, La Jolla, CA) containing the GDP-fucose transporter cDNA obtained from either the LADII or healthy lymphoblastoid cell lines. After 48 hours, transfected fibroblasts were plated on coverslips for 1 week. Cells were washed, fixed in 3.7% formaldehyde, and permeabilized with 0.1% Triton X100 (Fischer Scientific) in PBS. Cells were then stained with biotinylated AAL (2 μg/mL) in PEB, washed extensively, and incubated in a solution containing a 1:500 dilution of Cy5-conjugated streptavidin (Jackson Immunoresearch) in PEB. After a final wash, coverslips containing the stained cells were mounted on slides using Vectashield (Vector Laboratories, Burlingame, CA). All incubations were performed for 20 minutes at room temperature. Specificity of the staining with AAL was determined in preliminary experiments in which lectin binding to COS-7 cells could be completely blocked by the presence of 100 mM L-fucose in the staining solution and by the absence of AAL staining in nontransfected LADII fibroblasts. Samples were analyzed under a fluorescence microscope (Zeiss Axiophot 2; Zeiss Axiophot, Thornwood, NY), and images were acquired with an Orca-100 charge-coupled device (CCD) camera (Hamamatsu, Hamamatsu City, Japan) and analyzed with the OpenLab image software.
Intravital microscopy experiments
NOD/SCID animals were prepared for intravital microscopy of the cremaster muscle as previously described.24 Before the exteriorization of the cremaster muscle, the trachea was cannulated to allow spontaneous respiration, and a PE-10 catheter (Becton Dickinson) was inserted into the common left carotid artery to allow the injection of labeled cells. In some experiments, 107 nonlabeled human MNCs from each sample were injected immediately before the injection of labeled neutrophils to block potential sites of retention of human cells. CFSE-labeled neutrophils (5-20 × 106 cells) collected from the patient at different times of treatment, or from untreated healthy volunteers, were injected, and the cremasteric venules were visualized with an intravital microscope (MM-40; Nikon, Tokyo, Japan) equipped with a mercury fluorescence lamp and water immersion 10 × objective (Nikon; NA 0.3, water). Images were captured using an SIT camera (Hamamatsu) and a camera-controller (C2400; Hamamatsu) and were recorded using an S-VHS video recorder (SV0-9500MD; Sony, San Jose, CA). For analysis of the intravital experiments, hemodynamic parameters and numbers of rolling cells were calculated as described previously.23
Statistical analysis
All values are reported as mean ± SEM. Statistical significance was analyzed using the Student t test.
Results
New case of LADII
A 7-month-old boy of consanguineous Brazilian descent with microcephaly, multiple choroid plexus cysts, hypospadias, bilateral hydronephrosis, and developmental delay was examined for evaluation of recurrent infections, including 4 hospitalizations for fever, gastroenteritis, and severe cellulitis. There was no history of immunodeficiency in the family. Total white blood cell counts ranged from 30 000 to 83 000 cells/mL and absolute neutrophil counts from 6000 to 56 000 cells/mL. Fever episodes rapidly responded to antibiotic therapy, and blood cultures were always sterile. Immunologic evaluation of T-cell numbers and function, immunoglobulin levels, and CD11/18 expression were all within normal range. LADII was diagnosed by the absence of the sialyl Lewis X (sLeX) antigen, impaired E- and P-selectin binding, and presence of the Bombay blood type.
Cloning of the GDP-fucose transporter gene reveals a single-base deletion before the sixth transmembrane domain
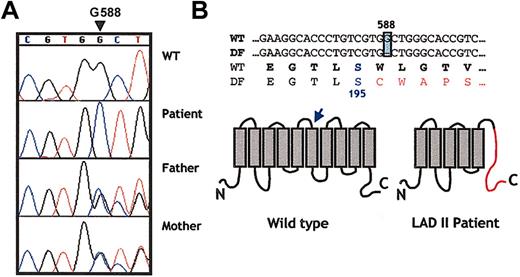
The gene responsible for the LADII syndrome has been recently shown to encode a putative Golgi GDP-fucose transporter whose structure predicts 10 transmembrane domains. Sequencing of the GDP-fucose transporter from the genome of the 2 known families of LADII revealed single and distinct missense mutations. The Turkish patient had a C>T transition at base 439, which led to the substitution of an arginine for a cysteine (Arg147Cys) in the fourth transmembrane domain. Three Arab patients exhibited a 923C>G transversion that replaced threonine for arginine (Thr308Arg) in the ninth transmembrane domain.21,22,25 To sequence the GDP-FucTP gene, we established an Epstein-Barr virus (EBV)–transformed cell line (DF1) from the patient's peripheral B cells. Full-length cDNA from theGDP-FucTP gene was amplified by RT-PCR and was subcloned into a bicistronic expression vector containing the GFP gene after the internal ribosomal entry site (IRES) sequence. Wild-typeGDP-FucTP was cloned from a lymphoblastoid line (B7) derived from a control subject and was subcloned into the same vector. The size and amount of GDP-FucTP mRNA expressed in DF1 were roughly similar to those of the control cell line, suggesting that the overall integrity of the patient's GDP-FucTP gene was preserved (data not shown). Sequencing of 2 independent subclones of GDP-FucTP from DF1 revealed a single nucleotide deletion at position 588 (ΔG588), whereas the sequence of the wild-type GDP-FucTP was consistent with that published.21 22 To further confirm these results, we sequenced amplicons from each of the 2 GDP-FucTP exons obtained from DNA extracts of the LADII DF1 cell line and primary skin fibroblasts and from the parent's peripheral blood leukocytes. As shown in Figure 1A, the patient was homozygous for ΔG588, whereas each parent was heterozygous. These results are consistent with an autosomal recessive transmission and strongly suggest that this single nucleotide deletion is responsible for the clinical syndrome exhibited in the patient. This deletion predicts an alteration of the open-reading frame of the protein after Ser195, introducing 34 random amino acids followed by a stop codon (Figure 1B) that likely renders the protein nonfunctional.
A single nucleotide deletion in position 588 (G588) in the GDP-fucose transporter gene of the new patient.
(A) Chromatograms show a partial sequence for the GDP-fucose transporter gene from a healthy donor, the LADII patient, and both parents. Note that G588 is absent in the patient, whereas both parents are heterozygotes in this position (overlaid sequence). (B) Partial primary sequence of the gene and the predicted protein region in which the deletion is found. A shift in the open-reading frame alters the protein sequence after Ser195 (arrow), as shown in the schematic representation of the predicted structure of the transporter. This frameshift creates a premature stop codon 34 residues after the mutation (shown in red in the right scheme).
A single nucleotide deletion in position 588 (G588) in the GDP-fucose transporter gene of the new patient.
(A) Chromatograms show a partial sequence for the GDP-fucose transporter gene from a healthy donor, the LADII patient, and both parents. Note that G588 is absent in the patient, whereas both parents are heterozygotes in this position (overlaid sequence). (B) Partial primary sequence of the gene and the predicted protein region in which the deletion is found. A shift in the open-reading frame alters the protein sequence after Ser195 (arrow), as shown in the schematic representation of the predicted structure of the transporter. This frameshift creates a premature stop codon 34 residues after the mutation (shown in red in the right scheme).
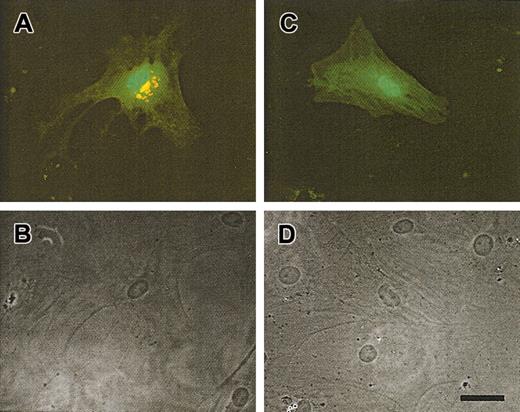
To further assess the function of the mutant gene, we transfected skin fibroblasts derived from the patient with either the wild-type or the mutant (ΔG588) forms of the GDP-FucTP gene. Transfected cells were detected by GFP expression (compare fluorescence [Figure2A,C] and Nomarski [Figure 2B,D] microscopy of the same field of view), and fucosylation activity was detected using AAL staining. As shown in Figure 2A, LADII fibroblasts transfected with wild-type cDNA exhibited strong perinuclear AAL staining, similar to that previously shown for fibroblasts from healthy donors or from LADII patients transfected with the wild-typeGDP-FucTP gene.21 In contrast, expression of the mutant cDNA in the patient's fibroblasts did not stain with the lectin (Figure 2C), suggesting that the mutant GDP-FucTPgene is responsible for the fucosylation defect observed in the patient.
Rescue in fucosylation of LADII fibroblasts by complementation with the full-length GDP-fucose transporter gene.
Skin fibroblasts obtained from the patient were transfected with a bicistronic vector containing a GFP reporter gene and the GDP-fucose transporter cDNA obtained from healthy (A-B) or LADII cells (C-D). Five days after transfection, LADII fibroblasts were stained with AAL to assess fucosylation (red). (A) Fibroblasts transfected (GFP-positive; green) with wild-type GDP-fucose transporter exhibit strong perinuclear staining, whereas (C) cells transfected with the ΔG588 transporter gene did not stain with AAL. The lower panels (B-D) show Nomarski optic images of the same fields as in panels A and C. Scale bar is approximately 50 μm.
Rescue in fucosylation of LADII fibroblasts by complementation with the full-length GDP-fucose transporter gene.
Skin fibroblasts obtained from the patient were transfected with a bicistronic vector containing a GFP reporter gene and the GDP-fucose transporter cDNA obtained from healthy (A-B) or LADII cells (C-D). Five days after transfection, LADII fibroblasts were stained with AAL to assess fucosylation (red). (A) Fibroblasts transfected (GFP-positive; green) with wild-type GDP-fucose transporter exhibit strong perinuclear staining, whereas (C) cells transfected with the ΔG588 transporter gene did not stain with AAL. The lower panels (B-D) show Nomarski optic images of the same fields as in panels A and C. Scale bar is approximately 50 μm.
In vitro and in vivo response to fucose therapy
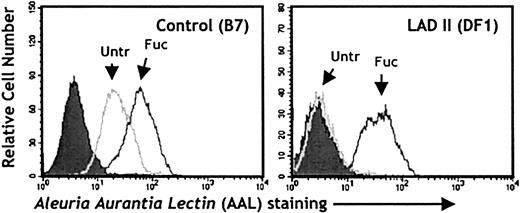
It has been reported that when fibroblasts, endothelial cells, and lymphoblastoid cell lines derived from LADII patients are grown in the presence of fucose, carbohydrate structures containing fucose can be induced on the cell surface.17,18 To evaluate the effect of fucose exposure on cells derived from our patient, we analyzed AAL binding to a B-lymphoblastoid cell line by flow cytometry. As shown in Figure 3, the control lymphoblastoid cell line (B7) bound AAL (left panel; Untr), and binding was further increased by exposure to 10 mM L-fucose in the medium for 5 days (left panel; Fuc). In contrast, the LADII lymphoblastoid cell line (DF1) displayed no significant binding at baseline (right panel; Untr), but, strikingly, the addition of L-fucose restored surface fucosylation to levels comparable to those found on control cells. A dose-response curve revealed that concentrations as low as 0.5 mM of L-fucose were sufficient to restore AAL binding (data not shown). These results are consistent with previous studies in cells derived from either Turkish or Arab patients,17 18 and they suggest that exposure to exogenous fucose can also restore fucosylation on LADII cells derived from this patient.
In vitro restoration of fucosylated glycoconjugates after fucose supplementation of a LADII lymphoblastoid cell line derived from the patient.
Control (B7; left panel) or LADII-derived (DF1; right panel) EBV-transformed lymphoblastoid cells were kept in culture for 5 days in the presence (Fuc) or absence (Untr) of 10 mM L-fucose. Fucosylated glycoconjugates on the cell surfaces were detected with the AAL lectin. Filled histograms represent control samples in which staining was carried out in the presence of 100 mM L-fucose.
In vitro restoration of fucosylated glycoconjugates after fucose supplementation of a LADII lymphoblastoid cell line derived from the patient.
Control (B7; left panel) or LADII-derived (DF1; right panel) EBV-transformed lymphoblastoid cells were kept in culture for 5 days in the presence (Fuc) or absence (Untr) of 10 mM L-fucose. Fucosylated glycoconjugates on the cell surfaces were detected with the AAL lectin. Filled histograms represent control samples in which staining was carried out in the presence of 100 mM L-fucose.
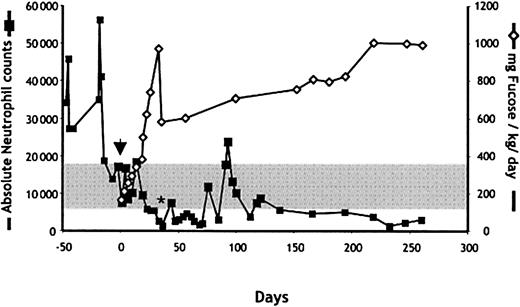
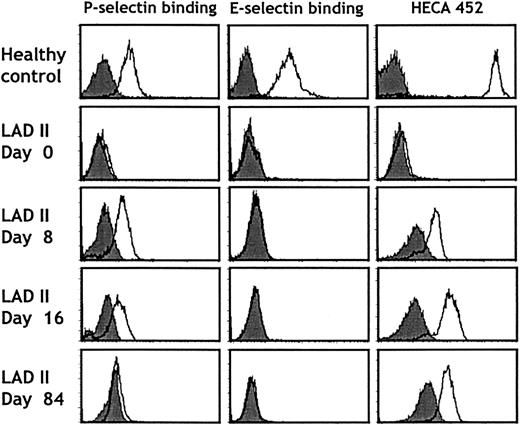
Because fucose therapy has previously been shown to be clinically effective in one patient with LADII,18 we initiated a treatment with fucose supplements. Oral administration of L-fucose was begun at a daily dose of 165 mg/kg divided 5 times per day and was increased progressively thereafter (Figure4). Within 2 weeks of the initiation of fucose treatment, neutrophil counts decreased to the normal range. To determine whether the clinical response correlated with improved selectin function, we assessed the expression of the HECA-452 epitope (which correlates with expression of sLeX) and the ability of neutrophils to bind P- and E-selectins in a fluid-phase assay. No sLeX or selectin binding was detected by fluorescence-activated cell sorter (FACS) before the initiation of fucose therapy (Figure 5; day 0). However, as early as 8 days after the initiation of fucose treatment, a significant increase in sLeX and P-selectin ligand expression was detected on the patient's neutrophils. Remarkably, E-selectin ligand expression could not be detected even after more than 230 days of treatment with fucose doses up to 1000 mg/kg per day (Figure 5). Although P-selectin binding progressively increased to reach levels close to those of healthy donor neutrophils (81% of the binding of control cells on day 62), sLeX expression increased more slowly and was still 20-fold lower by day 84 (Figure 5). These observations are in line with those obtained from the other LADII patient who responded to oral fucose,18 in which E-selectin ligands were detected much later than P-selectin ligands after fucose treatment, and they suggest a preferential incorporation of fucose in P-selectin glycoprotein ligands over E-selectin ligands.
Effect of fucose administration on circulating neutrophil counts in the LADII patient.
Fucose therapy was initiated on day 0 (arrow). The dose of L-fucose was sharply reduced on day 36 (to 580 mg/kg per day) in response to therapy-induced neutropenia (*). Neutrophil counts were determined using an automated cell counter. The gray area marks the limits of normal absolute neutrophil counts for 1 year of age.
Effect of fucose administration on circulating neutrophil counts in the LADII patient.
Fucose therapy was initiated on day 0 (arrow). The dose of L-fucose was sharply reduced on day 36 (to 580 mg/kg per day) in response to therapy-induced neutropenia (*). Neutrophil counts were determined using an automated cell counter. The gray area marks the limits of normal absolute neutrophil counts for 1 year of age.
Selectin binding and HECA 452 antigen expression on neutrophils before and during fucose therapy.
Neutrophils purified from a healthy donor (top panels) or the LADII patient (days 0-84) were analyzed for P- or E-selectin binding or HECA 452 antigen expression by flow cytometry using recombinant human P-selectin IgG or murine E-selectin IgM chimeras or the HECA 452 antibody. Filled histograms represent control stainings performed in the presence of 5 mM EDTA or rat IgG, respectively. Patterns similar to those on day 84 were obtained up to day 232 of treatment.
Selectin binding and HECA 452 antigen expression on neutrophils before and during fucose therapy.
Neutrophils purified from a healthy donor (top panels) or the LADII patient (days 0-84) were analyzed for P- or E-selectin binding or HECA 452 antigen expression by flow cytometry using recombinant human P-selectin IgG or murine E-selectin IgM chimeras or the HECA 452 antibody. Filled histograms represent control stainings performed in the presence of 5 mM EDTA or rat IgG, respectively. Patterns similar to those on day 84 were obtained up to day 232 of treatment.
Fucose therapy partially restores the ability of LADII neutrophils to roll in vivo
The marked improvement in neutrophil counts appeared to contrast with the selectin-binding assays, which only showed a partial rescue in selectin binding. To evaluate the ability of LADII leukocytes to roll in vivo, we isolated neutrophils from the LADII patient before and after fucose treatment. Healthy neutrophils were purified in parallel from healthy controls. Purified neutrophils were fluorescently labeled and injected through the carotid artery of immunodeficient NOD/SCID mice. Neutrophil behavior was then assessed in cremasteric venules. Under these conditions, rolling is almost entirely mediated by endothelial P-selectin.26 As shown in Figure6A, the interacting flux of LADII neutrophils, before and 8 days after fucose treatment, was low (2.3% ± 2.3% and 1.3% ± 1.3%, respectively) compared with the average observed with neutrophils from control donors (23.8% ± 5.6%). Strikingly, on day 16 after treatment, the percentage of interacting LADII neutrophils (29.8% ± 11%) was similar to that of controls. However, detailed analysis of videotapes demonstrated a clear difference in the quality of these interactions in that fucose-treated LADII neutrophils interacted briefly and for short distances compared with the longer distances and times achieved by healthy neutrophils, as depicted in the scattergram (Figure 6B). Thus these data suggest that though the behavior of fucose-treated neutrophils is clearly abnormal in vivo on day 16, a partial recovery of P-selectin ligands alone appears to be sufficient to restore leukocyte trafficking in LADII.
Effect of L-fucose therapy on rolling in vivo of LADII neutrophils in cremasteric venules of NOD/SCID mice.
Neutrophils purified from a healthy donor or the LADII patient were fluorescently labeled and injected through the carotid artery into NOD/SCID mice prepared for intravital microscopy of the cremaster muscle. (A) The percentage of interacting LADII neutrophils obtained on days 0 and 8 of treatment was greatly reduced compared with that of neutrophils from a healthy donor. On day 16, however, a sharp increase in the number of interactions of the patient's neutrophils was detected. Error bars represent mean ± SEM. n = 4-6 venules per mouse. * P < .01 compared with healthy or day 16. (B) Altered kinetics in neutrophil rolling on day 16. Detailed analysis of the time and length of each rolling event from healthy (▪) or LADII (○) neutrophils from day 16 reveals transient interactions of LADII neutrophils compared to those from a healthy donor.
Effect of L-fucose therapy on rolling in vivo of LADII neutrophils in cremasteric venules of NOD/SCID mice.
Neutrophils purified from a healthy donor or the LADII patient were fluorescently labeled and injected through the carotid artery into NOD/SCID mice prepared for intravital microscopy of the cremaster muscle. (A) The percentage of interacting LADII neutrophils obtained on days 0 and 8 of treatment was greatly reduced compared with that of neutrophils from a healthy donor. On day 16, however, a sharp increase in the number of interactions of the patient's neutrophils was detected. Error bars represent mean ± SEM. n = 4-6 venules per mouse. * P < .01 compared with healthy or day 16. (B) Altered kinetics in neutrophil rolling on day 16. Detailed analysis of the time and length of each rolling event from healthy (▪) or LADII (○) neutrophils from day 16 reveals transient interactions of LADII neutrophils compared to those from a healthy donor.
Fucose therapy induces autoantibodies against neutrophils and erythrocytes
After 35 days of fucose therapy, peripheral neutrophil counts decreased to levels below the adjusted normal counts (Figure 4, asterisk). We suspected that the overcorrection in the neutrophil counts might have resulted from a decreased leukocyte half-life, perhaps because of the generation of autoantibodies directed toward neoantigens expressed on the neutrophil surface. The dose of oral fucose was decreased from 975 mg/kg to 570 mg/kg per day, and the patient's serum was evaluated for the presence of antineutrophil antibodies. Samples of control and patient sera were incubated with purified neutrophils from healthy donors. Bound antibodies were detected using a Cy5-conjugated antihuman secondary antibody. As shown in Figure 7, serum from the fucose-treated LADII patient on day 41 strongly stained neutrophils by flow cytometry. Cytospin preparations revealed clustered-appearing membrane staining (Figure 7, lower inset) that was specific because no such staining was observed when neutrophils were incubated with control serum (Figure 7, upper inset). An independent reference laboratory (Neutrophil Serology Laboratory) confirmed these results and also demonstrated positive granulocyte agglutination assays (titer, 1:32) at the nadir of neutropenia (data not shown). Monoclonal antibody-specific immobilization of neutrophil antigens (MAIGA) revealed that the autoantibody was not directed against the known neutrophil autoantigens NA1, NA2, NB1, and MART (not shown). Western blot analyses of neutrophil lysates in our laboratory have failed to reveal further information about the nature of the antigen.
Appearance of antineutrophil activity in the serum of the LADII patient after L-fucose treatment.
Neutrophils purified from a healthy donor were stained with diluted (1:100) serum obtained from a healthy donor (control) or the LADII patient on day 41 of treatment (D41). Secondary antibody staining was used as control (closed histogram). Insets show immunofluorescence staining of cytospin preparations of neutrophils from the same samples used for fluorescence-activated cell-sorting (FACS) analysis.
Appearance of antineutrophil activity in the serum of the LADII patient after L-fucose treatment.
Neutrophils purified from a healthy donor were stained with diluted (1:100) serum obtained from a healthy donor (control) or the LADII patient on day 41 of treatment (D41). Secondary antibody staining was used as control (closed histogram). Insets show immunofluorescence staining of cytospin preparations of neutrophils from the same samples used for fluorescence-activated cell-sorting (FACS) analysis.
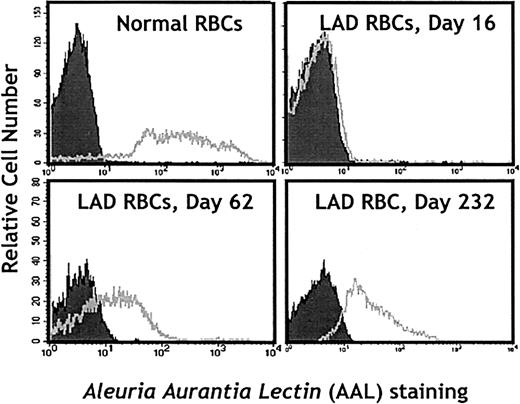
The possible induction by fucose therapy of fucosylated antigens on the surfaces of erythrocytes was also a major clinical concern. This could lead to hemolysis because LADII patients have natural antibodies against the H antigen (Bombay phenotype). Thus, we closely monitored hemoglobin, reticulocyte, and haptoglobin levels and the expression of fucosylated structures on the surfaces of the patient's erythrocytes using lectin staining. As shown in Figure8, no detectable AAL staining was observed on day 16 erythrocytes. However, starting from day 36 of fucose treatment up to the writing of this report (day 260), erythrocytes stained positively with AAL. In addition, erythrocytes collected on day 189 reacted with 2 of 3 polyclonal anti-H sources. Results of direct antiglobulin (Coombs) tests were always negative, and the patient never presented clinical evidence of autohemolysis. Taken together, these results, in contrast to those of a previous report,18 demonstrate that fucose therapy in LADII may induce the expression of neoantigens that represent a risk for autoimmune phenomena. Because of clinical concerns, fucose therapy has been dose adjusted to ensure an absolute neutrophil count of 1500 or more per microliter (Figure 4).
Neoexpression of fucosylated structures on erythrocytes from the LADII patient following L-fucose treatment.
Erythrocytes from a healthy donor (control) or the LADII patient on days 0, 62, and 232 of treatment were stained with the AAL lectin to detect fucosylated glycoconjugates on the cell surface.
Neoexpression of fucosylated structures on erythrocytes from the LADII patient following L-fucose treatment.
Erythrocytes from a healthy donor (control) or the LADII patient on days 0, 62, and 232 of treatment were stained with the AAL lectin to detect fucosylated glycoconjugates on the cell surface.
Psychomotor development is not improved by fucose therapy
In a manner similar to that for selectin ligands on leukocytes, psychomotor development was reported to improve in the patient with the Arg147Cys mutation18,21 but not in patients with Thr308Arg.19,22 We have not yet found evidence of psychomotor improvement in our patient who, currently 15 months of age, has only recently started to crawl and babble. In addition, our patient requires nasogastric tube feeding because of poor oropharyngeal coordination. Given that only 25% of the serum fucose concentrations is found in the cerebrospinal fluid,18 it is possible that psychomotor improvement in our patient is hampered by the limited doses of administered fucose, owing to the aforementioned complications.
Discussion
The 5 previously reported cases of LADII were of Middle Eastern descent.16 Four Arab patients from Israel and one Turkish patient from Germany shared similar characteristic clinical features, including severe mental retardation, profound leukocytosis with recurrent infectious episodes, defective selectin ligand formation with normal β2 integrin function, and Bombay phenotype on erythrocytes. The Bombay phenotype [deficiency in Fuc α (1,2) linkages forming the H antigen] and the absence of the sLeX antigen [in which Fuc α (1,3) linkage is a critical component] suggested that these patients had a broad defect in fucose metabolism and not a specific deficit in a fucosyltransferase.9 A subsequent study27 indeed showed impaired import of GDP-fucose in LADII-derived Golgi-enriched vesicles, a step required before high-energy fucose is incorporated into various glycoconjugate acceptors by fucosyltransferases.28 29
Major advances have recently been made in the therapy and understanding of this disease. The first came from the observation that exogenous fucose could overcome the fucosylation defect in culture17,27 and subsequently in a patient.18Treatment of the Turkish LADII subject with high doses of oral fucose produced a rapid lowering in the peripheral neutrophil counts, which coincided with the induction of selectin ligand expression on leukocytes but, remarkably, did not induce the expression of the H antigen on erythrocytes. In addition, improved psychomotor development was seen as a result of fucose therapy. The fact that none of the Arab patients responded to fucose treatment19 20 suggested that LADII in the Arab kindred had a different molecular basis.
The recent cloning of a putative GDP-fucose transporter, whose predicted structure encompasses 10 transmembrane domains, brought a molecular explanation for this differential response because Arab and Turkish patients exhibited distinct mutations at the GDP-fucose transporter locus. The Turkish subject had a point mutation (Cys439Thr) causing the replacement of arginine 147 by a cysteine (Arg147Cys) in the fourth transmembrane domain of the transporter, whereas the GDP-fucose transporter gene from 3 Arab patients contained a Cys923Gly mutation leading to a missense change from threonine to arginine (Thr308Arg) in the ninth transmembrane domain.21,22,25 It was thought that the Arg147Cys mutation might lead to a reduced affinity of the transporter for GDP-fucose that could be compensated by elevating cytosolic GDP-fucose levels, thus explaining the clinical response in the Turkish patient.29 This hypothesis was supported by kinetic studies of GDP-fucose import into Golgi preparations in which both Arab- and Turkish-derived cell extracts showed a reduced maximal velocity (Vm) of the saturable transport component, but only Golgi vesicles from the Turkish patient displayed a nonsaturable component.20 27
Given that the patient described in this report came from a different ethnic background, we suspected that we might find a novel mutation. Indeed, sequencing of cDNA and genomic DNA from our patient and his parents revealed a single base deletion (ΔG588) before the sixth transmembrane domain, producing a shift in the open-reading frame and a translation of 34 additional amino acids before a stop codon (Figure 1). Thus, at best, a severely truncated GDP-fucose transporter is expressed in our patient. Although our experiments using AAL staining suggest that the mutated transporter is nonfunctional, the limited sensitivity of this assay precludes a definitive conclusion on this issue. Surprisingly, the cells derived from our LADII patient and the clinical disease strongly responded to exogenous fucose. In a manner similar to that in the patient exhibiting the Arg147Cys mutation,18 neutrophil counts of our patient rapidly normalized, and the levels of soluble P-selectin binding to neutrophils reached 50% of that of control cells by day 40. Given the robust response to exogenous fucose in the presence of the truncated transporter and the lack of structural homologies between ΔG588 and Arg147Cys, we would argue that the residual transport activity might be mediated by alternative sugar transporter(s) whose activity cannot fully compensate for the mutated high-affinity transporter. Structure–function studies and gene knockout experiments will be useful to clarify whether ΔG588 is indeed a null mutation and whether other fucose transport activities remain when the GDP-FucTP is completely eliminated.
The results of the selectin ligand assays suggested that only a partial restoration of P-selectin ligand activity can fully restore leukocyte homeostasis in LADII. We have correlated these in vitro assays with the behavior of fucose-treated LADII neutrophils in vivo using intravital microscopy. Our results show that despite a rapid and complete normalization of peripheral neutrophil counts, individual neutrophils still displayed marked defects in their ability to interact with P-selectin in cremasteric venules. These results also underscore the limitations in the sensitivity of fluid-phase binding assays because little difference was found in vitro between days 8 and 16 of fucose treatment (Figure 5), whereas significant improvement in the neutrophil interactions with the vessel wall (and sustained clinical response) were observed in vivo after day 8.
It is interesting that in contrast to P-selectin ligands, E-selectin ligands were never induced in our patient. In the report by Marquardt et al,18 E-selectin ligand expression required approximately a 2.5-fold higher dose of L-fucose (1665 mg/kg per day) than that needed to induce P-selectin ligands. These results suggest a preferential fucosylation of P-selectin ligands in neutrophils and that a higher Golgi concentration of fucose may be required for the fucosylation of E-selectin ligands. Preferential fucosylation has been described at the fucosyltransferase level. For example, the 2 known leukocyte fucosyltransferases (FucTIV and FucTVII) exhibit a differential preference for distal α2,3-sialyated acceptors (FucTVII more than FucTIV) important for selectin ligands.30 In addition, studies using murine neutrophils suggest that FucTVII preferentially fucosylates P-selectin glycoprotein ligand (PSGL-1) whereas FucTIV displays a preference for E-selectin ligand-1 (ESL-1).31 The lack of acquisition of functional E-selectin ligands in our patient might also be explained by other possibilities, including a difference in the kinetics of the mutated GDP-fucose transporter, the lower doses of fucose administered to our patient, or the behavior of alternative sugar transporters in the Golgi.
Importantly, we found that the induction of fucosylation in our patient produced 2 potentially serious complications. The first was the appearance of autoimmune neutropenia that likely originated from the induction of neoantigens on the surfaces of neutrophils. The neutropenia responded to a reduction of the fucose intake (Figure 4), which lends support to a cause-and-effect relationship between these 2 phenomena. The second complication was the documented expression of fucosylated glycans on the surfaces of erythrocytes. Because the patient has a Bombay phenotype (absence of H antigen and presence of anti-H antibodies), the expression of the H antigen could potentially produce severe autoimmune hemolysis. Although we observed that fucose therapy also induced the expression of fucosylated structures (as detected by AAL) including the H antigen (as detected by specific antisera), no evidence of hemolysis was observed (as assessed by reticulocyte counts or haptoglobin and hemoglobin levels), and the patient never required any transfusion. These potentially severe complications highlight the importance of close monitoring during fucose therapy. Future structure–function analyses of the GDP-fucose transporter will be useful to understand the mechanisms behind the variable response to fucose therapy.
We thank Drs Anjali Kumar and John Lowe for providing reagents, Dr Marion E. Reid at the New York Blood Center for confirming the Bombay typing and evaluation of red cell antigens, and Dr Yande Kuang for providing the B7 lymphoblastoid cell line. We also thank Dr Luis Martinez and Goutham Narla for help with molecular biology and Drs Steven Simonte and Yoshio Katayama for help in obtaining blood samples from the patient and healthy donors, respectively.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-09-2840.
Supported by National Institutes of Health grants FDA1697, AI-48693, and AI-46732 (C.C.R.) and DK-56638 and HL-69438 (P.S.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul S. Frenette, Department of Medicine, Mount Sinai School of Medicine, Box 1079, One Gustave L. Levy Place, New York, NY 10029; e-mail: paul.frenette@mssm.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal