Despite some exciting new leads in molecular pathogenesis, AIDS-defining primary effusion lymphoma (PEL) remains a fatal malignancy. The lack of substantial progress in the management of PEL demands innovative treatment approaches. Targeting intracellular molecules critical to cell survival is one unexplored strategy for treating PEL. Here we show that inhibition of signal transducer and activator of transcription–3 (STAT3) leads to apoptosis in PEL cells. STAT3 is constitutively phosphorylated in PEL cell lines BC-1, BCBL-1, and VG-1. Transduction of dominant-negative STAT3 and pharmacological STAT3 inhibition caused caspase-dependent cell death. Although STAT3 activation is known to induce expression of Bcl-2 family proteins, PEL cell apoptosis was independent of Bcl-2, Bcl-XL, or Mcl-1 protein expression. Instead, STAT3 inhibition induced transcriptional repression of survivin, a recently identified inhibitor of apoptosis. Forced overexpression of survivin rescued VG-1 cells from apoptosis induced by STAT3 inhibition. Our findings suggest that activated STAT3 signaling directly contributes to malignant progression of PEL by preventing apoptosis, acting through the prosurvival protein survivin. Since constitutive STAT3 activation and survivin expression have been widely documented in different types of cancers, their linkage may extend to many malignancies and be critical to their pathogenesis.
Introduction
Since the advent of highly active antiretroviral therapy for HIV infection, life expectancy has improved substantially for HIV-infected individuals in whom virus replication can be successfully suppressed.1 Despite significant efforts to improve its outcome, non-Hodgkin lymphoma (NHL) remains a lethal complication of AIDS.2,3 AIDS-related NHLs present a predilection for widespread, extranodal disease; involvement of the central nerve system; frequent Epstein-Barr virus (EBV) infection; and poor responses to dose-intensive chemotherapy with high relapse rates.4-7 Among AIDS-related NHLs, primary effusion lymphoma (PEL) generally has an extremely aggressive clinical course with a median survival of only 3 months.8 PEL usually presents as a lymphomatous effusion in body cavities, and most cases exhibit dual infection with EBV and Kaposi sarcoma–associated herpesvirus (KSHV).9,10 Pleural and abdominal effusions from patients with AIDS-PEL contain various inflammatory cytokines.11,12 Among them, interleukin 10 (IL-10) and, to a lesser degree, viral IL-6 (vIL-6), the KSHV homolog of human IL-6,13 serve as autocrine growth factors for PEL cells.14 Vascular endothelial growth factor (VEGF), although not known to be an autocrine growth factor, is produced at a high level by PEL cells that also express VEGF receptor–1.15 Noteworthy, IL-10, vIL-6, and VEGF all induce phosphorylation of signal transducer and activator of transcription–3 (STAT3) for intracellular signaling.16-18 Ligand-dependent activation of STAT proteins is associated with organ development, cell proliferation, and differentiation, while constitutive activation of STATs often results in growth deregulation.19,20 STAT3 protein is constitutively activated in many human cancer cells, and aberrant STAT3 signaling is implicated as an important process in malignant progression.20 However, the mechanisms by which STAT3 contributes to oncogenesis have not been identified. Here we show that constitutive phosphorylation of STAT3 plays a critical role in promoting survival of KSHV+ PEL cells and that inhibition of STAT3 activity results in reduced protein expression of survivin, a member of the inhibitor of apoptosis protein (IAP) family.21 Our findings provide evidence that survivin directly contributes to malignant progression of PEL and that targeting survivin by STAT3 inhibition may provide a novel therapeutic approach for this aggressive lymphoma.
Materials and methods
Cell lines
The PEL cell lines VG-1 (a generous gift from Dr David Scadden, Harvard Medical School, Boston, MA) and BCBL-1 are infected with KSHV, and BC-1 is dually infected with KSHV and EBV.15,22 K562 and TF-1 are erythroleukemic cell lines that are negative for KSHV/EBV. Daudi is an EBV+/KSHV− Burkitt lymphoma cell line. BAF130 cell line is a murine BAF-B03–derived cell line expressing transfected human gp130 (a generous gift from Dr Masashi Narazaki, National Cancer Institute, Bethesda, MD). These cells were maintained as described previously.15 22-25
Western blotting and reverse transcriptase–polymerase chain reaction (RT-PCR)
After incubation with AG490 (Calbiochem, San Diego, CA) or 0.1% dimethyl sulfoxide (DMSO) (Sigma Chemical, St Louis, MO) (solvent) for 24 hours, cells were washed with ice-cold phosphate-buffered saline (PBS), and cell pellets were suspended in standard radioimmunoprotein assay (RIPA) buffer containing proteinase inhibitor cocktails (Roche Molecular Biochemicals, Mannheim, Germany). Western blotting was performed as described previously.13 To detect STAT3 phosphorylation, BAF130 cells stimulated with vIL-6 were used as a positive control. Recombinant vIL-6 was prepared as a fusion protein of maltose-binding protein (MBP) and amino acids 22 through 204 of vIL-6 (MBP–vIL-6).13 Antibodies (Abs) for Bcl-2, Mcl-1, Bcl-XL, p-STAT3, STAT3, and α-actin were purchased from Santa Cruz Biotechnology (CA), and an Ab for survivin was purchased from R&D Systems (Minneapolis, MN). Ab detection was performed by standard electrogenerated chemiluminescence (ECL) techniques (Perkin-Elmer, Boston, MA).
RT-PCR was performed as previously described,15 with the use of the following primer pairs: (1) vIL-6 forward, 5′-ATGTGCTGGTTCAAGTTGTGGTCTC-3′; reverse, 5′-TCACGTCGCTCTTTACTTATCGTG-3′; (2) viral FLICE-inhibitory protein forward, 5′-ATACAAGCCGGCACCATGGCC-3′; reverse, 5′-CTATGGTGTATGGCGATAGTGCC-3′; (3) viral cyclin forward, 5′-CACCCTGAAACTCCAGGC-3′; reverse, 5′-GATCCGATCCTCACATAGCG-3′; (4) latency-associated nuclear antigen (LANA) forward, 5′-GCAGTCTCCAGAGGTCTTCTC-3′; reverse, 5′-CGGAGCTAAAGAGTCTGGTG-3′; and (5) viral survivin forward, 5′-CAGCTTGAGTCAGTTTAGCACTGG-3′; reverse, 5′-GTACTACAGCTCTCAGGCCAATGT-3′. The quality of RNA was confirmed in all samples by parallel RT-PCR for glyceraldehyde-3-phosphate dehydrogenase (GAPDH).15
Construction and transfection of a dominant-negative STAT3-expressing vector
The cDNA of STAT3D, a dominant-negative form of murine STAT3 in which Phe424 and Phe435 of STAT3 are replaced with alanines26 (a kind gift from Dr Koichi Nakajima, Osaka City University, Japan), was inserted into the EcoRI site of the pEGFP–C2 vector (BD Biosciences Clontech, Palo Alto, CA). This construct expresses STAT3D as a fusion protein to the C-terminus of enhanced green fluorescence protein (EGFP). Plasmid vectors were transfected into cells with the use of Lipofectamine 2000 (Invitrogen) and transferrin (Sigma Chemical). Cells were harvested 2 and 3 days after transfection for the performance of Western blotting and apoptosis assays. Sorting of EGFP+ population was performed by means of the FACSVantage SE cell sorter (BD Biosciences, Sunnyvale, CA).
Apoptosis assay
Cells were washed with ice-cold PBS, resuspended in annexin V binding buffer (BD Pharmingen, San Diego, CA), and subsequently stained with annexin V–fluorescein isothiocyanate (FITC) or annexin V–phycoerythrin (PE) (BD Pharmingen) according to the manufacturer's recommendation. Propidium iodide (PI) was used as an indicator of cell viability. To examine the effect of tyrphostin AG490, dead cells were removed with the use of LSM lymphocyte separation medium (ICN, Aurora, OH), and the remaining cells were incubated at 5 × 105/mL in 12-well plates (2 mL per well) with AG490 or 0.1% DMSO for 48 hours. In the assays in which the caspase pathway was examined, the cell-permeable caspase inhibitor carbobenzoxy-valyl-alanyl-aspartyl(β-methyl ester-fluoromethy ketone (Z-VAD-fmk) (Calbiochem) was added at 100 μM to the cell suspension before the induction of apoptosis. Data acquisition and analysis were performed by means of a fluorescence microscope (Eclipse E600; Nikon, Melville, NY) or a FACScalibur cytofluorimeter with CELLQuest software (BD Biosciences).
DNA synthesis assay
Cells were cultured in 96-well microwell plates at 104 cells per well in RPMI 1640 with 10% fetal bovine serum (FBS) and graded concentrations of AG490 in an equal volume of drug solvent DMSO for 24 hours. DNA synthesis was measured by [3H]-thymidine incorporation (0.5 μCi [18.5 kBq] per well) (Perkin-Elmer) during the last 6 hours of culture. All assays were performed in triplicate cultures.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared as described previously.27 Protein concentrations were determined and normalized by the addition of extraction buffer. Electrophoretic mobility shift assays were performed as previously described.28 An oligonucleotide probe (5′-AGCTTAGGTTTCCGGGAAAGCAC-3′) corresponding to the IL-6 response element of the intercellular adhesion molecule–1 (ICAM-1) promoter29 was end-labeled with the use of polynucleotide kinase and 32P-γ–adenosine triphosphate (ATP). Competitive inhibition experiments were performed with the use of a 50-fold molar excess of unlabeled oligonucleotide. For supershift assays, prepared extracts were incubated with rabbit polyclonal Abs against STAT1 and/or STAT3 (Santa Cruz Biotechnology) for 60 minutes prior to addition of labeled probe. Samples were then electrophoresed through a 6% nondenaturing polyacryamaide gel and dried, and STAT-DNA complexes were detected by autoradiography.
Cytokine measurements
Cells were suspended at 1 × 106/mL in RPMI 1640 supplemented with 10% FBS, and cultured for 24 hours in the presence of AG490 or 0.1% DMSO. Conditioned media were tested by enzyme-linked immunosorbent assay (ELISA) with the use of human VEGF, IL-6, and IL-10 Quantikine kits (R&D Systems), following the manufacturer's instructions. The human IL-6 ELISA does not detect vIL-6.12 ELISA for KSHV vIL-6 was performed as described previously.12
Assays of reporter gene activity
The human survivin promoter activity was examined by 2 types of reporter gene systems: EGFP and heat-stable secreted alkaline phosphatase (SEAP). The human survivin promoter reporter constructs were derived from a 1.6-kb genomic fragment containing the 5′ region of the survivin gene upstream of the ATG translational start codon. Specific gene amplification by PCR was performed on genomic DNA isolated from human peripheral blood mononuclear cells with the following primers: psurvivinS-AseI, 5′-GGTGATTAATATATGAGTATTCTTTGTACT-3′; psurvivin AS- HindIII, 5′-AGGGAAGCTTCTTGAATGTAGAGAT-3′. After digestion with AseI and HindIII, the 1.6-kb fragment corresponding to positions −1512 to +70 of the human survivin promoter was purified, and the AseI-HindIII site of pEGFP-N3 vector (BD Biosciences Clontech), encoding the cytomegalovirus promoter, was replaced with the human survivin promoter. The construct DNA sequence was confirmed by cycle sequencing by means of the ABI PRISM dye terminal cycle sequencing kit (Applied Biosystems, Foster City, CA). To construct SEAP2–psurvivin, p-SEAP2–basic vector (BD Biosciences Clontech) was digested with XhoI, blunt-ended, and digested with HindIII. The humansurvivin promoter fragment was excised from plasmid psurvivin–EGFP by AseI digestion, blunting, andHindIII digestion, and was subsequently subcloned into the p-SEAP2–basic vector. The structure of psurvivin-SEAP was confirmed by restriction enzyme mapping. Transient transfection of psurvivin–EGFP, SEAP2–psurvivin, p-SEAP2–basic, and p-SEAP2–promoter (BD Biosciences Clontech) was performed with the use of Lipofectamine 2000 and transferrin. After 24 hours, cells were suspended at 5 × 105/mL in RPMI 1640 supplemented with 10% FBS, and cultured for additional 48 hours in the presence of AG490 or 0.1% DMSO. EGFP levels were determined by flow cytometry. SEAP assays were performed with the use of supernatants heated at 65°C for 30 minutes to inactivated endogenous alkaline phosphatase. The SEAP activity was measured by using a colorimetric assay based on hydrolysis of the para-nitrophenyl phosphate.
Forced overexpression of survivin in PEL cells
To construct a survivin-expressing vector, survivin cDNA was amplified by RT-PCR with the use of the following primers: survivin S–BamHI, 5′-GGCGGATCCATGGGTGCCCCGACGT-3′; survivin AS-XbaI, 5′-CCGTCTAGAGGCCTCAATCCATGGCA-3′. After digestion with BamHI and XbaI, this fragment was subcloned into the BamHI-XbaI site of pEF/Bsd vector (Invitrogen). The construct DNA sequence was confirmed by cycle sequencing. The vector was transfected into VG-1 cells by means of GenePORTER transfection reagent (Gene Therapy Systems, San Diego, CA). After selection with Blasticidin S (Invitrogen), stable survivin-transfected and control vector–transfected clones from each cell line were used for subsequent assays.
Results
Dominant-negative STAT3 induces apoptosis in PEL cells
Aberrant STAT activation is found in many malignancies, and constitutive activation of STAT3 is associated with cellular transformation, including B-cell transformation by EBV.19,20,30 To examine STAT3 activation in KSHV-infected cells, Western blot analysis was performed for PEL cell lines BC-1, BCBL-1, and VG-1. BAF130 cells, representing murine BAF-B03 cells stably transfected with human gp130, are known to proliferate in response to v-IL-6.31 Phosphorylation of STAT3 was detected in BAF130 cells stimulated with recombinant vIL-6 (Figure1A). Without exogenous cytokine stimulation, STAT3 phosphorylation was documented in all 3 PEL cell lines. Consistent with a previous report,23 phosphorylated STAT3 was not detected in Bcr-Abl–transformed K562 cells although unphosphorylated STAT3 was expressed abundantly in these cells.
(A) Tyrosine phosphorylation of STAT3 in KSHV+ PEL cells.
Cell lysates (2 × 106 cell equivalents per lane) from BAF130 cells (positive control), K562 cells (negative control), and PEL cell lines were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting. BAF130 cells were stimulated with 1 μg/mL MBP–vIL-6 at 37°C for 10 minutes before lysis. Phosphorylated STAT3 was visualized with the use of phospho-specific Ab recognizing Tyr705-phosphorylated STAT3. The same filter was stripped and blotted with an Ab for unphosphorylated STAT3, followed by restripping and reblotting with an Ab to α-actin. Data shown are representative of 3 independent experiments. (B) EGFP-STAT3D–expressing and control EGFP-expressing vectors were transfected into PEL and K562 cells. At 3 days after transfection, cells were stained with annexin V–PE and counted under a fluorescent microscope. The percentage of apoptosis indicates the fraction of annexin V–PE+ population within EGFP-expressing cells. Data represent the mean (± standard deviation [SD]) of 3 experiments. (C) EGFP+ VG-1 cells were sorted 2 days after transfection, and cultured in fresh medium for 1 day, followed by staining with annexin V–PE. Most cells expressing EGFP-STAT3D were positive for annexin V. Original magnification × 320. (D) At 2 and 3 days after transfection, VG-1 and K562 cells were stained with PI and analyzed by flow cytometry. A minimum of 5000 EGFP+ cells were analyzed for each sample. Shown is a representative of 4 experiments performed. FSC indicates forward scatter.
(A) Tyrosine phosphorylation of STAT3 in KSHV+ PEL cells.
Cell lysates (2 × 106 cell equivalents per lane) from BAF130 cells (positive control), K562 cells (negative control), and PEL cell lines were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting. BAF130 cells were stimulated with 1 μg/mL MBP–vIL-6 at 37°C for 10 minutes before lysis. Phosphorylated STAT3 was visualized with the use of phospho-specific Ab recognizing Tyr705-phosphorylated STAT3. The same filter was stripped and blotted with an Ab for unphosphorylated STAT3, followed by restripping and reblotting with an Ab to α-actin. Data shown are representative of 3 independent experiments. (B) EGFP-STAT3D–expressing and control EGFP-expressing vectors were transfected into PEL and K562 cells. At 3 days after transfection, cells were stained with annexin V–PE and counted under a fluorescent microscope. The percentage of apoptosis indicates the fraction of annexin V–PE+ population within EGFP-expressing cells. Data represent the mean (± standard deviation [SD]) of 3 experiments. (C) EGFP+ VG-1 cells were sorted 2 days after transfection, and cultured in fresh medium for 1 day, followed by staining with annexin V–PE. Most cells expressing EGFP-STAT3D were positive for annexin V. Original magnification × 320. (D) At 2 and 3 days after transfection, VG-1 and K562 cells were stained with PI and analyzed by flow cytometry. A minimum of 5000 EGFP+ cells were analyzed for each sample. Shown is a representative of 4 experiments performed. FSC indicates forward scatter.
To investigate the role of STAT3 in PEL cell survival, we constructed a vector that expresses STAT3D, a dominant-negative form of STAT3,26 as a fusion protein of EGFP. Tyrosine-phosphorylated STAT dimers translocate to the nucleus and bind specific DNA elements leading to transcriptional activation.19 Since the DNA-binding site is mutated, STAT3D can act as a specific competitive inhibitor of wild-type STAT3.26 When PEL cells were transfected with this construct, the expression of dominant-negative STAT3 was reflected by the intensity of EGFP. Expression of EGFP-STAT3D induced significant apoptotic cell death in PEL cells. The occurrence of apoptosis was measured by fluorescence-activated cell sorter (FACS) analysis of plasma membrane annexin V binding.32 At 3 days after transfection, up to 89% of STAT3D-expressing PEL cells underwent apoptosis while STAT3D transfection minimally increased the annexin V+ population in K562 cells (Figure 1B). EGFP itself was not toxic to PEL cells since fewer than 5% of cells expressing EGFP alone were apoptotic. The vast majority of VG-1 cells expressing EGFP-STAT3D (green) were positive for annexin V (red) (Figure 1C). This observation was further supported by FACS analysis demonstrating that most of the cells in the EGFP-STAT3D+ population shifted to the small-sized/PI+ fraction (Figure 1D). Similar results were obtained in BC-1 and BCBL-1 cells (data not shown). By contrast, K562 cells expressing EGFP-STAT3D continued to grow for more than 2 months in the presence of G418 (data not shown). These results suggest that constitutive STAT3 activation is critical to PEL cell survival.
Pharmacological STAT3 inhibition induces apoptosis in PEL cells
We examined the effect of pharmacological STAT3 inhibition on PEL cell growth. Tyrphostin AG490 is described as a Janus kinase 2 (JAK2) inhibitor leading to suppression of constitutively activated STAT3 molecules in acute leukemias and multiple myelomas.32 33 We looked for this effect of AG490 in PEL. An EMSA using an oligonucleotide probe corresponding to the IL-6 response element of the ICAM-1 promoter documented constitutive DNA binding activity in BC-1 cells (Figure2A). Supershift assays identified these DNA-binding complexes as homodimers and heterodimers of STAT1 and STAT3 (Figure 2B). AG490 treatment caused disappearance of the bands corresponding to STAT3/3 and STAT1/3, but not STAT1/1 (Figure2A), suggesting that AG490 elicits a selective inhibitory effect on STAT3 activation.
Effect of AG490 on PEL cell lines.
The tyrphostin AG490 inhibits constitutive activation of STAT3 and DNA synthesis in PEL cell lines. (A) Inhibition of constitutively activated STAT3 by AG490. BC-1 cells were incubated with 100 μM AG490 or DMSO (solvent) for 24 hours, and equal amounts of nuclear extracts (2 μg per lane) were incubated with a 32P-labeled oligonucleotide probe corresponding to the IL-6 response element and analyzed by EMSA. BAF130 cells were stimulated with 1 μg/mL MBP–v-IL-6 at 37°C for 10 minutes before lysis. Excess cold oligonucleotide competition (× 50) completely eliminated this binding activity (data not shown). Shown is a representative experiment of 3 performed. (B) Supershift assay was performed by adding an isotype control, anti-STAT1 and/or anti-STAT3 Abs, to a nuclear extract from BC-1 cells. (C) TF-1 cells (1 × 104 cells per well) were incubated with granulocyte-macrophage colony-stimulating factor (GM-CSF) (1 ng/mL) or human IL-6 (50 ng/mL) in the presence of AG490 for 24 hours, including a final 6-hour pulse with [3H]-thymidine. K562 and PEL cells were cultured under the same conditions but without exogenous cytokines. DMSO concentration was adjusted to a final 0.1% in all cultures. DMSO at this concentration did not affect the growth of these cell lines. The assay was performed in triplicate cultures. The results represent the mean percentage (± SD) of radioactivity compared with cultures in DMSO alone: SDs at most points were within 2% of each mean value. Shown is a representative experiment of 4 performed. (D) AG490 induction of apoptosis in PEL cells. Cells were treated with AG490 or 0.1% DMSO for 48 hours, stained with annexin V–FITC, and analyzed for apoptosis by flow cytometry. Marker positioning is based on fluorescence of controls, and data shown are from a representative experiment of 3 performed. The percentages of annexin V–FITC–binding cells are shown.
Effect of AG490 on PEL cell lines.
The tyrphostin AG490 inhibits constitutive activation of STAT3 and DNA synthesis in PEL cell lines. (A) Inhibition of constitutively activated STAT3 by AG490. BC-1 cells were incubated with 100 μM AG490 or DMSO (solvent) for 24 hours, and equal amounts of nuclear extracts (2 μg per lane) were incubated with a 32P-labeled oligonucleotide probe corresponding to the IL-6 response element and analyzed by EMSA. BAF130 cells were stimulated with 1 μg/mL MBP–v-IL-6 at 37°C for 10 minutes before lysis. Excess cold oligonucleotide competition (× 50) completely eliminated this binding activity (data not shown). Shown is a representative experiment of 3 performed. (B) Supershift assay was performed by adding an isotype control, anti-STAT1 and/or anti-STAT3 Abs, to a nuclear extract from BC-1 cells. (C) TF-1 cells (1 × 104 cells per well) were incubated with granulocyte-macrophage colony-stimulating factor (GM-CSF) (1 ng/mL) or human IL-6 (50 ng/mL) in the presence of AG490 for 24 hours, including a final 6-hour pulse with [3H]-thymidine. K562 and PEL cells were cultured under the same conditions but without exogenous cytokines. DMSO concentration was adjusted to a final 0.1% in all cultures. DMSO at this concentration did not affect the growth of these cell lines. The assay was performed in triplicate cultures. The results represent the mean percentage (± SD) of radioactivity compared with cultures in DMSO alone: SDs at most points were within 2% of each mean value. Shown is a representative experiment of 4 performed. (D) AG490 induction of apoptosis in PEL cells. Cells were treated with AG490 or 0.1% DMSO for 48 hours, stained with annexin V–FITC, and analyzed for apoptosis by flow cytometry. Marker positioning is based on fluorescence of controls, and data shown are from a representative experiment of 3 performed. The percentages of annexin V–FITC–binding cells are shown.
We then examined the effects of AG490 using 2 cell lines, K562 and TF-1, in which JAK2 plays a primary role for cell growth. K562 cells display constitutive phosphorylation of JAK2 that is critical to Bcr-Abl malignant transformation.34 TF-1 is a multifactor-dependent erythroleukemia cell line that exhibits rapid JAK2 phosphorylation in response to GM-CSF or IL-6, followed by STAT1/3/5 activation.24 When incubated with AG490 for 24 hours, K562 cells displayed decreased DNA synthesis in a dose-dependent manner (Figure 2C). TF-1 cells stimulated with exogenous GM-CSF or IL-6 showed a sensitivity to AG490 inhibition similar to that of K562 cells (Figure 2C). Of note, PEL cell lines exhibited a much greater sensitivity to AG490 than K562 cells or TF-1 cells (Figure 2C). This reduction in DNA synthesis correlated directly with the inhibitory effects of AG490 on DNA-binding activity (Figure 2A).
We next examined whether AG490 can promote apoptotic cell death. Following 48-hour exposure to AG490, PEL cells displayed a marked increase in annexin V binding measured by flow cytometry, with up to 78% of cells undergoing apoptosis (Figure 2C). By contrast, only a modest increase in annexin V+ cells was documented in K562 cells, which harbor constitutively activated JAK2,34in spite of the fact that AG490 caused 70% reduction in [3H]-thymidine incorporation in these cells. These results suggest that AG490 induces mainly a cytostatic effect in K562 cells and a cytotoxic effect in PEL cells.
KSHV-derived proteins and autocrine growth factors
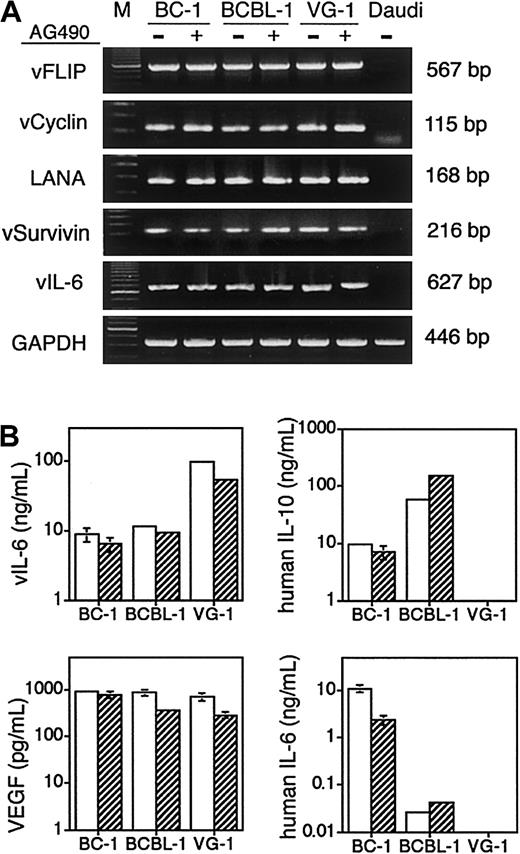
PEL is characterized by its consistent infection with KSHV. An important issue is whether STAT3 inhibition suppresses presumed oncogenic functions of KSHV, leading to apoptosis of PEL cells. KSHV encodes several proteins that regulate the proliferation and survival of infected cells. Some viral proteins are expressed only in the lytic phase, such as the viral homolog of Bcl-2 (open reading frame 16 [orf 16]), whereas others are expressed in latently infected PEL cells.35 Viral FLICE–inhibitory protein (orf K13/orf 71) protects cells from Fas-mediated apoptosis, and viral cyclin (orf 72) inactivates the tumor-suppressor function of retinoblastoma protein.36,37 Latency-associated nuclear antigen (orf 73) deregulates the retinoblastoma and p53 proteins.38Recently, the viral homolog of a spliced variant of survivin has been identified within KSHV orf K7.39 The orf K7 (viral survivin) is expressed in latently infected PEL cells,35and its protein product inhibits Fas-mediated apoptosis.39By RT-PCR, no significant reduction of message from these viral genes was detected after incubation with AG490 (Figure3A). In addition, expression of vIL-6 (orf K2) and viral survivin was not increased by AG490. Both vIL-6 and viral survivin are expressed constitutively in KSHV-infected PEL cells, but their expression is further induced in the lytic phase.12 35 These results suggest that AG490 does not induce KSHV lytic replication or regulate latent genes that can modulate cell cycle and survival.
Effects of AG490 on expression of KSHV-encoded genes and cytokines in PEL cells.
(A) AG490 minimally affects expression of antiapoptotic proteins encoded by KSHV. Cells were treated with 100 μM AG490 or DMSO for 24 hours. RT-PCR analysis was performed with the use of DNase-treated total RNA (0.25 μg for each reaction) for KSHV viral FLICE-inhibitory protein (vFLIP) (orf K13/orf 71), v-cyclin (orf 72), latency-associated nuclear antigen (LANA) (orf 73), v-survivin (orf K7), and v-IL-6 (orf K2). KSHV−/EBV+ Daudi cells were used as a negative control. The quality of RNA was confirmed by parallel RT-PCR amplification for GAPDH. (B) Regulation of growth factor secretion by AG490. Cells were treated with 100 μM AG490 or 0.1% DMSO for 24 hours, and specific ELISAs were performed on culture supernatants. Mean (± SD) protein levels per 106 live cells are shown from 3 independent experiments: SDs lower than 5% of the mean values are not visible in the bar graphs.
Effects of AG490 on expression of KSHV-encoded genes and cytokines in PEL cells.
(A) AG490 minimally affects expression of antiapoptotic proteins encoded by KSHV. Cells were treated with 100 μM AG490 or DMSO for 24 hours. RT-PCR analysis was performed with the use of DNase-treated total RNA (0.25 μg for each reaction) for KSHV viral FLICE-inhibitory protein (vFLIP) (orf K13/orf 71), v-cyclin (orf 72), latency-associated nuclear antigen (LANA) (orf 73), v-survivin (orf K7), and v-IL-6 (orf K2). KSHV−/EBV+ Daudi cells were used as a negative control. The quality of RNA was confirmed by parallel RT-PCR amplification for GAPDH. (B) Regulation of growth factor secretion by AG490. Cells were treated with 100 μM AG490 or 0.1% DMSO for 24 hours, and specific ELISAs were performed on culture supernatants. Mean (± SD) protein levels per 106 live cells are shown from 3 independent experiments: SDs lower than 5% of the mean values are not visible in the bar graphs.
We examined whether STAT3 inhibition modified the secretion of growth factors that play a pathogenetic role in PEL (Figure 3B). Secretion of vIL-6, an autocrine growth factor for some PEL cells, was not modified by AG490 in BC-1 or BCBL-1 cells but was reduced by 50% in VG-1 cells. Since vIL-6 expression is up-regulated during KSHV replication, this result further suggests that AG490 does not promote KSHV lytic replication in PEL cells. Production of IL-10, another autocrine growth factor for some PEL cells, was increased by 3-fold in BCBL-1 cells, perhaps reflective of an autocrine IL-10 feedback response,16 but did not change in BC-1 cells. In VG-1 cells, IL-10 was not detectable, but vIL-6 levels were much higher than those of BC-1 and BCBL-1, suggesting that autocrine growth stimulation in VG-1 cells may rely on different cytokines from those in BC-1 and BCBL-1 cell lines. However, we have no evidence that a 50% reduction in vIL-6 production is detrimental to the survival of VG-1 cells. AG490 did not change VEGF production in PEL cells even though STAT3 plays a role in transcriptional regulation of the VEGF gene in other cells.40 Secretion of IL-6 was reduced by 75% in BC-1 cells treated with AG490, but IL-6 does not affect BC-1 cell proliferation14 so it is unlikely that this reduction mediates AG490-induced apoptosis. Together, these results suggest that growth factor modulation is not essential to AG490-induced apoptosis.
STAT3 inhibition reduces survivin expression
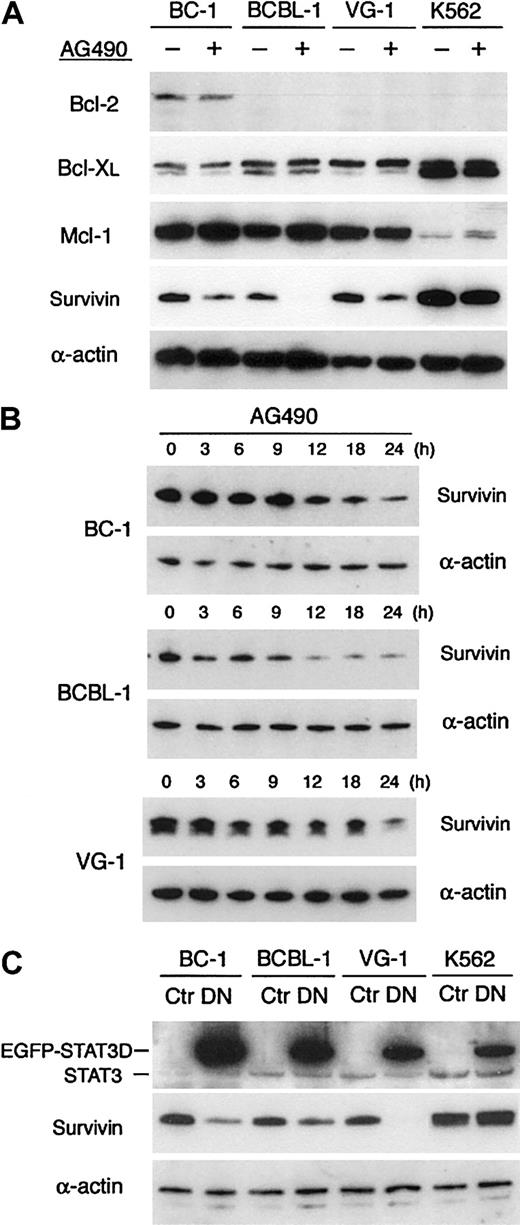
By regulating expression of the Bcl-2 family of antiapoptotic proteins in many cell types, STAT3 activation can inhibit apoptosis and promote cell proliferation. BCL-2 family members can protect cancer cells from apoptosis induced by a variety of stimuli, including anticancer agents.32,33,41 In PEL cells, however, we did not find diminished Mcl-1 and Bcl-XL expression following treatment with AG490 (Figure 4A). Bcl-2 was detectable only in BC-1 cells, and expression of this protein was not diminished by AG490 treatment. A novel modulator of the cell death/viability balance in cancer was recently identified as survivin, a member of the IAP family.21 It is noteworthy that expression of survivin was significantly reduced in AG490-treated PEL cells. By contrast, minimal reduction of survivin expression was observed in K562 cells. In PEL cells, inhibition of survivin expression was noted within 12 hours after AG490 treatment (Figure 4B). Suppression of survivin expression was also induced by dominant-negative STAT3 (Figure 4C). Transduction of EGFP-STAT3D was associated with reduced survivin expression in PEL cells but not in K562 cells. These results suggest that STAT3 inhibition induces a selective down-regulation of survivin expression in PEL cells.
(A) Cells were treated with 100 μM AG490 or 0.1% DMSO for 24 hours, and equal amount of cell lysates (25 μg per lane) were subjected to SDS-PAGE. Western blotting was performed to detect Bcl-2, Bcl-XL, Mcl-1, and survivin protein. Data shown are representative of 3 independent experiments. (B) Kinetic analysis of survivin protein expression. Cells were treated with 100 μM AG490 for the indicated time periods and analyzed for expression of survivin protein. Data shown are representative of 2 independent experiments. (C) Dominant-negative STAT3 reduces survivin expression. Cells were transfected with plasmid vectors for expression of EGFP-STAT3D or EGFP. At 2 days later, EGFP+ cells were sorted and lysed by RIPA buffer, and equal amounts of protein (30 μg per lane) were subjected to SDS-PAGE. Western blotting was performed for STAT3, survivin, and actin. Data shown are representative of 2 independent experiments. Ctr indicates control; DN, dominant-negative STAT3.
(A) Cells were treated with 100 μM AG490 or 0.1% DMSO for 24 hours, and equal amount of cell lysates (25 μg per lane) were subjected to SDS-PAGE. Western blotting was performed to detect Bcl-2, Bcl-XL, Mcl-1, and survivin protein. Data shown are representative of 3 independent experiments. (B) Kinetic analysis of survivin protein expression. Cells were treated with 100 μM AG490 for the indicated time periods and analyzed for expression of survivin protein. Data shown are representative of 2 independent experiments. (C) Dominant-negative STAT3 reduces survivin expression. Cells were transfected with plasmid vectors for expression of EGFP-STAT3D or EGFP. At 2 days later, EGFP+ cells were sorted and lysed by RIPA buffer, and equal amounts of protein (30 μg per lane) were subjected to SDS-PAGE. Western blotting was performed for STAT3, survivin, and actin. Data shown are representative of 2 independent experiments. Ctr indicates control; DN, dominant-negative STAT3.
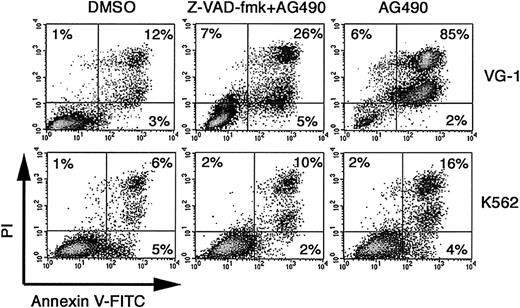
Recent studies suggest that survivin protects cells from apoptosis by inhibiting caspase proteases directly or indirectly.42Cleavage and activation of caspases plays a central role in the initiation and execution of apoptosis.42 To examine if AG490-induced apoptosis is a caspase-dependent phenomenon, PEL cells were incubated with the broad-spectrum caspase inhibitor Z-VAD-fmk. AG490-mediated killing of VG-1 cells was significantly reduced in the presence of Z-VAD-fmk (Figure 5). A similar effect was noted in BC-1 and BCBL-1 cells (data not shown). Together, these results indicate that AG490-induced PEL cell death is mediated mainly by a caspase-dependent pathway, and raise the possibility that STAT3 signaling may contribute to the prevention of programmed cell death by inducing survivin expression.
Inhibition of AG490-induced apoptosis by the caspase inhibitor Z-VAD-fmk.
Cells were incubated with 100 μM Z-VAD-fmk for 1 hour and were then treated for 48 hours with 100 μM AG490 or 0.1% DMSO. Cells were stained with annexin V–FITC and PI, and analyzed for apoptosis by flow cytometry. Shown is a representative of 2 experiments performed.
Inhibition of AG490-induced apoptosis by the caspase inhibitor Z-VAD-fmk.
Cells were incubated with 100 μM Z-VAD-fmk for 1 hour and were then treated for 48 hours with 100 μM AG490 or 0.1% DMSO. Cells were stained with annexin V–FITC and PI, and analyzed for apoptosis by flow cytometry. Shown is a representative of 2 experiments performed.
Transcriptional suppression of survivin gene expression by AG490
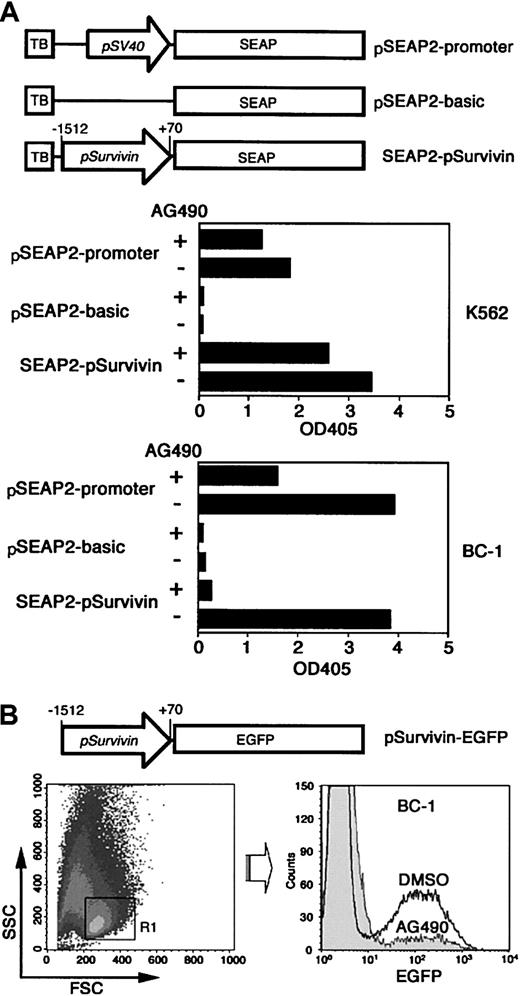
To determine if the AG490-induced decrease in survivin protein expression is due to repression of survivin promoter activity, a SEAP reporter construct was made. The 1.6-kb survivin promoter fragment (positions −1512 to +70) was cloned into a SEAP reporter plasmid, designated SEAP2–psurvivin (Figure6A). The pSEAP2–promoter is a positive control reporter vector that contains the SV40 early promoter upstream of the SEAP gene. After transfection (72 hours), alkaline phosphatase activity was detected in culture supernatants of both K562 and BC-1 cells transfected with pSEAP2–promoter. Equivalent or higher levels of alkaline phosphatase activities were detected in cells transfected with SEAP2–psurvivin, whereas minimal activity was detected in cells transfected with the vector lacking a promoter. AG490 suppressedsurvivin promoter activity significantly in BC-1 cells but only slightly in K562 cells. The alkaline phosphatase activity was suppressed more than 90% in BC-1 cells transfected with SEAP2–psurvivin. By contrast, AG490 reduced alkaline phosphatase activity by approximately 50% in BC-1 cells transfected with SEAP2-promoter even though a large proportion of BC-1 cells underwent apoptosis.
Induction of transcriptional suppression of the human
survivin gene by AG490. (A) The survivin promoter, ligated into the SEAP reporter vector pSEAP2–basic to create the construct SEAP2–psurvivin, was transiently transfected into K562 and BC-1 cells. The pSEAP2–basic and the pSEAP2–promoter that contains the simian virus 40 (SV40) early promoter upstream of the SEAP gene were transfected in the same manner. At 24 hours after transfection, cells were suspended at 5 × 105/mL and cultured for additional 48 hours in the presence of AG490 or 0.1% DMSO. The SEAP activities were determined by ELISA. Data represent the mean of triplicate assays. Shown is a representative of 2 experiments performed. TB indicates transcription blocker. (B) The reporter construct psurvivin–EGFP, which contains the survivin promoter upstream of the EGFP gene, was tranfected into BC-1 cells. Cells were treated with 100 μM AG490 or 0.1% DMSO for 48 hours. Live cells were gated by forward (FSC) and side scattering (SSC), and fluorescence intensity was determined on the basis of 5 × 104 live cells analyzed. Shown is a representative of 4 experiments performed.
Induction of transcriptional suppression of the human
survivin gene by AG490. (A) The survivin promoter, ligated into the SEAP reporter vector pSEAP2–basic to create the construct SEAP2–psurvivin, was transiently transfected into K562 and BC-1 cells. The pSEAP2–basic and the pSEAP2–promoter that contains the simian virus 40 (SV40) early promoter upstream of the SEAP gene were transfected in the same manner. At 24 hours after transfection, cells were suspended at 5 × 105/mL and cultured for additional 48 hours in the presence of AG490 or 0.1% DMSO. The SEAP activities were determined by ELISA. Data represent the mean of triplicate assays. Shown is a representative of 2 experiments performed. TB indicates transcription blocker. (B) The reporter construct psurvivin–EGFP, which contains the survivin promoter upstream of the EGFP gene, was tranfected into BC-1 cells. Cells were treated with 100 μM AG490 or 0.1% DMSO for 48 hours. Live cells were gated by forward (FSC) and side scattering (SSC), and fluorescence intensity was determined on the basis of 5 × 104 live cells analyzed. Shown is a representative of 4 experiments performed.
To confirm the effect of AG490 on the live cell population, we used another reporter plasmid that contains the survivin promoter upstream of the EGFP gene. BC-1 cells were transfected with this reporter plasmid, and EGFP expression in the live cell population was examined after 48-hour incubation with AG490 or DMSO. Flow cytometry analysis revealed a marked reduction of EGFP expression in live PEL cells by AG490 (Figure 6B), and the vast majority of the live cells underwent apoptosis in the following 24 hours (data not shown). We conclude from these results that AG490 induces repression of the humansurvivin promoter in PEL cells, and suggest that constitutively activated STAT3 promotes transcriptional activation of the human survivin gene, providing PEL cells with a survival advantage.
Forced expression of survivin rescues PEL cells from AG490-induced apoptosis
To examine whether survivin repression is responsible for PEL cell apoptosis induced by STAT3 inhibition, we established stable clones of VG-1 cells transfected with a survivin construct driven by the human elongation factor–1α subunit. After selection of stable transfectants, survivin-transfected as well as control vector–transfected cells were incubated with AG490. Expression of survivin protein was retained after AG490 treatment in survivin-transfected VG-1 cells, but was markedly reduced in control vector–transfected cells (Figure 7A). In DNA synthesis assays, enforced expression of survivin rendered VG-1 cells significantly less sensitive to AG490 (Figure 7B). After 48-hour incubation with AG490, annexin V+ cells increased significantly in control vector–transfected but not in survivin-transfected cells (Figure 7C). Similar results were obtained in BCBL-1 cells transfected with a survivin expression vector (data not shown). These results show that survivin can counteract apoptosis induced by AG490 in PEL cells, and provide evidence that constitutive activation of STAT3 sustains growth and survival of PEL cells, at least in part, through stimulation of survivin expression.
Effect of ectopic overexpression of survivin on VG-1 cells.
Ectopic overexpression of survivin rescues VG-1 cells from apoptosis induced by AG490. (A) Survivin-transfected and control vector–transfected VG-1 cells were treated with AG490 for 24 hours, and whole cell lysates (25 μg per lane) were subjected to SDS-PAGE. Western blotting demonstrates that survivin expression is unchanged in survivin-transfected but is reduced in control vector–transfected cells after AG490 treatment. (B) Cells were incubated with the indicated concentrations of AG490 for 24 hours, including a final 6-hour pulse with [3H]-thymidine. The results represent the mean percentage (± SD) of radioactivity as compared with cultures in 0.1% DMSO alone. Shown is a representative experiment of 3 performed. (C) Effects of enforced survivin expression on AG490-induced programmed cell death. After incubation with AG490 or 0.1% DMSO for 48 hours, cells were stained with annexin V–FITC and analyzed for apoptosis by flow cytometry. AG490 induced marked increase of the annexin V+ population in vector-transfected control cells but not in survivin-transfected cells. Data shown are representative of 3 independent experiments. The percentage of annexin V–FITC-binding cells are shown.
Effect of ectopic overexpression of survivin on VG-1 cells.
Ectopic overexpression of survivin rescues VG-1 cells from apoptosis induced by AG490. (A) Survivin-transfected and control vector–transfected VG-1 cells were treated with AG490 for 24 hours, and whole cell lysates (25 μg per lane) were subjected to SDS-PAGE. Western blotting demonstrates that survivin expression is unchanged in survivin-transfected but is reduced in control vector–transfected cells after AG490 treatment. (B) Cells were incubated with the indicated concentrations of AG490 for 24 hours, including a final 6-hour pulse with [3H]-thymidine. The results represent the mean percentage (± SD) of radioactivity as compared with cultures in 0.1% DMSO alone. Shown is a representative experiment of 3 performed. (C) Effects of enforced survivin expression on AG490-induced programmed cell death. After incubation with AG490 or 0.1% DMSO for 48 hours, cells were stained with annexin V–FITC and analyzed for apoptosis by flow cytometry. AG490 induced marked increase of the annexin V+ population in vector-transfected control cells but not in survivin-transfected cells. Data shown are representative of 3 independent experiments. The percentage of annexin V–FITC-binding cells are shown.
Discussion
Via autocrine production of growth factors or constitutive activation of intracellular signaling pathways, tumor cells can gain independence of exogenous growth factors.43 Unlike normal cells and tissues, constitutively activated STATs are detected in a wide variety of human cancer cells, and aberrant activation of STAT proteins is often associated with cellular transformation by various oncoproteins.19,20 Particularly in lymphoid cells, a link between STAT3 activation and regulation of Bcl-2 family protein expression has been demonstrated.32,33,41 STAT3 was required for Bcl-2 induction in pro-B cells,44 and forbcl-x gene expression in U266 myeloma cells.32Inhibition of STAT3 signaling resulted in apoptosis and decreased Mcl-1 expression in large granular lymphocyte leukemias.33 More recently, inhibition of STAT3 signaling in B-NHL cells by endogenous IL-10 neutralization resulted in reduced Bcl-2 expression and sensitization to cytotoxic drugs.41 Here we show that STAT3 inhibition in PEL cells induced apoptosis without down-regulation of Bcl-2, Mcl-1, or Bcl-XL expression. Instead, expression of the IAP family protein survivin was significantly reduced by STAT3 inhibition, and forced expression of survivin rescued PEL cells from AG490-induced apoptosis. In contrast to other lymphoid malignancies in which STAT3 inhibition sensitized cells to Fas-mediated apoptosis and to cytotoxic agents,32,33 41STAT3 inhibition by itself resulted in PEL cell death, suggesting that STAT3 is a desirable therapeutic target for PEL.
Survivin has been implicated in both cell cycle control and apoptosis resistance.42 Survivin promoter exhibits typical M phase inducible transactivation,45 and interference with the expression of survivin causes caspase-dependent cell death in the G2/M phase of the cell cycle.46 The half-life of survivin protein is only 30 minutes, and the ubiquitin-proteasome pathway is believed to mediate degradation of this protein.47 Here, we demonstrate that inhibition of STAT3 phosphorylation by AG490 results in transcriptional repression of the human survivin promoter activity. STAT3 plays a key role in G1-to-S phase cell cycle transition,44 and survivin displays cell cycle–regulated expression that peaks in the G2/M phase.45 Hence, reduced expression of survivin might simply be the result of inhibited cell cycle progression when STAT3 is inactivated. In a recent report, fibroblasts transformed by a persistently activated form of STAT3 presented increased survivin expression, which was down-regulated by interferon-γ–induced G1 arrest.48In another system, survivin message, undetectable in quiescent normal endothelial cells, was present in dividing cells stimulated by IL-11, which activates the STAT3 pathway.49 These observations support the notion that cell cycle progression is essential to survivin expression. However, a cell cycle–independent transcriptional effect is the most likely explanation for the reduced survivin expression in acute myeloid leukemias when phosphatidylinositol-3 kinase is inhibited.50 In the present study, STAT3 inhibition did not reduce survivin expression in K562 cells, but AG490 had a significant cytostatic effect in these cells, presumably by suppression of JAK2. In PEL cells, survivin expression may not be principally regulated through cell cycle controls because c-MYC is constitutively absent from these cells,10 and the c-mycpromoter is the target for STAT3 regulation of cell cycle progression.44 51
Among molecules of the IAP family, survivin exhibits the most restricted expression in adult tissues.42 Only occasional normal adult human or mouse tissues express survivin, whereas most of the common human cancers do.21,42 Selectivity of expression and biological importance render survivin an attractive target for treatment, but the 3-dimensional structure of survivin reveals no obvious opportunity for drug design.42 52 Disruption of STAT3 activation by tyrphostin AG490 may provide a new therapeutic approach for targeting survivin and treating AIDS patients with PEL. Since constitutive STAT3 activation and survivin expression are both commonly detected in cancers, their linkage may extend far beyond PEL.
We thank Drs Robert Yarchoan, Koichi Akashi, and Masashi Narazaki for helpful comments; Mike Klutch and George Poy for their help in DNA sequencing; Dr William Telford for his technical advice in FACS analysis; and Dr Veena Kapoor for cell sorting.
Prepublished online as Blood First Edition Paper, October 3, 2002; DOI 10.1182/blood- 2002-07-2130.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yoshiyasu Aoki, Experimental Transplantation and Immunology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 10 Center Dr 12N226, Bethesda, MD 20892-1907; e-mail: aokiy@mail.nih.gov.

![Fig. 1. (A) Tyrosine phosphorylation of STAT3 in KSHV+ PEL cells. / Cell lysates (2 × 106 cell equivalents per lane) from BAF130 cells (positive control), K562 cells (negative control), and PEL cell lines were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting. BAF130 cells were stimulated with 1 μg/mL MBP–vIL-6 at 37°C for 10 minutes before lysis. Phosphorylated STAT3 was visualized with the use of phospho-specific Ab recognizing Tyr705-phosphorylated STAT3. The same filter was stripped and blotted with an Ab for unphosphorylated STAT3, followed by restripping and reblotting with an Ab to α-actin. Data shown are representative of 3 independent experiments. (B) EGFP-STAT3D–expressing and control EGFP-expressing vectors were transfected into PEL and K562 cells. At 3 days after transfection, cells were stained with annexin V–PE and counted under a fluorescent microscope. The percentage of apoptosis indicates the fraction of annexin V–PE+ population within EGFP-expressing cells. Data represent the mean (± standard deviation [SD]) of 3 experiments. (C) EGFP+ VG-1 cells were sorted 2 days after transfection, and cultured in fresh medium for 1 day, followed by staining with annexin V–PE. Most cells expressing EGFP-STAT3D were positive for annexin V. Original magnification × 320. (D) At 2 and 3 days after transfection, VG-1 and K562 cells were stained with PI and analyzed by flow cytometry. A minimum of 5000 EGFP+ cells were analyzed for each sample. Shown is a representative of 4 experiments performed. FSC indicates forward scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood-2002-07-2130/3/m_h80433825001.jpeg?Expires=1769147053&Signature=wljHFmdiwmt6jaysmGYuzT4QjOwDBxTJHyL2B8jAnuRybnBsrifD73r3TkrdXikXjw997tT0a774Tsn2dVODBdFzknQum6cVR2NIX8vIN-YfFOkL5-fQWdHkIippWkoCyKc71RjxUk-5T8jzNriM4JBv8CHr4E0uezjyqZdd8SLQ1YHoQZqZF2zMcOiQunqZJmqdrRWXFHr8kr-~d6E2nrQIpXDMIzC-SkxQG-8-qga5AeK8l4VcugbirFAVi3hFD9PeojCwgAGr9qHHkNs7ElQcfts0GNcojLU1YGn0PYKdTZbilkIQclXC6qsc2aK7UMi6QtBNHNIo3FSuOn~MBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Effect of AG490 on PEL cell lines. / The tyrphostin AG490 inhibits constitutive activation of STAT3 and DNA synthesis in PEL cell lines. (A) Inhibition of constitutively activated STAT3 by AG490. BC-1 cells were incubated with 100 μM AG490 or DMSO (solvent) for 24 hours, and equal amounts of nuclear extracts (2 μg per lane) were incubated with a 32P-labeled oligonucleotide probe corresponding to the IL-6 response element and analyzed by EMSA. BAF130 cells were stimulated with 1 μg/mL MBP–v-IL-6 at 37°C for 10 minutes before lysis. Excess cold oligonucleotide competition (× 50) completely eliminated this binding activity (data not shown). Shown is a representative experiment of 3 performed. (B) Supershift assay was performed by adding an isotype control, anti-STAT1 and/or anti-STAT3 Abs, to a nuclear extract from BC-1 cells. (C) TF-1 cells (1 × 104 cells per well) were incubated with granulocyte-macrophage colony-stimulating factor (GM-CSF) (1 ng/mL) or human IL-6 (50 ng/mL) in the presence of AG490 for 24 hours, including a final 6-hour pulse with [3H]-thymidine. K562 and PEL cells were cultured under the same conditions but without exogenous cytokines. DMSO concentration was adjusted to a final 0.1% in all cultures. DMSO at this concentration did not affect the growth of these cell lines. The assay was performed in triplicate cultures. The results represent the mean percentage (± SD) of radioactivity compared with cultures in DMSO alone: SDs at most points were within 2% of each mean value. Shown is a representative experiment of 4 performed. (D) AG490 induction of apoptosis in PEL cells. Cells were treated with AG490 or 0.1% DMSO for 48 hours, stained with annexin V–FITC, and analyzed for apoptosis by flow cytometry. Marker positioning is based on fluorescence of controls, and data shown are from a representative experiment of 3 performed. The percentages of annexin V–FITC–binding cells are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood-2002-07-2130/3/m_h80433825002.jpeg?Expires=1769147053&Signature=UF94nxdTsqgmYlsq3New8dUUgrbLOWX4fyCun5aeICtzFfabcS30d7-zSrV6QeNsZkOCpjuS~qx4SWZJNjYEiZIWw5wwH20o7VzgnYrTIcYaX0~vnPJxpTr1pK-u7yQ6nIntYl-z1CWxCJov0Thr7szOpWrQOYwzVnvjfl~OzpLTrNbXriLqa9UqC0xMSAzECT2K-qVPjT4~GM-QZhYERbAEGCLCLWrEFlHZ~L0T~cTeHiibb-3qiTwRpNyFrba7hz6oblI1psK~bZ8BLLkz~iC-e4oxQNhK7ddTFt9EMtIx7qcp3kJ0Nlr5MJsIij45d5b4jL~yfibOF9T7wI~c~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Effect of ectopic overexpression of survivin on VG-1 cells. / Ectopic overexpression of survivin rescues VG-1 cells from apoptosis induced by AG490. (A) Survivin-transfected and control vector–transfected VG-1 cells were treated with AG490 for 24 hours, and whole cell lysates (25 μg per lane) were subjected to SDS-PAGE. Western blotting demonstrates that survivin expression is unchanged in survivin-transfected but is reduced in control vector–transfected cells after AG490 treatment. (B) Cells were incubated with the indicated concentrations of AG490 for 24 hours, including a final 6-hour pulse with [3H]-thymidine. The results represent the mean percentage (± SD) of radioactivity as compared with cultures in 0.1% DMSO alone. Shown is a representative experiment of 3 performed. (C) Effects of enforced survivin expression on AG490-induced programmed cell death. After incubation with AG490 or 0.1% DMSO for 48 hours, cells were stained with annexin V–FITC and analyzed for apoptosis by flow cytometry. AG490 induced marked increase of the annexin V+ population in vector-transfected control cells but not in survivin-transfected cells. Data shown are representative of 3 independent experiments. The percentage of annexin V–FITC-binding cells are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood-2002-07-2130/3/m_h80433825007.jpeg?Expires=1769147053&Signature=D3EOFfs8YHaPCdCMRe1SkTyDSTKqBYQkNZucN~4Uo~VWlbIvZSWWzxS2hk1z9QF8qs~xwh0qRR3s3kwu2ONbJKaMN87lL2oFI44ogJNcRcwR9~MOvBw-OHIKq9UxMPmhME8C1CxBwy5hyat9W056a2TN616LsWnQFXtkJziD3sppW2xHgxbjTcs7Nun~1E3Vv9tc7aqz2IXgZOgc20YpH82r2vaNK1lwtJAwoVLw5ezqLnMVGtyqnvUlo6z6v6y8vrzObB~0USRuvrxZyCPCj8KQCZgCbhAEdIPMK-T-3yDYy9BnF-~8CfHsEYfIG8WFFpP3RxZUicEVEfXOkVgj9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal