The expression/function of vascular endothelial growth factor (VEGF) receptors (VEGFR1/Flt1 and VEGFR2/KDR/Flk1) in hematopoiesis is under scrutiny. We have investigated the expression of Flt1 and kinase domain receptor (KDR) on hematopoietic precursors, as evaluated in liquid culture of CD34+ hematopoietic progenitor cells (HPCs) induced to unilineage differentiation/maturation through the erythroid (E), megakaryocytic (Mk), granulocytic (G), or monocytic (Mo) lineage. KDR, expressed on 0.5% to 1.5% CD34+ cells, is rapidly downmodulated on induction of differentiation. Similarly, Flt1 is present at very low levels in HPCs and is downmodulated in E and G lineages; however, Flt1 is induced in the precursors of both Mo and Mk series; ie, its level progressively increases during Mo maturation, and it peaks at the initial-intermediate culture stages in the Mk lineage. Functional experiments indicate that Mk and E, but not G and Mo, precursors release significant amounts of VEGF in the culture medium, particularly at low O2 levels. The functional role of VEGF release on Mk maturation is indicated by 2 series of observations. (1) Molecules preventing the VEGF-Flt1 interaction on the precursor membrane (eg, soluble Flt1 receptors) significantly inhibit Mk polyploidization. (2) Addition of exogenous VEGF or placenta growth factor (PlGF) markedly potentiates Mk maturation. Conversely, VEGF does not modify Mo differentiation/maturation. Altogether, our results suggest that in the hematopoietic microenvironment an autocrine VEGF loop contributes to optimal Mk maturation through Flt1. A paracrine loop involving VEGF release by E precursors may also operate. Similarly, recent studies indicate that an autocrine loop involving VEGF and Flt1/Flk1 receptors mediates hematopoietic stem cell survival and differentiation.
Introduction
During megakaryocytic (Mk) differentiation, Mk precursors switch from a mitotic to an endomitotic process characterized by DNA duplication without cytokinesis. This still poorly understood process leads to the formation of large polyploid cells with polylobulated nuclei that, in turn, give rise to platelets by cytoplasm fragmentation.1 2
The major regulator of Mk development, Mpl ligand/thrombopoietin (TPO), acts at all stages of megakaryocytopoiesis: commitment and proliferation of hematopoietic progenitor cells (HPCs), polyploidization of Mk precursors, and final maturation, including the formation of membrane demarcations and platelet production (reviewed in Kaushansky,1 Zucker-Franklin and Kaushansky,2Zimmet and Ravid,3 Cramer et al4). However, despite these properties, TPO fails to induce in vitro a level of Mk polyploidization comparable to that observed in vivo.5-7Addition of either single or combined cytokines (ie, kit ligand, interleukin-3, interleukin-6) to TPO-containing cultures, although improving Mk proliferation, negatively affects cytoplasmic maturation and polyploidization.5,6 Similarly, although erythropoietin (Epo) is considered the main growth factor stimulating erythropoiesis, additional cytokines are required at early and late erythroid (E) stages.8
Vascular endothelial growth factor (VEGF) is a key factor for proliferation and survival of endothelial cells.9-11 The VEGF family, including VEGF/VEGF-A, -B, -C, -D, and -E,10-12 as well as the placenta growth factor (PlGF),13 mediates angiogenic signals to endothelial cells through the binding with tyrosine kinase receptors designated VEGFR-1/Flt1, VEGFR-2/KDR/Flk1, and VEGFR-3/Flt-4.14 VEGF is the ligand of both Flt1 and kinase domain receptor (KDR) and consists of several isoforms generated by alternative splicing of a single mRNA precursor (VEGF121, 145, 165, 189, or 206), which differ in their molecular mass and their biologic properties, such as the ability to bind heparin or heparinlike molecules on cell surface.10,15 VEGF expression is enhanced spatially and temporally and is associated with physiologic events leading to angiogenesis in vivo, and its production is potentiated by hypoxia.16
Studies on gene knockout mice demonstrated the physiologic role of VEGF and its receptors, as central regulators of the development of vascular and hemopoietic tissues. Flt1 knockout causes a selective defect in the assembly and organization of vasculature.17Lack of either VEGF or KDR gene causes major defects in both vasculogenesis and blood island formation,18-21 suggesting the existence in embryonic life of a bipotent stem cell (SC) for hematopoietic and endothelial lineages, the hemangioblast.
In postnatal life, both Flt1 and KDR are expressed at low levels on CD34+ HPCs.22-27 More important, the small fraction of CD34+ cells expressing KDR displays multifunctional properties; ie, this cell subset comprises primitive hematopoietic cells,25 endothelial precursors,26 and hemangioblasts.27 VEGFRs are also expressed on hematopoietic precursors and terminal cells28: monocytes express Flt1,29 which mediates their migration in response to VEGF,30 whereas mature terminal Mk cells express KDR.23,28 Moreover, E precursors and Mk cells are able to produce and release VEGF,31,32 whose level is potentiated by TPO.33
Our studies in CD34+ cell unilineage differentiation culture demonstrate that Flt1 is expressed on monocytic (Mo) and Mk precursors; furthermore, Mk and E but not granulocytic (G) and Mo precursors release VEGF, particularly at low O2 levels. Labeling and Western blotting analysis with anti-Flt1 antibodies indicate that VEGF binding to early-intermediate Mk precursors occurs through Flt1. More important, treatment of Mk precursor cells with molecules inhibiting VEGF activity negatively affects their maturation, whereas exogenous VEGF promotes Mk development. Conversely, addition of exogenous VEGF to Mo unilineage cultures does not modify monocytic-macrophage differentiation/maturation.
Materials and methods
Hematopoietic growth factors (HGFs) and culture media
Recombinant human interleukin 3 (rhIL-3), granulomonocytic colony-stimulating factor (rhGM-CSF), and rhIL-6 were supplied by the Genetics Institute (Cambridge, MA); erythropoietin (rhEpo) was supplied by Amgen (Thousand Oaks, CA); flt-3 ligand (rhFL) was supplied by Immunex (Seattle, WA); rhG-CSF and rhM-CSF were purchased from R&D Systems (Minneapolis, MN); and thrombopoietin (rhTPO) was purchased from PeproTech (Rocky Hill, NJ). Iscoves modified Dulbecco medium (IMDM; GIBCO, Grand Island, NY) was prepared weekly before each purification experiment.
HPC purification
Adult peripheral blood (PB) was obtained from 20- to 40-year-old healthy male donors after informed consent. After a Ficoll gradient was performed as described by Testa et al,34 PB mononuclear cells were washed 3 times with phosphate-buffered saline (PBS) containing 2 mM EDTA (ethylenediaminetetraacetic acid) and centrifuged twice for 10 minutes at 20°C at 1400 rpm and once at 800 rpm to eliminate platelets. PB HPCs were purified by using the CD34 MultiSorter Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) as recommended by the manufacturer's instructions. CD34+fraction, selected by micro beads conjugated with anti-CD34 monoclonal antibodies (MoAbs), was further purified by passage through a second column. The purity of CD34+ cell population, 96% to 98%, was estimated by staining with antiphycoerythrin (PE)–conjugated anti-CD34 MoAb and by clonogenetic assay performed as previously reported.34
Unilineage HPC liquid suspension culture
HPCs were seeded at 5 × 104 cells/mL in liquid suspension culture and induced to specific differentiation by addition of appropriate HGFs combinations34-38: (1) in fetal calf serum–negative (FCS−) E culture,35 very low doses of IL-3 (0.01 U/mL) and GM-CSF (0.001 ng/mL), and saturating level of Epo (3 U/mL); (2) in FCS− G culture,34 low amounts of IL-3 (1 U), GM-CSF (0.1 ng), and saturating amount of G-CSF (500 U); (3) in FCS+ Mo culture36 IL-6 (1 ng) and saturating amounts of both FL (100 ng) and M-CSF (500 U); (4) in FCS−Mk culture,37 saturating doses of TPO (100 ng). All cultures were incubated in a 5% CO2, 5% O2, 90% N2 humidified atmosphere.
In some experiments, Mk precursors were cultured at suboptimal doses of TPO, alone or in combination with 100 ng/mL of either recombinant human VEGF165 or recombinant human PlGF or soluble VEGFRs/Fc (Flt1 and KDR) chimera proteins fused with the Fc region of immunoglobulins or 5 μg/mL neutralizing anti-Flt1 antibody (all from R&D Systems) that were added every 3 days. Culture conditions also included incubation at either 5% or 20% O2 and at 24-hour shifting from either 20% or 5% O2 to 1% O2.
Analysis of hematopoietic-differentiating precursors
Morphologic analysis.
Cells were collected at different days of culture, cytocentrifuged onto glass slides, and identified by morphologic analysis after staining with May-Grünwald-Giemsa (Sigma, St Louis, MO). Statistical analysis was performed by using t test for unpaired samples.
DNA staining.
Mk ploidy was analyzed by flow cytometry after DNA staining with propidium iodide (PI) according to the procedure described by Dolzhanskiy et al.39 Cells were washed and resuspended in Mk medium (cold Ca++- and Mg++-free PBS, 13.6 mM/L sodium citrate, 3% bovine serum albumin [BSA]) containing 0.5% Tween-20 for 30 minutes to permeabilize cell membranes. Then, an equal volume of Mk medium containing 0.5% Tween-20 and 2% paraformaldehyde was added. After 5 minutes at 4°C, cells were pelleted, resuspended in freshly prepared PI (50 μg/mL in 0.1% citrate saline buffer containing 1% BSA), and stored overnight in the dark at 4°C. The following day, cells were incubated with 50 μg/mL RNAse A for 30 minutes at room temperature in the dark and analyzed by flow cytometry using a MODFIT software (Verity Software House, Topsham, ME) equipped with a system to eliminate cell doublets from the analysis.
Flow cytometric analysis.
Phenotype of differentiating cells was analyzed by using MoAbs (Becton Dickinson, unless otherwise indicated) directly conjugated with either fluorescein isothiocyanate (FITC) or PE: (1) antiglycophorin A (Immunotech, Marseilles, France) for E culture, (2) anti-CD11b or -CD15 for G cells, (3) anti-CD14 for Mo cells, and (4) anti-CD61, -CD41a, -CD62 for megakaryocytes. Cells were incubated for 45 minutes at 4°C in the presence of proper amounts of specific MoAb. After 3 washes with cold PBS containing 0.1% BSA, cells were resuspended in PBS/2.5% formaldehyde and analyzed for their fluorescence emission by flow cytometry using a FACScan Lysis II program (Becton Dickinson).
Enzyme-linked immunosorbent assay
Either VEGF or P1GF concentration in cell culture supernatants was measured by using human immunoassays (R&D System) specific for either the soluble isoforms VEGF121 and VEGF165 or PlGF, respectively. For cell culture supernatants a sensitivity of 5 ng/mL could be achieved.
Reverse transcriptase (RT) PCR
Total RNA was extracted from 2 to 5 × 104 cells by the CsCl gradient technique in the presence of 12 μgEscherichia coli rRNA as a carrier. RT was performed according to the manufacturer's instructions (Boehringer, Mannheim, Germany) and normalized for β2-microglobulin (20 polymerase chain reaction [PCR] cycles). To evaluate VEGFR gene expression, aliquots of RT-RNA were amplified by 35 to 40 cycles of PCR, blotted onto nylon membranes, and hybridized with a specific probe. The following synthetic oligonucleotides were used: Flt1 forward 5′-GATACTCGACTTCC TCTGAA-3′, reverse 5′-ATCAAACATGGAGGTGGCATT-3′, probe 5′-GACTACATGCCAA TCAATGC-3′; KDR forward 5′-AGACTTTGAGCATGGAAG-3′, reverse 5′-CCATTCCACC AAAAGATG-3′, probe 5′-CATTATGACAACACAGCAGGAATCAGTCAG-3′; β2-microglobulin forward 5′-AACCACGTGACTTTGTCACAGC-3′, reverse 5′-CTGCTCAGATA CATCAAACATG-3′, probe GTGGGATCGAGACATGTAAGCAGC.
Flt1 Western blotting analysis
Flt1 analysis was performed on total cell membrane fraction obtained from 2 × 105 HeLa or K562 cell lines or megakaryocytes from either day 3 or day 10 of culture. Cells were washed with PBS and lysed for 30 minutes at 4°C with a buffer containing 20 mM Tris (tris(hydroxymethyl)aminomethane) pH 7.4, 2 mM EDTA, 0.1 μg/mL phenylmethylsulfonil fluoride, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 1 μg/mL pepstatin A, and 1 mM sodium orthovanadate. Lysates were centrifuged at 1000g for 5 minutes at 4°C to separate supernatant from nuclear pellet. Supernatant was thus centrifuged at 30 000gfor 1 hour to produce a nucleus-free membrane fraction (membrane). Pellet (nuclei) was washed with a solution containing 60 mM octylglucoside and 1% Triton X-100 to recover any particulate fraction left (washed). The washed and membrane fractions were pooled before protein quantification.
Samples containing equal amounts of protein were separated on 7.5% sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Nonspecific binding of antibody to the membrane was blocked by 1-hour incubation in 5% powdered skim milk in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% Tween-20). After 3 washes with TBST, membranes were incubated for 1 hour with the primary antibody antihuman flt1 (goat polyclonal; R&D Systems) diluted 1:125 in TBST containing milk and subsequently for 1 hour with antigoat horseradish peroxidase-conjugated secondary antibody (diluted 1:4000) (DAKO). Immunoreactivity was detected by using an enhanced chemiluminescence (ECL) detection kit (Pierce, Rockford, IL).
Flt1 flow cytometry and immunohistochemical analysis
Cells, resuspended in 1% BSA-PBS, were incubated for 30 minutes at 4°C with either 1 μg/mL anti-Flt1 MoAb KM173029 or, as a negative control, purified mouse IgGs1. After 2 washes the cells were reacted with PE-labeled F(ab2) antimouse-immunoglobulin G (IgG) goat antibody at 4°C for 30 minutes and analyzed by flow cytometry after 3 additional washings.
For immunocytochemical detection, cytospin preparation of Mk or Mo cells were fixed 10 minutes at 4°C with 1% paraformaldehyde, permeabilized 5 minutes with 0.1% Triton X-100/PBS, and then incubated with an appropriate dilution of anti-Flt1 MoAb. Slides were then processed by using a cell and tissue staining kit (R&D Systems), based on an AP-BCIP/NBT detection system. Negative controls were incubated with purified mouse IgGs.
Results
Morphologic and immunophenotypic characterization of differentiating hematopoietic precursors in unilineage culture
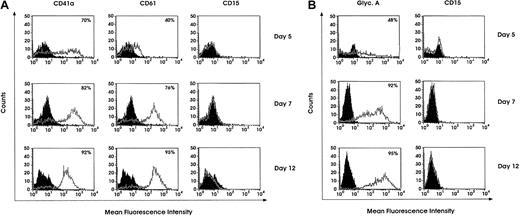
The detailed features of Mk, E, Mo, and G differentiation/maturation in unilineage cell cultures have been reported.34-38 Purified HPCs grown in liquid suspension culture supplemented with specific growth factor combinations (see “Materials and methods”) undergo a gradual wave of differentiation/maturation selectively along the Mk, E, G, or Mo lineages, thus giving rise to virtually pure unilineage cell populations. Confirming previous studies,34-38fluorescence analysis showed a progressive decrease of CD34 expression coupled with a gradually increasing expression of CD41a and CD61 in Mk cultures (Figure 1A), glycophorin A in E cells (Figure 1B), and CD15 and CD14 in G and Mo cultures, respectively (data not shown). Particularly, morphologic analysis of Mk cultures confirmed a gradual wave of maturation along the Mk pathway up to terminal cells. Specifically, in day 5 culture most cells represent Mk precursors, exhibiting a monolobated nucleus. At day 8, approximately 60% of cells are still monolobated, whereas the remaining ones exhibit 2 or 4 nuclear lobes. At day 12, 50% or more of megakaryocytes show 2 to 4 or more nuclear lobes and a highly granular cytoplasm. In E culture we confirmed the presence of a wave of selective erythroblast maturation, coupled with a gradual increase in the expression of erythroid-specific markers; eg, glycophorin A at day 12 was expressed on 95% of cells (Figure 1B). In both Mk and E cultures, we excluded the presence of contaminating G and Mo cells by labeling with anti-CD15 and -CD14 MoAbs (Figure 1A-B and results not presented); the lack of contaminating cells was confirmed by morphologic analysis (not shown).
Flow cytometry analysis of Mk- and E-differentiating cells.
Day 5, 7, or 12 differentiating Mk and E cells were analyzed for lineage-specific marker expression. Mk cells were labeled with anti-CD41a, -CD61, or -CD15 FITC-conjugated MoAbs (A), and E cells were labeled with PE-conjugated antiglycophorin A MoAb or FITC-conjugated CD15 MoAb (B).
Flow cytometry analysis of Mk- and E-differentiating cells.
Day 5, 7, or 12 differentiating Mk and E cells were analyzed for lineage-specific marker expression. Mk cells were labeled with anti-CD41a, -CD61, or -CD15 FITC-conjugated MoAbs (A), and E cells were labeled with PE-conjugated antiglycophorin A MoAb or FITC-conjugated CD15 MoAb (B).
VEGF is produced by Mk and E precursors, particularly at low O2 levels
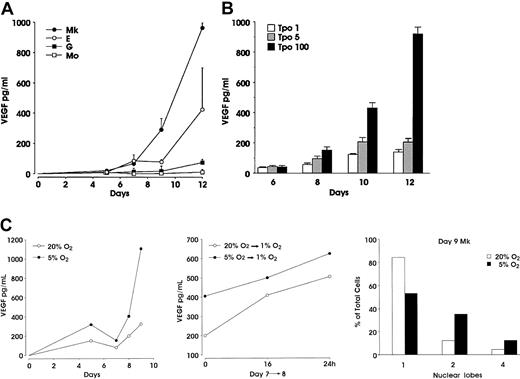
Using the unilineage cell cultures described earlier, we investigated the ability of differentiating Mk, E, Mo, and G cells to produce VEGF, from the stage of immature precursors to that of mature terminal cells (Figure 2A). Significant amounts of VEGF were released in the culture media of Mk and E precursors. VEGF was detectable starting from day 7 at a level of approximately 80 pg/mL in both E and Mk cultures and increased during maturation, the values being always higher in the latter ones. At terminal maturation, VEGF concentration was approximately 1000 and 500 pg/mL in Mk and E cultures, respectively. Conversely, VEGF protein measured in supernatants from Mo and G cultures at all stages of maturation was always within background levels (Figure 2A). In both Mk and E differentiation the values of PlGF concentration, measured at the same days of culture, were always below the detectable level (ie, < 10 pg/mL, data not shown). Moreover, megakaryocytes grown in the presence of either 1 or 5 ng/mL TPO released a significantly lower amount of VEGF, as compared with 100 ng/mL TPO cultures (Figure 2B).
VEGF concentration in supernatants from unilineage cultures.
(A) Enzyme-linked immunosorbent assay performed at the indicated days in supernatant from HPCs differentiating through Mk, E, G, or Mo lineages. (B) VEGF concentration evaluated in supernatants from Mk cells cultured in the presence of 1, 5, or 100 ng/mL TPO (mean ± SEM from 3 independent experiments). (C) Effect of oxygen tension on VEGF release and ploidy: HPCs induced to unilineage Mk differentiation at either 5% or 20% O2 tension were analyzed for VEGF release (top panel) and ploidy (bottom panel); an aliquot of day 7 megakaryocytes from both culture conditions was switched to 1% O2 (middle panel). A representative experiment of 3 is shown.
VEGF concentration in supernatants from unilineage cultures.
(A) Enzyme-linked immunosorbent assay performed at the indicated days in supernatant from HPCs differentiating through Mk, E, G, or Mo lineages. (B) VEGF concentration evaluated in supernatants from Mk cells cultured in the presence of 1, 5, or 100 ng/mL TPO (mean ± SEM from 3 independent experiments). (C) Effect of oxygen tension on VEGF release and ploidy: HPCs induced to unilineage Mk differentiation at either 5% or 20% O2 tension were analyzed for VEGF release (top panel) and ploidy (bottom panel); an aliquot of day 7 megakaryocytes from both culture conditions was switched to 1% O2 (middle panel). A representative experiment of 3 is shown.
Because it is well known that VEGF expression is regulated by hypoxia,40 41 we evaluated VEGF production in supernatants from Mk-differentiating cells grown at either 5% or 20% O2 tension. Mk cells grown at 5% O2 released higher levels of VEGF throughout the unilineage culture, as compared with 20% O2 controls (Figure 2C, top panel). To definitely link VEGF production to O2 tension, we further stressed the growth condition by switching day 7 Mk precursors from either 5% or 20% to 1% O2: the VEGF level in supernatants from 1% O2 megakaryocytes collected after 16 and 24 hours revealed a further increase of VEGF concentration, as compared with the level measured in the parental cultures (Figure 2C, middle panel). Interestingly, despite the same proliferation rate (data not shown), morphologic analysis indicated that Mk polyploidization was enhanced in 5% O2 versus 20% O2 culture condition. At day 9, in 5% O2 Mk cultures, 53%, 35%, and 12% of cells showed a monolobated, bilobated, and tetralobated nuclei, respectively, whereas megakaryocytes cultured at 20% O2 were 84% monolobated, 12% bilobated, and only 4% with tetralobated nuclei (Figure 2C, bottom panel).
Expression of VEGFRs on HPCs and differentiating hematopoietic precursors
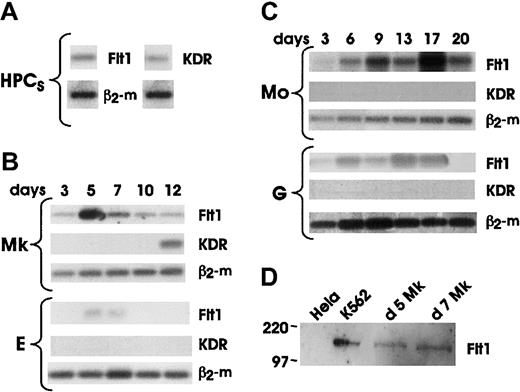
We analyzed the mRNA expression of VEGFRs on both HPCs and differentiating hematopoietic cells (Figure 3A-C). Our results showed that both Flt1 and KDR were expressed at very low levels in quiescent HPCs. KDR was rapidly downmodulated in all lineages soon after the addition of specific differentiating growth factors, and selectively reexpressed in advanced Mk maturation.23 28 The expression of Flt1 mRNA was lineage specific. Very low or undetectable levels of Flt1 mRNA were observed in either differentiating precursors or mature cells of G and E lineages. In contrast, a sustained expression of Flt1 mRNA was observed during all stages of Mo differentiation. Finally, Flt1 mRNA was induced during the initial-intermediate stages of Mk differentiation with a peak expression at day 5 to day 7 of culture and a subsequent decline to lower levels.
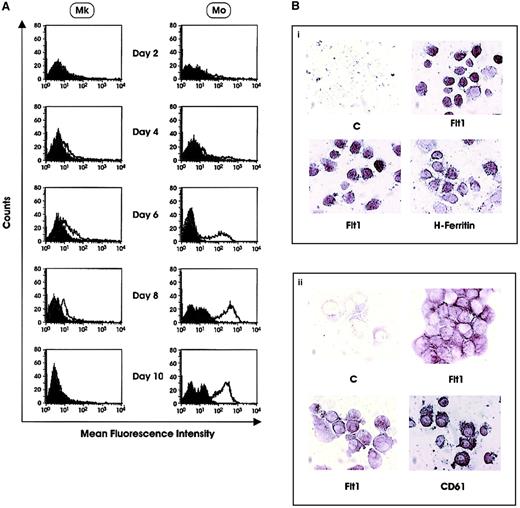
The expression of Flt1 was confirmed by Western blotting analysis of day 5 and day 7 Mk precursors. Proteins were isolated from the cell membrane fraction of Mk precursors, K562 and HeLa cell lines. The 2 latter cell lines were used as positive and negative controls, respectively (Figure 3D). A similar pattern of Flt1 expression was observed at the protein level by both flow cytometry and immunohistochemistry. Flow cytometric analysis of cells labeled with anti-Flt1 MoAb 173029 showed (1) no positive cells in HPCs differentiating along the E and G lineage, respectively; (2) a progressive increase in cells differentiating to along the Mo lineage; and (3) an increase during the initial stages of Mk differentiation with peak expression at day 6-7, followed by a decline at later stages of maturation (Figure 4A and data not shown). It is noteworthy that the positivity displayed by Mk is significantly lower than that shown by Mo precursors. These observations were confirmed by immunocytochemistry on day 6 Mk precursors, which were 95% positive for CD61 expression, while showing membrane reactivity for Flt1 on more than 60% of cells (Figure 4B). In parallel, we observed a marked reactivity of monocytes with anti-Flt1 MoAb (Figure 4B). It is worth mentioning that both Mk and Mo cells showed positivity on cell membrane as well as in cytoplasmic vesicles.
KDR and Flt1 mRNA and protein expression.
Representative results of mRNA expression on freshly purified HPCs (A); HPC-generated precursors differentiating and maturing through the Mk and E (B) and Mo and G (C) pathways in unilineage cultures. Aliquots of reverse transcribed RNA from 2 to 5 × 104 cells were collected at the indicated days and amplified by 40 PCR cycles based on β2-microglobulin normalization. (D) Western blot analysis of 50 μg/lane protein lysates from day 5 and day 7 megakaryocytes; K562 and HeLa cell lines were used as positive and negative control, respectively.
KDR and Flt1 mRNA and protein expression.
Representative results of mRNA expression on freshly purified HPCs (A); HPC-generated precursors differentiating and maturing through the Mk and E (B) and Mo and G (C) pathways in unilineage cultures. Aliquots of reverse transcribed RNA from 2 to 5 × 104 cells were collected at the indicated days and amplified by 40 PCR cycles based on β2-microglobulin normalization. (D) Western blot analysis of 50 μg/lane protein lysates from day 5 and day 7 megakaryocytes; K562 and HeLa cell lines were used as positive and negative control, respectively.
Flt1 protein expression.
(A) Flow cytometry analysis of Flt1 expression in HPCs differentiating along the Mk and Mo lineages. Results of a representative experiment are shown. (B) Immunocytochemical analysis of Flt1 in (i) Mo and (ii) Mk cells. Panels labeled “C” are negative controls. (Bi) Flt1 panels show expression in PB monocytes. (Bii) Flt1 panels show expression in day 6 Mk precursors.
Flt1 protein expression.
(A) Flow cytometry analysis of Flt1 expression in HPCs differentiating along the Mk and Mo lineages. Results of a representative experiment are shown. (B) Immunocytochemical analysis of Flt1 in (i) Mo and (ii) Mk cells. Panels labeled “C” are negative controls. (Bi) Flt1 panels show expression in PB monocytes. (Bii) Flt1 panels show expression in day 6 Mk precursors.
VEGF enhances Mk polyploidization
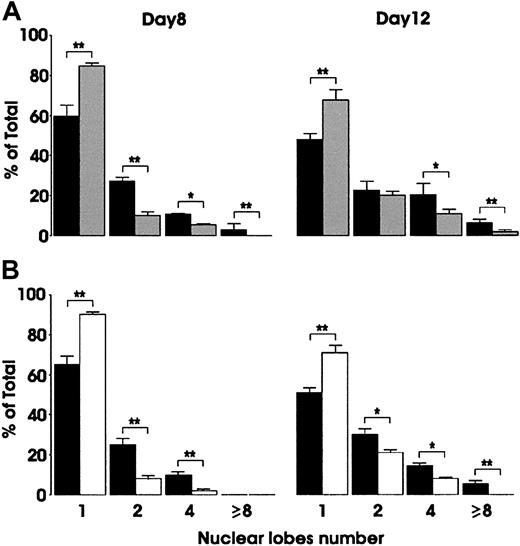
To investigate the possible role of VEGF in hematopoietic differentiation, we evaluated the effect of either soluble Flt1 and KDR receptors or exogenous VEGF on unilineage differentiation cultures. The addition in Mk cultures of 100 ng/mL of VEGF-neutralizing molecules, as Flt1/Fc, resulted in a significant reduction of the number of Mk nuclear lobes, as evaluated by morphologic analysis performed at day 8 and day 12 of culture (Figure 5A). Similar results were obtained by using KDR/Fc chimeric molecule (data not shown). We also evaluated the effect of an anti-Flt1–neutralizing monoclonal antibody. As expected, the results obtained after anti-Flt1 treatment on Mk polyploidization were comparable to those observed on megakaryocytes treated with VEGFRs/Fc-soluble molecules (Figure 5B). In all culture conditions, phenotypical analysis of day 8 and day 12 megakaryocytes did not reveal changes in the expression of specific differentiation markers such as CD41a, CD61, and CD62 (data not shown). In parallel, we have explored a possible effect of Flt1 blocking on Mo differentiation: the addition of VEGF or VEGFR-1 blocking reagents failed to modify the proliferation and the rate of Mo maturation (data not shown).
Effect of VEGFR-1/Fc or anti–VEGFR-1 MoAb on Mk polyploidization.
Cells were grown in the presence of TPO alone (▪), or combined with either 100 ng/mL VEGFR-1/Fc (░) (A) or 2 μg/mL neutralizing anti–VEGFR-1 MoAb (■) (B). Morphologic evaluation of nuclear lobe number was performed at day 8 and day 12 of culture. Mean ± SEM values of 3 independent experiments. *P < .05; **P < .01.
Effect of VEGFR-1/Fc or anti–VEGFR-1 MoAb on Mk polyploidization.
Cells were grown in the presence of TPO alone (▪), or combined with either 100 ng/mL VEGFR-1/Fc (░) (A) or 2 μg/mL neutralizing anti–VEGFR-1 MoAb (■) (B). Morphologic evaluation of nuclear lobe number was performed at day 8 and day 12 of culture. Mean ± SEM values of 3 independent experiments. *P < .05; **P < .01.
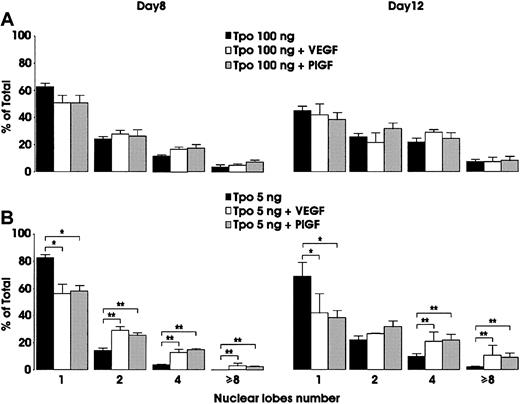
In view of the results obtained by using neutralizing VEGF molecules, we investigated the effect of exogenous VEGF addition. In Mk cultures grown in the presence of 100 ng/mL TPO, the addition of VEGF exerted a weak acceleration of Mk polyploidization (Figure6A). However, both morphologic evaluation and flow cytometry analysis of DNA content revealed that addition of VEGF to megakaryocytes grown in the presence of 5 ng/mL TPO significantly potentiated their polyploidization (Figure 6B and Figure7). A similar degree of polyploidization was observed when Mk cultures were performed in the presence of low concentrations of TPO (5 ng/mL) and 100 ng/mL of recombinant human PlGF (Figure 6B). However, addition of exogenous VEGF to Mo cultures did not modify the proliferation and maturation of Mo precursors (data not shown).
Effect of exogenous VEGF on Mk polyploidization.
Cells were grown in the presence of either 100 (A) or 5 (B) ng/mL TPO, alone or combined with 100 ng/mL exogenous VEGF or PlGF. Evaluation of nuclear lobe number was performed at day 8 and day 12 of culture. Mean ± SEM values of 3 independent experiments. *P < .05; **P < .01.
Effect of exogenous VEGF on Mk polyploidization.
Cells were grown in the presence of either 100 (A) or 5 (B) ng/mL TPO, alone or combined with 100 ng/mL exogenous VEGF or PlGF. Evaluation of nuclear lobe number was performed at day 8 and day 12 of culture. Mean ± SEM values of 3 independent experiments. *P < .05; **P < .01.
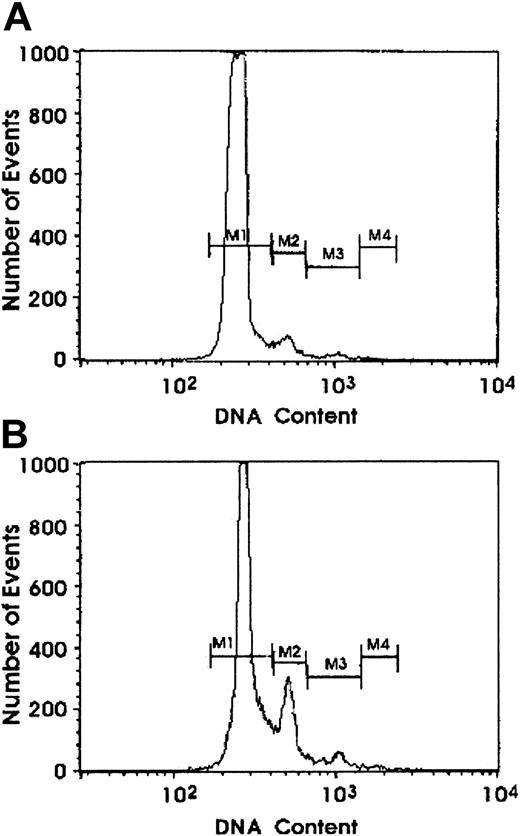
Effect of exogenous VEGF on Mk DNA content.
Ploidy was evaluated by flow cytometry analysis on MK grown in the presence of 5 ng/mL TPO alone (A) or combined with 100 ng/mL VEGF (B). At day 7 of culture, cells were stained with PI. A representative experiment of 3 is shown.
Effect of exogenous VEGF on Mk DNA content.
Ploidy was evaluated by flow cytometry analysis on MK grown in the presence of 5 ng/mL TPO alone (A) or combined with 100 ng/mL VEGF (B). At day 7 of culture, cells were stained with PI. A representative experiment of 3 is shown.
Discussion
Previous studies reported that human Mk and E cells express and release VEGF.31-33 Here, we extend those observations to document that VEGF is sustainedly produced by Mk and E precursors from early differentiation through late maturation.
It has been demonstrated that erythropoiesis is stimulated by hypoxia.40,41 Studies indicate that gene targeting of hypoxia-inducible factors causes a pronounced defect in multilineage hematopoiesis, particularly in the erythroid compartment.42 We observed that, in unilineage E culture, a low oxygen level (5% O2) not only exerted a positive effect on expansion and maturation of erythroid precursors but also increased VEGF release in the culture supernatants. Similarly, Mk cells cultured at 5% O2 released higher amounts of VEGF, as compared with those produced at 20% O2. Interestingly, hypoxic Mk cells displayed a significant increase in the mean ploidy despite an unmodified proliferation rate.
These results correlate with the expression of VEGFRs on differentiating hematopoietic cells. By using an RT-PCR approach, we showed that KDR is expressed at a low level in quiescent HPCs but is downmodulated during the early stages of HPCs differentiation, except for selective reinduction in late Mk cells. In contrast, Flt1 expression, detected at low level in quiescent HPCs, was selectively induced during Mo and Mk differentiation, whereas it was downmodulated to undetectable levels in the E and G lineages. Flt1 protein was expressed at elevated levels in the Mo lineage up to the terminal stage of maturation and at lower levels in the Mk lineage during the initial-intermediate but not terminal stages of development.
Our studies raise the possibility that autocrine and paracrine VEGF produced by Mk and E precursors targeting the Flt1 receptor on Mk-developing cells is of functional significance. Consistent with this hypothesis, morphology and DNA content analysis indicated a significant reduction of Mk ploidization in cells cultured in the presence of VEGF-neutralizing molecules. Conversely, the addition of exogenous VEGF or PlGF to the culture exerted a stimulatory effect on Mk ploidization. The finding that exogenous VEGF potentiates the effect of that secreted by Mk cells is expected, because the amount of endogenous VEGF released by megakaryocytes is relatively low and the biologic effects induced by this growth factor are concentration dependent.19Interestingly, bone marrow stroma cells produce higher amounts of VEGF than that measured in supernatants from cultured megakaryocytes and erythroblasts (our unpublished observations, 2002). It is also noteworthy that the developmental effect of VEGF is specifically observed on Mk precursors but not on the Mo series.
The positive action of VEGF on Mk maturation was more pronounced at low doses of TPO. Because normal in vivo TPO levels are also low (50-100 pg/mL),43 it may be suggested that under normal conditions VEGF and TPO positively interact to stimulate Mk maturation.
Furthermore, our findings indicate that hypoxia induces VEGF production by Mk cells, resulting in a stimulus of Mk maturation and platelet production. Several lines of evidence suggest that this mechanism may operate in vivo at the bone marrow level. In normal guinea pigs, rats, and mice, short-term hypoxia is followed by enhanced platelet production, leading to increased platelet counts44-47; because this phenomenon is not coupled with an increment in Mk production,48 it may be mediated by enhanced Mk maturation. These observations were confirmed in humans: exposure to high altitude hypoxic stimulation resulted in an increase of VEGF production49 and platelet numbers,50 without a rise of TPO levels.50 It is, therefore, suggested that VEGF may synergize with TPO to stimulate Mk maturation not only under normal conditions but also during hypoxia and perhaps in other physiopathologic conditions.
Although our data indicate that the stimulatory action of VEGF on Mk polyploidization is mediated via Flt1 binding, the biochemical pathway underlying this effect has not been elucidated. In this context, involvement of mitogen-activated protein kinase (MAPK) may be considered, because activation of MAPK in response to VEGF has been observed in endothelial cells,13,15 and TPO-induced activation of MAPK seems to play a role in Mk endomitosis.51-53
Interestingly, autocrine production of VEGF is pivotal for survival and differentiation of adult HSCs, as indicated in a conditional VEGF knockout model.54 Specifically, VEGF acts through both Flt1 and KDR/Flk1 (as shown by rescue of HSC differentiation on addition of VEGF mutants interacting selectively with either Flt1 or Flk1); additionally, an intracellular autocrine loop has been suggested.54 In line with these findings, VEGF administration in mice stimulates the differentiation of multiple hematopoietic lineages by a direct action on primitive progenitors55 and promotes hematopoiesis via recruitment of vasculogenic and hematopoietic stem cells.56
It is apparent, therefore, that autocrine-paracrine loops involving VEGF-Flt1 and/or VEGF-Flk1 may functionally operate in multiple steps of hematopoiesis and endothelial cell growth: these include HSC survival and differentiation, Mk development, monocyte mobilization, and endothelial cell growth. Hypothetically, we propose that Mk and E precursors, together with stromal cells, establish specific VEGF gradients in the bone marrow microenvironment, which may mediate the autocrine/paracrine functional loops indicated earlier. In this complex model, the present observations originally suggest that autocrine-paracrine VEGF release stimulates Mk maturation via the Flt1 receptor.
We thank Dr M. Shibuya for the generous gift of anti-Flt1 monoclonal antibody clone 1730. We thank M. Blasi, M. Fontana, and A. Zito for secretarial assistance and graphics.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-07-2184.
Supported by “Progetti di ricerca intramurali” of the Istituto Superiore di Sanità. No. 1057 (C.C.) and No. 1065 (U.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cesare Peschle, Thomas Jefferson University, Kimmel Cancer Institute, Bluemle Life Sciences Building, Room 609, 233 South 10th St, Philadelphia, PA 19107-5541; e-mail:cesare.peschle@mail.tju.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal