Abstract
Recent studies in tumor immunology indicate that malignant cells frequently express normal testicular-specific proteins. Because these proteins show restricted normal tissue distribution, they are usually highly immunogenic and may be potential targets for immunotherapy. In the present study, we have used a pair of sequence-specific primers in reverse transcription–polymerase chain reaction (RT-PCR) and sequence analysis to demonstrate that the X-linked gene encoding SPAN-Xb is expressed in multiple myeloma and other hematologic malignancies such as chronic lymphocytic leukemia (CLL), chronic myeloid leukemia (CML), and acute myeloid leukemia (AML). RT-PCR analysis demonstrates that SPAN-Xb is a cancer/testis antigen and shows a restricted normal tissue expression. It is not expressed in any normal tissue except testis. SPAN-Xb recombinant protein was produced and used in enzyme-linked immunosorbent assay (ELISA) and Western blot analysis. High-titer immunoglobulin G (IgG) antibodies, of IgG3 or IgG2 subclass, against SPAN-Xb were detectable in the sera of these patients. In contrast, SPAN-Xb mRNA or antibodies could not be detected in any of the healthy donors. There was a good correlation betweenSPAN-Xb gene expression and B-cell immune responses. These results suggest the in vivo immunogenicity of the SPAN-Xb protein. The presence of high-titer IgG responses suggests that the B-cell responses are likely to have been generated with CD4 T-cell cognitive help. Based on these data, we conclude that SPAN-Xb is a novel member of the family of cancer/testis antigens aberrantly expressed by, and capable of inducing, immune responses in patients with multiple myeloma and other hematologic malignancies.
Introduction
Recent advances in tumor immunology suggest the potential immunogenicity of various tumors in the autologous setting. In addition, an array of antigenic targets on tumor cells has been defined using cytotoxic T-lymphocyte (CTL) clones against tumor cells, either through screening target cells transfected with recombinant tumor complementary DNA (cDNA) libraries1 or biochemical purification of peptides eluted from the major histocompatibility complex (MHC) molecules.2 More recently, our group3 and others4 5 have shown that it is also possible to identify these novel tumor proteins without the need to establish tumor-reactive CTL clones using serologic screening of expression tumor cDNA library (SEREX). Unfortunately, all these methods are extremely labor intensive.
Through the technologies described above, it is apparent that neoplastic cells often aberrantly express normal testicular proteins. These proteins collectively form the new class of tumor antigens called cancer/testis antigens (CTAs). Because of the blood–testis barrier that limits contact between testicular and immune cells and the lack of human leukocyte antigen (HLA) class 1 expression on the surfaces of germ cells, the testis is an immune-privileged site. The restricted normal tissue distribution of CTAs to only the testis suggests that only tumor cells will be targeted during CTA immunotherapy; thus, these proteins are ideal candidates for tumor vaccines. We previously found that normal testicular proteins were often expressed at the mRNA level in hematologic malignancies.6,7 In addition, we3 and others8 also found that patients with hematologic malignancies produced high-titer immunoglobulin G (IgG) responses against tumor-derived proteins, supporting their in vivo immunogenicity and their potential use as targets for immunotherapy of hematologic malignancies.
Through the use of bio-informatics that involved an extensive search in the GenBank database for testicular-specific genes, followed by computer software prediction of the immunogenicity of the gene products, we have identified Sperm protein 17 (Sp17) as a novel CTA in multiple myeloma (MM)7 and ovarian cancer.9We have also successfully generated, from healthy volunteers and patients with cancer, HLA-class 1–restricted Sp17-specific CTLs that could lyse Sp17-positive MM10-12 and ovarian cancer cells.9 In the present study, we describe the successful identification of another testicular-specific protein, SPAN-Xb, aberrantly expressed in MM and other hematologic malignancies. We demonstrated that SPAN-Xb,13 a spermatid-specific protein, was frequently expressed and elicited immune responses in vivo in these patients. Our findings represent the first demonstration of SPAN-Xb expression and in vivo immune responses to the protein in patients with MM and other hematologic malignancies.
Materials and methods
Materials
Clinical materials consisting of tumor cells, sera, and bone marrow were obtained from patients with hematologic malignancies or from healthy donors. All clinical materials were obtained after informed consent and with approval from the local ethics committee. Samples from presentation and relapse were included. Genomic DNA and RNA were prepared from these samples. All samples were cryopreserved at −80°C until they were used in experiments.
Reverse transcription–polymerase chain reaction
Total RNA was extracted from cells using the Tri-reagent (Sigma, St Louis, MO). All RNA specimens were first treated with DNase I (Ambion, Austin, TX) to remove genomic DNA contamination. First-strand cDNA was synthesized from 1 μg total RNA with random hexamer primers using the GeneAmp RNA PCR Core Kit (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer's recommendation. The polymerase chain reaction (PCR) primers were as follows: for 5′SPANXb PCR, 5′-GCG GAT CCA TGG GCC AAC AAT CC-3′; for 3′ SPANXb PCR, 5′-GCA AGC TTT TGC TAC TTT TTA GG-3′. They amplify a cDNA of 328 base pairs (bp). PCR was performed by means of 35 amplification cycles at an annealing temperature of 55°C. Positive control amplification contained a plasmid with the SPAN-Xb cDNA amplified from human testis RNA by reverse transcription (RT)–PCR and a negative control contained all the PCR reaction mixture except for substitution of cDNA by water. RNA integrity in each sample was checked by the amplification of a 615-bp β-actin gene segment with primers 5′-GGC ATC GTG ATG GAC TCC G-3′ and 5′-GCT GGA AGG TGG ACA GCG A-3′ at an annealing temperature of 67°C. Successful removal of genomic DNA contamination was confirmed in each sample by amplification of the RNA without RT reaction. PCR products were visualized on an ethidium bromide agarose gel for a DNA band of the expected size. All results were confirmed in 2 independent RT-PCRs. Specificity of all the PCR products was further confirmed by BglI restriction enzyme digest.
Cloning and sequence analysis of SPAN-Xb
PCR products were cloned by the TA cloning system (Invitrogen, La Jolla, CA). Briefly, the PCR products were ligated into the pCR II TA cloning site that is flanked by 2 EcoRI restriction sites. Competent Escherichia coli (INVαF' cells) were transformed by heat-shock method and plated for ampicillin and blue-white color selection on agar plates containing 5-bromo-6-chloro-3-indolyl-β-d-galactopyranoside. Recombinant plasmid DNA was extracted and purified for nucleotide sequence analysis by an automated DNA sequencer.
Generation and purification of SPAN-Xb recombinant protein
Full-length SPAN-Xb cDNA was isolated and amplified from normal testicular RNA by RT-PCR. Following gel purification of the PCR products, the cDNA was cloned into pQE30 vector (Qiagen, Valencia, CA) between the BamHI and HindIII sites to produce a recombinant fusion protein of SPAN-Xb that contained a 6-histidine (6-His) peptide at the N-terminal of the protein. This strategy allowed affinity purification of the recombinant protein in a nickel Sephadex column. In addition, the 6-His tag was chosen instead of other N-terminal tag because 6-His tag is generally poorly immunogenic; therefore, it would not be expected to contribute to the immunogenicity of the fusion protein. The recombinant plasmid was transformed intoE coli and selected on agar plates for ampicillin resistance. The correct reading frame of SPAN-Xb cDNA in pQE30 was confirmed by DNA sequencing. To generate the recombinant protein, a recombinant clone was expanded in liquid culture and induced by 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) for 4 hours. Recombinant SPAN-Xb protein was harvested from E coli lysate by sonication. Following passage through the Ni-NTA affinity column and numerous rounds of washing, the protein was eluted. Successful generation of recombinant SPAN-Xb protein was confirmed on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) by Coomassie blue staining and Western blot analysis using an antibody directed at the N-terminal 6-His tag (Qiagen).
Enzyme-linked immunosorbent assay
Antibodies directed at SPAN-Xb protein were detected in patient sera using an in-house (enzyme-linked immunosorbent assay) ELISA system. Briefly, 96-well flat-bottom microtiter plates were coated with the purified recombinant SPAN-Xb protein at a concentration of 5 μg/mL. After 4-hour adherence of the antigen to the plate at 37°C, the wells were washed and then blocked with 3% bovine serum albumin in phosphate-buffered saline (PBS) at 37°C for 2 hours. Patient sera were diluted 1:1000 with the blocking buffer and dispensed into the wells in triplicate and allowed to bind overnight at 4°C. Alkaline phosphatase–conjugated goat antihuman IgG (Sigma, St Louis, MO) was then added to each well (1:1000 dilution in the blocking buffer). After 2 hours of incubation at room temperature,p-nitrophenylphosphate solution was added to each well and incubated at room temperature for color development. Twenty-five microliters 2 N NaOH was added to stop the reaction. Color intensity was measured on a microplate reader (Molecular Devices, Sunnyvale, CA) and was analyzed using the Softmax data analysis program. In each experiment, 2 different controls were set up: one consisted of wells coated with a control E coli–derived recombinant 6-His fusion protein, and another consisted of wells coated with PBS before the addition of the blocking buffer. All results were confirmed in 2 independent experiments.
Western blot analysis
Purified SPAN-Xb protein was fractionated in a 12% SDS–polyacrylamide gel and was transferred onto a nitrocellulose membrane. Successful generation of SPAN-Xb protein was confirmed using an antibody directed at the histidine tag. SPAN-Xb antibodies in the patient sera (1:1000 dilution) were detected by a goat antihuman IgG alkaline phosphatase conjugate. Antibody binding was visualized by reaction with the Western Blue-stabilized substrate (Promega, Madison, MI). An equal amount of another recombinant fusion protein derived fromE coli was used as the negative control.
Results
SPAN-Xb gene expression in MM and other hematologic malignancies
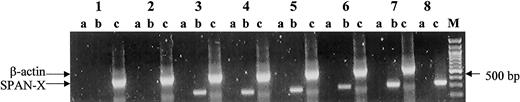
Using a pair of sequence-specific primers in RT-PCR, we first determined SPAN-Xb gene expression in tumor cells from patients with hematologic malignancies. SPAN-Xb transcripts could be detected in 6 (20%) of 30 patients with MM, 3 (33%) of 9 patients with chronic lymphocytic leukemia (CLL), 2 (29%) of 7 patients with chronic myeloid leukemia (CML), and 1 (50%) of 2 patients with acute myeloid leukemia (AML). In contrast, transcripts encoding SPAN-Xb could not be detected in any of the 20 peripheral blood samples or 14 bone marrow samples from healthy donors (P = .001) (Figure1). SPAN-Xb gene expression was observed regardless of the sex of the patients. One PCR product from each disease type was randomly picked for sequence analysis. In all 4 disease types, nucleotide analysis confirmed SPAN-Xb sequence with no evidence of nucleotide or sequence mutation. Because there was a BglI internal restriction site within the SPAN-Xb cDNA but not within SPAN-Xa or CTp11, we also used restriction digest withBglI to determine whether the PCR products in all the positive specimens were in fact SPAN-Xb and not the closely related genes of SPAN-Xa and CTp11. Unlike testicular PCR products in which there was only partial digest of the DNA, suggesting the possible coamplification of SPAN-Xa or CTp11, all positive specimens completely digested with BglI enzymes (Figure2). These results therefore indicate the aberrant expression of SPAN-Xb in MM and other hematologic malignancies. In this sample of limited size, the expression of theSPAN-Xb gene did not appear to correlate with the stage of the disease because positive results were equally distributed between specimens obtained from patients in early- and late-stage disease. In addition, the SPAN-Xb gene was also equally distributed between patients with newly diagnosed disease and patients who experienced disease relapse following therapy.
RT-PCR analysis of the SPAN-Xb gene in patients with hematologic malignancy using a pair of sequence-specific primers.
SPAN-Xb mRNA could be detected only in patients with hematologic malignancy and not in the peripheral blood or bone marrow of healthy donors. Lane 1, peripheral blood from a healthy donor; lane 2, bone marrow from a healthy donor; lanes 3-6, tumor cells from patients with AML (lane 3), CLL (lane 4), CML (lane 5), and MM (lane 6); lane 7, normal testis RNA; lane 8, positive control amplification using a plasmid containing SPAN-Xb cDNA. M indicates molecular marker; lane a, PCR of DNase I-treated RNA (ie, PCR without RT); lane b, PCR of RNA that underwent RT; and lane c, control amplification for β-actin gene fragment.
RT-PCR analysis of the SPAN-Xb gene in patients with hematologic malignancy using a pair of sequence-specific primers.
SPAN-Xb mRNA could be detected only in patients with hematologic malignancy and not in the peripheral blood or bone marrow of healthy donors. Lane 1, peripheral blood from a healthy donor; lane 2, bone marrow from a healthy donor; lanes 3-6, tumor cells from patients with AML (lane 3), CLL (lane 4), CML (lane 5), and MM (lane 6); lane 7, normal testis RNA; lane 8, positive control amplification using a plasmid containing SPAN-Xb cDNA. M indicates molecular marker; lane a, PCR of DNase I-treated RNA (ie, PCR without RT); lane b, PCR of RNA that underwent RT; and lane c, control amplification for β-actin gene fragment.
Restriction digest of the PCR products.
Normal testis (lane 1), AML (lane 2), CML (lane 3), CLL (lane 4), and MM (lane 5) with BglI enzyme showing the specificity of the PCR products. M indicates molecular marker; a, mock digestion; and b,BglI digestion.
Restriction digest of the PCR products.
Normal testis (lane 1), AML (lane 2), CML (lane 3), CLL (lane 4), and MM (lane 5) with BglI enzyme showing the specificity of the PCR products. M indicates molecular marker; a, mock digestion; and b,BglI digestion.
We previously reported the expression of Sp177 and the lack of coexpression of Sp17 with another CTA,MAGE-C1,14 in MM. Hence, we next determined whether SPAN-Xb was coexpressed with Sp17 in MM because CTA coexpression may provide the opportunity for the design of a polyvalent CTA vaccine for this disease. Using an RT-PCR method described previously,7 we found that Sp17 was in fact coexpressed in 6 of 6 SPAN-Xb+ samples from patients with MM. The expression of SPAN-Xb was, however, not solely linked to Sp17 because Sp17 transcripts were also detected in 3 of 20 SPAN-Xb− MM samples.
SPAN-Xb Is a CT antigen
Because tissue specificity is a vital consideration in the choice of an antigen for immunotherapy, we proceeded to determine the expression of SPAN-Xb transcripts in total RNA derived from a panel of normal tissues. By RT-PCR, we demonstrated that SPAN-Xb mRNA showed restricted normal tissue expression and that the transcripts were detected only in normal testis (Figure3). These results confirm that SPAN-Xb is a CT antigen.
RT-PCR analysis.
Analysis of RNA from a panel of normal tissue showing SPAN-Xb transcripts in only normal testis.
RT-PCR analysis.
Analysis of RNA from a panel of normal tissue showing SPAN-Xb transcripts in only normal testis.
Antibodies against SPAN-Xb can be detected in patients with MM and other hematologic malignancies
Although SPAN-Xb mRNA is frequently detected in patients with MM and other hematologic malignancies, it remained to be determined whether the mRNA was translated into SPAN-Xb protein that could be targeted by a tumor vaccine. An indirect method to support the in vivo translation of SPAN-Xb mRNA to protein is to determine whether antibodies against SPAN-Xb can be detected in these patients because immune responses against a protein only occur after antigenic stimulation by the protein. To determine whether SPAN-Xbgene expression was associated with the development of immune responses against SPAN-Xb protein, we first generated a recombinant SPAN-Xb protein that would be suitable for use in ELISA and Western blot analysis. We isolated and amplified the full-length coding cDNA sequence of SPAN-Xb from normal testicular RNA and expressed the recombinant protein in E coli as a fusion protein of SPAN-Xb with an N-terminal 6-His tag. After protein induction with isopropylthioglucose (IPTG), the recombinant protein was confirmed by SDS-PAGE followed by Coomassie blue staining and Western blotting. The recombinant protein was highly insoluble and was, therefore, dissolved in urea solution. Recombinant SPAN-Xb protein displayed a molecular size of approximately 20 kDa and was visualized in Western blot analysis by anti-His tag antibody (Figure4). Coomassie blue staining of the gel indicated that the purified recombinant protein was more than 95% pure (Figure 4).
Successful generation of SPAN-Xb recombinant protein fromE coli.
(A) Coomassie blue staining of a 12% SDS-polyacrylamide gel, under reduced conditions, showing purities of different aliquots of recombinant SPAN-Xb protein. (B) Western blot analysis of different aliquots of recombinant SPAN-Xb protein using anti-His tag antibodies, confirming the successful generation of recombinant SPAN-Xb protein. M indicates protein marker; lanes 1-2, recombinant protein from 2 different fractions of the eluates; lane 3, lysate of flow-through from washing; lane 4, lysate after passage through affinity column; lanes 5-6, different aliquots of recombinant E coli lysates; lane 7, lysate after 4 hours of protein induction; and lane 8, lysate at time 0 of protein induction.
Successful generation of SPAN-Xb recombinant protein fromE coli.
(A) Coomassie blue staining of a 12% SDS-polyacrylamide gel, under reduced conditions, showing purities of different aliquots of recombinant SPAN-Xb protein. (B) Western blot analysis of different aliquots of recombinant SPAN-Xb protein using anti-His tag antibodies, confirming the successful generation of recombinant SPAN-Xb protein. M indicates protein marker; lanes 1-2, recombinant protein from 2 different fractions of the eluates; lane 3, lysate of flow-through from washing; lane 4, lysate after passage through affinity column; lanes 5-6, different aliquots of recombinant E coli lysates; lane 7, lysate after 4 hours of protein induction; and lane 8, lysate at time 0 of protein induction.
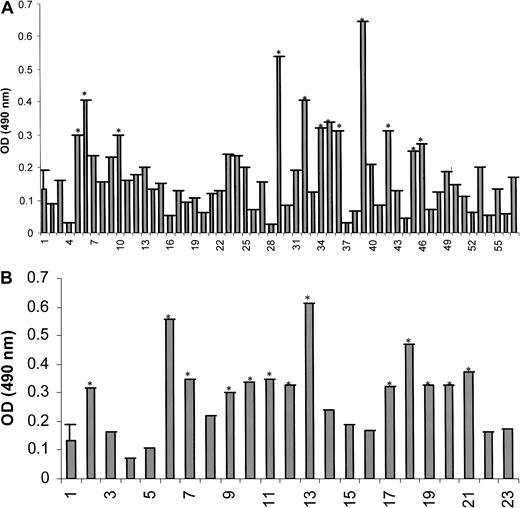
The purified SPAN-Xb protein was then used in an ELISA system to detect circulating antibodies against SPAN-Xb in the sera of patients with MM and other hematologic malignancies. We first established the basal signals in the ELISA system using sera from 24 healthy donors (mean ± SD at OD490nm = 0.1325 ± 0.059). Using the mean ± 2 SD from these 24 sera as the cutoff signal intensity at OD490nm, we found that high-titer IgG antibodies against SPAN-Xb protein were frequently detectable by ELISA in patients with MM and hematologic malignancies (Figure 5A-B). IgG antibodies against SPAN-Xb protein were detected by ELISA at a serum dilution of 1:1000 in 9 (20%) of 42 MM, 12 (60%) of 20 CML, 3 (33%) of 9 CLL, and 1 of 2 AML serum samples. These sera were tested in high dilution of 1:1000 to improve the specificity of the antibodies. In contrast, none of the sera from the 24 healthy donors were positive for SPAN-Xb antibodies. Binding of the antibodies from these sera to SPAN-Xb protein was specific because no signal was observed in any of the samples in the 2 sets of control wells in the ELISA system (all showed signal intensities at OD490nm of less than 0.010).
To further confirm the presence of SPAN-Xb antibodies, we carried out Western blot analysis applying these sera to the recombinant SPAN-Xb protein or to a control recombinant 6-His fusion protein also derived from E coli. Not surprisingly, because Western blot analysis is less sensitive than ELISA, not all ELISA-positive samples produced positive signals in Western blot analysis (Table 1) (Figure6). However, all ELISA-negative samples produced negative signals in Western blot analysis. Based on the results of ELISA, the proportions of patients with detectable IgG antibodies against SPAN-Xb in these diseases correlated with the proportions of PCR-positive samples.
Western blot (WB) analysis of serum samples
| Diagnosis, no. analyzed . | ELISA+/WB+ . | ELISA+/WB− . | ELISA−/WB+ . | ELISA−/WB− . |
|---|---|---|---|---|
| AML, 1 | 1 | 0 | 0 | 0 |
| CLL, 5 | 2 | 0 | 0 | 3 |
| CML, 13 | 6 | 6 | 0 | 1 |
| MM, 7 | 3 | 4 | 0 | 0 |
| Donor, 6 | 0 | 0 | 0 | 6 |
| Diagnosis, no. analyzed . | ELISA+/WB+ . | ELISA+/WB− . | ELISA−/WB+ . | ELISA−/WB− . |
|---|---|---|---|---|
| AML, 1 | 1 | 0 | 0 | 0 |
| CLL, 5 | 2 | 0 | 0 | 3 |
| CML, 13 | 6 | 6 | 0 | 1 |
| MM, 7 | 3 | 4 | 0 | 0 |
| Donor, 6 | 0 | 0 | 0 | 6 |
ELISA analysis of diluted sera (1:1000) showing the presence of antibodies directed at SPAN-Xb in patients with hematologic malignancies but not in healthy donors.
(A) ELISA for SPAN-Xb antibodies in patients with lymphoproliferative disorders (sample 1, mean ± 2 SD from 24 healthy donors; samples 2-45, MM; samples 46-57, CLL). (B) ELISA for SPAN-Xb antibodies in patients with myeloproliferative disorders (sample 1, mean ± 2 SD from 24 healthy donors; samples 2-21, CML; samples 22-23, AML). *Samples with signals in excess of mean ± 2 SD from 24 healthy donors.
ELISA analysis of diluted sera (1:1000) showing the presence of antibodies directed at SPAN-Xb in patients with hematologic malignancies but not in healthy donors.
(A) ELISA for SPAN-Xb antibodies in patients with lymphoproliferative disorders (sample 1, mean ± 2 SD from 24 healthy donors; samples 2-45, MM; samples 46-57, CLL). (B) ELISA for SPAN-Xb antibodies in patients with myeloproliferative disorders (sample 1, mean ± 2 SD from 24 healthy donors; samples 2-21, CML; samples 22-23, AML). *Samples with signals in excess of mean ± 2 SD from 24 healthy donors.
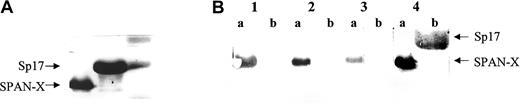
Western blot analysis to determine the specificity of the anti–SPAN-Xb antibodies.
(A) Coomassie blue staining of a 12% SDS-polyacrylamide gel showing the loading of SPAN-Xb recombinant protein and a control recombinant protein Sp17 (M indicates protein marker; lane a, SPAN-Xb; and lane b, Sp17). (B) Western blot analysis using diluted sera (1:1000) showing the presence of antibodies directed at SPAN-Xb and not Sp17 in patients with hematologic malignancies. Lane 1 indicates serum from patient with CML; lane 2, serum from patient with CLL; lane 3, serum from patient with AML; and lane 4, anti–6-His tag monoclonal antibodies. (Lane a, SPAN-Xb recombinant protein; lane b, Sp17 recombinant protein.)
Western blot analysis to determine the specificity of the anti–SPAN-Xb antibodies.
(A) Coomassie blue staining of a 12% SDS-polyacrylamide gel showing the loading of SPAN-Xb recombinant protein and a control recombinant protein Sp17 (M indicates protein marker; lane a, SPAN-Xb; and lane b, Sp17). (B) Western blot analysis using diluted sera (1:1000) showing the presence of antibodies directed at SPAN-Xb and not Sp17 in patients with hematologic malignancies. Lane 1 indicates serum from patient with CML; lane 2, serum from patient with CLL; lane 3, serum from patient with AML; and lane 4, anti–6-His tag monoclonal antibodies. (Lane a, SPAN-Xb recombinant protein; lane b, Sp17 recombinant protein.)
Given that the results of restriction digest with BglI indicated the selective expression of SPAN-Xb and not SPAN-Xa or CTp11, our results support the in vivo immunogenicity of SPAN-Xb protein in patients with MM and other hematologic malignancies. Because SPAN-Xb mRNA was detected in these patients, the presence of IgG antibodies suggests the in vivo translation of SPAN-Xb mRNA into SPAN-Xb protein that was capable of eliciting B-cell responses in patients with cancer.
To determine the nature of the B-cell responses, we determined the IgG subclass of the SPAN-Xb antibodies from 22 patients (7 MM, 12 CML, 2 CLL, and 1 AML) using subclass-specific antihuman globulins in the ELISA system. We found that SPAN-Xb antibodies were either of IgG2 or IgG3 subclass (Table 2). No IgG1 or IgG4 was detected.
Correlation between gene expression and immune response
In 19 patients (2 AML, 9 CLL, 7 CML, and 1 MM), paired tumor samples and sera were available to determine the correlation betweenSPAN-Xb gene expression (GE) and B-cell immune response (IR) within individual patients. There was a close correlation between GE and IR, except in 2 patients with CML—one was GE+ without IR and the other was IR+ without GE (Table 3).
Discussion
As a result of the molecular events often associated with the malignant transformation of normal cells, neoantigens are produced from gene amplification, mutation, or the formation of fusion genes that arise from chromosomal translocation. These gene products are potentially immunogenic and may serve as targets of tumor vaccines. In addition to neoantigens, normal proteins that are aberrantly expressed in tumor cells are also candidates for immunotherapy. In particular, normal testicular proteins aberrantly expressed on tumor cells are excellent targets. As a result of their being from an immune-privileged site, these proteins are normally highly immunogenic because the immune effector cells reactive against them are not subjected to thymic selection. Furthermore, because of the restricted normal tissue distribution, immunotherapy targeting testicular proteins is expected to be safe and without any nonspecific side effects. To isolate CTAs, various strategies have been investigated. They include applying patient sera to screen expression cDNA libraries constructed from either tumor RNA4 or normal testicular RNA.15Identification and characterization of these proteins and immune responses against the proteins are appropriate.
Because of the extreme labor intensity of plaque screening in SEREX, we have recently adopted an approach that relies on screening the GenBank database for testicular-specific proteins and then using the available bio-informatics to predict the immunogenicity of these proteins in vivo. Through this approach, another CTA, SPAN-Xb, has been identified for MM and other hematologic malignancies. The gene encoding SPAN-Xb is localized to Xq27.1 by fluorescence in situ hybridization,13 and the gene product is a spermatid-specific nuclear protein. It shows high degrees of homology at the nucleotide level (93%) and the protein level (86%) to CTp11, which is 98% and 97% homologous to the SPAN-Xa nucleotide and peptide sequences, respectively.16 CTp11 is aberrantly expressed by cancer cells from patients with melanoma and with kidney, prostate, and bladder cancer.17 However, the expression of CTp11, SPAN-Xa, or SPAN-Xb in hematologic malignancies had never been investigated. Our results indicate that SPAN-Xb transcripts are frequently aberrantly expressed in MM and other hematologic malignancies. The specificity of our findings was confirmed by nucleotide sequence analysis and restriction enzyme digest of the PCR products. Although the mechanisms leading to the aberrant expression of CTAs, such as SPAN-Xb, are unclear, it is likely a result of promoter demethylation that so often occurs in association with malignant cell transformation.18
SPAN-Xb expectedly demonstrates a restricted normal tissue expression, as determined by RT-PCR analysis. Therefore, provided the transcripts encoding SPAN-Xb are translated to an immunogenic protein, SPAN-Xb could be a potential target for immunotherapy of these diseases. Our work with Sp177 9-12 illustrates the immunogenicity and translational potential of aberrantly expressed normal testicular proteins in these diseases.
Given that previous works by us3 and others8demonstrated the ability of tumor-derived proteins to elicit B-cell immune responses in the autologous hosts in hematologic malignancies, we proceeded to determine whether high-titer IgG against SPAN-Xb could be detected in these patients. To do this, we first generated a SPAN-Xb recombinant protein from E coli. This was achieved through the cloning of the SPAN-Xb cDNA as a fusion gene to produce a recombinant SPAN-X protein that contained a 6-His tag at the N-terminal of the fusion protein. This strategy facilitated the purification of the recombinant protein by affinity column. We successfully generated the recombinant fusion protein from E coli. Surprisingly, the recombinant protein displayed on SDS-PAGE a molecular weight of approximately 20 kDa. This is identical to that obtained by an expression system using eukaryotic cells,16 although the open reading frame (ORF) predicts the protein to be approximately 11 kDa. The discrepancy in the molecular weight of recombinant SPAN-Xb protein was previously attributed to a possible posttranslational glycosylation of the protein.16 However, the recombinant protein we produced from a prokaryotic expression system was still larger than the predicted molecular weight, even though prokaryote-derived proteins are usually not glycosylated. Therefore, we suggest that the apparently higher molecular weight of the protein on SDS-PAGE may be attributed to the poorly insoluble nature of the protein. SPAN-Xb recombinant protein is bacteria derived. To exclude immune responses caused by contaminating bacterial antigens that might have been present in the recombinant protein preparation, we included in all experiments a control recombinant 6-His fusion protein that was also prepared from the E coli lysate.
Using the recombinant protein in ELISA and Western blot analysis, B-cell responses, in the form of high-titer IgG, against SPAN-Xb protein occur frequently in patients with MM and other hematologic malignancies and not in healthy donors. Sequence analysis of the PCR products from these malignant cells did not show any mutation within the SPAN-Xb cDNA. Therefore, the immune responses were directed at the wild-type SPAN-Xb protein. Because immune responses against a protein only occur after exposure to the protein, we infer that SPAN-Xb transcripts expressed in tumor cells were likely translated to SPAN-X protein. Interestingly, the proportions of patients expressing theSPAN-Xb gene were nearly the same as the proportions of patients with detectable antibodies against SPAN-Xb. These results further support the notion that SPAN-Xb is not only expressed at the mRNA level but is also translated to its protein in vivo. In addition, they also support the in vivo immunogenicity of the protein in patients with cancer. Most important, because B-cell immune responses to an antigen, including tumor antigens such as NY-ESO-1, are usually generated only if the antigen is also immunogenic to T cells, the presence of high-titer IgG responses in these patients suggests that SPAN-Xa is potentially a target for T-cell–based immunotherapy of these malignant diseases.
The presence of in vivo immune responses against SPAN-Xb protein does not necessarily suggest antitumor effects. To further characterize the nature of the immune responses, we determined the SPAN-Xb IgG subclasses in these patients. Surprisingly, 2 distinct types of immune responses were observed: IgG2 antibodies suggestive of a Th2-type immune response and IgG3 antibodies suggestive of a Th1-type immune response. The distribution of the IgG subclass did not appear to be disease related because it was equally divided among patients of different disease types.
Although mRNA level generally correlates with protein level, exceptions frequently occur, ranging from total lack of translation, despite an abundance of mRNA, to high levels of protein without significant up-regulation of mRNA.19 Such discrepancies may explain the results we obtained in one CML patient in whom the SPAN-Xb transcript was not detected by RT-PCR but in whom antibodies against SPAN-Xb protein were present. The close correlation observed betweenSPAN-Xb gene expression and immune responses suggests the in vivo translation of the SPAN-Xb gene in these diseases. Three of 4 patients with gene expression mounted antibody responses against SPAN-Xb protein. The ability to mount immune responses to an antigen depends on numerous factors, including the patient's age, HLA-type, and concurrent medications such as immunosuppressive agents. Therefore, any one of these factors could have led to the lack of immune responses observed in the one CML patient despiteSPAN-Xb gene expression. In contrast, neither gene expression nor immune response was detected in any of the healthy donors.
In conclusion, SPAN-Xb is another novel CTA in MM and other hematologic malignancies. Its gene expression in these diseases is associated with the frequent development of antibody responses against SPAN-Xb protein. Although SPAN-Xb is a nuclear-associated protein and will not be amenable to targeting by monoclonal antibodies, SPAN-Xb could be a target for T cells because most high-titer IgG B-cell responses occur with cognitive help from T cells. Interestingly, despite the frequent presence of circulating high-titer IgG against SPAN-Xb, it has not been previously isolated using conventional SEREX approaches in CML3 8 or MM (S.H.L. et al, unpublished data, 2001), suggesting that careful use of bio-informatics could complement the other antigen isolation methods currently used.
We thank Bart Barlogie (University of Arkansas for Medical Sciences, Little Rock) for providing some of the samples from patients with multiple myeloma used in the present study.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-06-1930.
Supported by grants from the National Institutes of Health/National Cancer Institute (RO1 CA88434), the Cancer Treatment Research Foundation, and the Mary Kay Ash Charitable Foundation.
Z.W. and Y.Z. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Seah H. Lim, Biotherapy and Stem Cell Transplant Program, Don and Sybil Harrington Cancer Center, 1500 Wallace Blvd, Amarillo, TX 79106; e-mail:slim@harringtoncc.org.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal