Abstract
The differentiation of eosinophils from hematopoietic precursors and their subsequent maturation, chemotaxis, and activation is primarily regulated by interleukin-5 (IL-5). To examine the effect of chronic IL-5 exposure on hematopoiesis, IL-5 transgenic (IL-5trg) mice and wild-type BALB/c (WT) mice were examined. In comparison to WT mice, a significant alteration in bone marrow hematopoiesis was observed in IL-5trg mice. Although the total number of myeloid progenitors in the bone marrow of IL-5trg mice was not significantly altered, the number of long-term culture-initiating cells (LTC-ICs) was 1.5-fold lower than that observed in WT mice. Furthermore, IL-5trg mice failed to demonstrate hematopoietic activity in long-term bone marrow cultures, which correlated with a significant decrease in the number of bone marrow mesenchymal/stromal progenitor (MSP) cells in these mice. In comparison to WT mice, a 10-fold decrease was observed in the number of fibroblast colony-forming units (CFU-Fs) in IL-5trg bone marrow. Hematopoietic activity of IL-5trg bone marrow cells was rescued by cultivation on preestablished layers of bone marrow-derived stromal cells. However, in contrast to bone marrow, increased hematopoietic activity was observed in the spleen and peripheral blood of IL-5trg mice. Likewise, the numbers of LTC-ICs and granulocyte-macrophage, macrophage, eosinophil, B-lymphocyte progenitors in the peripheral blood and spleen of IL-5trg mice were approximately 20-fold higher than in WT mice. A significant increase in CFU-F numbers was also observed in the spleens of IL-5trg mice compared with WT mice. Overall, our results suggest that constitutive overexpression of IL-5 can potentially induce colonization of spleen with MSP cells, which provides the necessary microenvironment for establishment of hematopoiesis in extramedullary sites.
Introduction
Several cytokines, including interleukin-3 (IL-3), IL-5, and granulocyte macrophage colony-stimulating factor (GM-CSF), participate in the generation and maturation of eosinophils in the bone marrow. Among these, IL-5 is the major cytokine involved in the generation and survival of eosinophils.1-3 IL-5 plays at least 2 roles in the generation of blood-borne eosinophils. It stimulates bone marrow CD34+ progenitor cells to differentiate into eosinophils as well as induces the mobilization of eosinophils from the bone marrow into the bloodstream.1,2,4,5 The release of mature eosinophils from the bone marrow is a multistep process, involving the detachment of eosinophils from the bone marrow microenvironment, the migration of eosinophils across the bone marrow sinus endothelium, and the release of eosinophils from the luminal surface of the endothelium.6 Once released, eosinophils traffic freely through the bloodstream and recruit to sites of allergic inflammation.7,8 During episodes of allergen challenge, increased numbers of mature eosinophils and IL-5–sensitive progenitors yielding eosinophil-bearing colonies have been observed in experimental mice. Likewise, intraperitoneal administration of IL-5 leads to profound eosinophilia in mice, whereas treatment with monoclonal antibodies (mAbs) against IL-5 receptor (IL-5R)9or IL-510 leads to normalization of elevated eosinophilia. In a number of human studies, increased expression of IL-5 is associated with an elevated number of CD34+eosinophil-basophil progenitors (Eos/B-CFUs) as well as immature and mature eosinophils in the bone marrow and peripheral blood.11 Furthermore, chronic exposure to IL-5 resulted in up-regulation of IL-5Rα expression on CD34+/45+ progenitor cells followed by their emigration from the bone marrow.11 Although these studies demonstrate the importance of IL-5 in eosinophilia, it is not clear whether bone marrow alone, or establishment of extramedullary sites of hematopoiesis, is likely to play an important role in IL-5–induced eosinophilopoiesis. For instance, in addition to increased number of IL-5–induced eosinophil progenitors and CD34+ cells in the bone marrow of patients with idiopathic hypereosinophilic syndrome (HES), allergic inflammation, and parasitic diseases, as well as allergen-challenged, parasitized, and IL-5 transgenic animals,10,12-17 extramedullary foci of IL-5–induced eosinophilopoiesis have also been described.4,18,19 The extramedullary hematopoietic foci were of the eosinophil lineage and contained, in addition, a large number of proliferating hematopoietic stem/progenitor cells (HSPCs).18 However, the cellular mechanisms involved in extramedullary hematopoiesis have not been well characterized.
We have attempted to study the generation and mobilization of eosinophil progenitors to extramedullary sites as a pathophysiologic mechanism underlying the systemic response to overexpression of IL-5. We observed that constitutive overexpression of IL-5 under the regulation of the T-cell promoter CD2 by IL-5trg BALB/c mice leads to mobilization of mesenchymal/stromal progenitor (MSP) cells from the bone marrow to the spleen. By providing a hematopoietic-supportive microenvironment, MSP cells attract HSPCs, resulting in establishment of extramedullary sites of hematopoiesis. Our observations suggest that in diseases associated with chronic exposure to elevated levels of IL-5, eosinophils can potentially also be generated outside of the bone marrow by the migrating progenitor cells.
Materials and methods
Mice
Age- and sex-matched BALB/c (WT) mice were obtained from Jackson Laboratory and maintained under standard laboratory conditions. IL-5trg mice (under the regulation of the CD2 promoter on a CBA background) were backcrossed into the BALB/c background for 10 generations and used in the present study. Animals up to the age of 6 months were used in all experiments.
Cells
Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (Walkersville, MD) and cultured according to the manufacturer's instructions. S17, a bone marrow-derived stromal cell line (kindly provided by Dr Helmut Ponta, Institute for Genetics, Forshungszentrum Karlsruhe, Germany), was cultured in RPMI supplemented with 10% fetal calf serum (FCS).20 To obtain bone marrow cells, femurs and tibias were dissected from killed mice, and the marrow was flushed out with RPMI supplemented with 5% FCS. Bone marrow cells were washed and maintained on ice until use. To prepare a suspension of spleen cells, spleens were removed from mice and rubbed through cotton tissue to ensure a single cell suspension. Recovered cells were aspirated through a 25G needle, washed twice, and maintained on ice until use. Peripheral blood was obtained from IL-5trg and WT mice to determine differential leukocyte counts, which were determined by staining with Wright-Giemsa dye (Fisher Scientific, Pittsburgh, PA), and values are expressed as percentage of total cells. The number of MSP cells derived from bone marrow cells was evaluated by using the colony-forming unit (CFU) culture technique originally described by Friedenstein et al21 and is detailed in “CFU assay.”
Long-term bone marrow cultures (LTBMCs)
Myeloid LTBMCs were established as described.22 In brief, freshly isolated bone marrow cells (106 cells/mL) were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 20% horse serum (StemCell Technologies, Vancouver, BC, Canada) and 10−6 M hydrocortisone (Sigma, St Louis, MO) in 6-well plates at 37°C in a humid atmosphere containing 5% CO2. Cultures were fed weekly by changing half of the culture medium. Nonadherent cells were collected from the culture medium, counted, and assayed for CFUs. Viability of cells was monitored by trypan blue exclusion. Only cells with more than 99% viability were used for all experiments.
CFU assay
Bone marrow cells from mice or nonadherent cells from LTBMCs were plated on semisolid methylcellulose supplemented with 30% FCS, 1% bovine serum albumin (BSA), 10−4 M 2-mercaptoethanol (ME), and 2 mM L-glutamine (StemCell Technologies). For CFU-granulocyte-erythrocyte-megakaryocyte-macrophage (GEMM) or CFU-granulocyte-macrophage (GM), cells were cultured at a concentration of 104 cells/mL in the presence of stem cell factor (SCF), IL-6, and erythropoietin (Epo), or 10 ng/mL GM-CSF, respectively. For CFU-Eos assay, cells were cultured at a concentration of 5 × 105 cells/mL in the presence of 50 ng/mL IL-5, whereas cells for CFU-B assay were grown at a concentration of 5 × 104 cells/mL in the presence of IL-7. To evaluate the number of CFU-Fs, cells at a concentration of 106/mL were cultured in Dexter conditions. Plates were cultured at 37°C in 5% CO2 atmosphere. Colonies were counted under the microscope after 7 days of culture.
Long-term-culture initiating cell assay (LTC-IC)
The assay was performed according to manufacturer's instruction (StemCell Technology). Briefly, cell populations to be examined were plated in DMEM supplemented with 20% horse serum and 10−6M hydrocortisone in limiting dilution into 96-well plates containing the stromal cell line S17. Cultures were fed weekly, and the number of wells containing colonies was evaluated after 14 days of culture.23
HSPC isolation
HSPCs were isolated by using a kit (StemCell Technology) containing a cocktail of purified biotinylated mAbs directed against CD2, CD45R, Ter119, and F4/80 designed to be used with antibiotin antidextran tetrameric antibody complexes and purified by magnetic cell separation. Briefly, bone marrow cell suspensions were incubated with first antibody cocktail for 15 minutes at 4°C. After washing, bone marrow cells were incubated with biotin tetramers for 15 minutes followed by incubation with magnetic beads for 15 minutes. Thereafter, cells were applied to a magnetic column. The unbound cells were collected, washed, and assayed.
Transwell migration assay
The assay was performed in duplicate by using 5-μm pore filters (Transwell 24-well cell clusters; Costar, Boston, MA). HUVECs were grown to 100% confluency on filters in HUVEC supportive medium (Clonetics). HSPCs (105) suspended in 100 μL migration medium (Ultraculture with 0.5% BSA) were loaded into the upper well of each Transwell filter chamber. Thereafter, filters were transferred into the lower wells containing 600 μL migration medium supplemented with 10 μg stromal-derived factor (SDF-1). The plates were incubated at 37°C in 5% CO2. After 4 hours of incubation, the upper wells were carefully removed, and the cells in the lower wells were collected and assayed for the presence of CFUs.
Flow cytometry
The cell surface expression of murine CXCR4 on HSPCs was determined by flow cytometry. Briefly, 5 × 105 cells were incubated with specific antibody (Research Diagnostics, Flanders, NJ) at a concentration of 10 μg/mL for 30 minutes at 4°C and washed with fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS], 2% FCS, 0.1% BSA, 0.01% NaN3) 2 times. Thereafter, cells were incubated with fluorescein isothiocyanate (FITC)–labeled secondary antibody for 30 minutes. Fluorescence was analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) according to standard procedures.
Statistical analysis
The results are expressed as the mean ± SD (error bars). Statistical significance was determined by using the Studentt test.
Results
LTBMCs from IL-5trg mice demonstrate weak hematopoietic activity
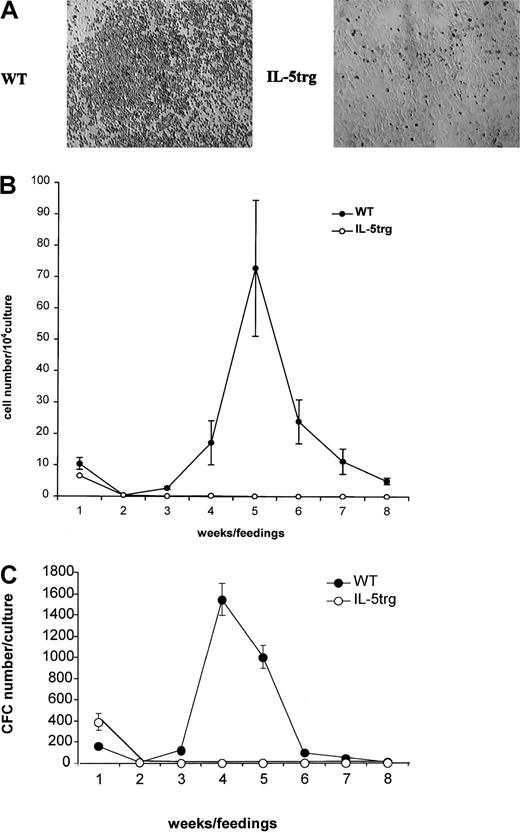
To determine the effect of chronic exposure to IL-5 on bone marrow hematopoiesis, myeloid LTBMCs from IL-5trg and WT BALB/c mice (up to 6 months of age) were established. Although LTBMCs from IL-5trg mice formed a confluent adherent layer, they failed to develop any areas of active hematopoiesis (“cobblestone areas”) (Figure1A). In contrast, LTBMCs established from WT mice demonstrated active hematopoiesis with well-formed cobblestone areas (Figure 1A). The production of nonadherent cells in LTBMCs, which contain a mixed population of mature cells and their hematopoietic progenitors at different stages of differentiation, is representative of the hematopoietic activity in LTBMCs. The number of nonadherent cells and hematopoietic progenitors produced in IL-5trg LTBMCs was significantly lower in comparison to LTBMCs from WT mice (P < .05; Figure 1B-C). These results are suggestive of insufficient hematopoiesis in IL-5trg LTBMCs. The lack of hematopoietic activity in IL-5trg LTBMCs could be a result of the decreased number of HPSCs maintained in LTBMCs from IL-5trg mice. Therefore, we examined the frequency of LTC-ICs in the adherent layer of IL-5trg and WT LTBMCs in the limiting dilution assay. Although no LTC-ICs were detected in the adherent layer of IL-5trg LTBMCs after 4 weeks of culture, frequency of LTC-ICs in 4-week LTBMCs established from WT mice was 40 ± 15 per culture (Figure 2A). These data suggest that IL-5trg bone marrow is either deficient in LTC-ICs or in cells that form hematopoietic-supportive microenvironment when cultured in vitro. Therefore, we next examined the number of hematopoietic and mesenchymal progenitors in the bone marrow of IL-5trg mice.
Hematopoietic activity of IL-5trg bone marrow in vitro.
LTBMCs (n = 3 per group) were established from IL-5trg and WT mice. (A) Photographs of adherent layers from IL-5trg and WT LTBMCs were taken after 4 weeks of culture. Areas of hematopoiesis are evident in WT but not in IL-5trg LTBMCs. Magnification ×10. (B) Half of the culture medium was harvested from LTBMCs after each feeding, and the number of nonadherent cells was calculated and expressed as mean ± SD. (C) Nonadherent cells harvested from IL-5trg and WT LTBMCs were plated at a concentration of 1 × 104 cells/mL in the presence of GM-CSF (10 ng/mL). After 7 days of culture, the number of colonies was counted, recalculated for the total number of nonadherent cells recovered from LTBMCs, and expressed as mean ± SD of the number of CFCs.
Hematopoietic activity of IL-5trg bone marrow in vitro.
LTBMCs (n = 3 per group) were established from IL-5trg and WT mice. (A) Photographs of adherent layers from IL-5trg and WT LTBMCs were taken after 4 weeks of culture. Areas of hematopoiesis are evident in WT but not in IL-5trg LTBMCs. Magnification ×10. (B) Half of the culture medium was harvested from LTBMCs after each feeding, and the number of nonadherent cells was calculated and expressed as mean ± SD. (C) Nonadherent cells harvested from IL-5trg and WT LTBMCs were plated at a concentration of 1 × 104 cells/mL in the presence of GM-CSF (10 ng/mL). After 7 days of culture, the number of colonies was counted, recalculated for the total number of nonadherent cells recovered from LTBMCs, and expressed as mean ± SD of the number of CFCs.
Hematopoietic and mesenchymal progenitors in IL-5trg bone marrow.
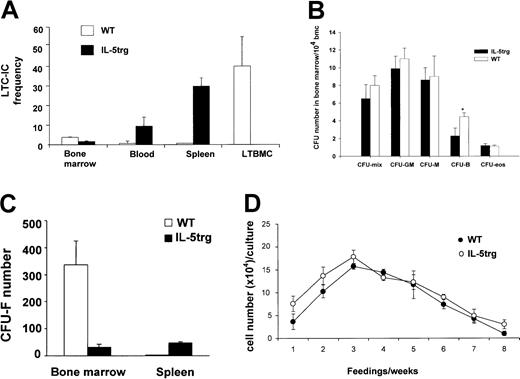
(A) Bone marrow, spleen, and peripheral blood cells were harvested from IL-5trg and WT mice (n = 5) and assayed for numbers of LTC-ICs in the limiting dilution assay in 3 independent experiments. Adherent layers from IL-5trg and WT LTBMCs (n = 3) were harvested after 4 weeks of culture and assayed for numbers of LTC-ICs. The LTC-IC frequency in the bone marrow (per 106 bone marrow cells), blood (per 1 mL), spleen (per whole organ), and LTBMC (per whole culture) from one representative experiment is shown as mean ± SD. (B) Bone marrow cell suspensions from IL-5trg and WT mice were plated into methylcellulose cultures in the presence of lineage-specific cytokines. GM-CSF or M-CSF was added to estimate numbers of granulocyte-macrophage– or macrophage-CFCs, and IL-5 (50 ng/mL) or IL-7 was added to estimate the numbers of eosinophil or B-lymphoid progenitors (*P < .01). Colonies were cultured in quadruples, calculated after 7 days of culture, and expressed as mean ± SD. (C) Bone marrow or spleen cells from IL-5trg and WT mice were cultured in Dexter media for 7 days (n = 3). Number of stromal cell colonies (CFU-Fs) was calculated under the microscope and expressed as mean ± SD of the number of CFU-F/whole spleen or femur for bone marrow. (D) Bone marrow cells harvested from IL-5trg and WT mice were plated on a 75% confluent layer of bone marrow-derived stromal cells. Half of the culture medium was harvested from the cultures at each feeding, and the number of nonadherent cells was calculated and expressed as mean ± SD.
Hematopoietic and mesenchymal progenitors in IL-5trg bone marrow.
(A) Bone marrow, spleen, and peripheral blood cells were harvested from IL-5trg and WT mice (n = 5) and assayed for numbers of LTC-ICs in the limiting dilution assay in 3 independent experiments. Adherent layers from IL-5trg and WT LTBMCs (n = 3) were harvested after 4 weeks of culture and assayed for numbers of LTC-ICs. The LTC-IC frequency in the bone marrow (per 106 bone marrow cells), blood (per 1 mL), spleen (per whole organ), and LTBMC (per whole culture) from one representative experiment is shown as mean ± SD. (B) Bone marrow cell suspensions from IL-5trg and WT mice were plated into methylcellulose cultures in the presence of lineage-specific cytokines. GM-CSF or M-CSF was added to estimate numbers of granulocyte-macrophage– or macrophage-CFCs, and IL-5 (50 ng/mL) or IL-7 was added to estimate the numbers of eosinophil or B-lymphoid progenitors (*P < .01). Colonies were cultured in quadruples, calculated after 7 days of culture, and expressed as mean ± SD. (C) Bone marrow or spleen cells from IL-5trg and WT mice were cultured in Dexter media for 7 days (n = 3). Number of stromal cell colonies (CFU-Fs) was calculated under the microscope and expressed as mean ± SD of the number of CFU-F/whole spleen or femur for bone marrow. (D) Bone marrow cells harvested from IL-5trg and WT mice were plated on a 75% confluent layer of bone marrow-derived stromal cells. Half of the culture medium was harvested from the cultures at each feeding, and the number of nonadherent cells was calculated and expressed as mean ± SD.
IL-5trg bone marrow is deficient of stromal progenitors
Frequency of LTC-ICs in the bone marrow of IL-5trg mice was 1.5-fold lower in comparison to WT mice (P = .014; Figure2A). The number of bipotent and monopotent myeloid progenitors in IL-5trg bone marrow was not significantly altered (P > .1). We, however, observed a 2-fold decrease in the numbers of B-lymphoid progenitors in IL-5trg bone marrow compared with WT bone marrow (P = .006; Figure 2B). Thus, these data suggest that the lack of hematopoietic activity in IL-5trg LTBMCs is not due to the absence of early hematopoietic progenitors. We next investigated hematopoiesis-supportive microenvironment in the bone marrow of IL-5trg mice. Hematopoiesis-supportive stromal cells that originate from mesenchymal progenitor cells were detected by CFU-Fs. Number of CFU-Fs in IL-5trg bone marrow was 10.5-fold lower in comparison to WT bone marrow (Figure 2C). Low numbers of stromal cells may result in insufficient maintenance of LTC-ICs during development of LTBMCs. Therefore, we cultured IL-5trg bone marrow cells on a preestablished layer of S17 bone marrow–derived stromal cells. As expected, when cultured on hematopoiesis-supportive stroma, IL-5trg bone marrow cells demonstrated significantly improved hematopoietic activity comparable to WT LTBMC cultures (Figure 2D).
Elevated level of IL-5 mobilizes HSPCs from the bone marrow to peripheral blood
We next examined the number of mature and progenitor cells in the peripheral blood of IL-5trg and WT mice. Although the differential counts of eosinophils in peripheral blood of IL-5trg mice were 15 times higher, the percentage of lymphocytes, monocytes, and neutrophils was slightly decreased compared with WT mice (Table1). Our data indicate that the total number of hematopoietic progenitors circulating in the peripheral blood of IL-5trg mice were 10- to 15-fold higher in comparison to WT mice (Table 2). Furthermore, the number of LTC-ICs in peripheral blood of IL-5trg mice was 15.5-fold higher than in WT mice (P < .01) (Figure 2A). These data suggest that the constitutive overexpression of IL-5 results in mobilization of HSPCs from the bone marrow and expansion in the periphery leading to establishment of extramedullary sites of hematopoiesis in mice.
Differential leukocyte count in IL-5trg and WT BALB/c mice
| . | Lymphocytes, % . | Eosinophils, % . | Neutrophils, % . | Monocytes, % . |
|---|---|---|---|---|
| IL-5trg | 53.2 ± 9 | 35.5 ± 7.2 | 8.8 ± 2.2 | 2.4 ± 0.66 |
| WT (BALB/c) | 65.6 ± 8.2 | 2.4 ± 1.6 | 27.6 ± 9.1 | 2.43 ± 0.3 |
| . | Lymphocytes, % . | Eosinophils, % . | Neutrophils, % . | Monocytes, % . |
|---|---|---|---|---|
| IL-5trg | 53.2 ± 9 | 35.5 ± 7.2 | 8.8 ± 2.2 | 2.4 ± 0.66 |
| WT (BALB/c) | 65.6 ± 8.2 | 2.4 ± 1.6 | 27.6 ± 9.1 | 2.43 ± 0.3 |
Peripheral blood cells were obtained from WT or IL-5trg mice (n = 4). The cells were collected by cytospin, fixed, and stained using Wright-Giemsa stain. Differential leukocyte counts are expressed as percentage of total cells.
Hematopoietic progenitors are present in blood of IL-5trg mice
| . | CFU-B . | CFU-GM . | CFU-M . | CFU-eos . | CFU-mix . |
|---|---|---|---|---|---|
| WT | 1 ± 0.1 | 1 ± 0.13 | 1 ± 0.15 | 0 ± 0 | 0 ± 0 |
| IL-5trg | 403 ± 67.6 | 465 ± 78 | 248 ± 41.6 | 434 ± 72.8 | 103 ± 14 |
| P | .02 | .007 | .007 | .005 | < .05 |
| . | CFU-B . | CFU-GM . | CFU-M . | CFU-eos . | CFU-mix . |
|---|---|---|---|---|---|
| WT | 1 ± 0.1 | 1 ± 0.13 | 1 ± 0.15 | 0 ± 0 | 0 ± 0 |
| IL-5trg | 403 ± 67.6 | 465 ± 78 | 248 ± 41.6 | 434 ± 72.8 | 103 ± 14 |
| P | .02 | .007 | .007 | .005 | < .05 |
Blood cells were harvested from IL-5trg and WT mice (n = 4) and plated into methylcellulose cultures in the presence of lineage-specific cytokines. GM-CSF or M-CSF was added to estimate numbers of granulocyte-macrophage- or macrophage-CFC, and IL-5 (50 ng/mL) or IL-7 was added to estimate the numbers of eosinophil or B-lymphoid progenitors. Colonies were cultured in quadruples, calculated after 7 days of culture, and expressed as mean ± SD.
Constitutive overexpression of IL-5 results in establishment of extramedullary sites of hematopoiesis
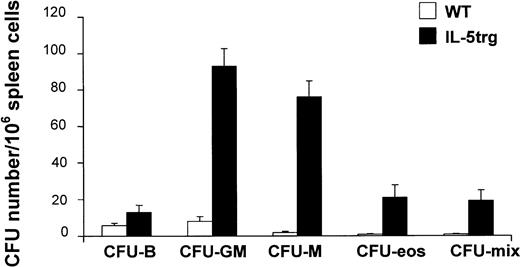
The size of spleens from IL-5trg mice was significantly larger (2- to 3-fold) in comparison to control WT mice (data not shown) and is in agreement with recent observations by Macias et al.24 We observed a 16-fold increase in CFU-F numbers in IL-5trg spleens in comparison to WT mice (P ≤ .01; Figure 2C). The marked increase in stromal progenitors in the spleens of IL-5trg mice was paralleled by a significant increase in the presence of committed hematopoietic progenitors as assessed with the use of a methylcellulose assay. Spleens of IL-5trg mice demonstrated a significant increase (10- to 40-fold) in numbers of CFU-mix, CFU-GM, CFU-M, and CFU-Eos as compared with spleens of WT mice (P < .001) (Figure3). Likewise, we found a 50-fold increase in the number of LTC-ICs in spleens of IL-5trg mice in comparison to WT mice (P = .007) (Figure 2A).
Extramedullary development of hematopoietic progenitor cells in IL-5trg mice.
Spleen cells were harvested from IL-5trg and WT mice and plated into methylcellulose cultures in the presence of lineage-specific cytokines. GM-CSF or M-CSF was added to estimate numbers of granulocyte-macrophage– or macrophage-CFCs, and IL-5 (50 ng/mL) or IL-7 was added to estimate the numbers of eosinophil or B-lymphoid progenitors. Colonies were cultured in quadruples, calculated after 7 days of culture, and expressed as mean ± SD.
Extramedullary development of hematopoietic progenitor cells in IL-5trg mice.
Spleen cells were harvested from IL-5trg and WT mice and plated into methylcellulose cultures in the presence of lineage-specific cytokines. GM-CSF or M-CSF was added to estimate numbers of granulocyte-macrophage– or macrophage-CFCs, and IL-5 (50 ng/mL) or IL-7 was added to estimate the numbers of eosinophil or B-lymphoid progenitors. Colonies were cultured in quadruples, calculated after 7 days of culture, and expressed as mean ± SD.
IL-5 does not affect transendothelial migration of HSPCs toward SDF-1
To determine whether IL-5 alters HSPC trafficking, we investigated the effect of IL-5 on the ability of HPSCs to transmigrate across an endothelial cell barrier toward SDF-1, a chemoattractant produced by bone marrow stroma, using a Transwell migration assay. An enriched population of HPSCs isolated from IL-5trg mice was incubated in the presence or absence of IL-5, and their ability to migrate across a monolayer of HUVECs in response to SDF-1 was compared with migration of HSPCs isolated from WT mice. The number of transmigrated cells isolated from WT and IL-5trg mice was similar. In addition, pretreatment of WT cells with IL-5 failed to significantly alter the percentage of migration of total cells from the upper well toward SDF-1 in comparison to untreated cells isolated from WT or IL-5trg HSPCs (Figure4A). Next, cells that emigrated in response to SDF-1 (in the presence or absence of IL-5) were recovered from the lower wells and cultured in methylcellulose media to enumerate the number of progenitor cells (Figure 4B). Furthermore, compared with the untreated WT cells, IL-5 pretreated HPSCs demonstrated unaltered migration toward SDF-1 as determined by lack of significant difference in the number of colony-forming cells (CFCs) in the 2 groups. We next examined if treatment with IL-5 alters the cell surface expression of SDF-1 receptor CXCR4. In line with the above observations, treatment of HSPCs with IL-5 for 24, 48, or 72 hours failed to alter the surface expression of CXCR4 (data not shown). Our results suggest that factors other than SDF-1 are likely to influence IL-5–mediated trafficking of HSPCs into extramedullary sites.
Effect of IL-5 on HSPC migration.
(A) Enriched populations of HSPCs were isolated from WT and IL-5trg mice. WT cells were incubated in media containing the cytokine cocktail (Flt-3 ligand, SCF, IL-6, IL-3, IL-11, thrombopoietin [Tpo]) with or without (control) IL-5. Cells were allowed to migrate toward SDF-1 across an endothelial monolayer for 4 hours. The number of migrated cells was calculated and expressed as a percentage of total cell number. (B) Migrated HSPCs were plated into methylcellulose cultures in the presence of the cytokine cocktail (IL-3, IL-6, SCF, Tpo). The numbers of colonies (n = 4 cultures per group) were counted, recalculated, and expressed as percentage of total migrated CFC mean ± SD.
Effect of IL-5 on HSPC migration.
(A) Enriched populations of HSPCs were isolated from WT and IL-5trg mice. WT cells were incubated in media containing the cytokine cocktail (Flt-3 ligand, SCF, IL-6, IL-3, IL-11, thrombopoietin [Tpo]) with or without (control) IL-5. Cells were allowed to migrate toward SDF-1 across an endothelial monolayer for 4 hours. The number of migrated cells was calculated and expressed as a percentage of total cell number. (B) Migrated HSPCs were plated into methylcellulose cultures in the presence of the cytokine cocktail (IL-3, IL-6, SCF, Tpo). The numbers of colonies (n = 4 cultures per group) were counted, recalculated, and expressed as percentage of total migrated CFC mean ± SD.
Discussion
IL-5 is produced by a number of cell types and is responsible for the maturation and release of eosinophils in the bone marrow. Peripheral and tissue eosinophilia are associated with a wide variety of inflammatory diseases, including, but not limited to, allergic asthma, HES, as well as host immunity to parasitic infections. The recruitment and localization of eosinophils to specific sites of tissue inflammation involve the engagement of cytokines, vascular and leukocyte adhesion molecules expressed both by the vascular endothelium and eosinophils, and chemoattractants that stimulate eosinophil migration.8 Previous studies have demonstrated that activation and recruitment of eosinophils into sites of inflammation is mostly mediated by IL-5.6 Furthermore, the differentiation and maturation of HSPCs into eosinophils and their release from the bone are primarily regulated by IL-5.6 Here, we demonstrate that constitutive overexpression of IL-5 in IL-5trg mice up to 6 months in age results in significant alterations in bone marrow hematopoiesis.
Increased levels of IL-5 were detected in plasma of IL-5trg mice (157.2 ± 16.8 pg/mL versus 2.2 pg/mL in WT mice, as measured by enzyme-linked immunosorbent assay [ELISA]). This is associated with elevated numbers of eosinophils in bone marrow, blood, and parenchymal organs. Earlier studies have demonstrated that these mice exhibit profound eosinophilia without significant changes in neutrophil, lymphocyte, and monocyte counts.12 We observed that the numbers of granulocyte-monocyte and eosinophil progenitors in bone marrow of IL-5 transgenic mice were not significantly different from those observed in WT mice. Although the number of LTC-ICs in the bone marrow of IL-5trg mice was slightly decreased (1.5-fold), bone marrow cells obtained from IL-5trg mice failed to establish a productive LTBMC. Hematopoietic activity of the IL-5trg bone marrow cells was restored by cultivating the cells on a pre-established stromal layer. These data are suggestive of the presence of low numbers of stromal progenitor cells in the bone marrow of IL-5trg mice that are required for providing the hematopoietic-supportive microenvironment. We further demonstrated that the number of stromal progenitor cells, as measured by CFU-F assay, was significantly decreased in the bone marrow of IL-5trg mice. Interestingly, bone marrow hematopoiesis was not significantly affected in IL-5trg mice. We speculate that the low number of residual MSP cells is sufficient to produce stromal cells and provide signals to mediate hematopoietic activity in the bone marrow of IL-5trg mice. In addition, other cells comprising the hematopoietic microenvironment, including macrophages, endothelial cells, reticular cells, and so forth, may also participate in supporting hematopoiesis in the bone marrow of IL-5trg mice. In contrast to the bone marrow, the number of CFU-Fs was appreciably increased in the spleen. In addition, we observed areas of ossification in the spleens of aged IL-5trg mice. This observation is supported by previously published findings that bone formation was observed in the spleen of IL-5trg mice.24 The overall mechanisms of MSP cell migration are largely unknown and very poorly understood. However, because both osteoblasts and stromal cells originate from a common mesenchymal stem cell,25 it is conceivable that MSP cells are mobilized into the spleen. Whether IL-5 directly modulates this migration to the spleen or is mediated through an indirect process via the regulation of other factor(s) remains unclear. Once mobilized, MSP cells may subsequently differentiate into osteoblasts and stromal cells in the spleen. Stromal cells provide a hematopoietic-supportive microenvironment for HSPCs, resulting in seeding of the spleen with circulating HSPCs and establishment of extramedullary sites of hematopoiesis. This is supported by our findings that IL-5 does not interfere with the ability of SDF-1, a chemokine produced by bone marrow–derived stroma, to mediate chemotaxis of HSPCs.
Overall there appears to be some controversy in establishing whether bone marrow alone, or extramedullary sites of hematopoiesis, can also function as additional important sites for IL-5–induced eosinophilopoiesis. In contrast to increased number of IL-5–induced eosinophil progenitors and CD34+ cells in the bone marrow,10,12,14,16,17 extramedullary foci of IL-5–induced eosinophilopoiesis have also been described.4,18,19Extramedullary eosinophilopoiesis has been observed in transgenic mice generated by using regulatory elements to drive T-cell expression of IL-5.4,26 Likewise, mice infected with Toxocara canis exhibit profound eosinophilia in extramedullary hematopoietic foci.18 The hematopoietic foci located in the liver of infected mice were of the eosinophil lineage and contained a large number of proliferating hematopoietic stem cells.18 These observations indicate that eosinophils can be generated outside of the bone marrow by differentiation from committed progenitors, suggesting that an extramedullary hematopoiesis-supportive microenvironment is provided under pathophysiologic conditions associated with elevated levels of IL-5.
To exclude a potential cytotoxic effect because of elevated levels of mature eosinophils observed in IL-5trg mice on progenitor cells and hematopoietic microenvironment, we stimulated eosinophilopoiesis in WT LTBMCs by treatment with IL-5 and monitored the formation of adherent layer, production of eosinophils, and hematopoietic activity of LTBMCs cultivated in the presence and absence of IL-5. In marked contrast to IL-5trg mice, treatment with IL-5 does not affect the formation of an adherent layer of LTBMCs nor areas of active hematopoiesis, but instead results in the production of increased numbers of nonadherent cells, including eosinophils and myeloid progenitors, as compared with untreated control cultures. These results are in line with our observation that an increase in endogenous IL-5 because of intraperitoneal ragweed immunization results in enhanced lymphophilopoiesis and eosinophilopoiesis in the bone marrow (S.K. et al, unpublished observations, April 1999). Thus, it is likely that in contrast to short-term allergen-induced up-regulation of IL-5, constitutive expression of IL-5 in IL-5trg mice results in induction of other factors mediating inhibition of bone marrow hematopoiesis.
Although it is conceivable that the high concentration of IL-5 in the peripheral blood of patients suffering from eosinophil-mediated tissue damage may directly or indirectly induce the recruitment of both eosinophils and early hematopoietic progenitors to sites of inflammation,19 the mechanisms involved in this process are not known. Our observations suggest that IL-5–mediated recruitment of HSPCs to tissue sites is not dependent on interactions with endothelial cells in blood vessels. Instead, it is likely that elevated levels of IL-5 induces a cascade of cellular events leading to an increased sensitivity of HSPCs to inflammatory cytokines, resulting in its facilitated and directed migration from the peripheral blood to sites of inflammation and a subsequent local induction of the terminal differentiation of HSPC toward eosinophilic lineage. In support of this hypothesis, studies have demonstrated the presence of an increased proportion of CD34+ progenitor cells expressing IL-5Rα, but not IL-3Rα or GM-CSFRα, in patients with dust (late phase) asthma 24 hours after allergen challenge, concurrently with the development of late asthmatic response.19
In summary, our studies demonstrate that sustained exposure to increased concentrations of IL-5 (in IL-5trg mice) leads to colonization of the spleen with bone marrow–derived stromal cells. This may provide the necessary microenvironment to support formation of the sites of extramedullary hematopoiesis. Our studies further demonstrate that exposure to IL-5 fails to alter the expression of SDF-1 receptor CXCR4. Instead, it is conceivable that sustained exposure to elevated levels of IL-5 results in the increased sensitivity of HSPCs to chemotactic factors that are elaborated at sites of tissue inflammation, leading to directed migration of HSPCs to sites of inflammation. Studies are currently under way to evaluate molecular mechanisms of IL-5–mediated HPSC recruitment to the sites of inflammation in vitro and in vivo.
We thank Dr Savita Rao for helpful suggestions and comments.
Prepublished online as Blood First Edition Paper, September 12, 2002; DOI 10.1182/blood-2002-03-0735.
Supported by National Institutes of Health grant RO1 AI35796 and Tobacco Related Disease Research Program grant 10RT-0171 (P.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
P. Sriramarao, Division of Vascular Biology, La Jolla Institute for Molecular Medicine, 4570 Executive Dr, San Diego, CA 92121; e-mail: rao@ljimm.org.

![Fig. 4. Effect of IL-5 on HSPC migration. / (A) Enriched populations of HSPCs were isolated from WT and IL-5trg mice. WT cells were incubated in media containing the cytokine cocktail (Flt-3 ligand, SCF, IL-6, IL-3, IL-11, thrombopoietin [Tpo]) with or without (control) IL-5. Cells were allowed to migrate toward SDF-1 across an endothelial monolayer for 4 hours. The number of migrated cells was calculated and expressed as a percentage of total cell number. (B) Migrated HSPCs were plated into methylcellulose cultures in the presence of the cytokine cocktail (IL-3, IL-6, SCF, Tpo). The numbers of colonies (n = 4 cultures per group) were counted, recalculated, and expressed as percentage of total migrated CFC mean ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/3/10.1182_blood-2002-03-0735/4/m_h80333792004.jpeg?Expires=1769176572&Signature=bAxZQiLMUJswiWmUAcXroXdY1LLkXcWQbTC3U8~vi~cI0FEGoeERKFksggPjkFhTGUEmX124eByaOC5TqeswKN3WzoKHx4v~0PGxR5hOUaxdP~ADmq-HOHz9LbLDEaeTJS-MRDDK2ZEDcMn44Ov~8fcNAHZQTTLdLVtzy3rk-H7RhZTHqDLaYJfqMQqhz467TyG5I17Ev2AchShdFrW88~3CiMwLM3sxM3wrqENkjboGgUKUQKKOi-kKBzGB6gSuJC19wBvm8-4yH3UE~qnxflrPuCqNifz7P2Mj201zaiRLj~daj9ulgpXpW1Pa9f6G6DhDHZNGQ1jC9HvTyHx~AA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal