Abstract
Neutrophils from patients with myelodysplastic syndrome (MDS) show a disturbed differentiation pattern and are generally dysfunctional. To study these defects in more detail, we investigated reactive-oxygen species (ROS) production and F-actin polymerization in neutrophils from MDS patients and healthy controls and the involvement of N-formyl-L-methionyl-L-lucyl-L-phenylaline (fMLP) and granulocyte macrophage–colony-stimulating factor (GM-CSF)–stimulated signal transduction pathways. Following fMLP stimulation, similar levels of respiratory burst, F-actin polymerization, and activation of the small GTPase Rac2 were demonstrated in MDS and normal neutrophils. However, GM-CSF and G-CSF priming of ROS production were significantly decreased in MDS patients. We subsequently investigated the signal transduction pathways involved in ROS generation and demonstrated that fMLP-stimulated ROS production was inhibited by the phosphatidylinositol 3 kinase (PI3K) inhibitor LY294002, but not by the MAPK/ERK kinase (MEK) inhibitor U0126. In contrast, ROS production induced by fMLP stimulation of GM-CSF–primed cells was inhibited by LY294002 and U0126. This coincides with enhanced protein kinase B (PKB/Akt) phosphorylation that was PI3K dependent and enhanced extracellular signal-regulated protein kinase 1 and 2 (ERK1/2) phosphorylation that was PI3K independent. We demonstrated higher protein levels of the PI3K subunit p110 in neutrophils from MDS patients and found that though the fMLP-induced phosphorylation of PKB/Akt and ERK1/2 could also be enhanced by pretreatment with GM-CSF in these patients, the degree and kinetics of PKB/Akt and ERK1/2 phosphorylation were significantly disturbed. These defects were observed despite a normal GM-CSF–induced signal transducer and activator of transcription 5 (STAT5) phosphorylation. Our results indicate that the reduced priming of neutrophil ROS production in MDS patients might be caused by a disturbed convergence of the fMLP and GM-CSF signaling routes.
Introduction
Myelodysplastic syndromes (MDS) are characterized by a differentiation defect in the multipotent stem cell compartment, resulting in a disturbed proliferation and differentiation of the erythroid, myeloid, or megakaryocytic lineage.1 With regard to the myeloid lineage, it has been shown that the intrinsic defect leads to granulocytopenia and to aberrant functioning of neutrophils. For instance, there have been reports indicating that reorganization of the actin cytoskeleton2 and the production of reactive-oxygen species (ROS)3 4 are affected in neutrophils from MDS patients.
Bacterial infections are the most common cause of death in MDS patients.5 The capacity of neutrophils to produce ROS such as O and H2O2 during the respiratory burst is essential for their bactericidal activity.6 ROS production can be stimulated by chemotactic factors, such as the bacterial peptide analog N-formyl-L-methionyl-L-lucyl-L-phenylalanine (fMLP).7Proinflammatory agents such as granulocyte macrophage–colony-stimulating factor (GM-CSF) or granulocyte–colony-stimulating factor (G-CSF) prime the fMLP-mediated respiratory burst but do not elicit ROS production on their own.8,9 This priming effect is not specific for ROS production, but it has been described for other cellular events, such as degranulation, migration, and adherence.10 11
Respiratory burst and additional neutrophil effector functions such as granule trafficking and phagocytosis are dependent on the organization of the actin cytoskeleton.12-15 There are 2 forms of actin, a monomeric form (G-actin) and a filamentous form (F-actin), that exist in equilibrium in the cell. On the stimulation of neutrophils with chemotactic factors, rapid conversion of G-actin to F-actin occurs.16 17 In contrast to ROS production, F-actin polymerization cannot be primed by proinflammatory factors.
Different signal transduction pathways have been identified that are critical for the cellular functions of neutrophils. Rac2, a member of the Rho family of small GTPases, acts as a molecular switch by cycling between an inactive guanosine diphosphate (GDP)–bound state and an active guanosine triphosphate (GTP)–bound state. Rac2 is involved in the reorganization of the actin cytoskeleton18-20 and the fMLP mediated respiratory burst, as demonstrated by experiments in Rac−/− mice and in patients with an inhibitory Rac2 mutation.21-24 In addition, phosphoinositide-3-OH kinase (PI3K) plays an important role in neutrophil ROS production. Several class 1 isoforms of PI3K have been identified; class 1Acatalytic subunits p110α, p110 β, and p110 δ associate with the regulatory subunit p85, whereas the class 1B catalytic subunit p110γ associates with p101. Evidence for PI3K involvement in neutrophil respiratory burst comes from studies with specific PI3K inhibitors.25-27 Furthermore, lipid products of PI3K associate with the phagocyte oxidase proteins p47phox and p40phox, 2 components of the neutrophil oxidase complex. The association of one of these lipids with p40phox has a regulatory effect on ROS production.28,29 Additional studies have revealed that extracellular signal-regulated protein kinase 1 and 2 (ERK1/2), a key member of the mitogen-activated protein kinase (MAPK) cascade, might be involved in the neutrophil respiratory burst. Inhibition of ERK activation by chemical inhibitors resulted in a reduction of fMLP-induced and GM-CSF–primed ROS production in human neutrophils in several studies.27 30
In this study, we further characterized the disturbed neutrophil responses in MDS and questioned which signal transduction pathways might be involved in aberrant neutrophil functioning. We demonstrated normal fMLP-mediated respiratory burst, F-actin polymerization, and Rac2 activation in neutrophils from MDS patients but found a strongly decreased priming effect of GM-CSF and G-CSF on ROS production. Analysis of signal transduction pathways revealed higher protein expression levels of the PI3K subunit p110 in neutrophils from MDS patients. Further examination also indicated altered phosphorylation levels of protein kinase B (PKB/Akt) and ERK1/2 in MDS patients.
Patients, materials, and methods
Reagents
N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP), phorbol 12-myristate 13-acetate (PMA), ferricytochrome c, and superoxide dismutase (SOD) were obtained from Sigma (St Louis, MO). Recombinant human GM-CSF was from Sandoz (Uden, The Netherlands), and r-metHuG-CSF was from Roche (Almere, The Netherlands). The PI3K inhibitor LY294002 was purchased from Alexis (Läufelfingen, Switzerland), and the MEK inhibitor U0126 was obtained from New England Biolabs (Beverly, MA). The GST-PAK-CD construct was a generous gift from Dr P. J. Coffer. Oregon Green 514-phalloidin and dihydro-rhodamine 123 (DHR123) were purchased from Molecular Probes (Eugene, OR). Polyclonal antibody against ERK1/2 (K23) and monoclonal antibody against PI3-kinase p110 (D-4) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibody against phosphorylated signal transducer and activator of transcription (STAT) 5A/B (Y694/Y699) was from Upstate Biotechnology (Lake Placid, NY), and antibodies against phosphorylated ERK1/2 (Thr202/Tyr204) and phosphorylated PKB/Akt (Ser473) were obtained from Cell Signaling Technology (Beverly, MA). Anti-Rac antibody was from Transduction Laboratories (Lexington, KY).
Patients
Fourteen patients with MDS were studied. MDS was classified as refractory anemia (RA) or RA with ringed sideroblasts (RARS) according to French-American-British (FAB) cooperative group criteria.31 Informed consent was obtained from all patients. The protocol was approved by the Human Subjects Review Board of the University Hospital Gröningen.
Isolation of neutrophils
Peripheral blood, anticoagulated with 0.32% sodium citrate, was obtained from healthy volunteers and patients with MDS. Neutrophils were isolated as described by Koenderman et al.32 In short, mononuclear cells were removed by centrifugation over Ficoll-Hypaque (Amersham, Uppsala, Sweden), and erythrocytes were lysed with ice-cold NH4Cl solution. Neutrophils were allowed to recover for 30 minutes at 37°C in RPMI 1640 (BioWhittaker, Verviers, Belgium) supplemented with 0.5% human serum albumin (HSA; CLB, Amsterdam, The Netherlands). Before stimulation, cells were resuspended in incubation buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 132 mM NaCl, 6 mM KCl, 1 mM MgSO4, 1.2 mM KH2PO4, 5 mM glucose, 1 mM CaCl2, 0.5% HSA). In all cases, the cell population isolated consisted of more than 95% neutrophils as determined by May-Grünwald-Giemsa staining.
F-actin polymerization assay
F-actin polymerization was measured as described by Nijhuis et al.33 In short, neutrophils (2.5 × 106cells/mL) were stimulated with 1 μM fMLP. At indicated time points, cells were fixed and permeabilized for 10 minutes at room temperature with ice-cold 3% formaldehyde in phosphate-buffered saline (PBS), containing 100 μg/mL lysophosphatidylcholine (Sigma, St Louis, MO). Polymerized F-actin was stained with 2.5 U/mL Oregon Green 514-phalloidin for 30 minutes at room temperature. Intracellular fluorescence was determined by flow cytometry (FACScalibur; Becton Dickinson Medical Systems, Sharon, MA).
Respiratory burst measurement
Production of H2O2 was measured as described by Mardiney et al,34 with some alterations. Briefly, neutrophils (2.5 × 106 cells/mL) were incubated with DHR123 for 15 minutes and were stimulated with 1 μM fMLP for 30 minutes. For priming experiments, cells were pretreated with 5 ng/mL GM-CSF, 2.5 ng/mL G-CSF, or both for 15 minutes before fMLP stimulation. When indicated, neutrophils were pretreated with signal transduction inhibitors. Stimulation of the neutrophils with fMLP was terminated by washing the cells with ice-cold PBS containing 1% HSA and placing them on ice. Oxidation of DHR123 to fluorescent rhodamine 123 was measured by fluorescence-activated cell sorter (FACS) analysis within 30 minutes.
Alternatively, O generation was measured by the superoxide dismutase-inhibitable reduction of ferricytochrome c, as described by Franssen et al.35 In short, GM-CSF–primed or unprimed neutrophils (1 × 106 cells/mL) were incubated in 96-well microtiter plates with ferricytochrome c at a final concentration of 0.856 mg/mL, either with SOD at a final concentration of 13.16 U/mL or with an equal volume incubation buffer. Cells were stimulated with 1 μM fMLP or 100 ng/mL PMA, and plates were scanned repetitively at 550 nm using an automated microplate reader (Thermomax; Molecular Devices, Sunnyvale, CA). Between readings, plates were kept at 37°C. Superoxide production was expressed as the difference in OD550 nm (ΔOD 550) between the ferricytochrome c reduction test in the absence and in the presence of SOD. Each test was performed in quadruplicate.
Rac activation assay
Activated Rac was precipitated using the CDC42 and Rac interactive binding (CRIB) domain of PAK (aa 56-272) as described previously.36 Briefly, neutrophils (1 × 106cells/mL) were stimulated with 0.1 μM fMLP for the indicated time and were lysed for 10 minutes in lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane] pH 7.4, 10% glycerol, 200 mM NaCl, 1% NP-40, 2 mM MgCl2, 2 mM sodium orthovanadate, and protease inhibitors [1 tablet Complete (Roche, Mannheim, Germany)/50 mL buffer]). Lysates were cleared by centrifugation, and GST-PAKcrib protein precoupled to glutathione-Sepharose beads (Pharmacia, Uppsala, Sweden) was added for 30 to 45 minutes at 4°C. Beads were washed 3 times with 1× lysis buffer and were boiled in Laemmli sample buffer. Bound proteins were separated by 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA). Activated Rac was detected by probing the membrane with anti-Rac antibodies and rabbit antimouse peroxidase-conjugated antibodies (DAKO, Copenhagen, Denmark) followed by enhanced chemiluminescence.
Western blotting
The amount of ERK and PI3-kinase p110 protein and the phosphorylation of STAT5, ERK, and PKB/Akt were determined by Western blotting. Neutrophils (2 × 106 cells/mL) were preincubated with inhibitors and stimulated with 1 μM fMLP or 5 ng/mL GM-CSF, as indicated in the figures. Stimulation was terminated by placing the cells on ice, subjecting them to immediate centrifugation, and resuspending the cell pellets in 1× Laemmli sample buffer. After boiling for 10 minutes, the proteins were separated on 10% SDS-PAGE and electrophoretically transferred to nitrocellulose membranes (Protran; Schleicher & Schuell, Dassel, Germany). Membranes were probed with antibodies against ERK1/2 (K23), PI3-kinase p110, phosphorylated STAT5A/B (Y694/Y699), phosphorylated ERK1/2, and phosphorylated PKB/Akt (Ser473) according to the manufacturer's protocols. Proteins were detected by enhanced chemiluminescence. The total amount of PKB/Akt present in the samples could not be quantified on membranes that were previously probed with antibodies against phosphorylated PKB/Akt because of high chemiluminescence of the phosphorylated protein. However, Western blot analysis of cell lysates from 3 MDS patients and 3 healthy volunteers run on one gel and probed with PKB/Akt antibody (Cell Signaling Technology, Beverly, MA) did not show differences in the amount of total PKB/Akt protein (data not shown). Quantification of phosphorylation levels was performed by densitometry of the films, using ImageMaster1D Elite (Pharmacia, Woerden, The Netherlands).
Statistical analysis
For respiratory burst measurements, differences in experimental values between healthy controls and MDS patients were calculated using the Mann-Whitney U test. Differences between paired samples were calculated using the Wilcoxon nonparametric test or the Studentt test for samples treated with signal transduction inhibitors. For comparison of PI3K p110 levels in MDS patients and healthy donors, densitometry values of p110 protein were corrected for the amount of ERK protein present in the samples, and differences between the normalized values were calculated using the Studentt test for unpaired samples. For quantification of phospho-PKB/Akt and phospho-ERK1/2 blots, differences in densitometry values of healthy controls as a percentage of that of MDS patients were calculated using the Student t test for paired samples. Data were expressed as mean ± SEM. P < .05 was considered significant.
Results
fMLP-stimulated F-actin polymerization and Rac2 activation are not disturbed in neutrophils from MDS patients
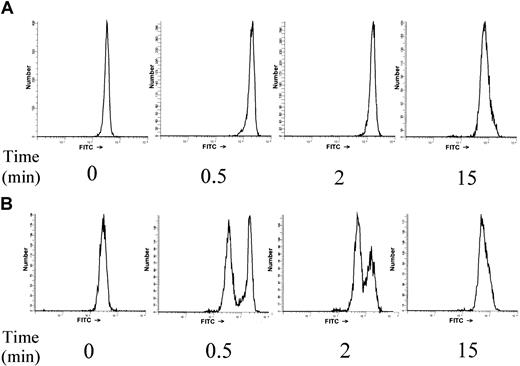
fMLP-induced F-actin polymerization was examined and compared in neutrophils from MDS patients and healthy controls (n = 8 for each group). On stimulation of neutrophils with 1 μM fMLP, a transient increase in the relative amount of F-actin was observed that was maximal within 30 seconds and declined after 2 to 5 minutes. The maximum relative increase of F-actin content in neutrophils from healthy subjects was 507% ± 22%. This was comparable to the maximum relative increase of F-actin in neutrophils from MDS patients (507 ± 52%; P = .963), though in MDS patients there was a wider variation in relative F-actin increase. The kinetics of F-actin polymerization in MDS neutrophils was similar to that of healthy volunteers (data not shown). Although patients and healthy controls showed no significant difference in the kinetics and relative amount of F-actin formed in response to fMLP, 3 MDS patients did have a subpopulation of neutrophils (21%, 48%, and 49% of the total population) that had virtually no detectable F-actin polymerization (Figure 1). These subpopulations could not be distinguished from the responsive neutrophil populations in dot-plot profiles because their scattering profiles were identical.
A subpopulation of neutrophils that does not polymerize F-actin in response to fMLP stimulation is present in some MDS patients.
Human neutrophils were stimulated with 1 μM fMLP for the indicated time. Cells were fixed and permeabilized, and F-actin was stained with 2.5 U/mL Oregon Green 514–phalloidin. Fluorescence histograms are shown for a representative healthy control (A) and one of the MDS patients with a nonresponsive neutrophil subpopulation (B).
A subpopulation of neutrophils that does not polymerize F-actin in response to fMLP stimulation is present in some MDS patients.
Human neutrophils were stimulated with 1 μM fMLP for the indicated time. Cells were fixed and permeabilized, and F-actin was stained with 2.5 U/mL Oregon Green 514–phalloidin. Fluorescence histograms are shown for a representative healthy control (A) and one of the MDS patients with a nonresponsive neutrophil subpopulation (B).
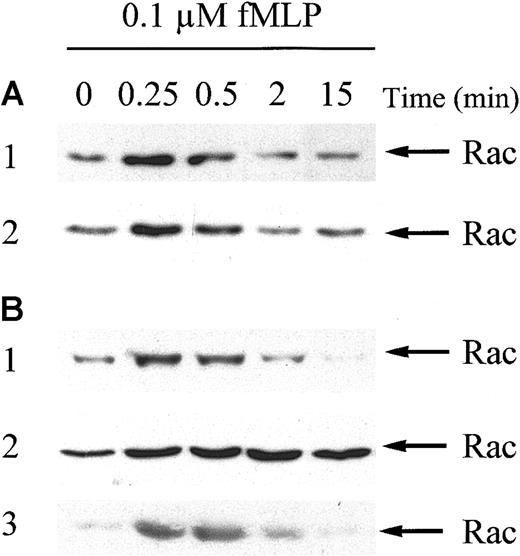
To confirm the normal fMLP response of MDS neutrophils at the molecular level, fMLP-stimulated Rac2 activation was studied by using a GST-beads pull-down technique. Figure2A demonstrates that maximal Rac2 activation in neutrophils from healthy subjects was achieved after 15 seconds of fMLP stimulation and declined to near baseline levels within 15 minutes. Similar results were obtained in neutrophils from MDS patients (n = 8), though there was some variation in the extent and time course of Rac2 activation among donors (Figure 2B). The mean increase in Rac2 activation after 15 seconds of fMLP stimulation was 729% ± 202% in neutrophils from healthy donors (n = 8) versus 658 ± 179% in neutrophils from MDS patients, as determined by densitometry of the films (P = .795; data not shown). Western blot analysis of whole cell lysates of neutrophils from 5 MDS patients and 3 healthy controls, run on one gel, demonstrated equal amounts of total Rac protein present in all individuals (data not shown).
Rac2 is activated in response to fMLP in neutrophils from healthy volunteers and MDS patients.
Neutrophils were stimulated with 0.1 μM fMLP for the indicated time. Stimulation was stopped by the addition of 3 × lysis buffer. Activated Rac2 was precipitated using GST-PAKcrib protein precoupled to glutathione-Sepharose beads. Proteins were separated by 15% SDS-PAGE and membranes were probed with Rac antibodies, followed by enhanced chemiluminescence. Rac activation was investigated in neutrophils from 8 MDS patients and 8 healthy controls. (A) Two representative blots from healthy subjects. (B) Three representative blots from MDS patients. Equal amounts of glutathione-Sepharose beads were loaded in all samples, as determined by Ponceau S staining of the membranes (not shown).
Rac2 is activated in response to fMLP in neutrophils from healthy volunteers and MDS patients.
Neutrophils were stimulated with 0.1 μM fMLP for the indicated time. Stimulation was stopped by the addition of 3 × lysis buffer. Activated Rac2 was precipitated using GST-PAKcrib protein precoupled to glutathione-Sepharose beads. Proteins were separated by 15% SDS-PAGE and membranes were probed with Rac antibodies, followed by enhanced chemiluminescence. Rac activation was investigated in neutrophils from 8 MDS patients and 8 healthy controls. (A) Two representative blots from healthy subjects. (B) Three representative blots from MDS patients. Equal amounts of glutathione-Sepharose beads were loaded in all samples, as determined by Ponceau S staining of the membranes (not shown).
Priming of the respiratory burst leads to an enhancement of superoxide radical production that is higher in healthy controls than in MDS patients
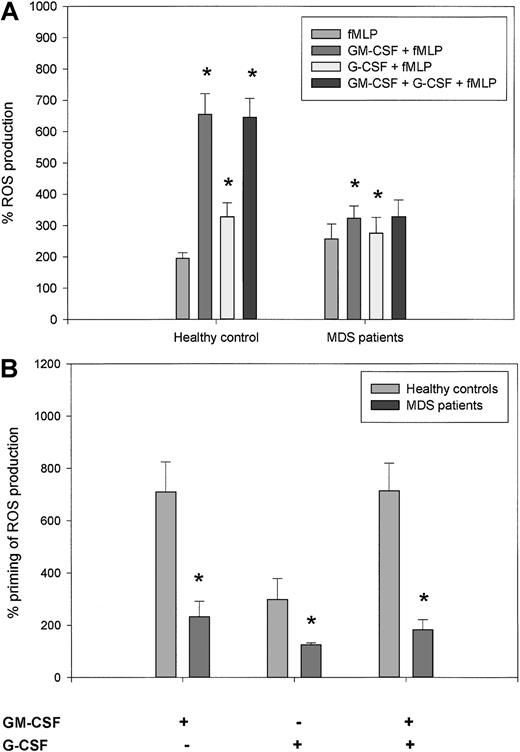
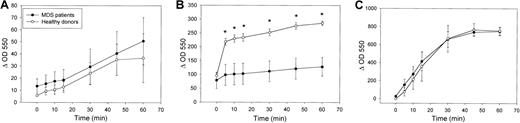
Given that the production of ROS is an essential antibacterial defense mechanism of neutrophils, we studied the amount of superoxide produced in neutrophils in response to fMLP and the enhancement of this response by priming with GM-CSF, G-CSF, or both. H2O2 production was calculated by expressing the fluorescence of rhodamine 123 in stimulated cells as a percentage of the fluorescence of unstimulated cells. As shown in Figure3A, fMLP stimulation induced the production of ROS to a similar level in neutrophils from healthy controls and MDS patients (196% ± 18% and 257% ± 48%, respectively; P = .753). In neutrophils from healthy donors, stimulation with GM-CSF or G-CSF alone did not lead to H2O2 production (data not shown). However, priming of the neutrophils with GM-CSF, G-CSF, or both, followed by fMLP stimulation, led to a significantly higher respiratory burst (656% ± 65%, 329% ± 44%, and 646% ± 60%, respectively) than when cells were stimulated with fMLP alone (P = .012 for all 3 groups). In neutrophils from MDS patients, the mean percentage increases in fluorescence intensity stimulated by 1 μM fMLP following GM-CSF and G-CSF priming were 323% ± 39% and 276% ± 50%, respectively, which was significantly higher than fMLP stimulation alone (P = .017 and P = .018, respectively). However, as shown in Figure 3B, the percentage increase in H2O2 production because of priming of neutrophils with GM-CSF, G-CSF, or both was significantly decreased in neutrophils from MDS patients than in healthy controls (P = .002, P = .012, andP = .003 respectively). These results indicate that whereas fMLP-triggered H2O2 production is normal in MDS patients, GM-CSF and G-CSF priming of the respiratory burst are considerably disturbed. These results were confirmed for O production by measuring ferricytochrome c reduction in the presence and absence of superoxide dismutase. Figure4A shows that there was no significant difference in O production in response to fMLP stimulation in neutrophils from healthy donors (n = 3) and MDS patients (n = 4). When neutrophils were primed with GM-CSF, subsequent fMLP stimulation triggered significantly higher O generation in neutrophils from healthy donors than in MDS patients (Figure 4B). In contrast, stimulation of O production by PMA, which is much slower in onset, was equal in neutrophils from MDS patients and healthy donors (Figure4C). These results indicate that priming of the generation of multiple ROS is greatly disturbed in neutrophils from MDS patients.
Priming of fMLP-stimulated H2O2production by GM-CSF and G-CSF is significantly decreased in neutrophils from MDS patients compared with healthy subjects.
Neutrophils were stimulated for 30 minutes with 1 μM fMLP with or without preincubation for 15 minutes with 5 ng/mL GM-CSF, 2.5 ng/mL G-CSF, or both. The production of H2O2 after stimulation was measured by FACS analysis and expressed as a percentage of the fluorescence in unstimulated cells. (A) Mean ROS production was calculated for 8 healthy subjects and 8 MDS patients. Asterisks represent significant differences in percentage H2O2 production in the primed groups versus the groups that were stimulated with fMLP alone (P < .05). (B) Respiratory burst-enhancing capabilities of GM-CSF, G-CSF, or both were calculated for each donor as follows: (GM-CSF + fMLP)/fMLP, (G-CSF + fMLP)/fMLP, and (GM-CSF + G-CSF + fMLP)/fMLP. The mean priming capacity of GM-CSF, G-CSF, or both on neutrophil respiratory burst is shown for 8 healthy subjects and 8 MDS patients. Differences between MDS patients and healthy controls were calculated using the Wilcoxon nonparametric tests (*P < .05).
Priming of fMLP-stimulated H2O2production by GM-CSF and G-CSF is significantly decreased in neutrophils from MDS patients compared with healthy subjects.
Neutrophils were stimulated for 30 minutes with 1 μM fMLP with or without preincubation for 15 minutes with 5 ng/mL GM-CSF, 2.5 ng/mL G-CSF, or both. The production of H2O2 after stimulation was measured by FACS analysis and expressed as a percentage of the fluorescence in unstimulated cells. (A) Mean ROS production was calculated for 8 healthy subjects and 8 MDS patients. Asterisks represent significant differences in percentage H2O2 production in the primed groups versus the groups that were stimulated with fMLP alone (P < .05). (B) Respiratory burst-enhancing capabilities of GM-CSF, G-CSF, or both were calculated for each donor as follows: (GM-CSF + fMLP)/fMLP, (G-CSF + fMLP)/fMLP, and (GM-CSF + G-CSF + fMLP)/fMLP. The mean priming capacity of GM-CSF, G-CSF, or both on neutrophil respiratory burst is shown for 8 healthy subjects and 8 MDS patients. Differences between MDS patients and healthy controls were calculated using the Wilcoxon nonparametric tests (*P < .05).
Priming of fMLP-stimulated O2 production by GM-CSF is significantly decreased in neutrophils from MDS patients compared with healthy subjects.
Freshly isolated neutrophils were (A) stimulated with 1 μM fMLP, (B) primed with 5 ng/mL GM-CSF for 15 minutes before fMLP stimulation, or (C) stimulated with 100 ng/mL PMA, and plates were scanned repetitively at 550 nm using an automated microplate reader. Superoxide production was expressed as the difference in OD550 nm (Δ OD 550) between the ferricytochrome c reduction test in the absence and in the presence of 13.16 U/mL SOD. Each test was performed in quadruplicate. Mean O2 production levels for 3 healthy volunteers and 4 MDS patients are shown. Differences between MDS patients and healthy controls were calculated using the Wilcoxon nonparametric test (*P < .05).
Priming of fMLP-stimulated O2 production by GM-CSF is significantly decreased in neutrophils from MDS patients compared with healthy subjects.
Freshly isolated neutrophils were (A) stimulated with 1 μM fMLP, (B) primed with 5 ng/mL GM-CSF for 15 minutes before fMLP stimulation, or (C) stimulated with 100 ng/mL PMA, and plates were scanned repetitively at 550 nm using an automated microplate reader. Superoxide production was expressed as the difference in OD550 nm (Δ OD 550) between the ferricytochrome c reduction test in the absence and in the presence of 13.16 U/mL SOD. Each test was performed in quadruplicate. Mean O2 production levels for 3 healthy volunteers and 4 MDS patients are shown. Differences between MDS patients and healthy controls were calculated using the Wilcoxon nonparametric test (*P < .05).
PI3K is involved in fMLP- and GM-CSF–stimulated respiratory burst and PKB/Akt phosphorylation in neutrophils from healthy donors
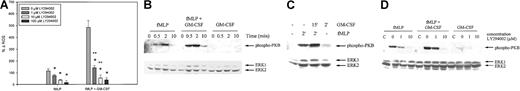
Our studies indicate that the priming effect of GM-CSF and G-CSF on the fMLP-induced respiratory burst is disturbed in MDS. To elucidate the signal transduction routes involved in the ROS production in healthy neutrophils, several chemical inhibitors of signal transduction pathways were used. First, we tested the effect of the specific PI3K inhibitor, LY294002, on the fMLP-stimulated respiratory burst in neutrophils, with or without GM-CSF pretreatment. Figure5A demonstrates that the fMLP-induced respiratory burst could be significantly inhibited at a concentration of 1 μM LY294002 (32% ± 8%; P = .021). Higher concentrations of LY294002 led to an even more pronounced reduction in fMLP-stimulated ROS production (66% ± 5% and 85% ± 8% for 10 and 100 μM LY294002, respectively; P < .05). The GM-CSF–primed respiratory burst was also significantly attenuated by preincubation of neutrophils with all concentrations of LY294002 tested. At concentrations of 1 and 10 μM LY294002, the reduction in fMLP-stimulated ROS production was significantly higher in the GM-CSF–primed group (70% ± 4% and 89% ± 4%, respectively) than in the unprimed group (P < .05). Incubation of neutrophils with 100 μM LY294002 led to an inhibition of the GM-CSF–primed ROS production of 93% ± 3%, which was statistically similar to that in the unprimed group.
Effect of LY294002 on ROS production and PKB/Akt phosphorylation in neutrophils from healthy volunteers.
(A) Isolated neutrophils were preincubated with 1, 10, or 100 μM LY294002 for 30 minutes and were stimulated for 20 minutes with 1 μM fMLP with or without priming with 5 ng/mL GM-CSF. ROS production was measured by FACS analysis. Results represent the mean increase in ROS produced after stimulation, compared with unstimulated cells, of 4 individual experiments. Significant reductions in respiratory burst by pretreatment of neutrophils with LY294002 are indicated by single asterisks (P < .05). Significant differences between the unprimed and the GM-CSF–primed groups in inhibition of ROS production by LY294002 are indicated with 2 asterisks (P < 0.05). (B) Neutrophils were stimulated with 1 μM fMLP, with or without priming with 5 ng/mL GM-CSF, or with GM-CSF alone. Serine-phosphorylated PKB/Akt was detected by Western blot analysis and immunodetection. ERK1/2 antibodies were used to determine equal loading (lower panel). (C) To better visualize GM-CSF–stimulated PKB/Akt phosphorylation, a longer exposure of another Western blot is shown. (D) Isolated human neutrophils were preincubated with increasing amounts of LY294002 for 30 minutes and were stimulated for 2 minutes with either 1 μM fMLP, with or without GM-CSF priming, or 5 ng/mL GM-CSF alone. Unstimulated cells were run as negative control (c). Cell lysates were analyzed by Western blotting, using an antibody against serine phosphorylated PKB/Akt (upper panel). Equal protein loading in the samples was confirmed by immunodetection of ERK1/2 (lower panel). A representative blot of 3 independent experiments is shown.
Effect of LY294002 on ROS production and PKB/Akt phosphorylation in neutrophils from healthy volunteers.
(A) Isolated neutrophils were preincubated with 1, 10, or 100 μM LY294002 for 30 minutes and were stimulated for 20 minutes with 1 μM fMLP with or without priming with 5 ng/mL GM-CSF. ROS production was measured by FACS analysis. Results represent the mean increase in ROS produced after stimulation, compared with unstimulated cells, of 4 individual experiments. Significant reductions in respiratory burst by pretreatment of neutrophils with LY294002 are indicated by single asterisks (P < .05). Significant differences between the unprimed and the GM-CSF–primed groups in inhibition of ROS production by LY294002 are indicated with 2 asterisks (P < 0.05). (B) Neutrophils were stimulated with 1 μM fMLP, with or without priming with 5 ng/mL GM-CSF, or with GM-CSF alone. Serine-phosphorylated PKB/Akt was detected by Western blot analysis and immunodetection. ERK1/2 antibodies were used to determine equal loading (lower panel). (C) To better visualize GM-CSF–stimulated PKB/Akt phosphorylation, a longer exposure of another Western blot is shown. (D) Isolated human neutrophils were preincubated with increasing amounts of LY294002 for 30 minutes and were stimulated for 2 minutes with either 1 μM fMLP, with or without GM-CSF priming, or 5 ng/mL GM-CSF alone. Unstimulated cells were run as negative control (c). Cell lysates were analyzed by Western blotting, using an antibody against serine phosphorylated PKB/Akt (upper panel). Equal protein loading in the samples was confirmed by immunodetection of ERK1/2 (lower panel). A representative blot of 3 independent experiments is shown.
An important downstream target of PI3K is PKB/Akt.37 38 As shown in Figure 5B (upper panel), fMLP induced PKB/Akt serine phosphorylation in a time-dependent manner. Phosphorylation was maximal within 2 minutes and declined to near basal levels after 10 minutes of stimulation. GM-CSF alone stimulated PKB/Akt phosphorylation levels to a much lower extent (Figure 5B). Longer exposure demonstrated that GM-CF–stimulated phosphorylation occurred at a maximum at 2 minutes (Figure 5C). Interestingly, PKB/Akt phosphorylation by fMLP could be enhanced by pretreatment of the neutrophils with GM-CSF.
Figure 5D demonstrates that the phosphorylation of PKB/Akt by stimulation of unprimed or GM-CSF–primed neutrophils with fMLP, or GM-CSF alone, could be partially inhibited by preincubation of the neutrophils with 1 μM LY294002 and completely inhibited with 10 μM. This indicates that the concentrations LY294002 used effectively inhibited PI3K activation. Our results demonstrate that fMLP stimulation of neutrophils leads to the production of reactive oxygen species at least partially by activating PI3K, and they imply that the priming effect of GM-CSF on the fMLP-induced neutrophil burst is completely dependent on the activation of PI3K.
GM-CSF–mediated enhancement of fMLP-stimulated respiratory burst is inhibited by preincubation of healthy neutrophils with the ERK inhibitor, U0126
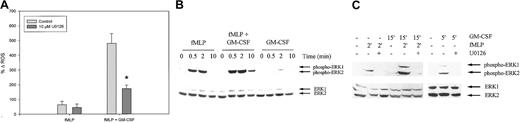
Next, we studied the role of the MEK/ERK pathway in the fMLP-induced respiratory burst in neutrophils by using the extremely potent, selective inhibitor of MEK1/2, U0126. Figure6A shows that unprimed fMLP-stimulated ROS production was not significantly reduced by preincubation of the neutrophils with U0126 (39% ± 20%; P = .554). However, GM-CSF priming of the respiratory burst could be significantly inhibited by 10 μM U0126 for 65% ± 1% (P = .021). Similar results were obtained when neutrophils were preincubated with 50 μM PD98059, another chemical MEK1/2 inhibitor (data not shown).
Effect of U0126 on ROS production and ERK1/2 phosphorylation in neutrophils from healthy volunteers.
(A) Isolated neutrophils were preincubated with 10 μM U0126 for 30 minutes and stimulated for 20 minutes with 1 μM fMLP with or without priming with 5 ng/mL GM-CSF. ROS production was measured by FACS analysis. Results represent the mean increase in ROS produced after stimulation, compared with unstimulated cells, of 4 individual experiments. Significant reduction of the respiratory burst by U0126 is indicated by an asterisk (P < .05). (B) Neutrophils were stimulated with 1 μM fMLP with or without priming with 5 ng/mL GM-CSF or with GM-CSF alone. Phosphorylation of ERK1/2 was detected by Western blot analysis and immunodetection. To confirm equal loading, the total amount of ERK1/2 was detected using the ERK1/2 K23 antibody (lower panel). A representative blot is shown of 3 independent experiments. (C) Neutrophils were preincubated with 10 μM U0126 for 30 minutes when indicated and were stimulated for the indicated amount of time with 1 μM fMLP, with or without priming with 5 ng/mL GM-CSF or with GM-CSF alone. Cell lysates were analyzed by Western blotting, using an antibody against phosphorylated ERK1/2 (upper panel). Equal protein loading in the samples was confirmed by immunodetection of ERK1/2 (lower panel).
Effect of U0126 on ROS production and ERK1/2 phosphorylation in neutrophils from healthy volunteers.
(A) Isolated neutrophils were preincubated with 10 μM U0126 for 30 minutes and stimulated for 20 minutes with 1 μM fMLP with or without priming with 5 ng/mL GM-CSF. ROS production was measured by FACS analysis. Results represent the mean increase in ROS produced after stimulation, compared with unstimulated cells, of 4 individual experiments. Significant reduction of the respiratory burst by U0126 is indicated by an asterisk (P < .05). (B) Neutrophils were stimulated with 1 μM fMLP with or without priming with 5 ng/mL GM-CSF or with GM-CSF alone. Phosphorylation of ERK1/2 was detected by Western blot analysis and immunodetection. To confirm equal loading, the total amount of ERK1/2 was detected using the ERK1/2 K23 antibody (lower panel). A representative blot is shown of 3 independent experiments. (C) Neutrophils were preincubated with 10 μM U0126 for 30 minutes when indicated and were stimulated for the indicated amount of time with 1 μM fMLP, with or without priming with 5 ng/mL GM-CSF or with GM-CSF alone. Cell lysates were analyzed by Western blotting, using an antibody against phosphorylated ERK1/2 (upper panel). Equal protein loading in the samples was confirmed by immunodetection of ERK1/2 (lower panel).
We subsequently examined the activation of the MEK/ERK pathway at the protein level. Phosphorylation of Thr202/Tyr204 of ERK1/2 was observed within 30 seconds of fMLP stimulation (Figure 6B). GM-CSF stimulation alone led to ERK1/2 phosphorylation at a maximum at 2 minutes, though at a much lower level than fMLP-stimulated ERK1/2 phosphorylation. Priming of neutrophils with GM-CSF, followed by fMLP stimulation, resulted in enhanced ERK1/2 phosphorylation compared with the effects of either fMLP or GM-CSF alone. The MEK1/2 inhibitor U0126, when added at a concentration of 10 μM, was capable of completely inhibiting ERK1/2 phosphorylation in unprimed and GM-CSF–primed neutrophils stimulated with 1 μM fMLP and in neutrophils stimulated with 5 ng/mL GM-CSF alone (Figure 6C). Together these data suggest that the MEK-ERK route is not involved in the unprimed ROS production but that it does play a role in the GM-CSF–priming effect of the respiratory burst.
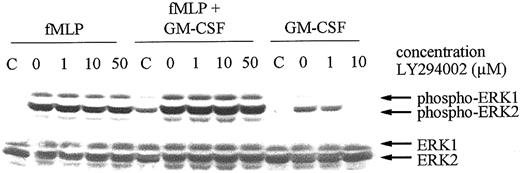
To establish the place of ERK in the signal transduction cascade, neutrophils were preincubated with LY294002 before fMLP or GM-CSF stimulation. As shown in Figure 7, ERK1/2 activation by fMLP, with or without GM-CSF priming, was not inhibited by preincubation of the cells with increasing concentrations of LY294002. In contrast, the ERK1/2 activation induced by GM-CSF stimulation alone was completely inhibited at 10 μM LY294002. These results indicate that whereas GM-CSF–mediated ERK1/2 phosphorylation is a downstream event of PI3K activation, the fMLP-stimulated ERK1/2 phosphorylation in GM-CSF–primed cells is PI3K independent.
Effect of LY294002 on the fMLP- and GM-CSF–induced phosphorylation of ERK1/2 in human neutrophils.
Neutrophils from a healthy volunteer were preincubated with the indicated concentrations of LY294002 for 30 minutes. Neutrophils were subsequently stimulated for 2 minutes with 1 μM fMLP alone, fMLP following priming of the neutrophils with 5 ng/mL GM-CSF, or with 5 ng/mL GM-CSF alone. Stimulation was stopped by the addition of 1 × Laemmli buffer to the pelleted neutrophils and by boiling the samples. Proteins were separated by SDS-PAGE, and Western blotting was performed using an antibody against phosphorylated ERK1/2 (upper panel). Lysates of unstimulated cells were run as negative controls (C). Equal loading was demonstrated by reprobing the blots with an antibody against ERK1/2 (lower panels).
Effect of LY294002 on the fMLP- and GM-CSF–induced phosphorylation of ERK1/2 in human neutrophils.
Neutrophils from a healthy volunteer were preincubated with the indicated concentrations of LY294002 for 30 minutes. Neutrophils were subsequently stimulated for 2 minutes with 1 μM fMLP alone, fMLP following priming of the neutrophils with 5 ng/mL GM-CSF, or with 5 ng/mL GM-CSF alone. Stimulation was stopped by the addition of 1 × Laemmli buffer to the pelleted neutrophils and by boiling the samples. Proteins were separated by SDS-PAGE, and Western blotting was performed using an antibody against phosphorylated ERK1/2 (upper panel). Lysates of unstimulated cells were run as negative controls (C). Equal loading was demonstrated by reprobing the blots with an antibody against ERK1/2 (lower panels).
Altered PI3K p110 protein levels and decreased PKB/Akt phosphorylation in neutrophils from MDS patients
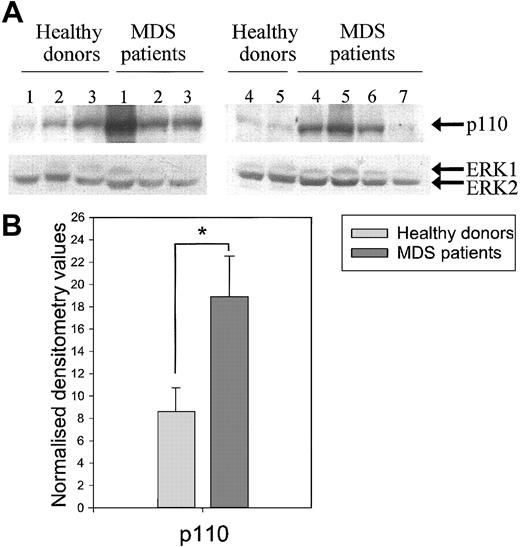
Because our results indicate involvement of PI3K in the priming of the respiratory burst and disturbed priming of this burst in neutrophils from MDS patients, we investigated the protein levels of the PI3K subunit p110 in neutrophils from healthy donors (n = 5) and MDS patients (n = 7). Figure 8 shows that though the p110 levels differed between individual donors (Figure8A), the amount of p110 present in the neutrophil lysates of MDS patients was significantly higher than in healthy volunteers after correction for ERK levels (Figure 8B, P < .05). These results indicate increased protein expression levels of the catalytically active p110 subunit in MDS neutrophils, possibly resulting in a disturbed regulation of the PI3K signaling pathway.
Increased PI3-kinase subunit p110 protein levels in neutrophils from MDS patients.
(A) Neutrophils from healthy donors (n = 5) and MDS patients (n = 7) were resuspended in 1 × Laemmli, and samples were boiled. Proteins were separated by SDS-PAGE, and Western blotting was performed using an antibody against PI3-kinase p110 (upper panel). Equal loading was demonstrated by reprobing the blots with an antibody against ERK1/2 (lower panel). (B) Protein levels were quantified by densitometry of the films. The p110 protein levels were corrected for ERK levels, and differences between the means of the normalized values were calculated between MDS neutrophils and healthy donors using the Studentt test for unpaired samples. Significant differences are indicated with an asterisk (P < .05).
Increased PI3-kinase subunit p110 protein levels in neutrophils from MDS patients.
(A) Neutrophils from healthy donors (n = 5) and MDS patients (n = 7) were resuspended in 1 × Laemmli, and samples were boiled. Proteins were separated by SDS-PAGE, and Western blotting was performed using an antibody against PI3-kinase p110 (upper panel). Equal loading was demonstrated by reprobing the blots with an antibody against ERK1/2 (lower panel). (B) Protein levels were quantified by densitometry of the films. The p110 protein levels were corrected for ERK levels, and differences between the means of the normalized values were calculated between MDS neutrophils and healthy donors using the Studentt test for unpaired samples. Significant differences are indicated with an asterisk (P < .05).
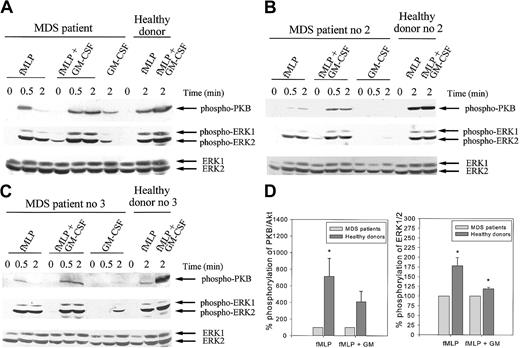
We subsequently examined the activation of PKB/Akt in neutrophils from MDS patients (n = 5). Isolated neutrophils were stimulated with fMLP with or without GM-CSF pretreatment or GM-CSF alone, and proteins were separated by SDS-PAGE. In each experiment, lysates of neutrophils from healthy controls, stimulated under the same experimental conditions, were run as controls. Time-curve experiments in neutrophils from MDS patients demonstrated maximal PKB/Akt phosphorylation after 30 seconds of fMLP stimulation and after 2 minutes of GM-CSF stimulation in most patients (Figure 9A-C). As in healthy donors, GM-CSF priming of the neutrophils from the MDS patients resulted in enhanced phosphorylation of PKB/Akt compared with the effects of fMLP or GM-CSF alone. Interestingly, in all cases studied, the total amount of phosphorylated PKB/Akt present after stimulation with fMLP with or without priming appeared lower in MDS neutrophils than in healthy controls (compare lanes 3 and 9 and lanes 6 and 10 in Figure 9A and lanes 3 and 11 and lanes 6 and 12 in Figure 9B-C). Quantification of the phosphorylation levels by densitometry indicated that the amount of phosphorylated PKB/Akt present in fMLP-stimulated neutrophil lysates was significantly higher in healthy donors than in MDS patients (P < .05). In GM-CSF–primed cells, fMLP stimulation also led to a higher phosphorylation of PKB/Akt in healthy donors than in MDS patients, though this was not significant, because of the high SEM (P = .073) (Figure 9D). Furthermore, a difference in the kinetics of phosphorylation was noted between MDS neutrophils and healthy controls. In 4 of 5 patients studied, the amount of fMLP-induced phosphorylation of PKB/Akt following GM-CSF priming was identical at 30 seconds and 2 minutes of stimulation, whereas in most healthy controls the phosphorylation level was significantly higher at 2 minutes than at 30 seconds (Figure 5B and data not shown). Protein loading was equal in all samples, as assessed by Western blotting for ERK1/2. These data indicate that in neutrophils from MDS patients, the kinetics and the maximal levels of PKB/Akt phosphorylation induced by fMLP stimulation and GM-CSF priming are affected.
Phosphorylation of PKB/AKT and ERK1/2 in response to fMLP and GM-CSF is decreased in MDS patients.
Neutrophils were isolated from whole blood from MDS patients and healthy controls as described. Cells were stimulated with 1 μM fMLP with or without priming with 5 ng/mL GM-CSF or with 5 ng/mL GM-CSF alone for the indicated time. Stimulation of the neutrophils was stopped by the boiling of samples in 1 × Laemmli buffer. Proteins were separated by SDS-PAGE, and PKB/Akt activation was detected by Western blotting, using antibodies against phosphorylated PKB/Akt (A-C, upper panels). The same blots were reprobed with an antibody against phosphorylated ERK1/2 (A-C, middle panels). ERK1/2 is shown in the lower panels and represents equal protein loading. The experiment was performed for 5 MDS patients and healthy controls. One patient was tested twice independently, with identical results. Results of 3 representative experiments are shown (A-C). (D) Phosphorylation of PKB/Akt and ERK1/2 was quantified by densitometry of the films. Level of phosphorylation of PKB/Akt and ERK1/2 in neutrophil lysates from healthy donors was expressed as a percentage of the phosphorylation observed in cell lysates from MDS patients, run on the same gel. Significant differences are indicated with asterisks (P < .05).
Phosphorylation of PKB/AKT and ERK1/2 in response to fMLP and GM-CSF is decreased in MDS patients.
Neutrophils were isolated from whole blood from MDS patients and healthy controls as described. Cells were stimulated with 1 μM fMLP with or without priming with 5 ng/mL GM-CSF or with 5 ng/mL GM-CSF alone for the indicated time. Stimulation of the neutrophils was stopped by the boiling of samples in 1 × Laemmli buffer. Proteins were separated by SDS-PAGE, and PKB/Akt activation was detected by Western blotting, using antibodies against phosphorylated PKB/Akt (A-C, upper panels). The same blots were reprobed with an antibody against phosphorylated ERK1/2 (A-C, middle panels). ERK1/2 is shown in the lower panels and represents equal protein loading. The experiment was performed for 5 MDS patients and healthy controls. One patient was tested twice independently, with identical results. Results of 3 representative experiments are shown (A-C). (D) Phosphorylation of PKB/Akt and ERK1/2 was quantified by densitometry of the films. Level of phosphorylation of PKB/Akt and ERK1/2 in neutrophil lysates from healthy donors was expressed as a percentage of the phosphorylation observed in cell lysates from MDS patients, run on the same gel. Significant differences are indicated with asterisks (P < .05).
Phosphorylation of ERK1/2 is decreased in neutrophils from MDS patients
Because we demonstrated that the MEK/ERK pathway is also involved in the GM-CSF–mediated priming of the respiratory burst, we compared the phosphorylation pattern of ERK1/2 after stimulation in neutrophils from healthy volunteers and MDS patients. FMLP-induced phosphorylation in MDS neutrophils could be enhanced by GM-CSF pretreatment in a manner similar to that in neutrophils from healthy controls (Figure 9A-C, middle panels). In all cases it was observed that the total amount of phosphorylated ERK1/2 present after 2 minutes of fMLP stimulation was slightly, but significantly, higher in GM-CSF–primed and unprimed neutrophils from healthy donors than in those from MDS patients (P = .013 and P = .010, respectively), though this was less pronounced than for phosphorylated PKB/Akt (Figure9D).
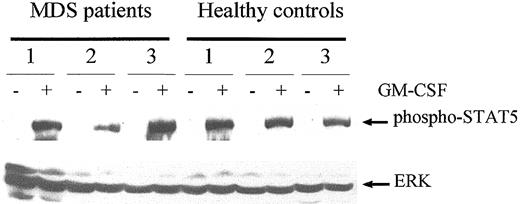
Finally, to exclude the possibility that the GM-CSF receptor in MDS is defective in general, we examined the capability of GM-CSF to induce tyrosine phosphorylation of STAT5. Figure10 shows that GM-CSF–induced STAT5 phosphorylation, though varying slightly between persons, did not differ significantly between MDS patients and healthy subjects. These data suggest that GM-CSF receptors on neutrophils from MDS patients are functional.
GM-CSF stimulates STAT5 activation in neutrophils from MDS patients and healthy controls to a similar extent.
Cell lysates from unstimulated neutrophils (−) and neutrophils that were stimulated for 10 minutes with 5 ng/mL GM-CSF (+) were analyzed for 3 MDS patients and 3 healthy volunteers. Western blot analysis was performed, and proteins were detected using an antibody against phosphorylated STAT5 (upper panel). Equal loading was confirmed by reprobing the blot with antibodies against ERK1/2.
GM-CSF stimulates STAT5 activation in neutrophils from MDS patients and healthy controls to a similar extent.
Cell lysates from unstimulated neutrophils (−) and neutrophils that were stimulated for 10 minutes with 5 ng/mL GM-CSF (+) were analyzed for 3 MDS patients and 3 healthy volunteers. Western blot analysis was performed, and proteins were detected using an antibody against phosphorylated STAT5 (upper panel). Equal loading was confirmed by reprobing the blot with antibodies against ERK1/2.
Discussion
The present study demonstrates that the responses of neutrophils from MDS patients are significantly disturbed with regard to GM-CSF and G-CSF priming of ROS production, whereas the fMLP-mediated cellular effects are not affected. In contrast to our findings, Carulli et al2 demonstrated a defect in the F-actin polymerization in neutrophils from MDS patients, but that applied especially to patients with refractory anemia (RA) with excess blasts (RAEB), RAEB in transformation (RAEB-t), and chronic myelomonocytic leukemia (CMML).2 In the present study, with patients with RA and RA with ringed sideroblasts (RARS), no significant decrease in actin polymerization was observed, though in 3 of 8 patients a neutrophil subpopulation was observed that did not polymerize F-actin in response to fMLP. However, respiratory burst measurements in these patients did not reveal separate neutrophil subpopulations; all neutrophils were capable of producing H2O2 following fMLP stimulation, indicating that the lack of F-actin polymerization was not attributed to an fMLP receptor defect. These results were further supported by our finding that Rac2 activation after fMLP stimulation was normal in neutrophils from MDS patients.
In contrast, the priming effects of GM-CSF and G-CSF on ROS production were severely disturbed in neutrophils from MDS patients. Previous studies by Zabernigg et al3 and Ohsaka et al4also demonstrated disturbed GM-CSF priming of the respiratory burst in neutrophils from most, but not all, MDS patients. In contrast to our study, Ohsaka et al4 found increased fMLP-induced O production in several patients. Although we demonstrated slightly higher O generation in response to fMLP in neutrophils from MDS patients compared with healthy donors, the difference was not significant. This discrepancy might be explained by the greater heterogeneity of the patient population reported in the study by Ohsaka et al.4 In accordance with Zabernigg et al,3 we found normal PMA-induced ROS production in neutrophils from MDS patients, indicating that the decreased respiratory burst observed after GM-CSF or G-CSF priming was not caused by a general incapability of MDS neutrophils to generate high levels of ROS.
The respiratory burst in neutrophils is produced by the reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase complex, which in its inactive state consists of flavocytochrome b558, stored in the membrane of specific granules, and the cytosolic components p47phox, p67phox, p40phox, and Rac2. On stimulation of the neutrophils, the expression of the flavocytochrome on the plasma membrane is up-regulated, and the cytosolic components are transported to the membrane.39,40 DeLeo et al41 have shown that lipopolysaccharide (LPS) priming of the neutrophil respiratory burst is accompanied by enhanced recruitment of flavocytochrome b558and association of p47phox, p67phox, and Rac2 with the plasma membrane. Recently, it has been described that PI3K binds and activates the NADPH complex, including p40phoxand p47phox, suggesting that it plays a major role in the respiratory burst.28,29 This is confirmed by our finding that the PI3K inhibitor LY294002 is capable of inhibiting fMLP-stimulated ROS production in unprimed and GM-CSF–primed neutrophils. To what extent the PI3K downstream target PKB/Akt is involved in the activation of the NADPH complex is unknown. Its involvement is often assumed as a result of the corresponding kinetics between PKB/Akt activation and respiratory burst, as measured by cytochrome c reduction in response to chemoattractants.42 Furthermore, as for ROS production, we show that the response of neutrophils to fMLP with regard to PKB/Akt phosphorylation can be primed by GM-CSF.
fMLP-induced p47phox phosphorylation has been shown to be mediated by ERK1/2.43 However, the role of ERK1/2 in the chemoattractant-stimulated respiratory burst is disputed. Some studies indicate that MAPK is at least partially responsible for fMLP-induced ROS formation,30,43-45 whereas in additional studies, no effect of ERK inhibitors on the neutrophil respiratory burst were observed.26,46 The present study demonstrates that fMLP-mediated ROS production is not significantly attenuated by the MEK inhibitor U0126. Moreover, it appeared that LY294002 at 1 and 10 μM significantly inhibited the fMLP-triggered respiratory burst without an effect on the fMLP-stimulated ERK1/2 phosphorylation in unprimed or GM-CSF–primed neutrophils, indicating a PI3K-independent activation of ERK. These data corroborate findings from Kodama et al,46who demonstrated that GM-CSF priming of ERK activity could not be inhibited by LY294002. However, ROS formation in response to fMLP in GM-CSF primed neutrophils was significantly inhibited by the MEK inhibitor U0126. These findings underscore the role of ERK in ROS production of primed granulocytes independent of the PI3K cascade and are in line with the recent findings of Hedin et al.47They demonstrate that ERK activation by Gi protein is regulated in part by a Ras- and a PI3K-independent pathway that might be modulated by tyrosine kinase. One of signaling cascades that might be involved in this process is the GTPase Rap, which can activate ERK independently of Ras.48
Limited studies have been focused on the signaling events in hematopoietic cells of MDS patients. STAT5 DNA binding in response to erythropoietin (EPO) was shown to be impaired in cells of the erythroid lineage.49,50 EPO exerts its STAT5-activating effect through the phosphorylation of Janus kinase 2 (JAK2),51,52suggesting a central role for JAK2 in the disturbed signaling in MDS. GM-CSF stimulation of neutrophils from MDS patients led to STAT5 tyrosine phosphorylation that was comparable to that in healthy controls, implying that GM-CSF receptor and JAK2 activation are normal in MDS. In contrast, a significant defect was observed with regard to PKB/Akt and ERK1/2 phosphorylation. fMLP-stimulated PKB/Akt phosphorylation in unprimed and GM-CSF–primed neutrophils was drastically lowered in MDS patients. Moreover, the kinetics of PKB/Akt phosphorylation were different than they were in healthy controls. These findings might be related to the altered p110 protein levels found in MDS neutrophils. The abundance of the catalytically active PI3K subunit p110 might result in a disturbed activation of the PI3K pathway. A recent study suggests that the overexpression of myr-p110 in murine fibroblasts, resulting in constitutive PI3K activation, does not lead to constitutive PKB/Akt activation but rather to a reduced ability of stimuli to induce the phosphorylation of PKB.53
Differences similar to those for PKB/Akt activation were observed for ERK1/2 phosphorylation, albeit to a lesser extent. Because the fMLP-mediated cellular responses were normal in MDS patients and GM-CSF could properly stimulate STAT5 activation, it is tempting to speculate that the convergence of fMLP and GM-CSF signaling is disturbed in MDS patients. It is conceivable that altered PI3K-PKB/Akt and ERK1/2 activation patterns found in MDS patients might lead to decreased priming of the respiratory burst by preventing proper assembly of the NADPH enzyme complex.
In conclusion, our studies indicate that PI3-kinase p110 protein levels are increased in neutrophils from MDS patients and that the phosphorylation of PKB/Akt and, to a lesser extent, ERK1/2 is disturbed in neutrophils from these patients. Further research will have to elucidate to what extent these alterations in signaling events are responsible for the disturbed neutrophil effector functions in MDS.
We thank all the patients and volunteers who participated in the study. We also thank Drs Paul J. Coffer and Evert Nijhuis for their assistance regarding technical procedures and for their helpful suggestions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
E. Vellenga, Department of Medicine, University Hospital Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail: e.vellenga@int.azg.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal