Previous studies have shown that approximately 20% of hemoglobin is lost from circulating red blood cells (RBCs), mainly during the second half of the cells' life span. Because hemoglobin-containing vesicles are known to circulate in plasma, these vesicles were isolated. Flow cytometry studies showed that most RBC-derived vesicles contain hemoglobin with all hemoglobin components present. The hemoglobin composition of the vesicles resembled that of old RBCs. RBC cohort studies using isotope-labeled glycine have been described, which showed a continuous presence of this label in hemoglobin degradation products. The label concentration of these products increased during the second half of the RBC life span, accompanied by a decrease within the RBC. It is concluded that the hemoglobin loss from circulating RBCs of all ages can be explained by shedding hemoglobin-containing vesicles. This loss occurs predominantly in older RBCs. Apparently the spleen facilitates this process since asplenia vesicle retention within RBCs of all ages has been described, accompanied by an increase in the percentage of total HbA1. The present study shows that in old RBCs of asplenic individuals, the decrease of hemoglobin content per cell such as seen in old RBCs of control individuals is absent due to an increase in the absolute amount of HbA1c and HbA1e2. It is concluded that hemoglobin-containing vesicles within old RBCs are “pitted” by the spleen.
Introduction
In previous studies we showed that approximately 20% of hemoglobin is lost from the circulating red blood cell (RBC).1,2 Moreover, it was clear that this loss increases substantially during the second half of the RBC life span.2 In accordance, RBC cohort studies using isotope-labeled glycine demonstrated a continuous presence of the label in hemoglobin degradation products with an increase during the second half of the RBC life span. Concomitantly, the label concentration in the RBC decreased,3,4 which indicates that hemoglobin is lost from RBCs of all ages, predominantly from older cells. As hemoglobin-containing vesicles are known to circulate in plasma,5 it appeared worthwhile to investigate their possible role in the process of hemoglobin loss. Vesicles were isolated from plasma obtained by pheresis and were studied by electron microscopy and flow cytometry. In addition, their hemoglobin composition was determined and compared with that of RBC fractions of different ages.
In individuals without a functioning spleen, the RBCs have an increased number of normally present small vacuoles (diameter < 300 nm) in combination with abnormally big vacuoles (diameter > 300 nm) in about 30% of the cells. Some of these vacuoles are known to contain hemoglobin.6 A previous study indicated a positive relation between the number of RBCs with big vacuoles (“pitted” cells), and the HbA1 percentage in RBC populations fractionated according to density.7 Likewise, the HbA1 percentage in whole blood was higher in asplenic individuals.
In order to evaluate the role of the spleen in this respect, we determined the hemoglobin content and composition of RBC populations fractionated according to volume and density in splenectomized individuals.
Materials and methods
Erythrocytes
Venous blood samples of 20 mL from 5 healthy regular blood donors and 5 splenectomized individuals who were not diabetic were collected in heparinized tubes (50 U/mL blood). Splenectomy was performed in the course of a staging laparotomy for malignant lymphoma. Chemotherapy had been administered in 2 individuals more than one year prior to blood sampling. In none of the patients has the lymphoma recurred.
Vesicles
Vesicles were isolated from plasma obtained by means of pheresis from the 5 healthy regular blood donors. After centrifugation at 8000 ×g at 4°C for 15 minutes, plasma was passed through an 0.8-μm pore size nitrocellulose filter MILLEX-AA 0.8 (Millipore, Milford, MA). The filtrate was centrifuged at 50 000 ×gat 4°C for 90 minutes. Control plasma was obtained from regular blood donations from 2 of the 5 healthy individuals. The isolated (red) pellet containing vesicles was examined with freeze-fracture electron microscopy, hemoglobin chromatography, and flow cytometry.
Electron microscopy
Freeze-fracture electron microscopy was performed as follows: glycerol was added to the samples up to a concentration of 25%. The samples were then frozen in a mixture of liquid and solid nitrogen, fractured in a Balzers freeze fracture device 301 (Balzers, Liechtenstein) and subsequently replicated with platina/carbon according to standard procedures.
Flow cytometry
The expression of glycophorin A (GPA) on and the presence of hemoglobin in vesicles were examined by flow cytometry (Beckman Coulter, Fullerton, CA). The following monoclonal antibodies were used: anti-GPA labeled with R-phycoerythrin (clone JC159, IgG1; DAKO, Glostrup, Denmark) and anti-Hb labeled with fluorescein isothiocyanate (FITC; clone 4B8, IgG2b; Wallac, Akron, OH). For each analysis 1 × 104 events were collected using Beckman Coulter software EXPO32 on the flow cytometer. In the forward scatter–side scatter dotplot a gate was set around the area with small particles. In order to identify the presence of hemoglobin, vesicles had to be blocked, fixed, permeabilized, and washed, respectively, by using a solution containing < 0.1% sodium azide, a solution containing buffered formaldehyde, a solution containing sodium dodecyl sulfate, and phosphate-buffered saline containing heparin and Triton X-100 (IQ Products, Groningen, The Netherlands).
RBC fractionation according to cell volume
RBC fractionation according to cell volume was performed using counterflow centrifugation in a Dijkstra Curame 3000 elutriation system (Dijkstra Vereenigde B.V., Lelystad, The Netherlands).
The separation was performed at a flow rate of 10 mL/min at 20°C. An isotonic buffer containing albumin and glucose (GASP) was used. The composition of the GASP buffer was 9 mM Na2HPO4, 1.3 mM NaH2PO4, 140 mM NaCl, 5.5 mM glucose, and 0.8 g/L bovine serum albumin (fraction V; Sigma Chemical, St Louis, MO), pH 7.4. Blood was diluted 1:3 with the GASP buffer, and 2 mL of this suspension was introduced into the buffer flow to the elutriation chamber. RBCs with increasing volume were eluted from the elutriation chamber at 6 different speeds of rotation from 2300 to 1700 rpm, yielding 6 fractions.
RBC fractionation according to cell density
RBC fractionation according to density was performed by means of a discontinuous Percoll gradient essentially as described by Rennie et al.8 The gradient was built up in 5 layers of 2 mL containing 80%, 73%, 68%, 65%, and 40% for healthy individuals and 80%, 67%, 64%, 61%, and 40% for splenectomized individuals (to achieve an even as possible distribution of RBCs in the fractions, in view of the lower mean cellular hemoglobin concentration [MCHC] of these individuals). In this way fractions with the lowest HbA1c percentage contained 2.5% and 2.1% of the total number of RBCs in healthy and asplenic individuals, respectively, whereas fractions with the highest HbA1c contained 0.4% and 2.9%, respectively. The Percoll solutions were buffered with a HEPES (N-(2-hydroxyethil)-piperazine-N'-2-ethanesulphonic acid) buffer containing 5% bovine serum albumin, 132 mM NaCl, 4.6 mM KCl, and 10 mM HEPES, pH 7.1.
RBCs were washed with GASP buffer and diluted with 1 volume of GASP buffer; 0.5 mL of this suspension was layered on the Percoll gradient. Separation was achieved after 15 minutes' centrifugation at 3000 rpm at room temperature. Four RBC fractions were collected by careful pipetting and then extensively rinsed with GASP buffer in order to remove the residual Percoll.
RBC fractionation
RBC fractionation was performed by means of counterflow centrifugation followed by Percoll gradient centrifugation, yielding 24 fractions of various ages, as published earlier.1
RBC parameters
The RBC parameters mean cellular volume (MCV) and MCHC were determined on a Technicon H1 (Bayer-Technicon, Tarrytown, NY). The MCV was directly measured. The MCHC was measured using the sideways scatter of the flow cytometry signal called CHCM. This CHCM was converted to mM. The value of the hemoglobin content per cell (MCH) was calculated as MCV × CHCM.
Hemoglobin fractionation
Hemoglobin fractionation was performed by means of ion exchange chromatography (Protein-Pak, SP8HR, Millipore). The elution buffers used were based on a buffer system as described by Jeppson et al.9 The composition of buffer A was 20 mM malonic acid and 1 mM NaCN, pH 5.9, in high-performance liquid chromatography (HPLC)–grade water. Buffer B had the same composition as buffer A with the addition of 380 mM NaNO3. Sodium cyanide was used as a stabilizer of hemoglobin. Before use, the buffers were passed through a 0.22-mm filter and degassed. The column was kept at 30°C. The hemoglobin components were separated with the use of a buffer gradient and monitored at 417 nm with a photodiode array detector; in this way an absorption spectrum at the top of each peak could be measured. Those spectra indicated the absence of oxidation of all hemoglobin components. The relative amount of each component was calculated from the peak area of that component at 417 nm divided by the total area of all components. With the use of this technique, 10 fractions were separated, as shown in Figure1. However, fraction HbA1dwas not always sufficiently separated from fraction HbA1c. In further analysis these 2 fractions were combined. Unknown components were found that eluted after HbA2. One component could chromatographically be discriminated from the others and was designated “X”; the other components as “Rest.” The absorption spectrum of “X” indicated the possibility of a heme-containing component. The absolute amount of all components in the RBCs was calculated from the relative amount times MCH. All data shown are the mean of the results of 5 healthy and 5 asplenic volunteers.
HPLC chromatogram of hemoglobin components.
Elution profile OD (—); percentage buffer (- - -).
HPLC chromatogram of hemoglobin components.
Elution profile OD (—); percentage buffer (- - -).
Statistical analysis
Significance was analyzed using the Student t test or Mann-Whitney U test. Regression analysis was done using second order linear regression, because goodness of fit was substantially better than using first order linear regression.
Results
Normal condition
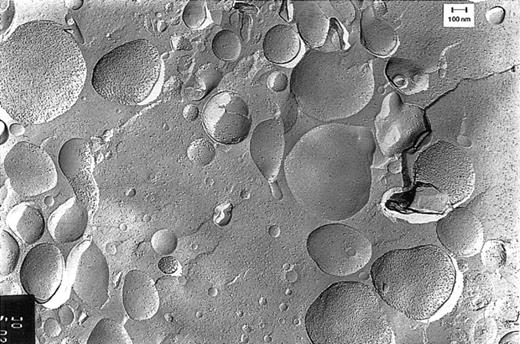
Freeze-fracture electron microscopy studies showed that the red-colored pellet obtained by centrifugation of pheresis plasma contained vesicles (Figure 2). As can be deduced from the density of the membranous particle distribution on the convex and concave fracture faces, there were relatively small vesicles (< 200 nm) with right-side-out– and inside-out–oriented membranes and vesicles with a wide variety in size (200-800 nm), with predominantly right-side-out membranes. This picture is not an artifact created by the plasmapheresis procedure, because vesicles from plasma isolated from regular blood donations without pheresis showed an identical picture.
Freeze-fracture electron microscopy of vesicles.
Vesicles were isolated from plasma obtained by pheresis from a healthy blood bank donor. The sidedness of the vesicles can be deduced from the density of the intramembranous particles on the convex and concave fracture faces of the RBC membranes. This density is 3 times higher on the protoplasmic than on the exoplasmic fracture face.10
Freeze-fracture electron microscopy of vesicles.
Vesicles were isolated from plasma obtained by pheresis from a healthy blood bank donor. The sidedness of the vesicles can be deduced from the density of the intramembranous particles on the convex and concave fracture faces of the RBC membranes. This density is 3 times higher on the protoplasmic than on the exoplasmic fracture face.10
Flow cytometry studies demonstrated that vesicles were derived mostly from RBCs (approximately 65% of the vesicles reacted with the RBC-specific antiserum against glycophorin A). One third of the vesicles were platelet derived (35% anti–CD41-FITC positive). After fixation and permeabilization, the percentage GPA-positive vesicles was significantly reduced to approximately 30%, irrespective of the presence of anti-Hb (Table 1). This can be explained by loss of GPA antigenicity during the procedure. Since all vesicles, GPA positive and/or Hb positive, are RBC derived (both GPA and Hb are specific markers), 74% (40.4 of 54.9) of all RBC-derived vesicles contain hemoglobin (Table2). Under the assumption that the presence of hemoglobin is unaffected by the fixation/permeabilization procedure, 62% (40.4 of 65.4) of all GPA-positive vesicles contain hemoglobin (Tables 1 and 2). From both percentages it can be concluded that most of the RBC-derived vesicles contain a detectable amount of hemoglobin.
Effect of fixation and permeabilization on GPA positivity of vesicles in plasma obtained by pheresis (n = 8)
| Vesicles . | Anti–GPA-PE positive (% ± SD) . |
|---|---|
| Before fixation/permeabilization | 65.4 ± 11.7* |
| After fixation/permeabilization | |
| In the absence of anti–Hb-FITC | 31.7 ± 5.9† |
| In the presence of anti–Hb-FITC | 29.8 ± 4.6† |
| Vesicles . | Anti–GPA-PE positive (% ± SD) . |
|---|---|
| Before fixation/permeabilization | 65.4 ± 11.7* |
| After fixation/permeabilization | |
| In the absence of anti–Hb-FITC | 31.7 ± 5.9† |
| In the presence of anti–Hb-FITC | 29.8 ± 4.6† |
Anti–GPA-PE negative vesicles were nearly all anti-CD41 positive.
Significantly different (P < .0002) from control vesicles (Student paired t test).
Flow cytometry after fixation and permeabilization of vesicles in plasma obtained by pheresis using anti–GPA-PE and anti–Hb-FITC (n = 8)
| Vesicles . | Percentage ± SD . |
|---|---|
| All GPA+ | 29.8 ± 4.6 |
| All Hb+ | 40.4 ± 6.4 |
| GPA+; Hb− | 14.5 ± 1.4 |
| GPA+; Hb+ | 15.3 ± 4.4 |
| GPA−; Hb+ | 25.1 ± 3.4 |
| GPA−; Hb− | 45.2 ± 6.2 |
| Vesicles . | Percentage ± SD . |
|---|---|
| All GPA+ | 29.8 ± 4.6 |
| All Hb+ | 40.4 ± 6.4 |
| GPA+; Hb− | 14.5 ± 1.4 |
| GPA+; Hb+ | 15.3 ± 4.4 |
| GPA−; Hb+ | 25.1 ± 3.4 |
| GPA−; Hb− | 45.2 ± 6.2 |
The composition of the hemoglobins found in the vesicles was determined and compared with results obtained from measurements in the RBC fractions with the lowest and highest HbA1c percentage, that is, the youngest and oldest RBC fractions, respectively (Table 3). From these comparisons it can be concluded that the hemoglobin composition of vesicles closely resembles that of old RBCs.
Hemoglobin components in young and old RBCs and vesicles obtained by plasmapheresis from control individuals
| Hb component . | Percentage of total hemoglobin ± SD . | ||
|---|---|---|---|
| Young RBCs3-150 (n = 5) . | Old RBCs3-151 (n = 5) . | Vesicles (n = 4) . | |
| HbA1 | 9.51 ± 2.483-152 | 16.71 ± 3.93 | 16.40 ± 2.69 |
| HbA1a | 0.85 ± 0.34 | 1.61 ± 0.75 | 1.28 ± 0.37 |
| HbA1b | 1.29 ± 0.62 | 2.03 ± 1.33 | 1.29 ± 0.17 |
| HbA1c | 4.07 ± 1.223-152 | 7.27 ± 0.63 | 6.83 ± 0.90 |
| HbA1e1 | 1.00 ± 0.78 | 1.99 ± 1.82 | 2.18 ± 1.38 |
| HbA1e2 | 2.30 ± 0.363-152 | 3.81 ± 0.493-152 | 4.90 ± 0.23 |
| HbA0 | 82.15 ± 3.34 | 74.76 ± 4.77 | 78.50 ± 3.24 |
| HbA2 | 3.94 ± 0.733-152 | 3.44 ± 0.693-152 | 2.62 ± 0.14 |
| HbF | 0.40 ± 0.18 | 0.43 ± 0.13 | 0.27 ± 0.11 |
| HbX + rest | 4.00 ± 2.17 | 4.66 ± 3.63 | 2.13 ± 0.59 |
| Hb component . | Percentage of total hemoglobin ± SD . | ||
|---|---|---|---|
| Young RBCs3-150 (n = 5) . | Old RBCs3-151 (n = 5) . | Vesicles (n = 4) . | |
| HbA1 | 9.51 ± 2.483-152 | 16.71 ± 3.93 | 16.40 ± 2.69 |
| HbA1a | 0.85 ± 0.34 | 1.61 ± 0.75 | 1.28 ± 0.37 |
| HbA1b | 1.29 ± 0.62 | 2.03 ± 1.33 | 1.29 ± 0.17 |
| HbA1c | 4.07 ± 1.223-152 | 7.27 ± 0.63 | 6.83 ± 0.90 |
| HbA1e1 | 1.00 ± 0.78 | 1.99 ± 1.82 | 2.18 ± 1.38 |
| HbA1e2 | 2.30 ± 0.363-152 | 3.81 ± 0.493-152 | 4.90 ± 0.23 |
| HbA0 | 82.15 ± 3.34 | 74.76 ± 4.77 | 78.50 ± 3.24 |
| HbA2 | 3.94 ± 0.733-152 | 3.44 ± 0.693-152 | 2.62 ± 0.14 |
| HbF | 0.40 ± 0.18 | 0.43 ± 0.13 | 0.27 ± 0.11 |
| HbX + rest | 4.00 ± 2.17 | 4.66 ± 3.63 | 2.13 ± 0.59 |
,
Fractions with lowest and highest HbA1c values, respectively.
Significantly different (P < .05) from vesicles (Mann-Whitney U test, one-tailed Pvalue).
Asplenia
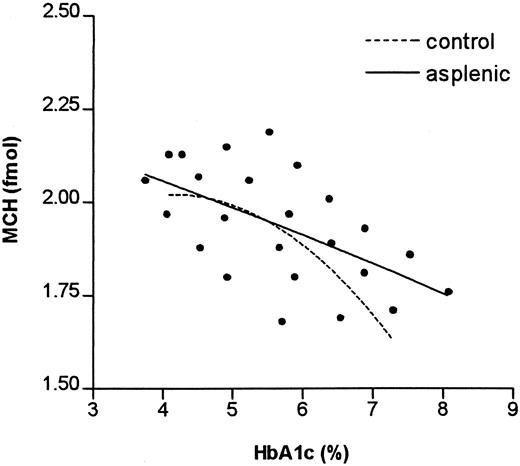
The MCH was determined in RBC fractions of different ages from control and asplenic individuals (Figure3). From this figure it can be deduced that the MCH in controls decreased from 2.0 to 1.6 fmol per cell (20%) and in asplenic individuals from 2.05 to 1.75 fmol per cell (15%). This difference of decrease in MCH is caused by the absence in asplenic individuals of the additional decrease in MCH as seen in older cells of control individuals.
The relationship between MCH and HbA1c in control and asplenic individuals.
The results of 24 fractions of asplenic individuals are shown. Each symbol represents the mean of 5 observations per fraction. The drawn line is the second order curve fit of the asplenic individuals; the broken line, that of the controls (individual values not given).
The relationship between MCH and HbA1c in control and asplenic individuals.
The results of 24 fractions of asplenic individuals are shown. Each symbol represents the mean of 5 observations per fraction. The drawn line is the second order curve fit of the asplenic individuals; the broken line, that of the controls (individual values not given).
A comparison of the hemoglobin composition of RBCs of asplenic individuals with that of RBCs of control individuals shows that in whole blood of asplenic individuals, the percentage of HbA1is increased, due mainly to an increase of HbA1c and HbA1e2, in combination with a lower percentage HbA0 (Table4).
Hemoglobin components in RBCs of control and asplenic individuals
| Hb component . | Percentage of total hemoglobin ± SD . | |
|---|---|---|
| Controls (n = 8) . | Asplenic individuals (n = 5) . | |
| HbA1 | 10.66 ± 0.98 | 11.84 ± 1.214-150 |
| HbA1a | 0.55 ± 0.10 | 0.69 ± 0.094-150 |
| HbA1b | 0.97 ± 0.30 | 0.90 ± 0.08 |
| HbA1c | 5.33 ± 0.40 | 5.86 ± 0.484-150 |
| HbA1e1 | 0.76 ± 0.27 | 0.78 ± 0.29 |
| HbA1e2 | 3.06 ± 0.23 | 3.61 ± 0.554-150 |
| HbA0 | 84.62 ± 1.28 | 82.27 ± 1.444-150 |
| HbA2 | 3.08 ± 0.39 | 3.38 ± 0.50 |
| HbF | 0.35 ± 0.17 | 0.23 ± 0.07 |
| HbX + rest | 1.38 ± 0.40 | 2.28 ± 0.674-150 |
| Hb component . | Percentage of total hemoglobin ± SD . | |
|---|---|---|
| Controls (n = 8) . | Asplenic individuals (n = 5) . | |
| HbA1 | 10.66 ± 0.98 | 11.84 ± 1.214-150 |
| HbA1a | 0.55 ± 0.10 | 0.69 ± 0.094-150 |
| HbA1b | 0.97 ± 0.30 | 0.90 ± 0.08 |
| HbA1c | 5.33 ± 0.40 | 5.86 ± 0.484-150 |
| HbA1e1 | 0.76 ± 0.27 | 0.78 ± 0.29 |
| HbA1e2 | 3.06 ± 0.23 | 3.61 ± 0.554-150 |
| HbA0 | 84.62 ± 1.28 | 82.27 ± 1.444-150 |
| HbA2 | 3.08 ± 0.39 | 3.38 ± 0.50 |
| HbF | 0.35 ± 0.17 | 0.23 ± 0.07 |
| HbX + rest | 1.38 ± 0.40 | 2.28 ± 0.674-150 |
Significantly different (P < .05) from controls (Mann-Whitney U test, one-tailedP value).
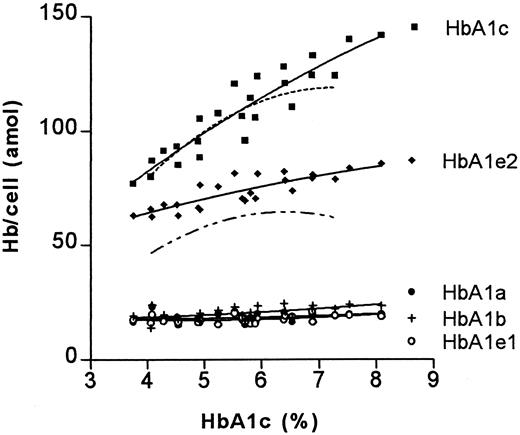
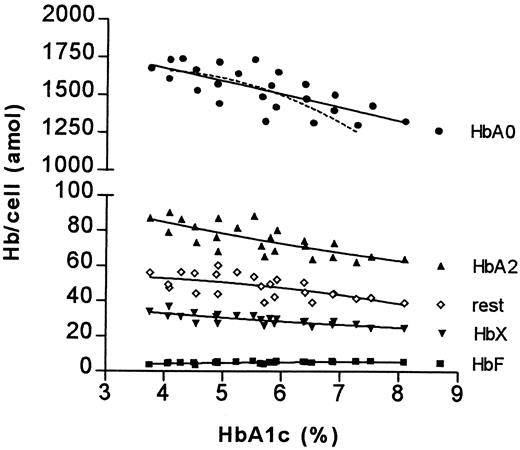
These relative differences appear to be RBC age dependent. When the absolute amounts of hemoglobin components in RBC fractions of different age were analyzed, it became evident that the increase of the HbA1c percentage as well as the decrease of the HbA0 percentage are caused only by changes in older cell fractions; the difference in HbA1e2 percentage less so (Figure4 and Figure5).
The relationship between HbA1c percentage and absolute amount of glycated hemoglobins.
For details see legend to Figure 3.
The relationship between HbA1c percentage and absolute amount of glycated hemoglobins.
For details see legend to Figure 3.
The relationship between HbA1c percentage and absolute amount of nonglycated hemoglobins.
For details see legend to Figure 3.
The relationship between HbA1c percentage and absolute amount of nonglycated hemoglobins.
For details see legend to Figure 3.
Examination of the changes in the absolute amounts of HbA1c, HbA1e2, and HbA0 in the youngest and oldest fractions of control and asplenic individuals indicates that the relative increase of MCH of old cells of asplenic individuals is mainly the result of an additional increase in the absolute amounts of HbA1c and HbA1e2 (Table5).
The absolute amounts of HbA1c, HbA1e2, and HbA0 in young and old RBCs of control (n = 5) and asplenic individuals (n = 5)
| Hb component . | Absolute amount (amol/cell ± SD) . | |||
|---|---|---|---|---|
| Young5-150 . | Old5-151 . | |||
| Control . | Asplenic . | Control . | Asplenic . | |
| HbA1c | 67.5 ± 16.8 | 87.1 ± 16.0 | 98.0 ± 5.5 | 141.7 ± 10.65-152 |
| HbA1e2 | 47.1 ± 5.8 | 62.7 ± 13.1 | 63.7 ± 6.9 | 85.5 ± 5.75-152 |
| HbA0 | 1691 ± 130 | 1735 ± 42 | 1251 ± 138 | 1329 ± 46 |
| Hb component . | Absolute amount (amol/cell ± SD) . | |||
|---|---|---|---|---|
| Young5-150 . | Old5-151 . | |||
| Control . | Asplenic . | Control . | Asplenic . | |
| HbA1c | 67.5 ± 16.8 | 87.1 ± 16.0 | 98.0 ± 5.5 | 141.7 ± 10.65-152 |
| HbA1e2 | 47.1 ± 5.8 | 62.7 ± 13.1 | 63.7 ± 6.9 | 85.5 ± 5.75-152 |
| HbA0 | 1691 ± 130 | 1735 ± 42 | 1251 ± 138 | 1329 ± 46 |
,
Fractions with lowest and highest HbA1c values, respectively.
Significantly different (P < .01) from control old RBCs (Mann-Whitney U test, two-tailedP value).
Discussion
Using a fractionation method that combines counterflow centrifugation (elutriation) with a discontinuous Percoll gradient fractionation, 24 fractions of different cell age could be obtained.1 As a marker of cell age the HbA1cpercentage, validated in iron-59 cohort studies,11-13 was used. By means of this marker it became evident that changes of MCV, MCH, and MCHC are age dependent.1,2 The MCV and MCH indeed decrease by 30% and 20%, respectively, whereas the MCHC increases by 15%.1,2 The decrease in MCH occurs mainly during the second half of the RBC life span. In accordance,15N-glycine and 14C-glycine studies show a continuous presence of the label in the hemoglobin degradation products stercobilin and urobilin, respectively, accompanied by a decrease of the label concentration within the RBC.3,4 A similar decrease was seen in studies featuring 59Fe as cohort label.11,12 Both the MCH and glycine studies indicate that hemoglobin loss from circulating RBCs predominantly occurs during the second half of the RBC life span. As hemoglobin-containing vesicles are known to circulate in plasma,5 we used various methods to analyze them. Irrespective of the way plasma was obtained, electron microscopy analysis showed that vesicles are heterogeneous in size. As to the origin of these vesicles, 2 different mechanisms seem to be involved: the large vesicles are predominantly pinched off, whereas the relatively small vesicles are pinched off as well as fragmented. Immunoblot data fail to demonstrate the presence of most cytoskeletal proteins such as spectrin but show the presence of integral membrane proteins such as band 3 (F.L.A.W., G.J.C.G.M.B., and J.M.W., manuscript in preparation). Examination by flow cytometry showed that approximately 65% of the vesicles are RBC derived and that most RBC-derived vesicles contain hemoglobin.
When the hemoglobin composition of vesicles was studied, we identified all hemoglobin components that are normally found within RBCs. The hemoglobin composition closely resembled that of old RBCs. The results from these vesicle studies suggest a continuous loss of hemoglobin in vesicles increasing during the second half of the RBC life span. This is in accordance with studies that show small-size vesicles (< 300 nm) within normal RBCs.6
In asplenia, not only a 4-fold increase in the number of these small vesicles can be found, but also abnormally big vacuoles (diameter > 300 nm) in about 30% of RBCs, some of which are known to contain hemoglobin.6 A previous study indicated a positive relationship between the number of RBCs with big vacuoles (“pitted” cells, as seen with phase-contrast microscopy) and the HbA1percentage in RBC populations fractionated according to density.7 Also, in this study the HbA1percentage of whole blood was increased. In order to clarify a possible relationship, we determined the MCH and hemoglobin composition in RBC populations fractionated according to volume and density in splenectomized individuals. Our present data show that the extra decrease of MCH observed in older fractions of control RBCs is absent in RBCs of asplenic individuals. This phenomenon must be attributed to a lesser decrease of HbA0 and an enhanced increase of HbA1c and HbA1e2 in these older fractions, which suggests retention of hemoglobin-containing vesicles.
In summary we conclude that circulating RBCs lose hemoglobin by vesiculation, especially from older cells, and that the spleen facilitates this latter process in analogy to the “pitting” of Heinz bodies.14
Preliminary observations suggest that rat RBCs lose hemoglobin in a comparable way by vesiculation. The resulting vesicles are rapidly cleared from the circulation chiefly by Kupfer cells in the liver and, to a lesser extent, by other parts of the mononuclear phagocyte system. As in rats, vesicles in humans probably also are rapidly eliminated from the circulation, presumably by an immunological process.15
Our findings do not only elucidate some aspects of the heterogeneous nature of RBC indices, but they also help to understand RBC survival studies in which hemoglobin is labeled either with a cohort label (glycine and iron) or a random label (sodium chromate). When considering the efficacy of RBC transfusions, it is obvious that considerable loss of hemoglobin from circulating donor cells is another reason why the effect of an RBC transfusion is less than one would expect. Furthermore, these findings should be taken into account while studying diabetic control, as already during normoglycemia, asplenia gives rise to an increase of the HbA1cpercentage.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-02-0500.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
J. M. Werre, Department for Bloodtransfusion and Transplantation Immunology, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail:werredehaas@planet.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal