Patients with severe acute chest syndrome (ACS) requiring endotracheal intubation and erythrocytopheresis are at increased risk for neurologic morbidity. This study examines patients with sickle cell disease who developed severe episodes of ACS, leading to endotracheal intubation, ventilatory support for respiratory failure, and erythrocytapheresis. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) studies, a neurologic examination by a pediatric neurologist, and cognitive testing were done in all patients. Five consecutive patients, aged 3 to 9 years, were identified with severe ACS. All patients developed neurologic complications resulting from ACS episodes, including seizures (n = 2), silent cerebral infarcts (n = 3), cerebral hemorrhage (n = 2), and reversible posterior leukoencephalopathy syndrome (n = 3). Children with severe ACS should have a magnetic resonance image of the brain, neurologic examination by a neurologist, and cognitive testing to detect the presence of neurologic morbidity.
Introduction
Acute chest syndrome (ACS) is one of the leading causes of hospitalization and mortality in patients with sickle cell disease.1 Children and adults with ACS are at an increased risk of respiratory failure requiring intubation and are subsequently at risk of overt strokes.2 In a large, prospective study of 538 children and adults, approximately 13% of patients with ACS had a severe course requiring mechanical ventilation.2 The rate of overt neurologic complications (seizures, altered mental status, and strokes) following an episode of ACS was 22% in adults and 8% in children.2 The main limitation of this study is that important clinical outcomes were not assessed, including silent cerebral infarcts and cognitive impairment. The identification of silent cerebral infarcts is particularly important because children with these lesions have a morbid condition associated with poor academic performance and low cognitive test scores.3Further, such children are at risk of subsequent overt strokes and/or further silent cerebral infarcts.4 5
Given the vulnerability of children with sickle cell disease for developing cerebral ischemic injury in the context of respiratory failure, coupled with the observation that silent cerebral infarcts are difficult to detect clinically,6 we elected to perform magnetic resonance imaging (MRI) of brain, obtain a neurologic evaluation by a pediatric neurologist, and obtain cognitive tests after patients were extubated.
Patients and methods
Institutional review board approval was obtained from Washington University School of Medicine. Consent was not obtained from the patients or families because all medical information obtained was identified as part of routine care and was present in their medical records. Patients' identifications were hidden. This case series examined all consecutive patients from 1996 to 2001 with hemoglobin SS diagnosed with ACS requiring endotracheal intubation and mechanical ventilation at St Louis Children's Hospital (SLCH), a tertiary care center. A diagnosis of ACS was defined by the radiologic finding of a new pulmonary radiodensity on chest radiograph in association with chest pain, fever, tachypnea, wheezing, or cough. Hospital records regarding the patients' neurologic status were reviewed, and all patients were examined by a pediatric neurologist (M.N.) following the ACS episode. MRI and magnetic resonance angiography (MRA) studies were obtained after stabilization of respiratory status, and all studies were reviewed by a single neuroradiologist, retrospectively, for the purpose of this study (R.M.). Silent cerebral infarcts and reversible posterior leukoencephalopathy syndrome (RPLS) were diagnosed on the basis of MRI findings and clinical symptoms.7,8 Silent cerebral infarct was defined in an individual with T2 hyperintensity on diffusion-weighted images (DWIs) and a decreased diffusion coefficient in regions of T2 hyperintensity without focal neurologic deficits lasting more than 24 hours' duration. RPLS was defined in an individual with an increased diffusion coefficient in areas of T2 hyperintensities on DWI in the context of clinical symptoms or physical findings associated with RPLS, including headache, seizures, visual disturbances, and altered level of consciousness.7 8 All patients underwent cognitive testing following discharge.
Case 1
Hospital course.
Patient 1, a 6-year-old boy with hemoglobin SS, presented with back pain, fever, and cough. On hospital day 2, the patient experienced progressive respiratory distress and ACS requiring erythrocytapheresis and intubation with aggressive ventilatory support and sedation for 17 days of his 21-day hospitalization. On day 13, the patient became hypertensive compared with age- and sex-matched children with sickle cell disease (90th percentile, 108 mm Hg; median, 100 mm Hg).9 During hospitalization, systolic blood pressures (SBPs) ranged from 102 to 158 mm Hg, with SBPs greater than 120 mm Hg from day 11 to day 20. Daily fluid intake averaged 2.35 L/m2 per day with a positive fluid balance of 0.5 L/m2 per day for 4 days prior to the onset of hypertension.
Neurologic examination.
Three days after extubation, on day 20, the patient developed a severe nonthrobbing headache. No focal neurologic signs or changes in level of consciousness were identified.
Imaging studies.
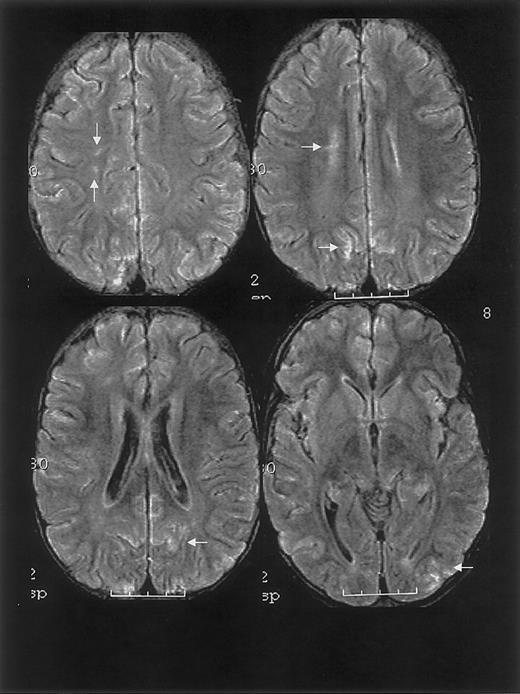
One year prior to ACS episode, the patient had a normal MRI study. MRI on hospital day 20 and 2.5 months following discharge revealed findings consistent with RPLS and silent cerebral ischemia (Figure 1).
MRI findings for case 1.
Multifocal areas of fluid-attenuated inversion recovery (FLAIR) T2-weighted signal abnormality in the subcortical white matter and overlying gray matter of the frontal, parietal, and occipital lobes (bottom).
MRI findings for case 1.
Multifocal areas of fluid-attenuated inversion recovery (FLAIR) T2-weighted signal abnormality in the subcortical white matter and overlying gray matter of the frontal, parietal, and occipital lobes (bottom).
Treatment and follow-up.
For approximately 5 years, the patient has received blood transfusion therapy with no evidence of subsequent stroke or neurologic morbidity.
Case 2
Hospital course.
Patient 2, a 4.5-year-old boy with hemoglobin SS, was readmitted to the hospital for abdominal and back pain 2 days following recent discharge. On hospital day 2, the patient developed respiratory distress, which progressed to acute respiratory distress syndrome (ARDS) requiring erythrocytapheresis and intubation with maximum ventilatory support and heavy sedation for 9 days of his 17-day hospitalization. On day 4, the patient became hypertensive (SBP, 170 mm Hg) temporally associated with receiving a blood transfusion and was persistently hypertensive (SBP range, 140-160 mm Hg) for 4 days when compared with the patient's baseline (110/70 mm Hg) and age- and sex-matched children with sickle cell disease (90th percentile, 110 mm Hg; median, 95 mm Hg).9 The patient was then treated with captopril. Prior to the onset of hypertension, daily average fluid intake was 2.4 L/m2 per day with a net positive fluid balance of 0.75 L/m2 per day for 5 days.
Neurologic examination.
On day 9, the patient developed generalized and multifocal seizures with postictal alteration in mental status. Neurologic evaluation revealed transient, mild right arm weakness and eye deviation to the left that resolved within 1 hour.
Imaging studies.
One year prior to ACS episode, the patient had normal MRI/MRA and transcranial Doppler studies. MRI on day 10 and 1 month following discharge revealed RPLS with superimposed ischemic white matter changes (Figure 2).
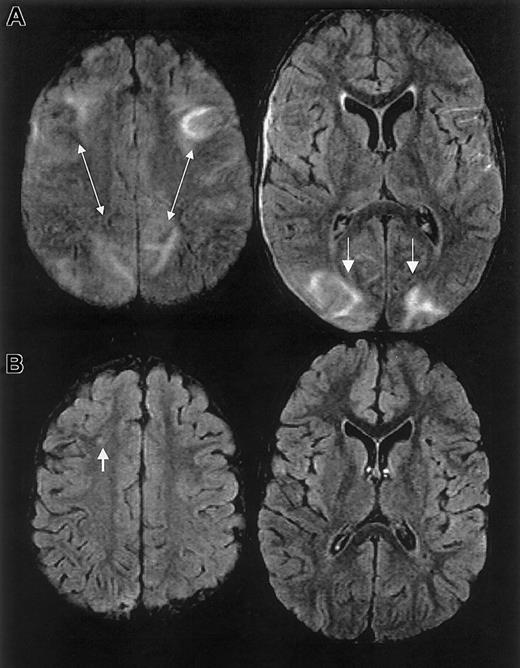
MRI findings for case 2.
Multifocal areas of signal hyperintensity predominantly limited to the cortex of the posterior parietal and occipital lobes on FLAIR T2-weighted images, including punctate areas of abnormality in the deep, periventricular white matter of the right frontal lobe and of the cortex of the right medial parietal lobe. MRI done 1 month following discharge revealed punctate lesions in the frontal lobe, representing subacute to chronic ischemic changes, but resolution of the T2 hyperintense lesions in the posterior distribution.
MRI findings for case 2.
Multifocal areas of signal hyperintensity predominantly limited to the cortex of the posterior parietal and occipital lobes on FLAIR T2-weighted images, including punctate areas of abnormality in the deep, periventricular white matter of the right frontal lobe and of the cortex of the right medial parietal lobe. MRI done 1 month following discharge revealed punctate lesions in the frontal lobe, representing subacute to chronic ischemic changes, but resolution of the T2 hyperintense lesions in the posterior distribution.
Treatment and follow-up.
The patient is receiving hydroxyurea therapy, antiseizure medication, and antihypertensive medication. The neurologic examination and MRI of the brain 8 months later have remained unchanged.
Case 3
Hospital course.
Patient 3, a 3-year-old boy with hemoglobin SS, presented with abdominal pain and decreased oral intake. Overnight, the patient developed increasing pain and ACS, which progressed further to ARDS and cardiorespiratory arrest. The patient was resuscitated and intubated requiring maximum ventilatory support and neuromuscular blockade for 11 days of his 31-day hospitalization. On hospital day 3, the patient received erythrocytapheresis. The patient was initially normotensive (SBP, 106 mm Hg) but on day 4 became hypertensive (SBP range, 120-159 mm Hg) for 8 days when compared with his baseline (106/60 mm Hg) and age- and sex-matched children with sickle cell disease (90th percentile, 104 mm Hg; median, 90 mm Hg).9Prior to the onset of hypertension, he received an average of 2.4 L/m2 per day of fluid and had an average net positive fluid balance of 1.1 L/m2 per day for 8 days. The patient was intermittently treated with furosemide.
Neurologic examination.
After extubation, the patient was slow to recover to his baseline level of alertness. Strength was reduced symmetrically to 2+/5. The patient was nonresponsive to visual confrontation and had difficulty focusing and following commands. By discharge, the patient was alert, active, and mobile; strength and speech were normal.
Imaging studies.
The initial clinical reading of the MRI, 1 day following endotracheal extubation, was bilateral ischemic infarcts and biparietal hemorrhages. The subsequent research reading of the initial MRI and follow-up MRI at 1 month and 15 months following discharge revealed findings consistent with RPLS complicated by hemorrhage; there was no evidence of infarction (Figure 3).
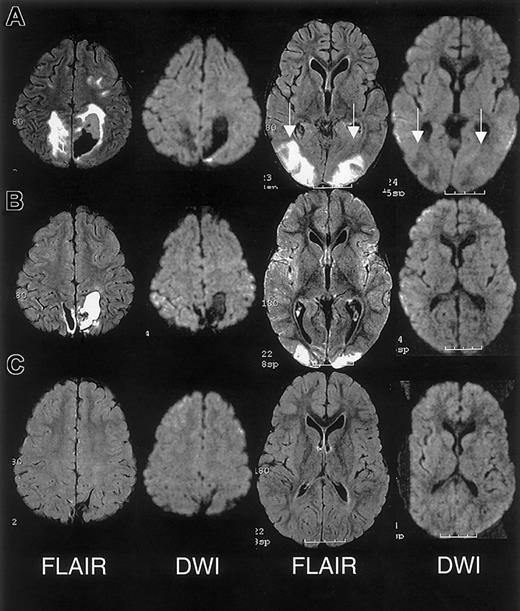
MRI findings for case 3.
(A) Extensive areas of FLAIR T2-weighted signal hyperintensity in the subcortical white matter and overlying gray matter of the posterior, frontal, and occipital lobes. Parenchymal hemorrhage was seen in the high parietal region predominantly on the patients's left. (B-C) Follow-up MRI scan 1 month and 15 months following discharge showed progressive evolution and resolution of the T2 signal hyperintensities with residual encephalomalacia in the left parietal region. These findings were consistent with reversible posterior leukoencephalopathy complicated by hemorrhage; there was no evidence of infarction.
MRI findings for case 3.
(A) Extensive areas of FLAIR T2-weighted signal hyperintensity in the subcortical white matter and overlying gray matter of the posterior, frontal, and occipital lobes. Parenchymal hemorrhage was seen in the high parietal region predominantly on the patients's left. (B-C) Follow-up MRI scan 1 month and 15 months following discharge showed progressive evolution and resolution of the T2 signal hyperintensities with residual encephalomalacia in the left parietal region. These findings were consistent with reversible posterior leukoencephalopathy complicated by hemorrhage; there was no evidence of infarction.
Treatment and follow-up.
The patient received blood transfusion therapy for 2 years, based on the clinical reading of cerebral infarction and severe ACS without any evidence of progressive neurologic disease on the basis of annual MRI examinations. The parents were given the option to continue blood transfusion therapy or to receive hydroxyurea therapy, and they chose the latter.
Case 4
Hospital course.
Patient 4, a 3.5-year-old girl with hemoglobin SS, presented with fever and abdominal pain. On hospital day 2, the patient developed ACS and ARDS requiring erythrocytapheresis, and the patient was intubated for 58 days of her 176-day hospitalization because of chronic respiratory failure. On day 59, the patient had a tracheostomy for prolonged ventilatory support. SBP ranged from 112 to 130 mm Hg for 16 days following erythrocytapheresis, and on day 18 the patient became markedly hypertensive (SBP range, 130-178 mm Hg) compared with baseline (118/80 mm Hg) and age- and sex-matched children (median, 90 mm Hg; 90th percentile, 100 mm Hg) for approximately 25 days. The patient was treated with nifedipine, captopril, and furosemide during periods of hypertension. During the 5 days prior to the onset of hypertension, the patient received an average of 1.6 L/m2 per day with a net positive fluid imbalance of approximately 0.5 L/m2 per day. Following tracheostomy, the patient was normotensive.
Neurologic examination.
On initial neurologic examination, tone proximally was reduced but increased at the ankles. She had markedly reduced endurance because of deconditioning and required intensive physical, occupational, and speech therapy. At discharge, on day 176, the neurologic examination was normal except for minimal delays in age-appropriate developmental skills.
Imaging studies.
MRI was recommended during hospitalization and declined by the family at that time. Four months following discharge, MRI revealed laminar cortical necrosis associated with incomplete infarction. MRA revealed an early moyamoya pattern.
Treatment and follow-up.
The patient has been receiving blood transfusion therapy for 4 months with no evidence of subsequent overt stroke. She continues to require ventilatory support at night and intermittently during the day.
Case 5
Hospital course.
Patient 5, a 9-year-old boy with hemoglobin SS, presented to the emergency department with abdominal pain. The next day, the patient experienced progressive pain in conjunction with worsening respiratory function. On day 3, because of ACS and ARDS, the patient received erythrocytapheresis and was intubated for 10 days of a 19-day hospitalization with maximal ventilatory support and heavy sedation. The patient received an average of 1.5 L/m2 per day and had a net fluid balance throughout his intensive care unit course. His blood pressure remained normal throughout hospitalization.
Neurologic examination.
On hospital day 15, the patient developed complex partial seizures with secondary generalization. Neurologic examination after the seizure episode revealed increased tone, reflexes, and clonus on the left side lasting more than 24 hours.
Imaging studies.
The initial clinical reading of the MRI, on hospital day 16, revealed a cerebral infarct in the frontoparietal area. Subsequent MRI reading for research purposes showed a subarachnoid hemorrhage without signs of infarction. MRA showed multifocal areas of narrowing of several vessels.
Treatment and follow-up.
Blood transfusion therapy was recommended, but the family declined. The patient has not had any signs of neurologic morbidity in the 3 years since his ACS episode.
Results
Five patients were admitted to the hospital for pain and developed ACS within 3 days of admission. Chest radiographs in all patients revealed progression to extensive multilobar involvement. All patients presented with oxygen saturation levels less than 92% on room air with a mean decrease from baseline of 9.4%. They subsequently required intubation, aggressive ventilatory support, and heavy sedation, and they experienced a prolonged hospitalization. All patients had hemoglobin levels less than 70 g/L (7.0 g/dL), with a mean decrease of 22.2 g/L (2.22 g/dL) from baseline and received multiple transfusions, including erythrocytapheresis to decrease hemoglobin S to less than 30%. The clinical features associated with the ACS episode are summarized in Table 1.
Course of acute chest syndrome
| Case no. . | Clinical symptoms . | Lowest Hgb, g/dL (change from baseline) . | O2 saturation on room air (change from baseline) . | Cognitive test scores . | Treatment . | Neurologic symptoms . | MRI findings . |
|---|---|---|---|---|---|---|---|
| 1 | Back pain, fever,*cough | 6.6 (−1.8) | 80% (−16%) | Verbal IQ, 86; performance IQ, 103 | Blood transfusion for 5 y | Frontal headache | RPLS, silent cerebral ischemia |
| 2 | Abdominal, back pain, respiratory distress | 3.7 (−2.7) | 92% (−6%) | Verbal IQ, 73; performance IQ, 73 | Hydroxyurea for 2 mo | Seizure, change in mental status | RPLS, with superimposed ischemia |
| 3 | Fever,*abdominal pain, cardiorespiratory arrest | 5.1 (−3.5) | 89% (−9%) | Verbal IQ, 79; performance IQ, 105 | Blood transfusion for 2 y; currently, on hydroxyurea | Delayed recovery to baseline alertness | RPLS, hemorrhage |
| 4 | Abdominal pain, fever,* respiratory distress | 6.5 (−2.1) | 89% (−11%) | Verbal IQ, 78; performance IQ, 100 | Blood transfusion for 4 mo | Dependent for locomotion and communication | Laminar necrosis and incomplete infarction |
| 5 | Chest, abdominal pain, fever,* respiratory distress | 6.9 (−1.0) | 90% (−5%) | General IQ, 85 | None | Complex partial seizure with secondary generalization | Frontal subarachnoid hemorrhage |
| Case no. . | Clinical symptoms . | Lowest Hgb, g/dL (change from baseline) . | O2 saturation on room air (change from baseline) . | Cognitive test scores . | Treatment . | Neurologic symptoms . | MRI findings . |
|---|---|---|---|---|---|---|---|
| 1 | Back pain, fever,*cough | 6.6 (−1.8) | 80% (−16%) | Verbal IQ, 86; performance IQ, 103 | Blood transfusion for 5 y | Frontal headache | RPLS, silent cerebral ischemia |
| 2 | Abdominal, back pain, respiratory distress | 3.7 (−2.7) | 92% (−6%) | Verbal IQ, 73; performance IQ, 73 | Hydroxyurea for 2 mo | Seizure, change in mental status | RPLS, with superimposed ischemia |
| 3 | Fever,*abdominal pain, cardiorespiratory arrest | 5.1 (−3.5) | 89% (−9%) | Verbal IQ, 79; performance IQ, 105 | Blood transfusion for 2 y; currently, on hydroxyurea | Delayed recovery to baseline alertness | RPLS, hemorrhage |
| 4 | Abdominal pain, fever,* respiratory distress | 6.5 (−2.1) | 89% (−11%) | Verbal IQ, 78; performance IQ, 100 | Blood transfusion for 4 mo | Dependent for locomotion and communication | Laminar necrosis and incomplete infarction |
| 5 | Chest, abdominal pain, fever,* respiratory distress | 6.9 (−1.0) | 90% (−5%) | General IQ, 85 | None | Complex partial seizure with secondary generalization | Frontal subarachnoid hemorrhage |
Fever is defined as temperature >38.5°C.
Prior to the ACS episode, none of the patients were diagnosed with hypertension or prior neurologic morbidity. Two children had normal MRI studies within 1 year of their severe ACS episode. One of 2 children also had a normal transcranial Doppler velocity measurement within a year of the episode. All 3 patients diagnosed with RPLS exhibited characteristic MRI changes, positive fluid balance, and hypertension immediately prior to focal neurologic findings and clinical features associated with RPLS (Table 2).
Neurologic symptoms in children with RPLS
| Case no. . | Headache . | Seizure . | Change in mental status . | Visual changes . | Elevated blood pressure . | Coexisting infarct . |
|---|---|---|---|---|---|---|
| 1 | + | − | − | − | + | + |
| 2 | − | + | − | − | + | + |
| 3 | − | − | + | + | + | − |
| Case no. . | Headache . | Seizure . | Change in mental status . | Visual changes . | Elevated blood pressure . | Coexisting infarct . |
|---|---|---|---|---|---|---|
| 1 | + | − | − | − | + | + |
| 2 | − | + | − | − | + | + |
| 3 | − | − | + | + | + | − |
indicates present; –, absent.
Discussion
This case series demonstrates that children with sickle cell disease and severe ACS requiring intubation and erythrocytapheresis are at high risk of neurologic morbidity, including silent cerebral infarcts, RPLS, and cerebral hemorrhage. Despite the high prevalence of silent cerebral infarcts (approximately 20%) in children with sickle cell anemia and the association with poor cognition and poor academic performance, no risk factor has been identified for silent cerebral infarcts.3,10 Conducting imaging studies to identify silent cerebral infarcts is important because children with prior silent cerebral infarcts are at an increased risk of subsequent overt stroke (8.1%) and silent cerebral infarcts (24.5%).5 11
The pathogenesis of RPLS in children with sickle cell disease, ACS, and respiratory failure is unclear. On the basis of the presentation of the patients in our case series, we postulate that children with sickle cell disease and hypoxemia, positive fluid balance resulting in hypertension, and poor cerebrovascular autoregulation12 are vulnerable to RPLS. Typically, RPLS is characterized by hypertension, headache, altered mental status, seizures, and/or visual changes associated with radiologic abnormalities, often reversible, in the white matter and occasionally gray matter.7,8 13 This syndrome has not previously been reported in children with sickle cell disease.
RPLS is difficult to distinguish from acute cerebral infarcts clinically and radiographically. The distinction is important because cerebral infarction implies irreversible damage and warrants blood transfusion therapy for an indefinite period. Conversely, RPLS is potentially reversible but may require judicious hypertensive management. In this case series, 2 patients were originally diagnosed as having cerebral infarcts according to the clinical reading of the MRI, one of whom was subsequently determined to have RPLS and the other with cerebral hemorrhage. Appropriate MRI techniques and interpretation play a key role in differentiating RPLS from cerebral infarct (Figure 4). Both RPLS and cerebral infarct present with T2-weighted hyperintensities. DWIs typically show hyperintense signal in cerebral infarcts, whereas DWIs in RPLS are ambiguous because of T2 shine-through effects.8 14 Producing images of the diffusion coefficient eliminates this ambiguity.
Characterization of T2-weighted hyperintensities on MRI of the brain distinguishing cerebral infarct from reversible posterior leukoencephalopathy syndrome (RPLS).
DWI indicates diffusion weighted image; DC, diffusion coefficient.
Characterization of T2-weighted hyperintensities on MRI of the brain distinguishing cerebral infarct from reversible posterior leukoencephalopathy syndrome (RPLS).
DWI indicates diffusion weighted image; DC, diffusion coefficient.
As with any case series, this study has limitations. We could not determine the time of a neurologic injury in children who were intubated and sedated. Thus, overt neurologic events may have been masked. Despite this limitation, all evidence would support that the neurologic events in the children occurred after endotracheal intubation and were temporally associated with their acute illness. We could not exclude the possibility that erythrocytapheresis increased the risk of silent cerebral infarcts in children who are acutely ill. At least one previous study (n = 6) has identified exchange transfusion, for the treatment of priapism, as a possible risk factor for overt strokes (3 of 6).15 Regardless of whether severe ACS or erythrocytapheresis is the primary risk factor for cerebral infarcts, when both events occur together, a MRI of the brain is warranted to guide patient management.
In summary, children with severe ACS requiring endotracheal intubation and erythrocytapheresis are at increased risk of significant neurologic morbidity and should undergo MRIs of the brain, neurologic evaluations by a neurologist, and cognitive assessments after their acute episode.
We thank Bradley Schlagger, MD, PhD, Jean Holowach Thurston, MD, and Rebecca Ichord, MD, for their valuable input.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-04-1183.
Supported by the Doris Duke Clinical Scholars Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael R. DeBaun, St Louis Children's Hospital, Campus Box 8116, One Children's Pl, St Louis, MO 63110; e-mail: debaun_m@kids.wustl.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal