Abstract
Granulocyte colony-stimulating factor (G-CSF) induces proliferation, maturation, and functional activities of myeloid progenitors and mature neutrophils through a specific receptor, the G-CSF-R. Different signals are mediated by distinct regions of the cytoplasmic domain of G-CSF-R, but the precise role of each region has not yet been fully clarified. We evaluated the involvement of Syk kinase, essential in mediating phagocytic signals by Fcγ receptors, in G-CSF–induced phagocytosis, using murine myeloid 32D cells transfected with wild-type (WT) human G-CSF-R (hG-CSF-R) or with a G-CSF-R mutant truncated at cytoplasmic amino acid 715. The G-CSF-R mutant lacks the immunoreceptor tyrosine-based activation motif (ITAM), putative binding site for Syk. Following treatment of WT hG-CSF-R transfectants with IgG-coated particles, there was a significant increase in phagocytosis in G-CSF–stimulated cells, in which Syk tyrosine phosphorylation occurred, paralleled by enhancement of its tyrosine kinase activity. In the mutant transfectants, no significant increase in phagocytosis or Syk tyrosine phosphorylation occurred after stimulation with G-CSF. We also demonstrated that tyrosine phosphorylation of the Src kinases Hck and Lyn occurs following G-CSF stimulation of cells expressing WT G-CSF-R, but that Hck is not phosphorylated in mutant G-CSF-R transfectants. The increase in phagocytosis following G-CSF stimulation cannot be attributed to a rapid de novo increase in expression of Fcγ receptors. G-CSF induced expression of Fcγ receptors only after prolonged stimulation. Our data provide evidence that the carboxy-terminal region of G-CSF-R plays a role in the phagocytosis of IgG-coated particles and that Syk and Hck kinase tyrosine phosphorylation is involved.
Introduction
Granulocyte colony-stimulating factor (G-CSF) induces proliferation and maturation of myeloid progenitors through a specific receptor (G-CSF-R).1 G-CSF also plays a role in promoting the function of mature granulocytes. Binding of the G-CSF-R by its ligand stimulates the activation of several distinct signal-transducing pathways.2 The specific cytoplasmic domains of the receptor responsible for triggering these pathways have been only partially elucidated.2, 3, 4, 5, 6, 7, 8, 9, 10 The carboxy-terminal region of the G-CSF-R is essential for induction of granulocytic maturation (through Stat3),11, 12, 13 cell survival,14 and inhibition of cell proliferation,15 whereas the membrane-proximal domain, mainly through Lyn and Jak kinases, mediates cellular proliferation.16,17 The existence of diseases such as severe congenital neutropenia (SCN), in which a part of the receptor is deleted,18, 19, 20 renders the determination of the functional specificity of G-CSF-R domains particularly meaningful.21,22
It has been demonstrated that Syk kinase is required for Fcγ receptor (FcγR)–mediated phagocytosis in monocytes/macrophages and that cotransfection of Syk kinase with FcγRI and its γ subunit or with FcγRIIA enhances phagocytosis in epithelial cells such as COS-1 or CHO cells.23, 24, 25, 26, 27, 28, 29, 30 It has also been demonstrated that Syk kinase is activated in cells treated with G-CSF31 and that neutrophils may display a high degree of phagocytic activity. To elucidate the role of G-CSF–induced Syk activation in granulocytic function, we analyzed the pattern of tyrosine phosphorylation of 32D myeloid cells transfected with wild-type (WT) G-CSF-R or with a naturally occurring truncated mutant of G-CSF-R (Δ715). This mutant of the G-CSF-R is derived from the granulocytes of a patient with SCN. The mutant gene contains a C>T substitution that introduces a TAG stop codon and thereby codes for a truncated G-CSF-R protein lacking 98 carboxy-terminal amino acids.32
Signaling through Syk kinase generally depends on the binding of Syk SH2 domains to phosphorylated tyrosines within the immunoreceptor tyrosine-based activation motif (ITAM) sequence, commonly located in the cytoplasmic domain of Ig gene superfamily receptors.33 The typical ITAM, as in the γ chain associated with FcγRI and FcγRIIIA, contains 2 YXXL sequences separated by 6 nonconserved amino acids. An ITAM-like sequence whose YXXL sequences are separated by 12 amino acids occurs in FcγRIIA and is required for phagocytosis by this receptor. An ITAM-like motif may be found between amino acid 727 and 747 of human G-CSF-R (hG-CSF-R; Figure 1) and is missing in the Δ715 mutant.31 We provide evidence that the receptor carboxy-terminal region containing this ITAM-like sequence is important for both Syk tyrosine phosphorylation and enhancement of phagocytic activity induced by ligation of G-CSF-R.
Structure of the cytoplasmic domain of WT G-CSF-R and the naturally occurring truncated mutant. The cytoplasmic domain of WT hG-CSF-R contains 3 cytokine receptor superfamily homology regions, designated box 1, box 2, and box 3 (gray boxes). The transmembrane domain of the receptor is represented as a black box. There are 4 tyrosine residues (Y) in the carboxy-terminal domain of G-CSF-R. The IL-3–dependent murine myeloid 32D cell line was transfected using a pLNCX retroviral expression vector containing human WT hG-CSF-R or the hG-CSF-R mutant (Δ715) cDNA. The putative ITAM-like motif, containing tyrosines 727 and 747, is indicated by the dark-gray box.
Structure of the cytoplasmic domain of WT G-CSF-R and the naturally occurring truncated mutant. The cytoplasmic domain of WT hG-CSF-R contains 3 cytokine receptor superfamily homology regions, designated box 1, box 2, and box 3 (gray boxes). The transmembrane domain of the receptor is represented as a black box. There are 4 tyrosine residues (Y) in the carboxy-terminal domain of G-CSF-R. The IL-3–dependent murine myeloid 32D cell line was transfected using a pLNCX retroviral expression vector containing human WT hG-CSF-R or the hG-CSF-R mutant (Δ715) cDNA. The putative ITAM-like motif, containing tyrosines 727 and 747, is indicated by the dark-gray box.
Materials and methods
Cell lines and growth factors
Experiments were carried out in 32D.cl3 cells, an interleukin 3 (IL-3)–dependent murine myeloid cell line that can undergo granulocytic terminal maturation. The subclone used for experiments lacked endogenous G-CSF-R expression, but retained the ability to mature. Cells were transfected by electroporation using a pLNCX retroviral expression vector containing human WT G-CSF-R cDNA or DA/human G-CSF-R (MT), a naturally occurring truncated form (Δ715) of the receptor (kindly provided by Prof I. P. Touw, Erasmus University, Rotterdam, The Netherlands). After transfection, cells were cultured in the presence of G418 and resistant clones expanded and tested for expression of the receptor. Both WT and MT G-CSF-R 32D cells were maintained in culture in RPMI 1640 medium plus fetal calf serum (FCS) 10% and murine IL-3, or 10% conditioned medium from the IL-3–producing murine WEHI 238 lymphoblastoid cell line.
hG-CSF-R expression
To check for hG-CSF-R expression, binding of the receptor to phycoerythrin (PE)–conjugated hG-CSF (Fluorokine, G-CSF–PE, R & D Systems, Minneapolis, MN) was monitored by flow cytometry. Cells were collected after culture in WEHI-conditioned medium, washed thoroughly, and incubated in the presence of hG-CSF–PE or in the presence of PE-conjugated streptavidin for 30 minutes on ice according to the manufacturer's protocol. After incubation, cells were washed, resuspended in cold buffer at the final concentration of 2.5 × 105 cells/200 μL, and immediately analyzed by flow cytometry.
Preparation of cell lysates
32D cells were incubated in RPMI 1640 medium in the absence of serum and growth factors for 18 hours. Cells were checked for viability by trypan blue exclusion (typically, > 85% of the cells were viable) prior to stimulation with recombinant hG-CSF (300 ng/mL; Amgen, Thousand Oaks, CA) for 15 minutes at 37°C. Stimulation was terminated by cooling the cell suspension on ice. Cells were lysed in 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.5, containing 1 mM EDTA (ethylenediaminetetra acetic acid), 150 mM NaCl, 1% Triton X-100, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 2 μg/mL pepstatin, 10 μg/mL TPCK (tosyl-L-phenylalanine-chloromethyl-ketone). Unsolubilized material was removed by centrifugation for 30 minutes at 12 000g at 4°C and protein concentration determined by the Bradford method.34
Immunoprecipitation and Western blotting
Cell lysates were incubated with anti-Syk or anti-Lyn antibodies for 90 minutes at 4°C and then adsorbed on protein A-Sepharose CL-4B (Pharmacia LKB, Uppsala, Sweden) for 30 minutes at 4°C. Immune complexes were washed 5 times with lysis buffer (see “Preparation of cell lysates”), eluted, and denaturated at 95°C for 5 minutes in Laemmli buffer. Lysates were loaded onto 8% and 7% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels, subjected to electrophoresis, and transferred electrophoretically onto nitrocellulose filters. The filters were then blocked and treated with appropriate monoclonal antibodies (mAbs): antiphosphotyrosine RC20 antibody (Transduction Laboratories, Lexington, KY), and anti-Syk, anti-Lyn, and anti-Hck mAbs (Santa Cruz Biotechnology, Santa Cruz, CA). After incubation with horseradish peroxidase (HPR)–conjugated species-specific secondary antibody (HPR-conjugated antimouse IgG, Boehringer-Mannheim, Indianapolis, IN, or HPR-conjugated antirabbit IgG, Chemicon, Temecula, CA), immune complexes were detected by the enhanced chemiluminescence reaction (Super Signal Ultra Chemiluminescent Substrate, Pierce, Rockford, IL). To ready the filter for probing with another primary antibody, filters were treated at 50°C for 30 minutes with stripping buffer containing 100 mM 2-mercaptoethanol, 62.5 mM Tris-HCl, pH 6.7, and 2% SDS.
In vitro tyrosine kinase assay
Anti-Syk immunoprecipitates of lysates obtained from WT and MT G-CSF-R 32D cells, stimulated or nonstimulated with G-CSF (300 ng/mL for 10 minutes), were assayed for protein tyrosine kinase (PTK) activity according to the nonradioactive protocol recommended by the manufacturer (Roche Molecular Biochemicals, Indianapolis, IN). This assay specifically detects the transfer of the γ-phosphate group from adenosine triphosphate (ATP) to a tyrosine of the provided biotin-labeled synthetic substrate peptide. Kinase activity is measured as absorbance at 405 nm in an enzyme-linked immunosorbent assay (ELISA) reader, after binding of antiphosphotyrosine-peroxidase antibodies to the phosphorylated-immobilized substrate. Results are expressed as enzyme activity-micromoles phosphate incorporated into the substrate per minute. Absolute values are determined relative to a phosphopeptide standard curve.
Phagocytosis
Dried sheep red blood cells (RBCs; Sigma, St Louis, MO) were reconstituted with water as recommended by the manufacturer prior to sensitization with the highest subagglutinating concentration of rabbit antisheep RBC antibody (UBI, Lake Placid, NY) at 37°C for 30 minutes. Sensitized RBCs were washed thoroughly and resuspended in phosphate-buffered saline (PBS) at the final concentration of 1 × 109/mL. Latex beads (1.1μm diameter; Sigma) were opsonized by incubation with FCS at 37°C for 60 minutes.
WT G-CSF-R 32D cells and MT G-CSF-R 32D cells were incubated at 37°C for 30 minutes with G-CSF (300 ng/mL) in the presence of sensitized RBCs (ratio, 100:1 RBCs/cells) or in the presence of preopsonized latex beads. Cells were briefly treated with hypotonic PBS to remove adherent RBCs. After cytospin, 32D cells were stained with May-Grünwald-Giemsa (MGG) and phagocytosed particles (RBCs or latex beads) scored by light microscopy (× 100). At least 100 cells were scored and ingestion of particles was expressed as the phagocytic index (PI, the number of RBCs or number of latex beads ingested per 100 32D cells). The above procedure was also used to examine phagocytosis in washed WT G-CSF-R and in MT G-CSF-R 32D cells that had been cultured for 3 days in the presence of G-CSF (100 ng/mL) to induce granulocyte maturation and potentially enhance phagocytosis.
To verify the role of Syk kinase in G-CSF phagocytic signaling, we also examined phagocytosis in normal neutrophils, in which the PI is higher than in 32D cells. Peripheral blood samples were obtained after informed consent from healthy donors and neutrophils isolated by density gradient separation (Ficoll Isopaque, Amersham, Little Chalfont, United Kingdom). Viable neutrophils were preincubated at 37°C for 60 minutes with the Syk tyrosine kinase inhibitor piceatannol (25 and 50 μg/mL) (Calbiochem, San Diego, CA). They were then subjected to 30 minutes' incubation with G-CSF and sensitized sheep RBCs (ratio, 100:1). Controls were piceatannol-treated neutrophils not treated with G-CSF/sensitized sheep RBCs and neutrophils not subjected to piceatannol but treated with G-CSF/sensitized sheep RBCs. Phagocytosis of RBCs by neutrophils was determined as described.
FcγR expression
The surface expression of FcγRI and FcγRII/III by WT G-CSF-R 32D cells and MT G-CSF-R 32D cells was analyzed by flow cytometry, using fluorescein isothiocyanate (FITC)–labeled antimouse FcγRI receptor mAbs (kindly provided by Dr P. Mark Hogarth, University of Melbourne, Victoria, Australia) and FITC-conjugated rat antimouse FcγRII/III mAb (Pharmingen, San Diego, CA). Cells were tested for FcγR expression before and after incubation with 300 ng/mL G-CSF (30 minutes) or 100 ng/mL G-CSF (3 days). Control antibody was isotype-matched antimouse IgG-FITC (Becton Dickinson, San Jose, CA). Results are expressed as mean fluorescence intensity (MFI), which is the ratio of the mean fluorescence channel of the labeled specific antibody to the control antibody channel.
Statistical analysis
Student t test (level of confidence 95%; df = 9 or 12) for paired and grouped data were applied to verify significant differences in PI.
Results
hG-CSF-R expression
The cell surface expression of the WT and MT receptors, both before and after hG-CSF binding, was monitored in all experiments by flow cytometry using PE-tagged G-CSF. As shown in Figure 2, 33% of WT receptor transfectants bound hG-CSF–PE (MFI, 1.34) and 76% of MT receptor transfectants bound hG-CSF–PE (MFI, 2.05), whereas only 7% (MFI, 1.01) of the parental 32D cells, which do not express human G-CSF-R, bound the fluorescent-tagged growth factor.
Binding of PE-conjugated hG-CSF to WT G-CSF-R or MT G-CSF-R transfectants. The fluorescence intensity of cells incubated with G-CSF–PE (solid lines) (or PE-conjugated streptavidin as control [dotted lines]) for 30 minutes on ice were analyzed by flow cytometry.
Binding of PE-conjugated hG-CSF to WT G-CSF-R or MT G-CSF-R transfectants. The fluorescence intensity of cells incubated with G-CSF–PE (solid lines) (or PE-conjugated streptavidin as control [dotted lines]) for 30 minutes on ice were analyzed by flow cytometry.
Tyrosine phosphorylation of Syk and Src family tyrosine kinases
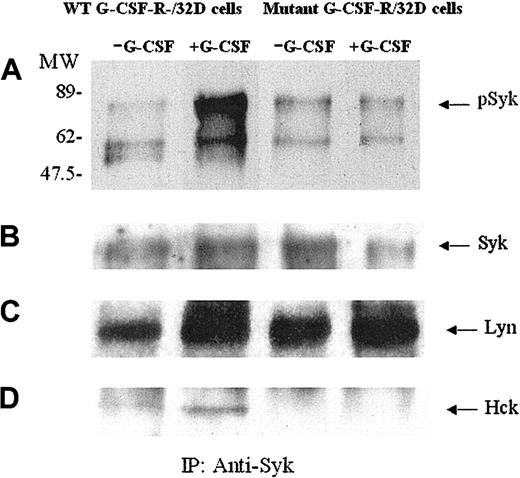
Previous reports have indicated the involvement of Syk kinase as well as other nonreceptor kinases in G-CSF signal transduction.9,31,35 We have further explored the activation and interaction of the components of the complex induced by G-CSF-R ligation. We first investigated whether G-CSF stimulation induced Syk tyrosine phosphorylation in 32D cells transfected with WT G-CSF-R. Cell lysates were immunoprecipitated with anti-Syk antibody and immunoblotted with antiphosphotyrosine antibody (Figure 3A). Stimulation for 10 minutes with G-CSF induced phosphorylation of Syk kinase in WT G-CSF-R transfectants, confirming an effect of G-CSF in Syk tyrosine phosphorylation.31 The naturally occurring truncated mutant of G-CSF-R lacks the carboxy-terminal region of the G-CSF-R cytoplasmic domain. Stimulation for 10 minutes with G-CSF failed to induce Syk phosphorylation in the 32D cell transfectants expressing the mutant receptor (Figure 3A lanes 3 and 4).
G-CSF–dependent Syk kinase phosphorylation occurs in WT G-CSF-R transfectants but not in transfectants expressing the truncated MT G-CSF-R. Murine 32D myeloid cells transfected with WT G-CSF-R or the truncated MT G-CSF-R, lacking the carboxy-terminal region of the receptor, were stimulated for 10 minutes with hG-CSF (300 ng/mL) and then lysed. SDS-PAGE was performed after immunoprecipitation of cell lysates with anti-Syk antibody. Blots were probed with antiphosphotyrosine antibody (A) and then stripped and reprobed with anti-Syk (B), anti-Lyn (C), or anti-Hck (D) antibodies. pSyk indicates phosphorylated Syk.
G-CSF–dependent Syk kinase phosphorylation occurs in WT G-CSF-R transfectants but not in transfectants expressing the truncated MT G-CSF-R. Murine 32D myeloid cells transfected with WT G-CSF-R or the truncated MT G-CSF-R, lacking the carboxy-terminal region of the receptor, were stimulated for 10 minutes with hG-CSF (300 ng/mL) and then lysed. SDS-PAGE was performed after immunoprecipitation of cell lysates with anti-Syk antibody. Blots were probed with antiphosphotyrosine antibody (A) and then stripped and reprobed with anti-Syk (B), anti-Lyn (C), or anti-Hck (D) antibodies. pSyk indicates phosphorylated Syk.
Syk tyrosine phosphorylation was not a function of receptor cell surface expression. Despite higher G-CSF-R cell surface expression, the cells expressing the MT receptor did not induce tyrosine phosphorylation of Syk. Thus, the ability to induce tyrosine phosphorylation of Syk (and enhance phagocytosis; see “Phagocytosis”) does not appear to be a direct function of receptor surface expression. Taken together, these experiments indicate that cytoplasmic sequences beyond amino acid 715 are important for G-CSF-R/Syk interaction.
Two additional tyrosine phosphorylated proteins were detected in cell lysates of G-CSF-R transfectants that had been immunoprecipitated with anti-Syk antibody. One was confirmed as the Src tyrosine kinase family–kinase Lyn p53/56, previously identified in Syk immunoprecipitates.31,36 Tyrosine phosphorylated Lyn coprecipitated with Syk for transfectants of both WT and MT G-CSF-R (Figure 3C). Tyrosine phosphorylation of Lyn increased following G-CSF stimulation as did the amount of Lyn that coimmunoprecipitated with Syk kinase (Figure 3C). Anti-Lyn immunoprecipitates of the same G-CSF transfectant cell lysates confirmed these observations.
A second phosphorylated protein detected in Syk kinase immunoprecipitates after G-CSF stimulation was identified as Hck kinase (Figure 3D). Recent reports indicate that Syk also coimmunoprecipitates with the SRC tyrosine kinase (SRTK) Hck37 and that Hck may have a role in phagocytosis. Unlike Lyn, Hck was not detected on antiphosphotyrosine immunoblots of Syk immunoprecipitates from mutant G-CSF-R–transfected cells. The coimmunoprecipitation and tyrosine phosphorylation of other Src kinases, such as Lck and Fyn, were not detected in Syk immunoprecipitates of WT receptor transfectants.
Tyrosine kinase assay
To further explore the involvement of Syk in G-CSF signaling suggested by the phosphotyrosine immunoblots, we evaluated tyrosine kinase activity in anti-Syk immunoprecipitates. The activity of Syk kinase (in terms of picomoles phosphate incorporated into the substrate peptide) is shown for immunoprecipitates of 32D cells before and after stimulation with G-CSF (300 ng/mL, 10 minutes; Figure 4). Syk kinase activity was significantly enhanced following G-CSF stimulation in WT/G-CSF-R–transfected 32D cells. Syk kinase activity in MT transfectants did not undergo a significant alteration following G-CSF stimulation.
Tyrosine kinase activity of Syk in lysates of 32D cells transfected with WT or MT G-CSF-R. 32D myeloid cells transfected with WT G-CSF-R or the truncated MT G-CSF-R were stimulated for 10 minutes with hG-CSF and then lysed. Cell lysates were immunoprecipitated with anti-Syk antibody. Activation of Syk kinase was analyzed in immunoprecipitates. Results obtained using nonradioactive kinase assays are expressed as picomoles phosphate. Results represent the means of triplicate experiments. Error bars represent SD.
Tyrosine kinase activity of Syk in lysates of 32D cells transfected with WT or MT G-CSF-R. 32D myeloid cells transfected with WT G-CSF-R or the truncated MT G-CSF-R were stimulated for 10 minutes with hG-CSF and then lysed. Cell lysates were immunoprecipitated with anti-Syk antibody. Activation of Syk kinase was analyzed in immunoprecipitates. Results obtained using nonradioactive kinase assays are expressed as picomoles phosphate. Results represent the means of triplicate experiments. Error bars represent SD.
Phagocytosis
Although it has been well documented that G-CSF promotes phagocytosis by neutrophils,37 the mechanism whereby the G-CSF-R contributes to phagocytosis is not well understood. The presence of Syk kinase and SRTKs, important in the transmission of the FcγR phagocytic signal,24, 25, 26, 27 in complex with the G-CSF-R prompted us to further examine the role of G-CSF-R in the phagocytic process. We investigated whether the cytoplasmic domain of the G-CSF-R is important for phagocytosis of IgG-coated particles in transfectants of the murine myeloid 32D cell line. The cell surface expression of G-CSF-R WT and MT was monitored before all the experiments by flow cytometry following G-CSF–PE binding. MT cells expressed a higher density of hG-CSF-R than did WT cells. Thus, the number of surface receptors cannot account for alterations in phagocytosis observed in our experiments.
In the absence of G-CSF stimulation, a very low but detectable level of phagocytosis was observed for the WT receptor (Tables 1, 2). After 30 minutes of exposure to G-CSF (300 ng/mL), the PI of cells incubated with IgG-coated sheep RBCs (EA) increased 2-fold (from 19 ± 5 to 40 ± 7; Table 1). Phagocytosis of opsonized latex beads was also significantly increased (from PI = 19 ± 9 to 51 ± 9 after G-CSF stimulation; Table 2). Nonopsonized EA or latex beads were not ingested. Stimulation (30 minutes) with G-CSF did not significantly modify the PI of MT G-CSF-R transfectants. For MT G-CSF-R transfectants, the PI for RBCs was 15 ± 6 for unstimulated cells and 19 ± 5 for stimulated cells (Table 1) and 14 ± 4 versus 16 ± 6 for opsonized latex beads (Table 2). Although the PI is low for 32D transfectants, the differences observed are statistically significant. The low efficiency of phagocytosis in 32D cells is most probably related to the functional and morphologic immaturity of this myeloid cell line (myeloblasts). Figure 5 illustrates the phagocytosis of RBCs by WT G-CSF-R (Figure 5B) but not by mutant G-CSF-R (Figure 5D) in cells stimulated with G-CSF. Note that although the mutant receptors appear to bind RBCs, mainly invagination (not ingestion) of IgG-coated particles occurs.
Phagocytosis of IgG-coated sheep RBCs by 32D cells transfected with WT G-CSF-R or truncated MT hG-CSF-R (▵715)
Experiment no. . | PI . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | WT hG-CSF-R . | WT + G-CSF* . | MT hG-CSF-R . | MT + G-CSF† . | |||
| 1 | 15 | 37 | 10 | 23 | |||
| 2 | 19 | 45 | 13 | 18 | |||
| 3 | 18 | 42 | 10 | 13 | |||
| 4 | 12 | 26 | 12 | 25 | |||
| 5 | 16 | 30 | 15 | 13 | |||
| 6 | 19 | 43 | 12 | 15 | |||
| 7 | 14 | 47 | 11 | 12 | |||
| 8 | 21 | 40 | 22 | 24 | |||
| 9 | 30 | 43 | 26 | 26 | |||
| 10 | 25 | 48 | 19 | 21 | |||
| Mean | 19 | 40 | 15 | 19 | |||
| SD | 5 | 7 | 6 | 5 | |||
| P | .000 | .06 | |||||
Experiment no. . | PI . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | WT hG-CSF-R . | WT + G-CSF* . | MT hG-CSF-R . | MT + G-CSF† . | |||
| 1 | 15 | 37 | 10 | 23 | |||
| 2 | 19 | 45 | 13 | 18 | |||
| 3 | 18 | 42 | 10 | 13 | |||
| 4 | 12 | 26 | 12 | 25 | |||
| 5 | 16 | 30 | 15 | 13 | |||
| 6 | 19 | 43 | 12 | 15 | |||
| 7 | 14 | 47 | 11 | 12 | |||
| 8 | 21 | 40 | 22 | 24 | |||
| 9 | 30 | 43 | 26 | 26 | |||
| 10 | 25 | 48 | 19 | 21 | |||
| Mean | 19 | 40 | 15 | 19 | |||
| SD | 5 | 7 | 6 | 5 | |||
| P | .000 | .06 | |||||
The phagocytic index (PI) values for nonopsonized RBCs, based on a mean of 3 exposures, were 0, 1, 1, and 1, respectively, for WT hG-CSF-R, WT + G-CSF, MT hG-CSF-R, MT + G-CSF.
WT hG-CSF-R + G-CSF, 300 ng/mL.
MT hG-CSF-R + G-CSF, 300 ng/mL.
Phagocytosis of latex beads by 32D cells transfected with WT G-CSF-R or truncated MT hG-CSF-R (Δ 715)
Experiment no. . | PI . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | WT hG-CSF-R . | WT + G-CSF* . | MT hG-CSF-R . | MT + G-CSF† . | |||
| 1 | 25 | 51 | 18 | 23 | |||
| 2 | 18 | 47 | 15 | 19 | |||
| 3 | 27 | 59 | 17 | 6 | |||
| 4 | 20 | 61 | 12 | 23 | |||
| 5 | 13 | 37 | 10 | 21 | |||
| 6 | 5 | 54 | 13 | 17 | |||
| 7 | 8 | 42 | 8 | 15 | |||
| 8 | 34 | 50 | 20 | 16 | |||
| 9 | 20 | 68 | 18 | 12 | |||
| 10 | 16 | 45 | 11 | 10 | |||
| Mean | 19 | 51 | 14 | 16 | |||
| SD | 9 | 9 | 4 | 6 | |||
| P | .000 | .186 | |||||
Experiment no. . | PI . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | WT hG-CSF-R . | WT + G-CSF* . | MT hG-CSF-R . | MT + G-CSF† . | |||
| 1 | 25 | 51 | 18 | 23 | |||
| 2 | 18 | 47 | 15 | 19 | |||
| 3 | 27 | 59 | 17 | 6 | |||
| 4 | 20 | 61 | 12 | 23 | |||
| 5 | 13 | 37 | 10 | 21 | |||
| 6 | 5 | 54 | 13 | 17 | |||
| 7 | 8 | 42 | 8 | 15 | |||
| 8 | 34 | 50 | 20 | 16 | |||
| 9 | 20 | 68 | 18 | 12 | |||
| 10 | 16 | 45 | 11 | 10 | |||
| Mean | 19 | 51 | 14 | 16 | |||
| SD | 9 | 9 | 4 | 6 | |||
| P | .000 | .186 | |||||
PI for nonopsonized latex beads was 0, 0, 0, 0, respectively, for WT hG-CSF-R, WT + G-CSF, MT hG-CSF-R, MT G-CSF.
WT hG-CSF-R + G-CSF, 300 ng/mL.
MT hG-CSF-R + G-CSF, 300 ng/mL.
Morphologic analysis of phagocytosis of IgG-coated sheep RBCs. Transfectants of WT G-CSF-R (A-B) or the truncated G-CSF-R MT (C-D) were incubated for 30 minutes with IgG-coated sheep RBCs in the presence (B,D) or in the absence (A,C) of G-CSF, 300 ng/mL. Stained with May-Grünwald-Giemsa. Original magnification, × 100.
Morphologic analysis of phagocytosis of IgG-coated sheep RBCs. Transfectants of WT G-CSF-R (A-B) or the truncated G-CSF-R MT (C-D) were incubated for 30 minutes with IgG-coated sheep RBCs in the presence (B,D) or in the absence (A,C) of G-CSF, 300 ng/mL. Stained with May-Grünwald-Giemsa. Original magnification, × 100.
As previously reported,38 extended culture in the presence of G-CSF induces granulocytic maturation of 32D transfectants. We next analyzed whether extended culture in the presence of G-CSF influenced phagocytosis of IgG-coated particles in hG-CSF–transfected 32D cells. Partial granulocytic maturation was obtained after 3 days of culture in the presence of G-CSF at 100 ng/mL. Specifically, after 3 days of G-CSF incubation, 24% of WT G-CSF-R 32D transfectants and 20% of MT receptor transfectants were morphologically mature neutrophils, that is, cells with polylobated or ring-shaped nuclei, and granulated cytoplasm (Figure 6). Further stimulation with G-CSF (at 300 ng/mL) while incubating 30 minutes with RBCs or opsonized latex beads increased the PI of 32D WT transfectants (PI = 14 ± 2 for nontreated versus 45 ± 8 for G-CSF treated; Table 3; Figure 6). Phagocytosis was not enhanced when mutant transfectants were further stimulated by G-CSF (PI = 17 ± 3 versus 16 ± 3; Table 3). Note also that baseline phagocytosis was comparable in control cells not exposed to G-CSF for long periods (PI = 19 ± 5 for WT and 15 ± 5 for MT; Table 1) and control cells cultured for 3 days in G-CSF (100 ng/mL; PI = 15 ± 2, and 17 ± 3 WT and MT, respectively; Tables 1 and 3).
Morphologic analysis of phagocytosis of IgG-coated sheep RBCs after maturation of 32D cells induced by G-CSF. Transfectants of WT G-CSF-R (A-B) or the truncated G-CSF-R mutant (C-D) were incubated for 30 minutes with IgG-coated sheep RBCs in the presence (B,D) or in the absence (A,C) of G-CSF, 300 ng/mL, after incubation for 3 days with G-CSF, 100 ng/mL, performed to obtain partial granulocytic maturation. Stained with May-Grünwald-Giemsa. Original magnification, × 100.
Morphologic analysis of phagocytosis of IgG-coated sheep RBCs after maturation of 32D cells induced by G-CSF. Transfectants of WT G-CSF-R (A-B) or the truncated G-CSF-R mutant (C-D) were incubated for 30 minutes with IgG-coated sheep RBCs in the presence (B,D) or in the absence (A,C) of G-CSF, 300 ng/mL, after incubation for 3 days with G-CSF, 100 ng/mL, performed to obtain partial granulocytic maturation. Stained with May-Grünwald-Giemsa. Original magnification, × 100.
Phagocytosis of IgG-coated sheep RBCs by 32D cells transfected with WT G-CSF-R or truncated MT hG-CSF-R (Δ 715) after G-CSF–induced differentiation
Experiment no. . | PI . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | WT hG-CSF-R . | WT + G-CSF* . | MT hG-CSF-R . | MT + G-CSF† . | |||
| 1 | 12 | 37 | 20 | 18 | |||
| 2 | 17 | 55 | 12 | 18 | |||
| 3 | 15 | 46 | 17 | 16 | |||
| 4 | 13 | 50 | 19 | 14 | |||
| 5 | 11 | 37 | 15 | 12 | |||
| Mean | 14 | 45 | 17 | 16 | |||
| SD | 2 | 8 | 3 | 3 | |||
| P | .0003 | .6213 | |||||
Experiment no. . | PI . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | WT hG-CSF-R . | WT + G-CSF* . | MT hG-CSF-R . | MT + G-CSF† . | |||
| 1 | 12 | 37 | 20 | 18 | |||
| 2 | 17 | 55 | 12 | 18 | |||
| 3 | 15 | 46 | 17 | 16 | |||
| 4 | 13 | 50 | 19 | 14 | |||
| 5 | 11 | 37 | 15 | 12 | |||
| Mean | 14 | 45 | 17 | 16 | |||
| SD | 2 | 8 | 3 | 3 | |||
| P | .0003 | .6213 | |||||
The PI for nonopsonized RBCs, based on the mean of 3 experiments, was 0, 0, 0, 0, respectively, for WT hG-CSF-R, WT + G-CSF, MT hG-CSF-R, MT + G-CSF.
WT hG-CSF-R + G-CSF, 300 ng/mL.
MT hG-CSF-R + G-CSF, 300 ng/mL.
To elucidate the role of Syk in G-CSF–induced phagocytosis, we determined the PI of neutrophils exposed to piceatannol at concentrations selective for Syk inhibition (Table 4). When neutrophils were challenged with G-CSF for 30 minutes in the absence of piceatannol, the PI of RBCs increased significantly, from 38 ± 17 to 97 ± 21. This large response was not observed for neutrophils preincubated with piceatannol and exposed to G-CSF in the presence of 25 μg/mL piceatannol (PI, 33 ± 7 to 42 ± 18). The inhibition of G-CSF–induced phagocytosis was also evident at 50 μg/mL piceatannol (PI, 17 ± 9 to 28 ± 26).
G-CSF–induced phagocytosis of IgG-coated sheep RBCs by neutrophils treated or untreated with piceatannol
. | PI . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure no. . | Untreated . | . | Piceatannol, 25 μg/mL . | . | Piceatannol, 50 μg/mL . | . | |||||
| . | Control . | G-CSF . | Control . | G-CSF . | Control . | G-CSF . | |||||
| 1 | 62 | 86 | 40 | 68 | 20 | 49 | |||||
| 2 | 29 | 75 | 36 | 29 | 28 | 53 | |||||
| 3 | 25 | 122 | 30 | 35 | 9 | 4 | |||||
| 4 | 35 | 105 | 25 | 36 | 10 | 7 | |||||
| Mean | 38 | 97 | 33 | 42 | 17 | 28 | |||||
| SD | 17 | 21 | 7 | 18 | 9 | 26 | |||||
| P | .03 | .29 | .29 | ||||||||
. | PI . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure no. . | Untreated . | . | Piceatannol, 25 μg/mL . | . | Piceatannol, 50 μg/mL . | . | |||||
| . | Control . | G-CSF . | Control . | G-CSF . | Control . | G-CSF . | |||||
| 1 | 62 | 86 | 40 | 68 | 20 | 49 | |||||
| 2 | 29 | 75 | 36 | 29 | 28 | 53 | |||||
| 3 | 25 | 122 | 30 | 35 | 9 | 4 | |||||
| 4 | 35 | 105 | 25 | 36 | 10 | 7 | |||||
| Mean | 38 | 97 | 33 | 42 | 17 | 28 | |||||
| SD | 17 | 21 | 7 | 18 | 9 | 26 | |||||
| P | .03 | .29 | .29 | ||||||||
The PI for nonopsonized RBCs, based on the mean of 3 experiments, was 0 and 1 for cells not exposed to piceatannol, respectively not stimulated and stimulated with G-CSF (300 ng/mL); and 0 and 0 both for unstimulated and G-CSF—stimulated cells, both in the presence of 25 μg/mL or 50 μg/mL piceatannol.
Baseline phagocytic activity was lowered as well, in a dose-dependent fashion by piceatannol. These observations clearly indicate that interfering with Syk activity profoundly affects G-CSF–induced phagocytosis.
FcγR expression
In the process of granulocytic maturation, myeloid cells acquire or enhance specific functions such as bacterial killing by phagocytosis. This process is mediated by FcγRs induced in the maturation process. We and others have demonstrated that FcγRI is induced in both human and murine polymorphonuclear cells (PMNs) under certain conditions such as exposure to G-CSF or interferon γ (IFN-γ).39 Mice lack the genes for the potentially stimulatory receptors FcγRIIA and FcγRIIIB40 and murine PMNs do not express FcγRIIIA, although this receptor is present on murine macrophages. Thus, of the FcγRs, only the nonphagocytic FcγR, FcγRIIB, is expressed constitutively on murine PMNs. We investigated whether de novo expression of the phagocytic FcγR, FcγRI, was a precipitating factor in the effect of G-CSF-R on IgG-mediated phagocytosis.
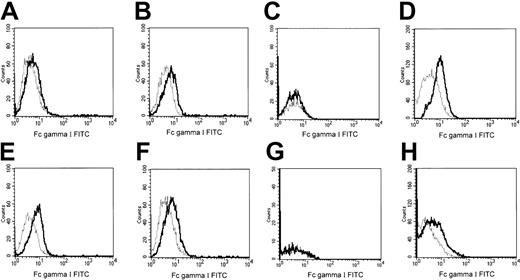
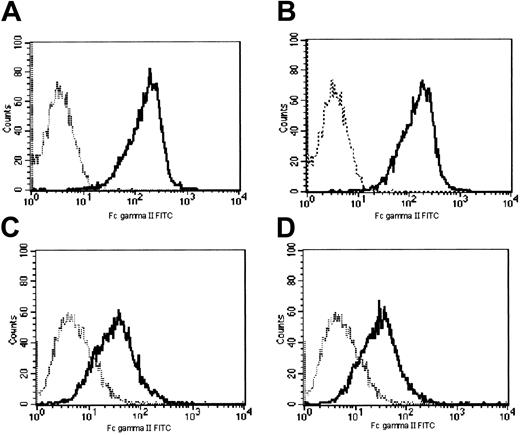
Cell surface expression of murine FcγR was assayed with anti-mFcγRI mAb and cell surface expression of murine FcγRII/III with anti-mFcγII/III mAb. FcγRI expression was extremely low and was not significantly induced by short exposure of WT or MT G-CSF-R 32D cells to G-CSF (Figure 7). Incubation of WT and MT G-CSF-R 32D transfectants with G-CSF for 30 minutes also did not modify expression of FcγRs recognized by mAb 2.4G (FcγRII/III; Figure 8). Therefore, the increase in phagocytosis following G-CSF stimulation cannot be attributed to a significant rapid de novo increase in surface expression of FcγRs.
Expression of FcγRI after exposure to G-CSF. FcγRI cell surface expression was evaluated by flow cytometry using FITC-conjugated rat antimouse FcγRI mAb (solid lines). (A-D) Cells transfected with WT G-CSF-R; (E-H) cells transfected with mutant G-CSF-R. In panels A, B, E, and F, cells were cultured for 30 minutes in the presence (B,F) or absence of G-CSF (A,E). In panels D and H cells were cultured for 3 days in the presence of G-CSF (100 ng/mL). Panels C and G depict the cells cultured in the absence of G-CSF for 3 days (+ 2.5% WEHI medium). Dotted lines indicate fluorescence of isotype-matched antimouse Ig-FITC–incubated cells.
Expression of FcγRI after exposure to G-CSF. FcγRI cell surface expression was evaluated by flow cytometry using FITC-conjugated rat antimouse FcγRI mAb (solid lines). (A-D) Cells transfected with WT G-CSF-R; (E-H) cells transfected with mutant G-CSF-R. In panels A, B, E, and F, cells were cultured for 30 minutes in the presence (B,F) or absence of G-CSF (A,E). In panels D and H cells were cultured for 3 days in the presence of G-CSF (100 ng/mL). Panels C and G depict the cells cultured in the absence of G-CSF for 3 days (+ 2.5% WEHI medium). Dotted lines indicate fluorescence of isotype-matched antimouse Ig-FITC–incubated cells.
Expression of FcγRII/III after a 30-minute exposure to G-CSF. FcγRII/III cell surface expression was evaluated by flow cytometry using FITC-conjugated rat antimouse FcγRII/III mAb (solid lines). (A-B) Cells transfected with WT G-CSF-R; (C-D) cells transfected with mutant G-CSF-R; (A,C) no G-CSF stimulation; (B,D) stimulation with G-CSF (300 ng/mL) for 30 minutes. Dotted lines indicate fluorescence of isotype-matched antimouse Ig-FITC–incubated cells.
Expression of FcγRII/III after a 30-minute exposure to G-CSF. FcγRII/III cell surface expression was evaluated by flow cytometry using FITC-conjugated rat antimouse FcγRII/III mAb (solid lines). (A-B) Cells transfected with WT G-CSF-R; (C-D) cells transfected with mutant G-CSF-R; (A,C) no G-CSF stimulation; (B,D) stimulation with G-CSF (300 ng/mL) for 30 minutes. Dotted lines indicate fluorescence of isotype-matched antimouse Ig-FITC–incubated cells.
We also examined whether G-CSF modifies expression of the phagocytic FcγR, FcγRI, in our cell model after longer exposure to the cytokine. In cells partially differentiated after exposure to G-CSF for 3 days, there was, as expected, a small but significant increase (P ≤ .001) in FcγRI expression in both WT and MT cells. For WT G-CSF-R 32D cells, the MFI of FcγRI was 1.05 after 30 minutes of G-CSF exposure and 1.75 after 3 days of exposure to the cytokine (Figure 7B and D, respectively); for MT cells, the MFI of FcγRI was 1.3 after 30 minutes of G-CSF treatment and 1.93 at day 3 of G-CSF exposure (Figure 7F and H, respectively). In terms of the PI, however, the response to G-CSF of cells maintained in G-CSF for 3 days (Table 3) was not significantly different from the response of cells exposed to G-CSF for only 30 minutes (Table 1). Note, however, that for WT cells at day 0, when cells had not been induced to mature, the PI of WT cells stimulated with G-CSF for 30 minutes was about 2-fold higher than that of controls (Table 1). After a further 30-minute challenge with 300 ng/mL G-CSF, the PI of WT cells exposed to G-CSF for 3 days was about 3-fold higher than that of cells not stimulated (Table 3). Thus, although we did not observe a significant change in the PI of WT cells in either the long-term G-CSF–treated or untreated cells, there does appear to be a trend to increased phagocytosis with the small but significant enhanced Fcγ expression observed in the partially mature granulocytic cells.
Discussion
In addition to promoting the production and maturation of neutrophils,1 G-CSF has an important stimulatory effect on IgG-mediated phagocytosis.41,42 We have demonstrated that stimulation of cells expressing WT hG-CSF-R by G-CSF enhances phagocytosis of IgG-coated particles, but that stimulation of 32D cells expressing the truncated mutant (Δ715) of the G-CSF-R does not. We made our observations in a murine myeloid cell line susceptible to terminal granulocytic maturation, a model to define the effects of G-CSF.38 The truncated mutant G-CSF-R was derived from granulocytes of a patient with SCN and lacks 98 carboxy-terminal amino acids.19
Studies to clarify the specific role of the G-CSF-R domains have indicated that the carboxy-terminal domain is essential in transducing signals for cell survival, cell maturation, and inhibition of cell proliferation, whereas the membrane proximal region is fundamental for mitogenic signals.8,22 Several specific tyrosine residues have been identified as responsible for these activities.9,43,44 Cells homozygous for truncated G-CSF-R have alterations in their growth and maturation response to G-CSF14 and lack the ability to activate Shc/Grb2/p140 complex formation after G-CSF exposure.9,44 Our data are consistent with and extend these studies.
Because the phagocytosis of IgG-coated particles is mediated by FcγRs, a possibility was that enhancement of phagocytosis after a short-term stimulation with the ligand was due to an increase in FcγR expression. Mice lack the genes for the FcγRs, FcγRIIA and FcγRIIIB, and FcγRIIIA is not expressed in mouse neutrophils.40 The phagocytic FcγR, FcγRI, is expressed at very low levels (or not at all) in resting neutrophils but is induced when PMNs are incubated with G-CSF.39 Thus, murine neutrophils constitutively express mainly the nonphagocytic FcγR, FcγRIIB. In our murine model, we did not observe a rapid induction of the FcγRs by G-CSF (Figures 4, 5). Furthermore, we demonstrated that culture for 3 days with G-CSF (which induced partial cell maturation) can induce some de novo expression of FcγRI, but that baseline phagocytosis was not sensitive to small changes in FcγRI expression. The observation that G-CSF increases phagocytosis of IgG-coated particles in cells with limited expression of the phagocytic FcγRs raised the question of how this effect is achieved.
Our data demonstrate that in cells expressing WT G-CSF-R, incubation with G-CSF is a stimulus to induce the rapid tyrosine phosphorylation of Syk, Lyn, and Hck kinases (Figure 3). Syk is a critical effector of immunoreceptor-mediated cell signaling in B and T lymphocytes and is essential for FcγR signaling in monocytes/macrophages.23, 24, 25, 26, 27 Syk binds via its 2 SH2 domains to conserved cytoplasmic sequences (ITAMs) in receptors and adaptor proteins. ITAMs contain tyrosines, which, after phosphorylation by Src kinases, may recruit Syk or ZAP-70 through their 2 SH2 domains and thereby initiate downstream signaling events.27 Syk protein tyrosine kinase is essential for FcγR signaling in monocytes/macrophages and neutrophils23, 24, 25, 26 and enhances FcγR-mediated phagocytosis in transfected epithelial cells.28,37 We observed tyrosine phosphorylation of Syk and induction of its tyrosine kinase activity following G-CSF ligation of WT but not MT G-CSF-R. The atypical ITAM motif between amino acids 727 and 747 in the carboxy-terminal region of G-CSF-R has been suggested as a potential phosphotyrosine site for Syk SH2 recognition.31 Our observation that activation of Syk induced by ligation of G-CSF-R is dependent on the availability of the carboxy-terminal region of the G-CSF-R in our G-CSF induction model for phagocytosis is consistent with this thesis.
One possibility is that G-CSF enhances FcγRI-mediated phagocytosis by reinforcing or potentiating the FcγRI signaling pathway through activation of Syk kinase following G-CSF-R activation. We have observed that overexpression of Syk in cells in which Syk expression is low enhances FcγRI/γ phagocytosis.28 Furthermore, in FcγR-mediated phagocytosis, the uptake of opsonized particles is driven by the actin-based cytoskeleton and Syk kinase has been observed to localize with actin at an early stage of particle engulfment.45
Although SRTKs have been considered to be responsible for initial FcγR tyrosine phosphorylation following receptor cross-linking, it has also been proposed that Syk itself can enhance ITAM phosphorylation after the first step of ITAM phosphorylation by other kinases.46 Following receptor ligation by ligand or antibody, phosphorylated ITAMs bind to Syk kinase. Syk/FcγR association may occur, however, under conditions other than immunologic stimulation.47 In platelets after thrombin-induced activation, Syk associates with FcγRIIA and becomes phosphorylated on tyrosine apparently before tyrosine phosphorylation of FcγRIIA. FcγRIIA tyrosine phosphorylation is inhibited at concentrations of piceatannol that specifically inhibit Syk but have no effect on SRTKs. These results suggest that Syk may participate in FcγR phosphorylation and lend support to the thesis that by providing additional phosphorylated FcγR ITAM sites, which in turn may bind and recruit other Syk molecules, Syk can amplify an initial weak FcγR signal.
Such a mechanism may be operative for enhanced phagocytosis following G-CSF–induced activation of Syk. By this mechanism, activated Syk could phosphorylate FcγRI/γ and thus amplify the phagocytic signal while bypassing a need for increased membrane expression of FcγRI for enhancement of phagocytosis. Of note, G-CSF-R clusters after G-CSF ligation and phosphotyrosine kinases (PTKs) including Syk and SRTKs are recruited into a signaling complex with WT G-CSF-R.31 Thus, another factor that may contribute to the enhancement of IgG-mediated phagocytosis following G-CSF-R ligation is the clustering of Syk molecules, providing high local concentrations of Syk kinase activity usually present in FcγRI receptor/Syk signaling complexes.48
The roles of the SRTKs Lyn and Hck in G-CSF–induced phagocytosis is unclear. Lyn kinase, which is essential for the G-CSF mitogenic signal,33 binds to the proline-rich region of the membrane proximal domain (box 1) of the G-CSF-R through its SH3 domain.36 The binding is constitutive.35 As expected, we observed phosphorylated Lyn in Syk immunoprecipitates from both WT and MT G-CSF-R cells and from both ligated and unligated receptors (Figure 3C). However, enhancement of Syk tyrosine phosphorylation and activation was appreciable only in lysates from cells expressing WT G-CSF-R after G-CSF ligation (Figures 3A and 4), suggesting that the association of phosphorylated Lyn with Syk is not sufficient for sustained Syk phosphorylation. The absence of phosphorylated Syk in cells expressing the MT G-CSF-R, with which Lyn is also constitutively associated, argues against a direct role for Lyn in Syk activation.
A more likely participant in Syk phosphorylation and G-CSF-R/Syk enhancement of phagocytosis in these cells is Hck kinase, another kinase of the Src family shown to be involved in G-CSF signaling.37 Hck, activated by G-CSF, is recruited to activated G-CSF-R, binding via its SH2 domain to phosphotyrosines. In our study, Hck tyrosine phosphorylation and its coprecipitation with Syk after G-CSF stimulation occurred in transfectants expressing WT G-CSF-R, but not in transfectants expressing the mutant receptor (Figure 3D). Thus, in these cells, G-CSF–induced tyrosine phosphorylation of Hck and Syk, and G-CSF enhancement of phagocytosis of IgG-coated particles all appear to share a dependency on sequences in the carboxy-terminal region of G-CSF-R.
In summary, our data stress the relevance of G-CSF as a stimulator of phagocytic activity in neutrophils via the specific activation of Syk. This function is dependent on the ITAM-like region of the G-CSF-R receptor in the carboxy-terminal domain.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-07-2271.
Supported in part by AIL-Firenze and NIH grants HL-27068 and HL-69498.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 2. Binding of PE-conjugated hG-CSF to WT G-CSF-R or MT G-CSF-R transfectants. The fluorescence intensity of cells incubated with G-CSF–PE (solid lines) (or PE-conjugated streptavidin as control [dotted lines]) for 30 minutes on ice were analyzed by flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-07-2271/5/m_h81134406002.jpeg?Expires=1769364342&Signature=MiJkQShB~qAzATaO52yK1pccD3aw9C0Izi7tYnn87rbMSvAZLoP6iPy0NLq2XeatrivzHqfFul9b4~obcLc-NLTNq9xh3RMqZ-fI9o5w7Hg1czUyV78Gz5-pymlPyasjpRZAv6K4ufKtya86XoBVufHfXuTOGT8qkc9L6-wEoMvqsVwe3waDvIdRXy63ojfegn~S~ruJeuUD6PJYbaWLDAv0wrHhXVe3e3ZBSF9s6a0OgMTfoLM6klIOdJJTAD2ypUBVlIvYpq4xPYAm7wcDWwHKr8Gec5rzr7kPTzHHXgvd2nRfczCmmYPAcja2yxEc1KUwcjOnkJcn3K-tGY3I2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal