Abstract
Macrophages and dendritic cells play an important role in regulating B-cell responses, including proliferation to antigens such as trinitrophenyl (TNP)—Ficoll and TNP-Brucella abortus. However, the mechanisms and molecule(s) that regulate these processes are relatively undefined. In this report, we show that human macrophages generated in vitro strongly costimulate proliferation of dense human tonsillar B cells ligated via their B-cell antigen receptor (BCR) but not proliferation via CD40. Similarly, dendritic cells also markedly enhance BCR-activated B-cell proliferation. Soluble molecule(s) are required for human macrophages to costimulate proliferation of B cells triggered via their BCR. Importantly, a TACI (trans-membrane activator and CAML interactor)—Fc fusion protein inhibits both macrophage- and dendritic cell (DC)—dependent BCR-activated B-cell proliferation, indicating a requirement for at least one of the known TACI ligands, BAFF and/or APRIL. Consistent with a major role for BAFF, macrophages release BAFF at levels sufficient to potently costimulate BCR-induced B-cell proliferation. In addition, BAFF is more than 100-fold more potent than APRIL in enhancing BCR-mediated human B-cell proliferation. Furthermore, immunodepletion of APRIL under conditions that prevent APRIL-mediated B-cell costimulation does not block macrophage enhancement of B-cell proliferation. Finally, there is no correlation between the high levels of a proliferation-inducing ligand (APRIL) expressed by macrophages compared with DCs and the similar abilities of macrophages and DCs to enhance BCR-stimulated B-cell proliferation. In summary, our results suggest that macrophage- and DC-derived B-cell—activating factor belonging to the TNF family (BAFF) represents a key molecule by which macrophages and DCs directly regulate human B-cell proliferative responses to T-cell—independent stimuli.
Introduction
Antibody responses to antigens can be broadly divided into 2 types; T-cell dependent (TD) and T-cell independent (TI).1 T-cell—dependent antibody responses require the tumor necrosis factor receptor (TNFR) homolog, CD40, a costimulatory molecule expressed on B cells and dendritic cells (DCs).2,3 Engagement of CD40 is mediated by CD154 (CD40 ligand), principally expressed on activated T cells.
Less is known about TI responses initiated by bacterial cell wall lipopolysaccharides (type 1 antigens) and repetitive polysaccharides (type 2 antigens). The splenic marginal zone (MZ) provides a primary line of defense against blood-borne pathogens, including encapsulated bacteria in mice.4, 5, 6 Anatomically, the MZ lies between the white and red pulp of the spleen and is composed of B cells, macrophages, and DCs.7 The MZ B cells are adjacent to the marginal sinuses and are among the first cell population encountered by blood-borne antigens. TI type 2 immune responses require MZ B cells. Thus, mice deficient in the tyrosine kinase Pyk-2 that lack splenic MZ B cells have defective antibody responses to the TI type 2 antigen, trinitrophenyl (TNP)—Ficoll.8 DCs localized within the MZ express phenotypic markers typically associated with macrophages and have been referred to as myeloid DCs.9 In vitro—derived myeloid DCs initiate humoral immune responses in mice to TI antigens, expressed by the intact gram-positive extracellular bacteria Streptococcus pneumoniae.10 Recently, a specific population of murine DCs that can promote the generation of antigen-specific plasmablasts from naive MZ B cells has been identified. These DCs are blood-derived, CD11clo DCs, which capture antigen and then migrate from the blood to the spleen.11 However, a supporting or accessory role for other cell types in regulating B cells such as macrophages is also possible. Indeed, bacteria-primed splenic murine macrophages also enhance MZ B-cell survival in vitro.11
Earlier in vitro studies suggested that the TI B-cell response in mice to TNP-haptenated antigens such as TNP-Ficoll, TNP-lipoprotein (LP), and TNP—Brucella abortus directly required macrophages.12, 13, 14, 15 Interestingly, interleukin 1 (IL-1) appeared to substitute for the requirement of macrophages in B-cell responses to TI antigens. In addition, IL-1—secreting cells reconstitute the response of macrophage-depleted splenic cells to TI antigens.15
A newly characterized tumor necrosis factor (TNF) ligand-receptor system has recently been implicated in the regulation of both TI and TD immune responses. B-cell—activating factor belonging to the TNF family (BAFF; also known as TALL-1, THANK, BlyS, and zTNF4) is a novel TNF ligand superfamily member that is a potent regulator of B-cell development and function.16, 17, 18, 19, 20 BAFF protein is expressed by myeloid lineage cells and exists as both cell surface—associated and soluble, released forms.19,21 BAFF binds to 3 distinct TNF receptors: TACI (trans-membrane activator and CAML interactor), BCMA (B-cell maturation antigen), and BAFF-R/BR3 (BLyS receptor 3).20,22, 23, 24, 25, 26 Two of these receptors, TACI and BCMA, also bind another TNF ligand that shares about 50% sequence homology in its extracellular region with BAFF, known as a proliferation-inducing ligand (APRIL), TRDL-1, or TALL-2.17,27, 28, 29, 30 Like BAFF, APRIL is also released as a biologically active protein from cells.31
Overexpression or injection of BAFF increases MZ B-cell numbers and serum immunoglobulin levels in mice.32 Furthermore, TACI-deficient mice have defective responses to TI-2 antigens.33,34 In addition to its role in T-cell—independent immune responses, BAFF also appears to modestly enhance T-cell—dependent immune responses.35
In this study, we investigated a potential role for human macrophages in directly enhancing proliferation of human tonsillar B cells. We show that human macrophages directly enhance proliferation of human B cells triggered via their B-cell antigen receptor (BCR) but not CD40, a process that requires soluble molecule(s). Moreover, we show that macrophage- and mono-DC—regulated BCR-induced human B-cell proliferation is inhibited by TACI-Ig (immunoglobulin), demonstrating a requirement for one or more of the TACI ligands, BAFF, and/or APRIL. Consistent with a major role for BAFF, macrophages released BAFF at doses sufficient to maximally costimulate BCR-induced B-cell proliferation. Moreover, immunodepletion of APRIL did not block macrophage enhancement of BCR-induced B-cell proliferation.
In summary, our studies using human cells suggest that macrophages directly regulate B-cell proliferation via the TNF ligand, BAFF, and support the idea that macrophages may play an important accessory role during TI immune responses.
Materials and methods
Materials
Purified soluble recombinant FLAG-tagged human BAFF (residues 141-285) and a recombinant TACI-Ig fusion protein consisting of the extracellular domain (amino acids 1-154) of human TACI fused to the Fc portion of the human IgGγ1 heavy chain were generous gifts from Dr Jane Gross (Zymogenetics, Seattle, WA). Purified recombinant human TACI-Ig was also purchased from Alexis (San Diego, CA). Equivalent results were observed with TACI-Ig from both sources. Recombinant soluble FLAG-tagged human APRIL (residues 105-250) and soluble human CD40 ligand (CD40L) were obtained from Alexis. A preservative-free rabbit anti-APRIL polyclonal serum (catalog no. 2415) was purchased from ProSci (Poway, CA). Rabbit anti-BAFF polyclonal serum (catalog no. 07-167) was obtained from Upstate Biotechnology (Lake Placid, NY). Mouse anti-human BAFF monoclonal antibodies (mAbs) were purchased from either Research Diagnostics (Flanders, NJ) or ID Labs (London, ON). Rabbit anti-p38 mitogen-activated protein kinase (MAPK) polyclonal serum (sc-535) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-human IgM F(ab′)2 fragments (anti-IgM) were purchased from Jackson ImmunoResearch (West Grove, PA). Macrophage colony-stimulating factor (M-CSF) and IL-4 were obtained from Research Diagnostics. Human granulocyte-macrophage colony-stimulating factor (GM-CSF) (Leukine/Sargramostim; Immunex, Seattle, WA) for research purposes was purchased from the University of Washington School of Medicine Pharmacy (Seattle).
Generation of CD14+ monocyte-derived human macrophages and dendritic cells
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral human blood of anonymous individual donors by centrifugation over Ficoll-Hypaque (Robbins Scientific, Sunnyvale, CA). T cells were depleted by rosetting with sheep erythrocytes. CD14+ cells were subsequently isolated by positive selection with magnetic anti-CD14 microbeads according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). For human monocyte—derived macrophages (subsequently referred to as “macrophages”), CD14+ cells (1 × 106/mL) were cultured in complete RPMI 1640 medium containing 100 ng/mL M-CSF at 37°C in a humidified incubator for 6 to 8 days. Macrophage preparations were more than 95% CD14+ and were also mannose receptor (MR)+, CD71+, and CD3–, and many of the cells morphologically appeared spindle-like and adherent. Human monocyte—derived DCs (subsequently referred to as “mono-DCs”) were generated in vitro from CD14+ cells cultured with GM-CSF (100 ng/mL), IL-4 (30 ng/mL), and 2-mercaptoethanol (50 μM) for up to 7 days and were more than 95% CD14– and strongly CD1a+.36
Isolation of dense human tonsillar B cells
Dense B lymphocytes were isolated from human tonsils derived from different individual donors by sheep erythrocyte rosetting to deplete T cells and Percoll density gradient centrifugation as described.37 These cells were more than 95% CD20+ and more than 70% naive IgD+CD38– B cells and were the source of all B cells used in this study.
B-cell proliferation assays
Macrophages, mono-DCs, or monocytes (0.3-5 × 104) or macrophage-conditioned culture medium (MCCM) were cultured for 72 hours with dense tonsillar B cells (2 × 105) in the presence of anti-IgM (10 μg/mL), CD40 (G28-5) mAb (250 ng/mL), or soluble human CD40L (100 ng/mL) in the presence of enhancer (1 μg/mL) as described by the manufacturer in 96-well flat-bottomed polystyrene culture plates (Becton Dickinson, Franklin Lakes, NJ). Cells were incubated for the final 16 to 24 hours with 0.0185 MBq (0.5 μCi) [3H]-thymidine. Incubations were performed in triplicate, and results are presented as the mean ± SD. In some experiments, macrophages, MCCM, mono-DCs, or soluble BAFF or APRIL were preincubated for at least 30 minutes at 37°C with either 10 μg/mL TACI-Ig or control-Ig fusion protein prior to coculture with B cells.
In some experiments, B cells and either macrophages or mono-DCs were cultured in separate compartments using Transwells (6.5 mm diameter, 0.4-μm tissue culture—treated polycarbonate membrane; Costar, Corning, NY). Macrophages or mono-DCs (7.5 × 104) in a volume of 0.1 mL in the upper compartment were cultured with B cells (2 × 105) in a volume of 0.6 mL in the lower chamber for 72 hours. Cells were pulsed for the final 16 to 24 hours with 0.0555 MBq (1.5 μCi) [3H]-thymidine. After 72 hours, B cells were divided into 3 wells of a flat-bottomed 96-well plate, and [3H]-thymidine incorporation was quantified by liquid scintillation counting. Results are presented as the mean ± SD of each triplicate.
Immunodepletion of macrophage-conditioned culture medium
Recombinant APRIL or MCCM was incubated for 3 hours at 4°C with constant mixing with either 0 to 10 μg/mL rabbit anti-human APRIL polyclonal IgG or normal rabbit IgG. Protein A—Sepharose beads (30 μL packed beads) were added for the final 90 minutes to bind immune complexes. After centrifugation at 14 000g for 5 minutes at 4°C, immunodepleted supernatants were sterile filtered (0.2 μm) and cocultured with B cells in the presence of anti-IgM (10 μg/mL) as described above.
Immunoblotting
Whole-cell lysates were prepared from approximately 5 × 106 cells as described.38 Lysates (50 μg protein) were resolved by 12.5% sodium dodecyl sulfate—polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose in non-SDS—containing transfer buffer (25 mM Tris (tris(hydroxymethyl)aminomethane), 0.2 M glycine, 20% methanol, pH 8.5). For APRIL immunoblotting, nitrocellulose membranes were blocked overnight at 4°C with blocking buffer (TBST [Tris-HCl, NaCl, Tween 20] plus 5% [wt/vol] nonfat dry milk) and subsequently incubated overnight at 4°C with 1 μg/mL rabbit anti-APRIL serum diluted in blocking buffer. As a control for protein loading, nitrocellulose membranes were reprobed with 0.25 μg/mL rabbit anti-p38 MAPK serum diluted in TBST containing 5% (wt/vol) bovine serum albumin (BSA).
BAFF ELISA
High-capacity 96-well enzyme-linked immunosorbent assay (ELISA) plates (MaxiSorp, Nunc, VWR International, Brisbane, CA) were coated overnight at 4°C with anti-human BAFF mAb (5 μg/mL) diluted in phosphate-buffered saline (PBS) as a capture antibody. Following blocking with PBS containing 1% (wt/vol) BSA for at least 2 hours at room temperature, culture supernatants or soluble BAFF as a standard were incubated overnight at 4°C with the anti-BAFF—coated 96-well plates. Samples were then incubated for 2 hours at room temperature with 2 μg/mL rabbit anti-human BAFF serum and finally with goat anti-rabbit horseradish peroxidase (HRP) serum (1:2500; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at room temperature. HRP enzyme activity was quantified using 3, 3′, 5, 5′-tetramethylbenzidine (TMB; Sigma, St Louis, MO) as a substrate at 450 nm. All values are the mean ± SD of triplicate samples.
BAFF and APRIL reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells using a guanidine isothiocyanate-based method (Isoquick kit; Orca Biosciences, Bothell, WA), followed by a 30-minute incubation at 37°C with 5 to 10 U RNase-free DNase (Promega, Madison, WI) to digest residual genomic DNA. Total RNA was further purified using a RNA clean-up protocol supplied with a RNeasy kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized from total RNA (1 μg) using oligo(dT) primers (0.5 μg) and Superscript II reverse transcriptase (200 U) exactly as described by the manufacturer (Invitrogen, Carlsbad, CA). APRIL, BAFF, and glyceraldehyde phosphate dehydrogenase (GAPDH) were amplified by PCR using the following gene-specific primers: APRIL (129 base pair [bp]), 5′-CCGATGCCCTGGAAGCCTGG-3′ and 5′-AGTCATCCTTGGAGGTGGCG-3′; BAFF (368 bp), 5′-TCACGCCTTACTTCTTGC-3′ and 5′-GACCCTGAACGGCACGCTTATT-3′; GAPDH (452 bp), 5′-ACCACAGTCCATGCCATC AC-3′ and 5′-TCCACCACCCTGTTGCTGTA. PCR reactions were performed using 1 × thermophilic buffer, 0.5 mM each deoxynucleoside triphosphate (dNTP), and 0.5 μM sense and antisense primers with 1 U Taq polymerase (Promega) under the following conditions: APRIL, 2 mM MgCl2, 52°C annealing temperature, 32 cycles; BAFF, 1 mM MgCl2, 50°C annealing temperature, 35 cycles; GAPDH, 1.5 mM MgCl2, 50°C annealing temperature, 35 cycles. PCR products were resolved on 2.5% or 3% (wt/vol) NuSieve 3:1 agarose (FMC BioProducts, Rockland, ME) in 1 × TBE (Tris Borate EDTA (ethylenediaminetetraacetic acid)) buffer.
Results
Myeloid lineage cells enhance BCR-stimulated human B-cell proliferation
Earlier studies have shown that monocytes and different populations of in vitro-generated human DCs directly enhance the proliferation of CD40-activated B cells.39, 40, 41 Because macrophages represent another discrete myeloid cell lineage that interacts with B cells particularly in the splenic MZ,7 we were interested in testing whether monocyte-derived macrophages (“macrophages”) may also directly increase human B-cell proliferation in vitro. Dense human tonsillar B cells (“B cells”) were stimulated with either anti-IgM, which crosslinks the BCR, or alternatively treated with soluble CD40 ligand (CD40L) or CD40 mAb. These serve as a surrogate for CD40L expressed on activated T cells and also stimulate both human B cells and macrophages.
In the absence of a costimulus, macrophages induced little increase in B-cell proliferation (Figure 1A). However, in the presence of anti-IgM, macrophages strongly enhanced BCR-induced B-cell proliferation up to 10-fold in a cell number—dependent manner. The enhanced proliferation of BCR-activated B cells by macrophages also correlated with increased numbers of trypan blue—negative B cells, suggesting that macrophages also increased B-cell survival (data not shown). In contrast, macrophages weakly augmented either CD40L- or anti-CD40—stimulated B-cell proliferation at low numbers of macrophages relative to B cells (Figure 1B). Surprisingly, the same high numbers of macrophages that strongly enhanced BCR-induced proliferation, significantly reduced both CD40L- and anti-CD40—induced thymidine incorporation into B cells (Figure 1B). Furthermore, MCCM also significantly inhibited anti-CD40—stimulated proliferation of B cells in a dose-dependent manner (data not shown).
Macrophages and mono-DCs strongly enhance BCR- but not CD40-stimulated B-cell proliferation. (A) Macrophages strongly increase BCR-activated B-cell proliferation. Macrophages were cultured with dense tonsillar B cells in the absence (□) or presence of goat anti-human IgM F(ab′)2 fragments (▪) for 72 hours. Cells were incubated for the final 16 to 24 hours with [3H]-thymidine. Incubations were performed in triplicate, and representative results are presented as the mean ± SD from 1 of 6 similar experiments. The mean fold maximal increase in BCR-induced B-cell proliferation by macrophages was 3.9 ± 1.5 in 6 independent experiments. (B) Effect of macrophages on CD40-induced B-cell proliferation. Macrophages were incubated with B cells in the absence (▵) or presence of soluble CD40L (▴), CD40 mAb (•), or an isotype control mAb (MOPC21, ○) for 72 hours. Cells were pulsed for the final 16 to 24 hours with [3H]-thymidine. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 similar experiments. (C) Dendritic cells strongly enhance BCR-activated B-cell proliferation. Mono-DCs were cultured with B cells in the absence (□) or presence of goat anti-human IgM F(ab′)2fragments (▪) for 3 days. Thymidine incorporation was quantified as described in panel A. Incubations were performed in triplicate, and data are from 1 of 3 similar experiments. The mean fold maximal enhancement of BCR-induced B-cell proliferation by mono-DCs was 4.0 ± 1.2 in 3 separate experiments. (D) Monocytes weakly increase BCR-stimulated B-cell proliferation. CD14+ monocytes were cultured with B cells in the absence (□) or presence of goat anti-human IgM F(ab′)2fragments (▪) for 72 hours. Thymidine incorporation was quantified as described in panel A. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 similar experiments. The mean fold maximal increase in BCR-induced B-cell proliferation by monocytes was 2.2 ± 1.0 in 3 independent experiments. (E) Effect of dendritic cells on CD40-induced B-cell proliferation. Mono-DCs were incubated with B cells in the presence of medium (□), CD40 mAb (•), or an isotype control MOPC21 mAb (○) for 72 hours. Cells were incubated for the final 16 to 24 hours with [3H]-thymidine. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 independent experiments.
Macrophages and mono-DCs strongly enhance BCR- but not CD40-stimulated B-cell proliferation. (A) Macrophages strongly increase BCR-activated B-cell proliferation. Macrophages were cultured with dense tonsillar B cells in the absence (□) or presence of goat anti-human IgM F(ab′)2 fragments (▪) for 72 hours. Cells were incubated for the final 16 to 24 hours with [3H]-thymidine. Incubations were performed in triplicate, and representative results are presented as the mean ± SD from 1 of 6 similar experiments. The mean fold maximal increase in BCR-induced B-cell proliferation by macrophages was 3.9 ± 1.5 in 6 independent experiments. (B) Effect of macrophages on CD40-induced B-cell proliferation. Macrophages were incubated with B cells in the absence (▵) or presence of soluble CD40L (▴), CD40 mAb (•), or an isotype control mAb (MOPC21, ○) for 72 hours. Cells were pulsed for the final 16 to 24 hours with [3H]-thymidine. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 similar experiments. (C) Dendritic cells strongly enhance BCR-activated B-cell proliferation. Mono-DCs were cultured with B cells in the absence (□) or presence of goat anti-human IgM F(ab′)2fragments (▪) for 3 days. Thymidine incorporation was quantified as described in panel A. Incubations were performed in triplicate, and data are from 1 of 3 similar experiments. The mean fold maximal enhancement of BCR-induced B-cell proliferation by mono-DCs was 4.0 ± 1.2 in 3 separate experiments. (D) Monocytes weakly increase BCR-stimulated B-cell proliferation. CD14+ monocytes were cultured with B cells in the absence (□) or presence of goat anti-human IgM F(ab′)2fragments (▪) for 72 hours. Thymidine incorporation was quantified as described in panel A. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 similar experiments. The mean fold maximal increase in BCR-induced B-cell proliferation by monocytes was 2.2 ± 1.0 in 3 independent experiments. (E) Effect of dendritic cells on CD40-induced B-cell proliferation. Mono-DCs were incubated with B cells in the presence of medium (□), CD40 mAb (•), or an isotype control MOPC21 mAb (○) for 72 hours. Cells were incubated for the final 16 to 24 hours with [3H]-thymidine. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 independent experiments.
Similar to macrophages, mono-DCs also potentiated BCR-induced B-cell proliferation (Figure 1C). Using CD14+ cells isolated from the same donor, in vitro—derived macrophages and mono-DCs enhanced anti-IgM—induced B-cell proliferation to similar degrees with a 4- to 6-fold enhancement in thymidine incorporation. In contrast, CD14+ monocytes weakly enhanced BCR-activated B-cell proliferation only 2-fold (Figure 1D).
Similar to our results with monocyte-derived macrophages (Figure 1B), mono-DCs also weakly enhanced CD40-stimulated proliferation of B cells about 2-fold at lower ratios of mono-DCs to B cells (Figure 1E). In contrast to macrophages, highest numbers of mono-DCs did not markedly inhibit anti-CD40—induced B-cell proliferation (Figure 1E).
Macrophages and mono-DCs costimulate B-cell proliferation via soluble factor(s)
We then tested whether the effect of macrophages on BCR-stimulated B-cell proliferation could be mediated by a soluble factor(s), by incubating MCCM with B cells in the presence or absence of anti-IgM. In the absence of BCR ligation, MCCM, like macrophages (Figure 1A), did not significantly increase B-cell proliferation (Figure 2A). However, in the presence of anti-IgM, MCCM strongly increased B-cell proliferation in a dose-dependent manner up to 5-fold (Figure 2A). This effect was independent of M-CSF in the culture media, which did not itself enhance BCR-induced B-cell proliferation (Figure 2A).
Macrophages and BCR ligation costimulate B-cell proliferation via soluble factor(s). (A) Macrophage-conditioned culture medium strongly enhances BCR-stimulated B-cell proliferation. Serial 2-fold dilutions of 7-day culture supernatants from macrophages (▪,□) or M-CSF—containing control supernatants (•,○) were cultured with B cells in either the presence (▪,•) or absence (□,○) of goat anti-human IgM F(ab′)2 fragments for 72 hours. [3H]-thymidine incorporation was quantified as described in Figure 1A. Incubations were performed in triplicate, and results are presented as the mean ± SD of each triplicate from a representative experiment. The mean fold maximal increase in BCR-induced B-cell proliferation by macrophage-conditioned culture medium was 3.8 ± 1.4 from 4 independent experiments. (B) Enhancement of BCR-induced B-cell proliferation by macrophages does not require direct cell-cell contact. Macrophages, BAFF, or APRIL in the upper compartment were cultured with B cells in the presence of goat anti-human IgM F(ab′)2 fragments in the lower chamber of a 24-well Transwell culture plate for 72 hours. For comparative purposes, macrophages were also cocultured in direct contact with B cells in a standard 24-well tissue culture plate under identical conditions. Cells were pulsed for the final 16 to 24 hours with [3H]-thymidine. [3H]-thymidine incorporation was quantified as described in Figure 1A. Incubations were performed in triplicate, and data are presented as the mean ± SD from 1 of 2 similar experiments. Similar results were also observed using mono-DCs. No statistical difference (P < .05) was observed between the control and Transwell groups for each of the 3 stimulated conditions (anti-IgM + BAFF, anti-IgM + APRIL, anti-IgM + macrophages).
Macrophages and BCR ligation costimulate B-cell proliferation via soluble factor(s). (A) Macrophage-conditioned culture medium strongly enhances BCR-stimulated B-cell proliferation. Serial 2-fold dilutions of 7-day culture supernatants from macrophages (▪,□) or M-CSF—containing control supernatants (•,○) were cultured with B cells in either the presence (▪,•) or absence (□,○) of goat anti-human IgM F(ab′)2 fragments for 72 hours. [3H]-thymidine incorporation was quantified as described in Figure 1A. Incubations were performed in triplicate, and results are presented as the mean ± SD of each triplicate from a representative experiment. The mean fold maximal increase in BCR-induced B-cell proliferation by macrophage-conditioned culture medium was 3.8 ± 1.4 from 4 independent experiments. (B) Enhancement of BCR-induced B-cell proliferation by macrophages does not require direct cell-cell contact. Macrophages, BAFF, or APRIL in the upper compartment were cultured with B cells in the presence of goat anti-human IgM F(ab′)2 fragments in the lower chamber of a 24-well Transwell culture plate for 72 hours. For comparative purposes, macrophages were also cocultured in direct contact with B cells in a standard 24-well tissue culture plate under identical conditions. Cells were pulsed for the final 16 to 24 hours with [3H]-thymidine. [3H]-thymidine incorporation was quantified as described in Figure 1A. Incubations were performed in triplicate, and data are presented as the mean ± SD from 1 of 2 similar experiments. Similar results were also observed using mono-DCs. No statistical difference (P < .05) was observed between the control and Transwell groups for each of the 3 stimulated conditions (anti-IgM + BAFF, anti-IgM + APRIL, anti-IgM + macrophages).
In addition, we compared the stimulatory effects of macrophages separated from B cells by a permeable membrane in Transwells with macrophages cocultured in direct contact with B cells in standard wells. Soluble forms of the TNF ligands BAFF and APRIL, which have been shown to augment BCR-induced B-cell proliferation,19,29 were added to the upper chamber of the Transwell separate from human B cells in the lower well as controls to demonstrate that the Transwell membrane was functional (Figure 2B). Macrophages separated from B cells enhanced BCR-dependent B-cell proliferation to the same level observed with macrophages cultured in direct contact with B cells (Figure 2B). Similarly, mono-DCs cultured apart from B cells also increased BCR-induced B-cell proliferation (data not shown). These data suggest that direct cell contact is not essential for macrophages and DCs to increase B-cell proliferation. Thus, the ability of both macrophages and mono-DCs to enhance BCR-dependent human B-cell proliferation is mediated by a soluble factor(s).
TACI-Ig inhibits macrophage- and mono-DC—induced costimulation of B-cell proliferation
Two novel, related TNF superfamily members, BAFF and APRIL, bind to TACI and can be blocked by soluble TACI-Ig.20 Myeloid lineage cells express the highest levels of BAFF.19,21 Although BAFF is constitutively expressed by macrophages as both membrane-associated and secreted forms, it is undetectable on the surface of myeloid DCs.21 However, a soluble form of BAFF is secreted by DCs.21 In contrast, little is known about APRIL protein expression in primary cells.27
To test whether BAFF and/or APRIL plays a role in macrophage-induced B-cell costimulation, we preincubated TACI-Ig with either macrophages or MCCM prior to coculture with B cells. Under experimental conditions in which TACI-Ig blocked BAFF-induced B-cell costimulation, TACI-Ig but not a control IgG1 fusion protein also completely inhibited macrophage-mediated B-cell proliferation (Figure 3A). Similarly, B-cell costimulation via anti-IgM and MCCM was also strongly blocked by a TACI-Ig but not a control IgG1 fusion protein (Figure 3A). Anti-IgM did not increase thymidine incorporation by macrophages themselves. This result excludes the possibility that proliferation of the macrophages accounts for the strong increase in BCR-induced B-cell proliferation by macrophages (Figure 3A). Furthermore, TACI-Ig, but not a control IgG1 fusion protein, also markedly inhibited mono-DC—enhanced BCR-induced B-cell proliferation (Figure 3B). These results show that BAFF and/or APRIL are required for macrophage- and DC-induced B-cell costimulation.
TACI-Ig inhibits macrophage- and mono-DC—induced costimulation of B-cell proliferation. (A) Enhancement of BCR-induced B-cell proliferation by macrophages requires a TACI ligand. Macrophages, 7-day conditioned macrophage culture supernatant, or BAFF was preincubated for at least 1 hour at 37°C with PBS, TACI-Ig, or a control-Ig fusion protein prior to coculture with B cells in the presence of goat anti-human IgM F(ab′)2 fragments. [3H]-thymidine incorporation assays were performed as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 independent experiments. (B) A TACI ligand is required for mono-DC—mediated increases in BCR-induced B-cell proliferation. Dendritic cells or BAFF were pretreated for 1 hour at 37°C with PBS, TACI-Ig, or a control immunoglobulin fusion protein prior to coculture with B cells in the presence of goat anti-human IgM F(ab′)2 fragments. [3H]-thymidine incorporation assays were performed as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 2 experiments. (C) TACI-Ig partially reduces anti-IgM—induced B-cell proliferation. B cells in the presence or absence of BAFF were preincubated for 1 hour at 37°C with PBS, TACI-Ig, or control-Ig fusion protein prior to culture in the presence of goat antihuman IgM F(ab′)2 fragments. [3H]-thymidine incorporation assays were performed as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD of triplicate incubations from 1 of 2 separate experiments.
TACI-Ig inhibits macrophage- and mono-DC—induced costimulation of B-cell proliferation. (A) Enhancement of BCR-induced B-cell proliferation by macrophages requires a TACI ligand. Macrophages, 7-day conditioned macrophage culture supernatant, or BAFF was preincubated for at least 1 hour at 37°C with PBS, TACI-Ig, or a control-Ig fusion protein prior to coculture with B cells in the presence of goat anti-human IgM F(ab′)2 fragments. [3H]-thymidine incorporation assays were performed as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 independent experiments. (B) A TACI ligand is required for mono-DC—mediated increases in BCR-induced B-cell proliferation. Dendritic cells or BAFF were pretreated for 1 hour at 37°C with PBS, TACI-Ig, or a control immunoglobulin fusion protein prior to coculture with B cells in the presence of goat anti-human IgM F(ab′)2 fragments. [3H]-thymidine incorporation assays were performed as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 2 experiments. (C) TACI-Ig partially reduces anti-IgM—induced B-cell proliferation. B cells in the presence or absence of BAFF were preincubated for 1 hour at 37°C with PBS, TACI-Ig, or control-Ig fusion protein prior to culture in the presence of goat antihuman IgM F(ab′)2 fragments. [3H]-thymidine incorporation assays were performed as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD of triplicate incubations from 1 of 2 separate experiments.
Myeloid cells express BAFF and APRIL mRNA and protein; regulation by cytokines and bacterial cell wall components
Because our results strongly suggest that a macrophage- and mono-DC—derived TACI ligand mediates B-cell costimulation, we next examined the expression of BAFF and APRIL in different human myeloid cells. Initially, RT-PCR was used to quantify relative mRNA levels. Both BAFF and APRIL were expressed at similar relative mRNA levels in macrophages, mono-DCs, and monocytes (Figure 4A, upper and middle panels). APRIL but not BAFF mRNA was also expressed by dense tonsillar B cells (data not shown). Reports indicate that BAFF can be processed into a truncated soluble and biologically active polypeptide.16,19,21 Thus, we quantified release of soluble BAFF using capture ELISA. Supernatants from M-CSF—derived macrophages contained BAFF in the range of 3 to 23 ng/mL during a 6- to 8-day incubation period from approximately 20 different donors (Figure 4B). Treatment of macrophages with either IL-10 or interferon-γ (IFN-γ) but not IL-4 enhanced BAFF levels approximately 2- to 6-fold compared with untreated controls, consistent with another study21 (Figure 4C). In addition, the gram-positive cell wall component, peptidoglycan, also moderately increased BAFF release 2- to 3-fold (Figure 4C).
Expression of BAFF and APRIL in myeloid lineage cells. (A) Myeloid lineage cells express comparable levels of BAFF and APRIL mRNAs. First-strand cDNA was synthesized from RNase-free DNase-digested total RNA using oligo(dT) primers and Superscript II reverse transcriptase. BAFF, APRIL, and GAPDH were amplified by PCR using gene-specific primers. PCR products were resolved on NuSieve 3:1 agarose. DNA molecular size markers are indicated in the far left lanes. (B-C) Macrophages constitutively release BAFF; cytokines and bacterial cell wall components induce BAFF release from macrophages. Anti-human BAFF mAb-coated ELISA plates were incubated with PBS/BSA for 2 hours at room temperature. After blocking, culture supernatants or soluble BAFF as a standard was incubated overnight at 4°C with anti-BAFF—coated plates. Samples were incubated for 2 hours at room temperature with rabbit anti-human BAFF polyclonal serum and subsequently with goat anti-rabbit HRP for 1 hour at room temperature. HRP enzyme activity was quantified using TMB as a substrate at 450 nm. All values are the mean ± SD of triplicate samples. (D-E) Macrophages but not mono-DCs or monocytes constitutively express high relative levels of APRIL protein. APRIL is induced by LPS and interferon-γ in mono-DCs. Cell lysates (50 μg total protein) from B cells, macrophages, mono-DCs, and monocytes were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with APRIL antiserum (upper panel) or reprobed with p38 MAPK antiserum (lower panel) as a control for protein loading.
Expression of BAFF and APRIL in myeloid lineage cells. (A) Myeloid lineage cells express comparable levels of BAFF and APRIL mRNAs. First-strand cDNA was synthesized from RNase-free DNase-digested total RNA using oligo(dT) primers and Superscript II reverse transcriptase. BAFF, APRIL, and GAPDH were amplified by PCR using gene-specific primers. PCR products were resolved on NuSieve 3:1 agarose. DNA molecular size markers are indicated in the far left lanes. (B-C) Macrophages constitutively release BAFF; cytokines and bacterial cell wall components induce BAFF release from macrophages. Anti-human BAFF mAb-coated ELISA plates were incubated with PBS/BSA for 2 hours at room temperature. After blocking, culture supernatants or soluble BAFF as a standard was incubated overnight at 4°C with anti-BAFF—coated plates. Samples were incubated for 2 hours at room temperature with rabbit anti-human BAFF polyclonal serum and subsequently with goat anti-rabbit HRP for 1 hour at room temperature. HRP enzyme activity was quantified using TMB as a substrate at 450 nm. All values are the mean ± SD of triplicate samples. (D-E) Macrophages but not mono-DCs or monocytes constitutively express high relative levels of APRIL protein. APRIL is induced by LPS and interferon-γ in mono-DCs. Cell lysates (50 μg total protein) from B cells, macrophages, mono-DCs, and monocytes were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with APRIL antiserum (upper panel) or reprobed with p38 MAPK antiserum (lower panel) as a control for protein loading.
APRIL protein was detected by immunoblotting cell lysates using an APRIL-specific polyclonal antiserum. APRIL was expressed as 2 distinct polypeptides of approximately 25 and 27 kDa (Figure 4D). The existence of multiple forms of APRIL is consistent with previous studies that identified 3 alternatively spliced human APRIL cDNAs (TRDL-1α, TRDL-1β, and TRDL-1γ) and that are predicted to encode proteins of 27.4, 25.7, and 27.1 kDa, respectively.28 Macrophages expressed high relative levels of the smaller APRIL polypeptide (APRIL-short, Figure 4D). This shorter APRIL species was not detected in unstimulated monocytes, mono-DCs, or B cells (Figure 4D). In contrast, the larger APRIL species (APRIL-long) was expressed at low levels in B cells, but not significantly expressed in monocytes, mono-DCs, or macrophages (Figure 4D). Consistent with a minor role for endogenously produced APRIL driving B-cell proliferation, a TACI-Ig but not a control-Ig fusion protein partially inhibited anti-IgM—stimulated B-cell proliferation (Figure 3C). Furthermore, B-cell and myelocytic leukemia tumor-derived cell lines (Raji, T5-1, HL-60, and THP-1) exclusively expressed high levels of the larger APRIL species (data not shown), consistent with reported mRNA expression data.27 Treatment of mono-DCs with either lipopolysaccharide (LPS) or IFN-γ markedly increased levels of the smaller APRIL species but not to the same levels expressed in macrophages (Figure 4E). Neither LPS nor IFN-γ further enhanced the constitutively very high levels of APRIL observed in macrophages.
BAFF strongly costimulates BCR- but not CD40-induced human B-cell proliferation
Because macrophages and mono-DCs express both BAFF and APRIL21 (Figure 4), we compared the effects of recombinant BAFF and APRIL on the proliferation of B cells. In the absence of a costimulus, soluble BAFF induced moderate increases in B-cell proliferation (Figure 5A). However, in the presence of anti-IgM, BAFF strongly costimulated B-cell proliferation more than 10-fold (Figure 5A). Importantly, BAFF levels (3 ng/mL) that maximally costimulated BCR-induced B-cell proliferation were within the range of BAFF levels constitutively released by macrophages (Figure 4B). Similar to BAFF, soluble recombinant APRIL also enhanced BCR-induced B-cell proliferation 2- to 3-fold, but the concentrations of APRIL required to costimulate B-cell proliferation were more than 50-fold higher compared with BAFF (Figure 5B). We also tested the effect of soluble BAFF on CD40L-induced B-cell proliferation. BAFF increased CD40L-induced B-cell proliferation less than 2-fold over a wide range of CD40L and BAFF concentrations (Figure 5C). These data correlate with our earlier results showing that macrophages and mono-DCs only weakly augment CD40-induced proliferation of B cells (Figure 1B).
MBAFF strongly enhances BCR- but not CD40-induced proliferation of B cells. (A) BAFF strongly augments BCR-activated B-cell proliferation. B cells were incubated with the indicated doses of BAFF in the presence (▪) or absence (□) of goat anti-human IgM F(ab′)2 fragments (10 μg/mL) for 72 hours. [3H]-thymidine incorporation was determined as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 4 experiments. (B) APRIL moderately increases BCR-induced B-cell proliferation. B cells were treated with APRIL in the presence (▪) or absence (□) of goat anti-human IgM F(ab′)2 fragments (10 μg/mL) for 72 hours. [3H]-thymidine incorporation was determined as described in Figure 1. Assays were performed in triplicate, and results are presented as the mean ± SD from 1 of 2 similar experiments. (C) Effect of BAFF on CD40 ligand-stimulated B-cell proliferation. B cells were treated for 72 hours with increasing doses of BAFF in the absence (□) or presence of soluble human CD40L at the following concentrations: 20 ng/mL (▪), 50 ng/mL (○), or 100 ng/mL (•). [3H]-thymidine incorporation was determined as described in Figure 1. Assays were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 similar experiments.
MBAFF strongly enhances BCR- but not CD40-induced proliferation of B cells. (A) BAFF strongly augments BCR-activated B-cell proliferation. B cells were incubated with the indicated doses of BAFF in the presence (▪) or absence (□) of goat anti-human IgM F(ab′)2 fragments (10 μg/mL) for 72 hours. [3H]-thymidine incorporation was determined as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 4 experiments. (B) APRIL moderately increases BCR-induced B-cell proliferation. B cells were treated with APRIL in the presence (▪) or absence (□) of goat anti-human IgM F(ab′)2 fragments (10 μg/mL) for 72 hours. [3H]-thymidine incorporation was determined as described in Figure 1. Assays were performed in triplicate, and results are presented as the mean ± SD from 1 of 2 similar experiments. (C) Effect of BAFF on CD40 ligand-stimulated B-cell proliferation. B cells were treated for 72 hours with increasing doses of BAFF in the absence (□) or presence of soluble human CD40L at the following concentrations: 20 ng/mL (▪), 50 ng/mL (○), or 100 ng/mL (•). [3H]-thymidine incorporation was determined as described in Figure 1. Assays were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 similar experiments.
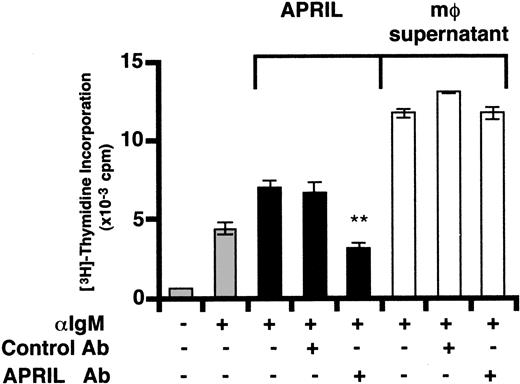
Macrophage-induced B-cell proliferation does not require APRIL
To further distinguish between a possible role for BAFF or APRIL, we immunodepleted APRIL using an APRIL antiserum. APRIL-dependent increases in BCR-induced B-cell proliferation were completely blocked by preincubation of purified recombinant APRIL with the APRIL antiserum but not control serum (Figure 6). In contrast, MCCM-mediated enhancement of anti-IgM—induced B-cell proliferation was unaffected following preincubation with anti-APRIL (Figure 6). These results together with the observation that APRIL is much less effective than BAFF in stimulating B cells and also that APRIL protein is not expressed in unstimulated DCs (Figure 4D-E, Figure 5), strongly suggest that BAFF is the major macrophage- and mono-DC—derived factor costimulating BCR-activated human B cells.
Immunodepletion of APRIL does not affect macrophage-induced costimulation of B cells. Macrophage-conditioned culture supernatant or recombinant human APRIL were incubated for 3 hours at 4°C with constant mixing with PBS, purified rabbit anti-human APRIL IgG, or control IgG (10 μg/mL). Protein A—Sepharose beads were added for the final 90 minutes to bind immune complexes. After centrifugation at 4°C, immunodepleted supernatants were sterile filtered and cocultured with dense tonsillar B cells in the presence of anti-human IgM F(ab′)2 fragments (10 μg/mL) as described in Figure 1. The asterisk indicates statistically significant differences between samples within the bracketed groups (P < .05).
Immunodepletion of APRIL does not affect macrophage-induced costimulation of B cells. Macrophage-conditioned culture supernatant or recombinant human APRIL were incubated for 3 hours at 4°C with constant mixing with PBS, purified rabbit anti-human APRIL IgG, or control IgG (10 μg/mL). Protein A—Sepharose beads were added for the final 90 minutes to bind immune complexes. After centrifugation at 4°C, immunodepleted supernatants were sterile filtered and cocultured with dense tonsillar B cells in the presence of anti-human IgM F(ab′)2 fragments (10 μg/mL) as described in Figure 1. The asterisk indicates statistically significant differences between samples within the bracketed groups (P < .05).
Discussion
These studies show that both human monocyte—derived macrophages and DCs directly enhance proliferation of BCR-activated human B cells via a soluble factor(s) (Figures 1, 2). Moreover, macrophage- and mono-DC—induced B-cell costimulation was inhibited by a TACI-Ig fusion protein (Figure 3), which binds 2 known ligands, BAFF and APRIL. Although macrophages express both BAFF and APRIL protein (Figure 4), immunodepletion of APRIL did not block macrophage-induced B-cell costimulation consistent with the finding that BAFF is the major factor required for the enhancement of BCR-induced B-cell proliferation by macrophages (Figure 6). Consistent with these results and as previously reported,21 BAFF was secreted by macrophages at levels sufficient to potently costimulate B-cell proliferation (Figures 4, 5). Furthermore, BAFF was at least 50-fold more potent than APRIL in augmenting anti-IgM—dependent proliferation of tonsillar B cells (Figure 5). Finally, there was no correlation between the high levels of APRIL protein expressed in macrophages compared with mono-DCs and the similar abilities of these 2 myeloid cell types to costimulate B-cell proliferation (Figures 1 and 4D). This finding is consistent with the model that APRIL does not play a major role in augmenting BCR-mediated B-cell proliferation. Indeed, a possible role for APRIL in regulating B-cell homeostasis in vivo remains to be shown, as APRIL-deficient mice die in utero.42 These results are also consistent with previous studies using mice deficient in various BAFF receptors, which strongly suggest a major positive regulatory role for BAFF-R, which binds only BAFF, but not TACI or BCMA, in BCR-induced B-cell costimulation.25,26,33,34,43
Our findings are consistent with a role in vivo for human macrophage— and DC-derived BAFF in TI type 2 humoral immune responses, in which macrophages and DCs located within the splenic MZ produce and secrete BAFF. Soluble BAFF may subsequently serve a supporting role in the generation of antigen-specific plasmablasts, as shown by a recent study in which bacteria-primed splenic macrophages and DCs provide a survival signal to MZ B cells.11
DCs play an established role in stimulating antigen-specific naive T cells to proliferate, secrete cytokines, and express CD40L. These activated T cells subsequently stimulate antigen-specific naive B lymphocytes to proliferate and differentiate into germinal center (GC) B cells or IgM-secreting plasma cells.44 Additional studies also showed that DCs directly enhance proliferation and immunoglobulin production of CD40-activated naive and memory B cells.39,41 Although IL-12 and soluble IL-6R-α chain (spg 80) are required for the differentiation of naive B cells into IgM-secreting plasma cells,40,45 the mechanism(s) by which DCs directly costimulate B-cell growth remain uncharacterized. The factor(s) described by Dubois et al39 that enhanced B-cell proliferation appeared to be both soluble and produced in a CD40-independent manner. Similarly, we found using Transwells that both DCs and macrophages enhance BCR-induced B-cell proliferation through the generation of a predominantly soluble factor(s) (Figure 2). Nevertheless, it is unlikely that BAFF is the DC-derived factor that enhances CD40-activated proliferation of human tonsillar B cells,39,41 because BAFF only weakly enhances CD40-induced proliferation of dense human tonsillar B cells (Figure 5C). In related studies, macrophages, which strongly enhanced BCR-induced proliferation (Figure 1A) and released BAFF at levels sufficient to potently costimulate B-cell proliferation (Figure 4B), inhibited CD40L-stimulated proliferation of B cells (Figure 1B, data not shown), suggesting that macrophages may differentially regulate B-cell responses to TI versus TD B-lymphocyte signals.
Although our results suggest that APRIL does not appear to play a major role in macrophage-induced costimulation of B cells (Figures 4, 5, 6), we show for the first time that APRIL protein is differentially expressed in myeloid lineage cells, with high constitutive levels observed in macrophages but not mono-DCs or monocytes (Figure 4D). Our results also indicate that APRIL expression may be regulated at the posttranscriptional level, because APRIL mRNA was present in all 3 myeloid cell lineages examined, yet high levels of protein were only observed in macrophages (Figure 4). In addition, APRIL may be regulated after translation via cleavage and secretion. It is currently unknown whether endogenous APRIL is processed and released by macrophages, although recent studies using APRIL-transfected HEK293 cells show that APRIL, like BAFF, can be released as a soluble and biologically active ligand.31 We were unsuccessful in attempts to establish an APRIL-specific ELISA using a panel of commercially available APRIL antisera to examine its possible release by macrophages and mono-DCs, as we and others have observed for BAFF21 (Figure 4B-C).
Although our studies were performed using dense human tonsillar human B cells, a subset of these B cells, known as subepithelial (SE) B cells, have many of the morphologic, phenotypic, and functional characteristics of splenic MZ B cells.46,47 These small, CD5– SE B cells represent about 5% to 10% of the total tonsillar B cells. Interestingly, SE B cells, but neither germinal center nor follicular mantle tonsillar B-cell subsets, specifically responded to the TI-II antigen, TNP-Ficoll, consistent with their identification as a tonsillar B-cell counterpart of splenic MZ B cells. Further experiments are necessary to examine whether distinct human tonsillar B-cell subsets (naive, germinal center, memory, and SE) differentially respond to BAFF.
In conclusion, our results demonstrate that monocyte-derived macrophages and DCs directly enhance BCR-mediated human B-cell proliferation via a soluble factor(s), which is specifically blocked by TACI-Ig.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-10-3123.
Supported by grants GM37905, DE13061, and RR00166 from the National Institutes of Health and a grant from Zymogenetics.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Jane Gross and colleagues (Zymogenetics Inc, Seattle, WA) for generously providing purified soluble recombinant FLAG-tagged human BAFF and TACI-Ig fusion protein. We are grateful to Drs Helen Floyd and Hiro Niiro for critical reading and comments during the preparation of this manuscript.

![Figure 1. Macrophages and mono-DCs strongly enhance BCR- but not CD40-stimulated B-cell proliferation. (A) Macrophages strongly increase BCR-activated B-cell proliferation. Macrophages were cultured with dense tonsillar B cells in the absence (□) or presence of goat anti-human IgM F(ab′)2 fragments (▪) for 72 hours. Cells were incubated for the final 16 to 24 hours with [3H]-thymidine. Incubations were performed in triplicate, and representative results are presented as the mean ± SD from 1 of 6 similar experiments. The mean fold maximal increase in BCR-induced B-cell proliferation by macrophages was 3.9 ± 1.5 in 6 independent experiments. (B) Effect of macrophages on CD40-induced B-cell proliferation. Macrophages were incubated with B cells in the absence (▵) or presence of soluble CD40L (▴), CD40 mAb (•), or an isotype control mAb (MOPC21, ○) for 72 hours. Cells were pulsed for the final 16 to 24 hours with [3H]-thymidine. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 similar experiments. (C) Dendritic cells strongly enhance BCR-activated B-cell proliferation. Mono-DCs were cultured with B cells in the absence (□) or presence of goat anti-human IgM F(ab′)2fragments (▪) for 3 days. Thymidine incorporation was quantified as described in panel A. Incubations were performed in triplicate, and data are from 1 of 3 similar experiments. The mean fold maximal enhancement of BCR-induced B-cell proliferation by mono-DCs was 4.0 ± 1.2 in 3 separate experiments. (D) Monocytes weakly increase BCR-stimulated B-cell proliferation. CD14+ monocytes were cultured with B cells in the absence (□) or presence of goat anti-human IgM F(ab′)2fragments (▪) for 72 hours. Thymidine incorporation was quantified as described in panel A. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 similar experiments. The mean fold maximal increase in BCR-induced B-cell proliferation by monocytes was 2.2 ± 1.0 in 3 independent experiments. (E) Effect of dendritic cells on CD40-induced B-cell proliferation. Mono-DCs were incubated with B cells in the presence of medium (□), CD40 mAb (•), or an isotype control MOPC21 mAb (○) for 72 hours. Cells were incubated for the final 16 to 24 hours with [3H]-thymidine. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-10-3123/5/m_h81134391001.jpeg?Expires=1767730716&Signature=mud9cm~oiZeSmm1acD7FCCIJ5qnqDb9EFew8ELkhWV9njJOADdiMCL8RkOc3P4PNdvwUMX423jQKUL8aCjmj9kcyegVQboRRXiEBus7ANF~Lo2QmO~CtvPKJauVftGz-zAWYrtmhiRi7FvHMNQeX7jjh2wBPFzZDWfqh3enXwYtpUAimqYaGXOI95W0mCyDgOjRWZvNHN9lTg-CNX7Y-Vi0n7yDfo2EHnifuicdQ0ssynMXmAfkECu2-MOUzLMRlM1v-3KL85s70ja-QmDmEuLcUVxtW6o1kNyT-IMTk5lK4rRiOK9jG8YFXWCWef1j9tBNQ9x-KEFdeLAXoKQTH3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Macrophages and BCR ligation costimulate B-cell proliferation via soluble factor(s). (A) Macrophage-conditioned culture medium strongly enhances BCR-stimulated B-cell proliferation. Serial 2-fold dilutions of 7-day culture supernatants from macrophages (▪,□) or M-CSF—containing control supernatants (•,○) were cultured with B cells in either the presence (▪,•) or absence (□,○) of goat anti-human IgM F(ab′)2 fragments for 72 hours. [3H]-thymidine incorporation was quantified as described in Figure 1A. Incubations were performed in triplicate, and results are presented as the mean ± SD of each triplicate from a representative experiment. The mean fold maximal increase in BCR-induced B-cell proliferation by macrophage-conditioned culture medium was 3.8 ± 1.4 from 4 independent experiments. (B) Enhancement of BCR-induced B-cell proliferation by macrophages does not require direct cell-cell contact. Macrophages, BAFF, or APRIL in the upper compartment were cultured with B cells in the presence of goat anti-human IgM F(ab′)2 fragments in the lower chamber of a 24-well Transwell culture plate for 72 hours. For comparative purposes, macrophages were also cocultured in direct contact with B cells in a standard 24-well tissue culture plate under identical conditions. Cells were pulsed for the final 16 to 24 hours with [3H]-thymidine. [3H]-thymidine incorporation was quantified as described in Figure 1A. Incubations were performed in triplicate, and data are presented as the mean ± SD from 1 of 2 similar experiments. Similar results were also observed using mono-DCs. No statistical difference (P < .05) was observed between the control and Transwell groups for each of the 3 stimulated conditions (anti-IgM + BAFF, anti-IgM + APRIL, anti-IgM + macrophages).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-10-3123/5/m_h81134391002.jpeg?Expires=1767730716&Signature=qlc9yRR05jSMszvg0Zu121OPb9kcvuX8Dt7ETQgR-UsEuf5KN1GAMvK-dxjY8Aiw1DE6M2R0nHy51naE-BzdorJg8SownBOukETKXKWlwnPvFgTVUH6LccBoAGKy1tpCJ9wM79mI9A1WMBnSBa2sYQjTj89zAh8IobH7LJzHSKllFefzsWbfelFqaKIIEkBmTH3Xn8KcPLY1AVesSbMPXasnHlJnsNctyucaOzjFBGTgRPvHvLrSChyVlfqj-GZiFj7VhCIHuWS0rGd0nulnYAXbhYiJejFBxbkJ9Wg2wK3Sdszokrgz305ytYqXep2lLJdQNAbYAlOMKqf4ovsjuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. TACI-Ig inhibits macrophage- and mono-DC—induced costimulation of B-cell proliferation. (A) Enhancement of BCR-induced B-cell proliferation by macrophages requires a TACI ligand. Macrophages, 7-day conditioned macrophage culture supernatant, or BAFF was preincubated for at least 1 hour at 37°C with PBS, TACI-Ig, or a control-Ig fusion protein prior to coculture with B cells in the presence of goat anti-human IgM F(ab′)2 fragments. [3H]-thymidine incorporation assays were performed as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 independent experiments. (B) A TACI ligand is required for mono-DC—mediated increases in BCR-induced B-cell proliferation. Dendritic cells or BAFF were pretreated for 1 hour at 37°C with PBS, TACI-Ig, or a control immunoglobulin fusion protein prior to coculture with B cells in the presence of goat anti-human IgM F(ab′)2 fragments. [3H]-thymidine incorporation assays were performed as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 2 experiments. (C) TACI-Ig partially reduces anti-IgM—induced B-cell proliferation. B cells in the presence or absence of BAFF were preincubated for 1 hour at 37°C with PBS, TACI-Ig, or control-Ig fusion protein prior to culture in the presence of goat antihuman IgM F(ab′)2 fragments. [3H]-thymidine incorporation assays were performed as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD of triplicate incubations from 1 of 2 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-10-3123/5/m_h81134391003.jpeg?Expires=1767730716&Signature=OTiK4dXGyNliCE8qnLWne2SRFNvd9fJ78XFbpxPD4FJkdPHKFYNIj~LoLiEYlgnCs8bB2rPQsMQKNFIOtcfx1tgFQ4PM4-wPkimXF-8TF~o0XReTAlp~qUuSY0gjZK~r1DhncGUn7KgPzCNIGD7v84WJbqu1R-SwCEYbqPaGLhsJFG7zXOlqhGY5oEicmcPdL3UwTC0wYlmFD0xPdV0IajZ3mqhpprBz-aN8oVBF3g0ZMDYBhfjwsfoBLB~7qB0BwWcWTEi6A89iHNnY3qkFJjGuSk9ADzGFNm~z92R83vxQ9-tDx8cNa8~hCzwje5HZAXmZu1UDYKzfS~LLMZhpPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. MBAFF strongly enhances BCR- but not CD40-induced proliferation of B cells. (A) BAFF strongly augments BCR-activated B-cell proliferation. B cells were incubated with the indicated doses of BAFF in the presence (▪) or absence (□) of goat anti-human IgM F(ab′)2 fragments (10 μg/mL) for 72 hours. [3H]-thymidine incorporation was determined as described in Figure 1. Incubations were performed in triplicate, and results are presented as the mean ± SD from 1 of 4 experiments. (B) APRIL moderately increases BCR-induced B-cell proliferation. B cells were treated with APRIL in the presence (▪) or absence (□) of goat anti-human IgM F(ab′)2 fragments (10 μg/mL) for 72 hours. [3H]-thymidine incorporation was determined as described in Figure 1. Assays were performed in triplicate, and results are presented as the mean ± SD from 1 of 2 similar experiments. (C) Effect of BAFF on CD40 ligand-stimulated B-cell proliferation. B cells were treated for 72 hours with increasing doses of BAFF in the absence (□) or presence of soluble human CD40L at the following concentrations: 20 ng/mL (▪), 50 ng/mL (○), or 100 ng/mL (•). [3H]-thymidine incorporation was determined as described in Figure 1. Assays were performed in triplicate, and results are presented as the mean ± SD from 1 of 3 similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-10-3123/5/m_h81134391005.jpeg?Expires=1767730716&Signature=Z1m8kLYiolo5PhpuP9oIg0yYyOjStn1HVwyxsou8E6jDHCtcDGATeppgX3AbQTfuok9SBiZKEY0QA6PfZdknottrIFvXQ5i2ywezmVphrEdfKE8VUbFqdk0xH8C2JYdOu46JWJUVwD7Kb8f-ES4I30cjUqypys1RqbfUfqKA~Pa674PXG7wPojECJePIzouPXod9~4pxQanHH~ttFlHd82h6~il6Fdh8-hy53x0CcgX1oyxdK7MZrM9AZqZ8XZ-hC66vWbKSfvFkRfF1F7ygUd5~roAnJmZCP7Ef6MlWfCF3RvQjIK~eC2SyXwQNsEhZXE81Mk66ZMnXzaNG0l6tiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal