Abstract
Progenitor B cells deficient in Pax5 are developmentally multipotent, suggesting that Pax5 is necessary to maintain commitment to the B-cell lineage. Commitment may be mediated, in part, by Pax5 repression of myeloid-specific genes. To determine whether Pax5 expression in multipotential cells is sufficient to restrict development to the B-cell lineage in vivo, we enforced expression of Pax5 in hematopoietic stem cells using a retroviral vector. Peripheral blood analysis of all animals reconstituted with Pax5-expressing cells indicated that more than 90% of Pax5-expressing cells were B220+ mature B cells that were not malignant. Further analysis showed that Pax5 completely blocked T-lineage development in the thymus but did not inhibit myelopoiesis or natural killer (NK) cell development in bone marrow. These results implicate Pax5 as a critical regulator of B- versus T-cell developmental fate and suggest that Pax5 may promote commitment to the B-cell lineage by mechanisms that are independent of myeloid gene repression.
Introduction
The generation of highly specialized cells within the hematopoietic system of mammals occurs through well-defined intermediates that have increasingly restricted developmental potential and little to no self-renewal ability. In one model, long-term self-renewing hematopoietic stem cells (LT-HSCs) differentiate into HSCs with more limited self-renewal activity and then into oligopotent progenitors restricted to the lymphoid and myeloid cell lineages.1, 2, 3 Common lymphoid progenitor (CLP) cells undergo additional lineage commitment events to generate progenitor cells restricted to the B-, T-, or natural killer (NK) cell pathways.
Null mutations in the genes encoding E2A, early B-cell factor (EBF1), and Pax5 (BSAP) have shown that these factors are necessary for the generation of B-lineage cells.4, 5, 6, 7 Loss of Pax5 resulted in a slightly later B-cell developmental block than seen in the E2A or EBF knock-out animals. However, in the absence of Pax5, progenitor B cells acquire the ability to differentiate into multiple hematopoietic lineages both in vitro and in vivo.8,9 The Pax5-deficient pro-B cells expressed both EBF and E2A, suggesting that these factors alone were not sufficient to irreversibly commit cells to the B-cell lineage without Pax5. Additional experiments showed that Pax5 might be functioning in progenitor B cells, in part, by repressing genes such as the macrophage colony-stimulating factor (M-CSF) receptor and myeloperoxidase, which are associated with differentiation along the myeloid lineage.8 Interestingly, the “de-differentiation” of Pax5—/— pro-B cells only occurred on removal of the cytokine interleukin 7 (IL-7) from in vitro cultures of Pax5-deficient pro-B cells, which may indicate a combined function for both IL-7 receptor signaling and Pax5 activity to determine irreversible commitment to the B-cell pathway.
Because the loss-of-function Pax5 experiments defined a critical role for Pax5 in the maintenance of B-cell identity, we wanted to test whether enforced expression of Pax5 in multipotential HSCs would be sufficient to restrict developmental potential to the B-cell lineage. To address this issue, we generated a retroviral vector that coexpressed Pax5 along with a green fluorescent protein (GFP) marker that was translated from an internal ribosome entry site (IRES). In this way, we were able to use GFP expression as an indicator of cells that also expressed Pax5. Transduced HSCs were transplanted into lethally irradiated mice and then analyzed for their contribution to the various blood cell lineages by flow cytometry (FACS). We found that the peripheral blood and spleens of all animals reconstituted with Pax5-expressing cells were almost entirely composed of B220+IgM+ B-lineage cells (approximately 90%), which was in contrast to animals reconstituted with cells expressing the control GFP vector. In the bone marrow, the initial stages of myeloid and NK cell development appeared unimpaired by expression of Pax5. However, Pax5 expression seemed to inhibit myeloid cell emigration from the bone marrow or induced apoptosis in later-stage myeloid cells in that only 5% to 10% of Pax5+GFP+ cells in the periphery expressed markers characteristic of mature myeloid cells. In contrast to myeloid development, the level of Pax5 expression from the retroviral vector was sufficient to completely block T-cell development. These results suggest that Pax5 plays a critical role in determining B- versus T-cell development fate.
Materials and methods
Plasmids
A Pax5 cDNA kindly provided by Dr M. Busslinger (Institute of Molecular Pathology, Vienna, Austria) was cloned into the EcoR1 site of the murine stem cell virus (MSCV) retroviral vector10 upstream of an IRES element and GFP. The control vector contained only the IRES and GFP sequences.
Bone marrow harvesting and culture
C57B/6-Ly-5.2 mice were injected intraperitoneally with 150 mg/kg body weight 5-fluorouracil (5-FU, catalog no. F6627; Sigma, St Louis, MO) dissolved in sterile phosphate-buffered saline (PBS). Four days later, mice were killed, and bone marrow was harvested from the femurs and tibias using a 25-gauge needle and sterile PBS. After a single cell suspension was obtained, red cells were lysed for 5 minutes on ice in 0.5 mL ACK (8.3 g ammonium chloride and 1.0 g potassium bicarbonate in 1 L distilled deionized water) per mouse equivalent of bone marrow. Cells were then washed in PBS, filtered, and then resuspended in Dulbecco modified Eagle medium (DMEM; Gibco-BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco-BRL, catalog no. 16141-079), penicillin 100 IU/mL, streptomycin 100 IU/mL (Gibco-BRL catalog no. 15070-063), 1 × nonessential amino acids, 1 × sodium pyruvate (1 mM), 1 × l-glutamine (2 mM; Gibco-BRL), and 50 μM β-mercaptoethanol (BME; Sigma). To induce stem cell cycling, cytokines were added to the culture media as follows: 5 ng/mL IL-6 (R&D Systems), 50 ng/mL stem cell factor (R&D Systems, Minneapolis, MN), and 1 × leukemia inhibitory factor (ESGRO, catalog no. ESG1107; Chemicon, Temecula, CO). Cells were prestimulated for 24 hours in a sterile incubator at 37°C in 5% CO2.
Retrovirus production and transduction
BOSC 23 cells11 (2 × 106) were plated on a 6-cm tissue-culture dish 1 day prior to calcium-phosphate transfection. Immediately before the transfection, fresh media containing 25 μM chloroquine diphosphate (Sigma catalog no. C6628) was added to the BOSC 23 cells. Plasmid DNA (20 μg) encoding for either the control GFP or Pax5 retroviruses was transfected into the BOSC 23 cells by standard calcium-phosphate transfection. The DNA-CaPO4 coprecipitate was incubated with the cells for between 2 and 6 hours before changing the media to remove the chloroquine. Twenty-four hours after transfection, the cells were irradiated (3000 rads) using a Cobalt irradiator. The prestimulated bone marrow cells were then plated on top of the irradiated, retroviral producer cells along with 4 μg/mL polybrene (hexadimethrine bromide; Sigma H-9268). Transduced cells were transplanted after 48 hours of coculture in the incubator.
Western blot analysis
To check retroviral expression of Pax5, supernatant from the retroviral packaging cells was harvested and added to NIH-3T3 cells together with 4 μg/mL polybrene. After 48 hours, cells were lysed, and a Western blot was performed using a goat polyclonal antibody to detect Pax5 (Santa Cruz) and enhanced chemiluminescence (ECL; Amersham). One million GFP+ myeloid lineage cells (Mac-1+Gr-1+) were FACS sorted from bone marrow of animals reconstituted with Pax5- or control GFP-expressing cells 8 weeks previously. Sorted cells were 98% pure, based on reanalysis.
Methylcellulose colony-forming assay
Bone marrow from 3 GFP and from 3 Pax5 mice reconstituted 12 weeks previously was harvested as described in “Bone marrow harvesting and culture.” From each mouse, 20 000 GFP+ cells were sorted into Iscoves modified Dulbecco medium (IMDM) supplemented with 10% fetal calf serum. Cells were plated into methylcellulose using MethCult 3434 media (Stem Cell Technologies, Vancouver, BC, Canada), which contains the recombinant cytokines, stem cell factor (SCF), IL-3, IL-6, and erythropoietin (EPO). Ten days after sorting, colonies of more than 50 cells were counted and examined for GFP+ expression using an inverted fluorescent microscope. Representative colonies were picked, and cells were cytospun onto microscopic glass slides. Wright-Giemsa (Sigma) staining was performed on the slides to examine cell morphology.
Transplantation
C57B/6-Ly-5.1 mice were lethally irradiated (950 rads) in a split dose spaced by 3 hours. Transduced cells were injected intravenously at a dose of approximately 1 to 4 × 106 cocultured cells per recipient animal. Mice receiving transplants were maintained on acidified water supplemented with neomycin sulfate (1.1 g/L; Sigma catalog no. N6386), polymyxinB sulfate (106 U/L; Sigma catalog no. P1004), and sulfamethoxazole/trimetoprim (400 mg/L).
Flow cytometric staining and analysis
Peripheral blood, spleen, and bone marrow single-cell suspensions were stained with antibodies after lysis of red blood cells. The phycoerythrin (PE)–conjugated antibodies used were anti-B220 (RA3-6B2), anti–Mac-1 (M1/70), anti–Gr-1 (RB6-8C5), anti-CD3 (KT31.1), anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti–Ter-119 (TER-119), anti-NK1.1 (Pharmingen), anti-kappa, anti-lambda (kindly provided by Dr John F. Kearney, University of Alabama, Birmingham); the biotinylated antibody used was anti–Ly-5.2 (Pharmingen; anti-CD45.2, clone 104); and the allophycocyanin (APC)–conjugated antibodies used were Streptavidin (Pharmingen) and anti-B220 (RA3-6B2). A FACS Calibur (Becton Dickinson) was used for data collection. Analysis was done using FloJo software (TreeStar, San Carlos, CA).
Results
Pax5-expressing cells in the periphery are almost entirely B220+
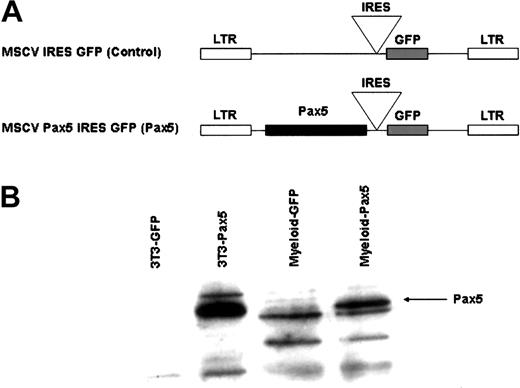
Because the loss-of-function Pax5 experiments defined a critical role for Pax5 in the maintenance of B-cell identity, we wanted to test whether enforced expression of Pax5 in multipotential HSCs would be sufficient to restrict developmental potential to the B-cell lineage. To address this issue, we generated a retroviral vector10 that coexpressed Pax5 along with a GFP marker that was translated from an IRES sequence (Figure 1A). In this way, we were able to use GFP expression as an indicator of cells that also expressed Pax5. Pax5 expression was confirmed in bone marrow myeloid lineage cells (Mac-1+Gr-1+) FACS-purified from reconstituted animals by Western analysis (Figure 1B). The presence of Pax5 in the myeloid lineage clearly demonstrates that Pax5 is coordinately expressed with GFP in the reconstituted animals.
Generation of retroviral constructs. (A) A Pax5 cDNA was cloned upstream of the IRES sequence in the parental retroviral vector, MSCV.10 (B) Retroviral expression of Pax5 in NIH-3T3 cells and FACS-sorted myeloid-lineage (Mac-1+Gr-1+) cells isolated from reconstituted animals 12 weeks after transplantation. NIH-3T3 extract was obtained by transduction of cells with control or Pax5-expressing retroviral vectors. One million myeloid-lineage cells were used per lane. Western analysis was done using an anti-Pax5 antibody (Santa Cruz Biotechnology) and ECL.
Generation of retroviral constructs. (A) A Pax5 cDNA was cloned upstream of the IRES sequence in the parental retroviral vector, MSCV.10 (B) Retroviral expression of Pax5 in NIH-3T3 cells and FACS-sorted myeloid-lineage (Mac-1+Gr-1+) cells isolated from reconstituted animals 12 weeks after transplantation. NIH-3T3 extract was obtained by transduction of cells with control or Pax5-expressing retroviral vectors. One million myeloid-lineage cells were used per lane. Western analysis was done using an anti-Pax5 antibody (Santa Cruz Biotechnology) and ECL.
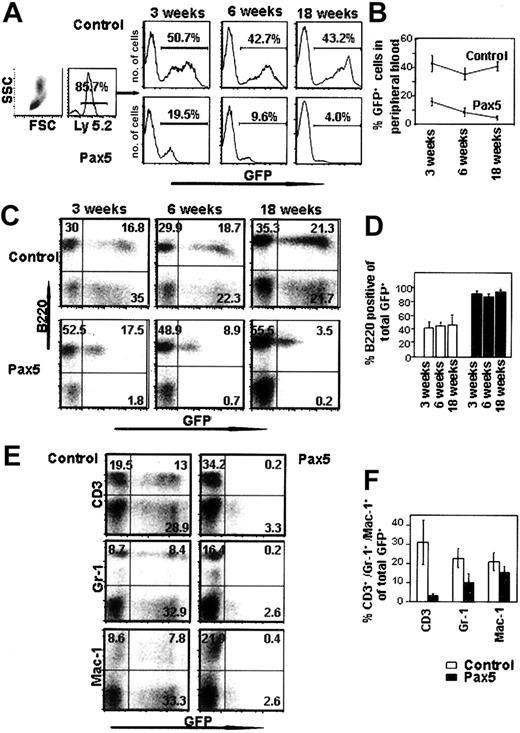
Bone marrow from C57B/6-Ly-5.2 mice treated with 5-FU 4 days previously to enrich for progenitor cells was harvested and transduced with control GFP- or Pax5-expressing retroviruses. Transduced cells were then transplanted into lethally irradiated, C57B/6-Ly-5.1 congenic animals and then analyzed for their contribution to the various blood cell lineages by FACS. Peripheral blood analysis of animals transplanted with Pax5-expressing cells showed that the percentage of Pax5+GFP+ cells decreased over time, perhaps indicating a gradual exhaustion of HSC potential induced by Pax5 or a reduced ability of Pax5-expressing HSCs to compete with wild-type HSCs in bone marrow (Figure 2A-B). The reduction in GFP chimerism over time seen in animals reconstituted with Pax5-expressing cells supports the conclusion that there was no malignant transformation induced by ectopic Pax5 expression in hematopoietic cells. Lineage analysis of the circulating leukocytes reconstituted with Pax5-expressing cells showed that the vast majority of Pax5+GFP+ cells were comprised of B220+IgM+ B-lineage cells (approximately 90%), which was in contrast to animals reconstituted with cells expressing the control GFP vector (Figure 2C-D). We stained for B220 because Pax5 is known to be a positive regulator of another pan–B-cell marker, CD19.12 Costaining of cells with B220 and either CD3, Gr-1, or Mac-1 showed that B220 was not inappropriately expressed on other blood cell types because of overexpression of Pax5 (data not shown). The phenotype we observed was consistent at all time points analyzed for 3 independent transplantation experiments (Figure 2D). In long-term reconstituted animals 18 weeks after transplantation, the mean percentage of B220+ cells was 93.5% of the transduced donor cells (compared with 46.3% in the control GFP-reconstituted animals). The difference in the percentage of GFP+ cells that are B220+ in the Pax5 animals versus the controls was statistically significant at all time points analyzed (P < .01). Pax5-expressing cells that were not B220+ included low percentages of myeloid lineage cells (Gr-1+Mac-1+ cells) and very low levels of CD3+ cells that represented approximately 2% to 3% of the total GFP+ cells in peripheral blood (Figure 2E-F). The observed difference in the level of GFP expression in the control GFP-expressing cells versus cells that coexpressed Pax5 was most likely due to enhanced transduction of bone marrow cells with the control vector, which generated titers that were typically 10-fold higher than the Pax5 vector (data not shown). As mentioned earlier, there may also have been some selection against HSCs that expressed very high levels of Pax5, which we cannot formally rule out.
Peripheral blood analysis of mice reconstituted with control GFP- or Pax5-expressing cells. (A) Leukocytes were gated by forward and side scatter and also for the Ly-5.2 donor cell marker. Histograms indicate the percentage of GFP+ donor cells from representative animals. (B) The proportion of GFP+ donor cells in the peripheral blood decreases with time in animals reconstituted with Pax5-expressing cells. Each time point is representative of 5 control GFP- and 7 Pax5-reconstituted animals (P < .01). (C) Donor cells (Ly-5.2+) were gated and then analyzed for the percentage of B220+ cells in the peripheral blood. Representative plots are shown. (D) Proportion of B lymphocytes among the donor-derived GFP+ cells in the peripheral blood. The data are representative of 5 control GFP- (□) and 7 Pax5-reconstituted (▪) animals for each time point analyzed (P < .01 at 3, 6, and 18 weeks). (E-F) The percentages of peripheral blood GFP+ cells that stain positive for CD3, Gr-1, and Mac-1 are indicated. Data are representative of 4 control GFP- and 7 Pax5-reconstituted animals analyzed at 18 weeks after transplantation.
Peripheral blood analysis of mice reconstituted with control GFP- or Pax5-expressing cells. (A) Leukocytes were gated by forward and side scatter and also for the Ly-5.2 donor cell marker. Histograms indicate the percentage of GFP+ donor cells from representative animals. (B) The proportion of GFP+ donor cells in the peripheral blood decreases with time in animals reconstituted with Pax5-expressing cells. Each time point is representative of 5 control GFP- and 7 Pax5-reconstituted animals (P < .01). (C) Donor cells (Ly-5.2+) were gated and then analyzed for the percentage of B220+ cells in the peripheral blood. Representative plots are shown. (D) Proportion of B lymphocytes among the donor-derived GFP+ cells in the peripheral blood. The data are representative of 5 control GFP- (□) and 7 Pax5-reconstituted (▪) animals for each time point analyzed (P < .01 at 3, 6, and 18 weeks). (E-F) The percentages of peripheral blood GFP+ cells that stain positive for CD3, Gr-1, and Mac-1 are indicated. Data are representative of 4 control GFP- and 7 Pax5-reconstituted animals analyzed at 18 weeks after transplantation.
B220+Pax5+ cells in the periphery are not malignant
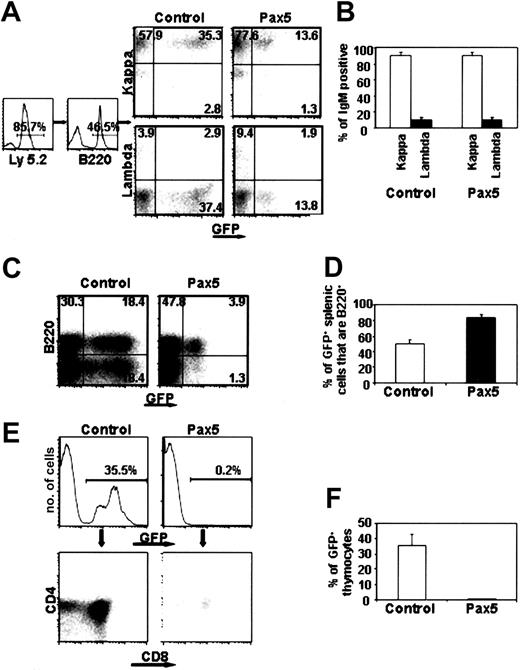
Although the percentage of Pax5+ B lymphocytes was relatively constant even at the earliest times after transplantation, it remained possible that Pax5 expression could have selectively expanded B-lineage cells. The fact that we observed no GFP control-reconstituted animals that exhibited such high percentages of donor B lymphocytes (n = 14) indicates that random retroviral insertions into the genome did not contribute to the phenotype seen in animals reconstituted with Pax5-expressing cells. To further investigate the possibility of clonal expansion within the B-cell compartment, peripheral blood and splenic mature B cells were analyzed for expression of immunoglobulin κ and λ light chains. The normal ratio of κ/λ-bearing B cells in C57B/6 animals is approximately 10:1, and we would expect this ratio to be altered if clonal expansion occurred in a Pax5-expressing cell.13 Among the donor-derived GFP+ B cells, the proportion of κ/λ-expressing cells was approximately 10:1 for both control GFP and Pax5-reconstituted animals (Figure 3A-B; n = 5 for control and n = 7 for Pax5). In addition, reverse transcription–polymerase chain reaction (RT-PCR) analysis using primers that amplified the rearranged D-J region of the immunoglobulin heavy chain locus failed to identify any abnormal expansion of a particular clone (data not shown). Therefore, the high percentage of B-lineage cells in animals reconstituted with Pax5-expressing cells was not due to malignancy. FACS analysis of the spleen in reconstituted animals also showed that 84% of donor-derived, Pax5+GFP+ cells were B220+IgM+ (Figure 3C-D and data not shown).
Analysis of lymphocyte development in spleen and thymus. (A-B) κ/λ light chain expression in peripheral blood of animals reconstituted for 12 weeks. The data are representative of 5 control GFP- and 7 Pax5-reconstituted animals. Percentages of cells within the gated quadrants are shown. (C) The percentage of B220+ B cells in spleen was analyzed in 3 control GFP- and 3 Pax5 animals at 12 weeks after transplantation. (D) The percentage of Pax5+GFP+ B lymphocytes in the spleens of reconstituted animals was statistically significant (P < .01). (E-F) Absence of Pax5-expressing thymocytes. The data are representative of 6 control GFP- and 6 Pax5-reconstituted animals analyzed at 20 weeks after transplantation.
Analysis of lymphocyte development in spleen and thymus. (A-B) κ/λ light chain expression in peripheral blood of animals reconstituted for 12 weeks. The data are representative of 5 control GFP- and 7 Pax5-reconstituted animals. Percentages of cells within the gated quadrants are shown. (C) The percentage of B220+ B cells in spleen was analyzed in 3 control GFP- and 3 Pax5 animals at 12 weeks after transplantation. (D) The percentage of Pax5+GFP+ B lymphocytes in the spleens of reconstituted animals was statistically significant (P < .01). (E-F) Absence of Pax5-expressing thymocytes. The data are representative of 6 control GFP- and 6 Pax5-reconstituted animals analyzed at 20 weeks after transplantation.
Absence of GFP+ T cells in the thymus of Pax5-reconstituted animals
In the absence of Pax5, cells previously committed to the B-cell fate acquire the ability to differentiate into mature T-lineage cells when adoptively transferred into Rag2-deficient animals.9,14 These results suggest that Pax5 may contribute to B-cell commitment by blocking development of T-lineage cells in the thymus or at the level of common lymphoid progenitor cells in the bone marrow.2 FACS analysis of the thymus in animals reconstituted with Pax5-expressing cells showed that developing T cells were essentially absent (Figure 3E-F). The mean percentage of Pax5+GFP+ cells was 0.38% in 6 animals that were analyzed at 20 weeks after transplantation. GFP control-reconstituted animals were 35% GFP+ when analyzed at the same time after transplantation (n = 6). When the few Pax5+GFP+ thymocytes were analyzed, there did not seem to be a block in development at any stage prior to CD4/CD8 expression in that the proportions of GFP+ CD4 and CD8 cells were similar to those found in the thymus of control GFP animals (Figure 3E). Even though the frequency of Pax5+GFP+ thymocytes was extremely low, levels of GFP fluorescence in the thymus of all reconstituted animals were approximately 8-fold higher than autofluorescent cells seen in analyses of non-reconstituted thymus (data not shown). This finding suggests that very few cells may escape Pax5-mediated inhibition of T-cell development or, alternatively, these cells could represent rare anomalous retroviral integration events that delete Pax5 during proviral integration.
Pax5 does not prevent commitment to the myeloid lineage
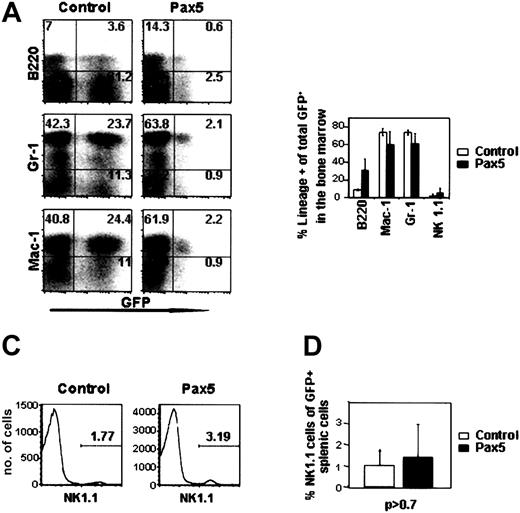
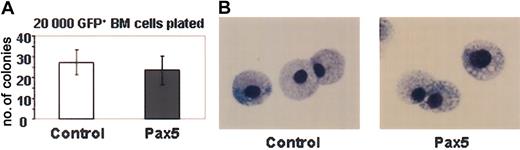
Previous studies have shown that myeloid-specific genes such as the M-CSF receptor and myeloperoxidase are negatively regulated by Pax5.8,9 Upon loss or inactivation of Pax5, and in the absence of IL-7 receptor signaling, these genes are de-repressed and Pax5—/— progenitor B cells acquire the ability to differentiate into multiple cell types, including macrophage and granulocytic cells in vitro and in vivo.8,9,15 We were, therefore, anticipating that Pax5 expression in multipotential progenitor cells would block myelopoiesis in the bone marrow. Contrary to our expectations, Pax5-expressing bone marrow cells efficiently differentiated into Mac-1+Gr-1+ cells in proportions that were similar to control GFP-reconstituted animals (Figure 4A-B). The greatly reduced percentage of Pax5-expressing granulocytic cells in the peripheral circulation (between 5%-10% of Pax5+GFP+ cells, Figure 2F) may be due to a block in granulocyte-macrophage (GM)–CSF responsiveness and increased sensitivity toward apoptosis in myeloid cells that ectopically express Pax5.16 In our experiments, we noted no significant difference in the number or type of myeloid colonies that were generated in methylcellulose on plating of 20 000 GFP+ bone marrow cells from animals reconstituted 12 weeks previously with control GFP- or Pax5-expressing cells (Figure 5). This finding indicates that Pax5 expression does not inhibit the differentiation of myeloid progenitor cells from more primitive cells in bone marrow, at least at the level of Pax5 expression obtained from the retroviral vector. Why Pax5-expressing myeloid cells did not accumulate in the periphery in our case is presently unclear. Because Pax5 is not normally expressed in myeloid-lineage cells17 (Figure 1B), our observations regarding myeloid development are only significant because they show Pax5 is not sufficient to repress the normal commitment mechanisms that activate myeloid development from multipotential progenitor cells.
Bone marrow analysis of control GFP- and Pax5-expressing cells. (A) Donor-derived bone marrow cells from representative animals were stained for B220, Gr-1, Mac-1, and NK1.1. Percentages of cells within the gated quadrants are shown. (B) Percentages of donor-derived GFP+ cells that express B220 (P < .10), Gr-1 (P < .22), Mac-1 (P < .24), and NK1.1. (P < .50). Data are representative of 3 control GFP- and 3 Pax5-reconstituted animals receiving transplants 12 weeks previously. (C-D) NK1.1 staining of spleen samples from animals receiving transplants 12 weeks before (n = 3 for both constructs, P > 0.7). The cells (histograms) shown are gated on viable, GFP+ splenocytes based on propidium iodide staining.
Bone marrow analysis of control GFP- and Pax5-expressing cells. (A) Donor-derived bone marrow cells from representative animals were stained for B220, Gr-1, Mac-1, and NK1.1. Percentages of cells within the gated quadrants are shown. (B) Percentages of donor-derived GFP+ cells that express B220 (P < .10), Gr-1 (P < .22), Mac-1 (P < .24), and NK1.1. (P < .50). Data are representative of 3 control GFP- and 3 Pax5-reconstituted animals receiving transplants 12 weeks previously. (C-D) NK1.1 staining of spleen samples from animals receiving transplants 12 weeks before (n = 3 for both constructs, P > 0.7). The cells (histograms) shown are gated on viable, GFP+ splenocytes based on propidium iodide staining.
Myeloid colony-forming assay of GFP+ bone marrow cells isolated from control GFP- and Pax5-expressing animals. (A) Number of colonies obtained from plating 20 000 GFP+ bone marrow cells (P < .56). Colonies of more than 50 cells were counted as positive after 10 days in methylcellulose. (B) Representative cytospins from the in vitro grown myeloid colonies stained with Wright-Giemsa. Original magnification, × 1000.
Myeloid colony-forming assay of GFP+ bone marrow cells isolated from control GFP- and Pax5-expressing animals. (A) Number of colonies obtained from plating 20 000 GFP+ bone marrow cells (P < .56). Colonies of more than 50 cells were counted as positive after 10 days in methylcellulose. (B) Representative cytospins from the in vitro grown myeloid colonies stained with Wright-Giemsa. Original magnification, × 1000.
Pax5 does not inhibit NK cell development in bone marrow or spleen
With respect to NK cell development in bone marrow, we analyzed 5 control GFP- and 5 Pax5-reconstituted animals and found no significant difference in the percentage of NK1.1+ cells in bone marrow (Figure 4B). Further analysis of NK1.1+ cells in the spleens of reconstituted animals also showed no significant difference in the frequencies of NK1.1+ cells at 12 weeks after transplantation (Figure 4C-D). This finding indicates that Pax5 expression at the level of common lymphoid progenitor cells does not inhibit NK or B-cell development in bone marrow but has a profoundly negative effect on the earliest stage of T-cell development.
Discussion
Our experiments show that Pax5 is a critical factor in establishing B- versus T-lymphoid identity. The promotion of B-cell development could be due to repression of T-cell specification and commitment, a block in homing of a T-cell precursor from the bone marrow to the thymus, and/or specific activation of the B-cell program in oligopotent lymphoid progenitor cells that express Pax5 in bone marrow. The latter explanation may be unlikely because B-cell development progresses to the Fraction B stage in the absence of Pax5.8 Interestingly, overexpression of EBF in hematopoietic stem cells results in a similar, although not identical, phenotype as that seen with Pax5, suggesting that EBF and Pax5 may act in overlapping downstream pathways to promote B-cell commitment (Z.Z. and C.A.K., unpublished observation, November 12, 2002). RT-PCR analysis of normal developmental intermediates from C57B/6 bone marrow, including long-term self-renewing HSCs,1 CLP cells,2 and Fraction A cells,18 showed that Pax5 is transcriptionally activated only in a subset of Fraction A cells (B220+CD19—NK1.1— cells). A total of 3 of 10 samples containing 5, double-sorted Fraction A cells were positive for Pax5 transcript after 2 rounds of nested PCR, whereas 100% of B220+CD19+ Fraction B cells were positive for Pax5 expression19 (data not shown). This finding indicates that Pax5 is probably not expressed in the developmental intermediate where lymphoid versus myeloid specification is occurring in vivo. In the subset of Fraction A cells that do express Pax5, our data suggest that these cells will be temporally restricted to differentiate along a B- or NK-cell pathway.
Curiously, Pax5 was not sufficient to block myeloid development when ectopically expressed in more primitive multipotent progenitor cells. These observations are in apparent contrast with experiments showing that a null mutation in Pax5 results in loss of commitment to the B-cell lineage and acquisition of developmental multipotentcy.8 One interpretation of these results is that the levels of Pax5 expression from our retroviral vector are not sufficient to block myeloid-specific gene expression but are sufficient to completely block T-cell development. The inability of Pax5 to block myelopoiesis was also observed in transgenic experiments in which Pax5 was knocked in to the Ikaros locus.20 Our data suggest that Pax5 likely functions at the level of oligopotent lymphoid progenitor cells to determine B- versus T-cell developmental fate. Because we do not observe Pax5 expression in CLP cells, other factors would act at that level to restrict switching to a myeloid fate in vivo. Downstream activation of Pax5 might then replace the myeloid lineage–repressive function of a factor that was no longer active as well as instructively act to specify B- versus T-cell developmental fate.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-10-3139.
Supported by grants R01DK55650 (C.V.C. and Z.Z.) and R01DK54766 (H.-G.K.) from the National Institutes of Health and in part by a Howard Hughes Faculty Development Award (53000281) (C.A.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Larry Gartland for expert assistance with the flow cytometer and for support from Dr Max Cooper and Dr John F. Kearney in the Division of Developmental and Clinical Immunology and Dr Meinrad Busslinger (IMP, Vienna, Austria) for helpful discussions and communicating results prior to publication. We also express our appreciation to Dr John F. Kearney for critical reading of the manuscript and for helpful suggestions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal