Abstract
In this study we report the molecular and functional characterization of very early interleukin 7 receptor α (IL-7Rα)+-CD79a+CD19– B-cell progenitors, produced by human CD34+CD19–CD10– cord blood cells grown in the presence of stromal cells and cytokines. Purified IL-7Rα+CD79a+CD19– cells transcribed the B-lymphoid specific genes E2A, EBF, TdT, Rag-1, had initiated DJH rearrangements, but almost lacked Pax-5 mRNA. When exposed to appropriate environmental conditions, these cells repressed B-cell genes and completely differentiated into CD14+ macrophages, CD56+ natural killer cells, and CD4high T cells. Retention of the DJH rearranged genes in both CD14+ and CD56+ cells unambiguously demonstrates that early B-cell genes, expressed prior to Pax-5, can be activated in a multipotent human progenitor cell whose final fate, including in non-B lineages, is determined by external signals.
Introduction
Early stages of human B-cell development can be identified by the expression of cytoplasmic and cell-surface markers and the ordered rearrangements of the V, D, and J gene segments that encode the variable domain of the immunoglobulin heavy-chain (IgH) locus.1 Thus, D→JH rearrangements in pro-B cells precede the V→DJH rearrangement in pre-B cells. The earliest B-cell precursors that carry a complete VDJH rearrangement have been identified by their intracellular expression of a μ chain without light (L) chain. These pre-B cells express the CD10 and CD19 antigens and the μ chain associated with a surrogate light chain coded by the λ5/14.1 and VpreB genes.2 More primitive pro-B cells recognized by their expression of CD34, CD19, and CD10 antigens carry a DJH rearrangement, which can be transcribed. More recently, CD19– cells have been isolated from fresh bone marrow, which expresses the B-cell receptor (BCR)–associated molecule CD79a or Vpre-B proteins and a DJH rearrangement.3, 4, 5, 6 This suggests that the B-cell program can be activated prior to the expression of the CD19 antigen and VDJH rearrangement. How multipotent stem cells progressively restrict their potential to a B-cell fate has been best studied in mice deleted of key control genes, such as E2A, EBF, and Pax-5.7, 8, 9 Absence of the first 2 arrest B-cell differentiation prior to the DJH rearrangement, whereas typical B220+c-Kit+ pro-B cells, which have initiated DJH rearrangement and express Rag-1, Rag-2, TdT, λ5, VpreB, E2A, and EBF, can be isolated from the bone marrow of Pax-5–/– mice. Despite their pro-B phenotype, these cells can differentiate into macrophages, osteoclasts, dendritic cells, natural killer (NK) cells, T cells, granulocytic cells and erythroid cells in vitro and in vivo, when exposed to the appropriate conditions.10,11 On the basis of these observations, it has been proposed that the B-cell program is first activated in a “multipotent” cell and that the function of Pax-5 is to repress the myeloid and T-cell genes and restrict cells to a B-cell fate.12,13 Even though this 2-step pattern has not been demonstrated using primary cells or wild-type mice, a close proximity exists between B-lymphoid, macrophages, and osteoclast lineages in healthy mice,14, 15, 16 perhaps controlled by the relative levels of Pu.1 and Pax-5.17,18
Investigating human B-cell differentiation is hampered both by the low proliferation rate of human pro-B cells8,19 and the small number of markers recognizing early-B cells, as opposed to those available in mice (B220, CD43, CD24, c-Kit, AA4.1).20, 21, 22 Furthermore, reproducible conditions to grow human B-cell progenitors in vitro have been described only recently. High numbers of CD34–CD19+CD10+sIgM– pre-B cells that express Pax-5, λ-like, and μ transcripts are obtained23 in cocultures of cord blood–derived CD34+ cells on murine feeders.23, 24, 25, 26, 27, 28 Human B-cell differentiation can also be obtained in vivo in NOD-SCID (nonobese diabetic severe combined immunodeficient) mice injected with human CD34+ cells.29,30
Our goal in the present study was to determine when the B-cell program was first activated in CD34+CD10–CD19– cells purified from human cord blood and induced to differentiate in vitro in the presence of the MS-5 murine feeders. Our major finding reported here is the identification of an early pro–B-cell progeny that expresses CD79a and the α chain of the interleukin 7 (IL-7) receptor (CDw127) but lacks CD10 and CD19. DJH, but not VDJH, rearrangements were present, and Pax-5 was only faintly expressed. Most strikingly, when exposed to appropriate cytokines, these cells differentiated into natural killer cells, T cells, and macrophages; the retention of the DJH signature in these non-B cells demonstrated that they originated in a progenitor, which had initiated a B-cell program. These findings thus suggest that B-lymphoid genes are first expressed in a human multipotent progenitor still able to adopt non–B-cell programs in response to lineage-specific environmental signals.
Materials and methods
Isolation and preparation of cells
Human umbilical cord blood was collected with the informed consent of the mother. Mononuclear cells were subjected to a standard CD34 immunomagnetic bead separation (Miltenyi Biotec, Auburn, CA) exactly as previously described.31 Bead-separated CD34+ cells (40%-80% pure) were used fresh or after cryopreservation. CD34+CD19–CD10– cells were sorted with an ELITE cytofluorometer (Beckman-Coulter, Miami, FL) equipped with an argon ion laser (Innova 70-4-Coherent radiation, Palo Alto, CA) tuned to 488 nm and operating at 500 mw. Cells were analyzed after cell sorting, and purity reached more than 98%. MS-5 cells were grown as previously described.23
Antibodies
The following monoclonal mouse immunoglobulin antibodies (MoAbs) to human antigens were used: CD1a-phycoerythrin (PE), CD7-fluorescein isothiocyanate (FITC), CD11b-FITC, CD14-FITC, CD19-PE-Cy5, CD34-FITC or PE-Cy5, CD34-PE-Cy5, CD36-FITC, CD38-FITC, CD45RA-FITC, CD45-FITC or-PE, CD56, CD79a-PE or PE-Cy5, CDw127-PE, HLA-DR-FITC (Beckman-Coulter). CD4-PE or PE-Cy5, CD8-FITC, CD10-FITC, CD14-FITC, CD45RA-FITC, CD64-FITC, CD79a-PE or -APC (allophycocyanin) were from Becton Dickinson (BDIS, San Jose, CA). CD115 (CSF-1-R, c-fms) was detected by indirect immunofluorescence with rat anti–CSF-1-R (Zymed, San Francisco, CA) followed by FITC-conjugated F(ab′)2 donkey antirat IgG (Jackson Immunoresearch, West Grove, PA). The intracellular expression of CD79a was determined using a cell permeabilization kit (Harlan Sera-Lab, Loughborough, United Kingdom).
Culture of CD34+CD19–CD10– cord blood progenitor cells
CD34+CD10–CD19– cells (5000/mL) were cultured in 24-well plates (Falcon, Becton Dickinson) precoated with a confluent layer of MS-5 cells in lymphoid conditions: RPMI 1640 (Gibco BRL) with 10% human AB serum (Institut Jacques BOY, Reims, France), 5% pretested fetal calf serum (FCS; HCC-6400; Stem Cell Technologies, Vancouver, BC, Canada), rhu-stem cell factor (SCF; 50 ng/mL; kindly provided by Amgen, Thousands Oaks, CA), rhu-IL-2 (5 ng/mL; Diaclone, Besançon, France), and rhu-IL-15 (1 ng/mL; Diaclone).28,32 Analysis of differentiated cells was performed on a FACSCalibur using CellQuest software (Becton Dickinson).
Assessment of the macrophage, natural killer cell, and T-cell differentiation of CD79a+-enriched CDw127+ early pro-B cells
The ability of CDw127+CD11b–CD36–CD19– cells (> 85% enriched in CD79a+ cells, thereafter designated as CD79a+-enriched CDw127+) to differentiate into B cells, NK cells, and granulocytic and monocytes-macrophages was analyzed in 24-well plates in 2 different conditions, both on MS-5 cell feeders.
B-cell and NK cell differentiations. CD79a+-enriched CDw127+ (2-4000/mL) cells were incubated exactly as described in “Cell sorting,” except that 50 ng/mL rhu-FLT3-L (Diaclone) was added to promote NK cell proliferation.
Monocyte/macrophage differentiation. Differentiation was evaluated on MS-5 cells in α-minimum essential medium (MEM) with 10% FCS, rhu-SCF (50 ng/mL), rhu-GM-CSF (granulocyte macrophage colony-stimulating factor) (20 ng/mL; PeproTech, Rocky Hill, NJ), and rhu-M (macrophage)–CSF (50 ng/mL; PeproTech).
All wells were incubated for 14 days at 37°C, 5% CO2, and fed weekly. Cells in both conditions were then counted and assessed for their expression of CD19, CD14, and CD56. CD14+ cells generated in “macrophage” conditions, CD56+ NK cells, and CD19+ B cells generated in “lymphoid” conditions were sorted by fluorescence-activated cells sorter (FACS). Cells were sorted twice to ensure more than 98% purity (see “Assessment of the macrophage, natural killer cell, and T-cell differentiation of CD79a+-enriched CDw127+ early pro-B cells”).
T-cell differentiation. Differentiation was evaluated in fetal thymic organ culture (FTOC) exactly as described previously in detail,33 using E14 embryonic thymic lobes from NOD-SCID mice. Each lobe was incubated with either 10 000 unfractionated day 14 cultured cells, CDw127+CD79a+CD19– or CDw127+CD19+ cells. Human cells were recovered after 30 days by mechanical disruption of the pooled thymic lobes and labeled with antihuman CD4-PE and CD8-FITC MoAbs and antihuman CD4-PE-Cy5 and CD1a-PE MoAbs.
Cell sorting
Cell sorting of CD79a+CD19–cells. Freshly sorted CD34+CD10–CD19– cord blood cells were cultured as described earlier. To sort CD79a-enriched CDw127+ cells at day 14, several wells (usually 10-15) were pooled, and nonadherent cells were removed and mixed with adherent cells mechanically dissociated (on average 107 cells were obtained). Because the anti-CD79a MoAb recognizes an intracellular epitope, it could not be used to sort viable cells. Therefore, to purify CD79a+ cells, we used a positive selection with the anti-CDw127 (α chain of the IL-7 receptor, IL-7Rα) antibody, which recognizes a high proportion of the CD79a+ cells (see “Results”) coupled with a negative selection excluding CD19+ B cells, and myeloid precursors, which expressed the CD11b, and/or the CD36+ antigens. Briefly, cells were incubated with MoAbs against CDw127-PE, CD19-PE-Cy5, and a mixture of CD36-FITC and CD11b-FITC. To sort cells, a small morphologic gate containing all CD79a+ cells was first selected by 2 parameter histograms side scatter (SSC) versus forward scatter (FSC). CDw127+ cells lacking CD11b and CD36 were selected and were further discriminated according to their expression of CD19+. CDw127+CD11b–CD36–CD19– cells were sorted twice to ensure a purity more than 98%. The proportion of CD79a+ cells among CDw127+-sorted cells was determined in each experiment.
Cell sorting of mature NK cells, B cells, and macrophages at day 28. CD14+, CD19+, and CD56+ mature cells generated by CDw127+CD11b–CD36–CD19– grown in vitro were sorted twice at day 28. Cells cultured in lymphoid and macrophage conditions were all labeled with anti–CD19-PE, anti–CD14-FITC, and anti–CD56-PE-Cy5.
DNA preparation and PCR analysis of immunoglobulin gene rearrangements
Cell fractions collected by cell sorting were resuspended in polymerase chain reaction (PCR) buffer 1 × (AmpliTaqGOLD buffer II; Applied Biosystems, Rotkreuz, Switzerland), 1.5 mM MgCl2, and 500 μg/mL proteinase K (Boehringer Mannheim). After 90 minutes of incubation at 56°C, proteinase K was subsequently inactivated by heating at 95°C for 10 minutes. PCR was performed in a volume of 50 μL containing 100 μM deoxynucleotide triphosphates (dNTPs), 1 μmol/L each primer, and 0.3 U AmpliTaqGOLD polymerase (Applied Biosystems). PCR conditions consisted of 10 minutes at 94°C and 38 cycles of 45 seconds at 94°C, 60 seconds at 65°C, 60 seconds at 72°C. Amplification of complete VDJH rearrangements was performed using a 5′ consensus framework 3 (FR3) primer (5′-GACACGGCCGTGTATTACTGTGC-3′)anda3′ consensus JH primer (5′-AACTGCAGAGGAGACGGTGAC-3′). For detection of incomplete DJH rearrangements, a mixture of consensus 5′ primers for each of the 7 main DH gene families34 was used (Table 1) with the same 3′ consensus JH primer used for detection of complete VDJH rearrangements.
Sequence of DH primers
. | Sense . |
|---|---|
| DA | TGTGTGACTACAGTAACTAC |
| DM | TCACAG (C/T)GGGTATAAC (C/T) GGA |
| DN | GTCACAGTGG (G/A) GTATAGCAGC |
| DLR | GTGTCACTGTGAG (A/G/C) ATATTGT |
| DQ52 | AGAACCACTGTGCTAACTGG |
| DK | TGTCAGACTGTGGTGGATA |
| DXP | TCTGTGTCACTGTGGTATTAC |
. | Sense . |
|---|---|
| DA | TGTGTGACTACAGTAACTAC |
| DM | TCACAG (C/T)GGGTATAAC (C/T) GGA |
| DN | GTCACAGTGG (G/A) GTATAGCAGC |
| DLR | GTGTCACTGTGAG (A/G/C) ATATTGT |
| DQ52 | AGAACCACTGTGCTAACTGG |
| DK | TGTCAGACTGTGGTGGATA |
| DXP | TCTGTGTCACTGTGGTATTAC |
Gene expression analyzed by reverse transcription (RT)–PCR
Sorted cells (104 to 3 × 104 cells) were lysed in 200 μL TRIzol (Life Technologies), and total RNA was removed by chloroform extraction (1:5 vol/vol). RNA was then reverse transcribed using random hexamers and the Superscript kit (Gibco BRL) according to the manufacturer's instructions.
The cDNA input for each population was normalized to obtain equivalent signals with the housekeeping ribosomal S14 gene. Moreover, the PCR process was optimized to ensure a linear amplification.
A volume of 15 pmol of each of the primers listed in Table 2 was used. To avoid DNA amplification, primers were chosen to span introns for each transcript. PCR conditions consisted of 94°C for 45 seconds, annealing at specific primer melting temperature for 1 minute, 72°C for 1 minute. The PCR products (10 μL) were analyzed by 1.5% agarose gel electrophoresis.
Sequence of primers used for RT-PCR
Probe . | Sense . | Antisense . | Annealing temperature, °C . | Cycles . |
|---|---|---|---|---|
| S14 | GGCAGACCGAGATGAATCCTCA | CAGGTCCAGGGGTCTTGGTCC | 65 | 29 |
| mb1 (CD79a) | GATGCCGGGGATGAATATGAAG | GCTCCCCTAGAGGCAGCGA | 60 | 33 |
| CD19 | TCACCGTGGCAACCTGACCATG | GAGACAGCACGTTCCCGTTACTG | 65 | 40 |
| CD10 | CTGTGAGAATGATCGCACTCTATG | GATTCCAGTGCATTCATAGTAATC | 65 | 35 |
| TdT | ACACGAATGCAGAAAGCAGGA | AGGCAACCTGAGCTTTTCAAA | 60 | 33 |
| EBF | CTCACTTTGAGAAGCAGCCGC | CATGTCACGTGGGTTTCCCGC | 65 | 32 |
| Pax5 | AGCAGGACAGGACATGGAGGA | ATCCTGTTGATGGAACTGACGC | 65 | 32 |
| Pu-1 | TGGAAGGGTTTCCCCTCGTC | TGCTGTCCTTCATGTCGCCG | 60 | 33 |
| Gata 3 | ACTCCTACATGGACGCGGC | GGTGGATGGACGTCTTGGAG | 60 | 35 |
| Rag-1 | GAGAGCAGAGAACACACTTT | CTTTTCAAAGGATCTCACCC | 55 | 32 |
| c-fms | CAGCACCAACAACGCTACCTTC | TCGAGGTTGAGGGTCAGGACTT | 65 | 35 |
Probe . | Sense . | Antisense . | Annealing temperature, °C . | Cycles . |
|---|---|---|---|---|
| S14 | GGCAGACCGAGATGAATCCTCA | CAGGTCCAGGGGTCTTGGTCC | 65 | 29 |
| mb1 (CD79a) | GATGCCGGGGATGAATATGAAG | GCTCCCCTAGAGGCAGCGA | 60 | 33 |
| CD19 | TCACCGTGGCAACCTGACCATG | GAGACAGCACGTTCCCGTTACTG | 65 | 40 |
| CD10 | CTGTGAGAATGATCGCACTCTATG | GATTCCAGTGCATTCATAGTAATC | 65 | 35 |
| TdT | ACACGAATGCAGAAAGCAGGA | AGGCAACCTGAGCTTTTCAAA | 60 | 33 |
| EBF | CTCACTTTGAGAAGCAGCCGC | CATGTCACGTGGGTTTCCCGC | 65 | 32 |
| Pax5 | AGCAGGACAGGACATGGAGGA | ATCCTGTTGATGGAACTGACGC | 65 | 32 |
| Pu-1 | TGGAAGGGTTTCCCCTCGTC | TGCTGTCCTTCATGTCGCCG | 60 | 33 |
| Gata 3 | ACTCCTACATGGACGCGGC | GGTGGATGGACGTCTTGGAG | 60 | 35 |
| Rag-1 | GAGAGCAGAGAACACACTTT | CTTTTCAAAGGATCTCACCC | 55 | 32 |
| c-fms | CAGCACCAACAACGCTACCTTC | TCGAGGTTGAGGGTCAGGACTT | 65 | 35 |
Results
Identification of a population of CDw127+CD79a+CD19– early B-cell progenitors produced in vitro by human CD34+CD10–CD19– cells
Cord blood (CB)–derived CD34+ purified cells were further depleted by cell sorting of both CD34+CD19+CD10+ pro-B cells and CD34+CD19–CD10+ cells, which have been shown to contain restricted lymphoid progenitors.27 In a series of 4 separate experiments, we cultured CD34+CD19–CD10– cells (5000 cells/well) for 3 weeks in the presence of confluent MS-5 feeders, SCF, IL-2, and IL-15. The mean (± SD) fold increase in the number of nucleated cells was 17.2 (± 7.5), 80 (± 29.4), and 210 (± 24) at days 7, 14, and 21, respectively.
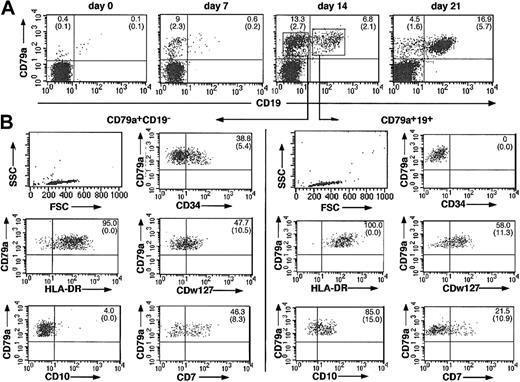
Kinetics of expression of CD79a, CD19, and other B-cell markers. At days 7, 14, and 21, we analyzed the expression of CD79a and CD19 (Figure 1). Less than 0.5% of input CD34+CD19–CD10– cells expressed CD79a. At day 7, CD79a+ cells represented 9.5% ± 2.3% (n = 4) of nucleated cells. This proportion increased to 20% at week 2 and remained stable thereafter (n = 11). At day 7, almost none of these CD79a+ cells coexpressed CD19; at day 14, 30% to 50% CD79a+ cells were CD19+ and more than 80% at day 21.
Immunophenotype of cells differentiated in vitro from CD34+CD10–CD19– human cord blood progenitors. (A) Kinetics of expression of the B-cell markers CD79a and CD19 on CD34+CD10–CD19– progenitors (day 0) incubated in stromal-based cultures with SCF, IL-2, and IL-15. Nonadherent and adherent cells were collected at days 7, 14, and 21; cells were first incubated with PE-Cy5–coupled anti-CD19 MoAb and then permeabilized and labeled with APC-coupled anti-CD79a MoAb. Limits for positivity and negativity were defined by incubating cells with nonimmune isotypic-matched immunoglobulin. (B) Staining profiles of CD79a+CD19– and CD79a+CD19+ cells at day 14. Nucleated cells collected from the cultures were subjected to 3-color labeling. Anti-CD79a and anti-CD19 were systematically included and associated to a third antibody recognizing HLADR, CD7, CD34, CD10, CDw127, and CD38 (not shown). Both CD79a+CD19– cells and the companion CD79a+CD19+ fraction were simultaneously analyzed for the expression of these markers using a FACScalibur and CellQuest software. Results are shown from 1 of 4 similar experiments with similar results. Mean (SD) percentages of at least 4 experiments are indicated.
Immunophenotype of cells differentiated in vitro from CD34+CD10–CD19– human cord blood progenitors. (A) Kinetics of expression of the B-cell markers CD79a and CD19 on CD34+CD10–CD19– progenitors (day 0) incubated in stromal-based cultures with SCF, IL-2, and IL-15. Nonadherent and adherent cells were collected at days 7, 14, and 21; cells were first incubated with PE-Cy5–coupled anti-CD19 MoAb and then permeabilized and labeled with APC-coupled anti-CD79a MoAb. Limits for positivity and negativity were defined by incubating cells with nonimmune isotypic-matched immunoglobulin. (B) Staining profiles of CD79a+CD19– and CD79a+CD19+ cells at day 14. Nucleated cells collected from the cultures were subjected to 3-color labeling. Anti-CD79a and anti-CD19 were systematically included and associated to a third antibody recognizing HLADR, CD7, CD34, CD10, CDw127, and CD38 (not shown). Both CD79a+CD19– cells and the companion CD79a+CD19+ fraction were simultaneously analyzed for the expression of these markers using a FACScalibur and CellQuest software. Results are shown from 1 of 4 similar experiments with similar results. Mean (SD) percentages of at least 4 experiments are indicated.
Next, we characterized in detail, in 4 separate experiments, the phenotype of CD79a+CD19– and CD79a+CD19+ B cells generated in culture (Figure 1). The CD79a+CD19– population fell within a discrete gate characterized by an intermediate FSC (300-400) and a very low SSC, which overlapped with that delineating CD79+CD19+ cells. At day 7, all CD79a+ cells expressed CD34 (not shown), but only 40% at day 14, and none thereafter, which confirmed that CD79a antigen appears prior to the CD19 on CD34+ immature precursors. The variation of 2 other antigens was also indicative of the immaturity of CD79a+CD19– cells; HLA-DR was highly expressed on CD79a+CD19+, whereas a few HLA-DRlow cells could be found in the CD79a+CD19– subset (Figure 1B). The CD7 antigen was highly expressed on 46.3% (± 8.3%) of CD79a+CD19– cells and clearly down-regulated on CD79a+CD19+ (21.5% ± 10.9%) (Figure 1B). Both CD19+ and CD10+ fractions expressed uniformly CD38 and CD45RA antigens (not shown). CD10 was not detectable on CD79a+CD19– cells, a finding which confirmed our previous finding that, in vitro, CD10 does not appear prior to CD19. Accordingly, all CD79a+CD19+ cells were CD10+ (n = 5) (Figure 1B). Because the α chain of the IL-7 receptor has been shown to be a key marker discriminating lymphoid- versus myeloid-committed murine early progenitors,21 we examined if the expression of IL-7Rα could be helpful in humans as well. Half of CD79a+CD19– and CD79a+CD19+ B-cell populations expressed moderate levels of IL-7Rα (CDw127) at day 14, but, interestingly, expression of this receptor was very transient: it was undetectable at day 7 and disappeared at day 18 (data not shown). Consistent with our own data, and as previously reported by others,23 CD79a+CD19+ B cells generated in vitro were not fully differentiated and did not express sIgM, CD20, or CD22 (not shown). In summary, these data suggest that in vitro cord blood–derived CD34+CD10–CD19– progenitors differentiated into an early CD34+CD79a+CD19– stage and further progressed into the CD34low/neg CD79a+-CDw127+CD19–, preceding the acquisition of CD19 in a CD34–CD79a+CD19+ stage.
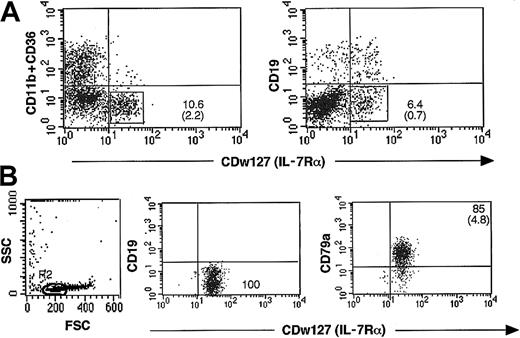
Sorting of cultured CD79a+-enriched CDw127+(IL-7Rα) CD19–B-cell progenitors. Because direct sorting of CD79a+ is precluded by the intracytoplasmic location of the epitope recognized by this MoAb, we took advantage of the expression of the α chain of the IL-7R on the cell surface of 50% CD79a+ cells. Positive selection of CDw127+ cells was coupled to a negative selection eliminating CD19+ B cells, CD11b+, and/or CD36+ myeloid precursor cells, some of which coexpressed CDw127 (Figure 2A). CDw127+CD11b–CD36–CD19– cells were sorted at day 14 of the culture; this timing was selected before it preceded the peak of proliferation of CD56+ NK cells, and at that time 50% of CD79a+CD19– cells expressed IL-7Rα, and all myeloid CD34+ cells can be eliminated according to their expression of CD36 and CD11b. CDw127+CD11b–CD36–CD19– represented 2% to 4% of nucleated cells at day 14. After 2 rounds of cell sorting, 85% ± 4.8% (n = 11) of sorted cells were CD79a+ (Figure 2B). The 15% of CDw127+ cells that were defined as CD79a– according to the upper limit of the negative control could not be distinguished by their FSC/SSC profile (Figure 2B) and most likely expressed low levels of this antigen. These cells lacked colony-forming activity in standard methylcellulose assays in contrast to the high cloning efficiency (6%) of unfractionated day 14 cells (data not shown) and exhibited a scatter profile typical of that of lymphoid cells (FSC/SSC).
Sorting procedure to enrich for CD79a+CD19– cells at day 14. (A) CD34+CD10–CD19– progenitors sorted from fresh or thawed cord bloods were incubated on MS-5 stromal cells with SCF, IL-2, and IL-15. After 2 weeks, all nonadherent and adherent nucleated cells from 10 to 14 wells were collected and labeled with anti–CDw127-PE, a mixture of anti-CD11b and anti–CD36-FITC-Cy5, and anti–CD19-PE-Cy5. Cells positive for the CD11b+ and CD36+ markers represented 50% of the population, and CDw127+ represented 10%, half of which coexpressed CD19. CDw127+CD11b–CD36–CD19– cells were sorted as detailed in “Materials and methods.” (B) Sorted cells were reanalyzed after labeling with anti-CD79a and CDw127 to evaluate their purity and morphologic profile. The panel on the right shows that more than 85% of the cells were CD79a+ and had a very low SSC and heterogeneous FSC values. All CD79a– contaminants fell into gate R2 indicated in the left panel.
Sorting procedure to enrich for CD79a+CD19– cells at day 14. (A) CD34+CD10–CD19– progenitors sorted from fresh or thawed cord bloods were incubated on MS-5 stromal cells with SCF, IL-2, and IL-15. After 2 weeks, all nonadherent and adherent nucleated cells from 10 to 14 wells were collected and labeled with anti–CDw127-PE, a mixture of anti-CD11b and anti–CD36-FITC-Cy5, and anti–CD19-PE-Cy5. Cells positive for the CD11b+ and CD36+ markers represented 50% of the population, and CDw127+ represented 10%, half of which coexpressed CD19. CDw127+CD11b–CD36–CD19– cells were sorted as detailed in “Materials and methods.” (B) Sorted cells were reanalyzed after labeling with anti-CD79a and CDw127 to evaluate their purity and morphologic profile. The panel on the right shows that more than 85% of the cells were CD79a+ and had a very low SSC and heterogeneous FSC values. All CD79a– contaminants fell into gate R2 indicated in the left panel.
CD19+ cells (all of which coexpressed CD79a) were sorted at day 14 or 21 in the same cultures and were used as positive controls in the next PCR experiments.
Molecular characterization of CD79a+-enriched CDw127+CD11b–CD36–CD19– cells
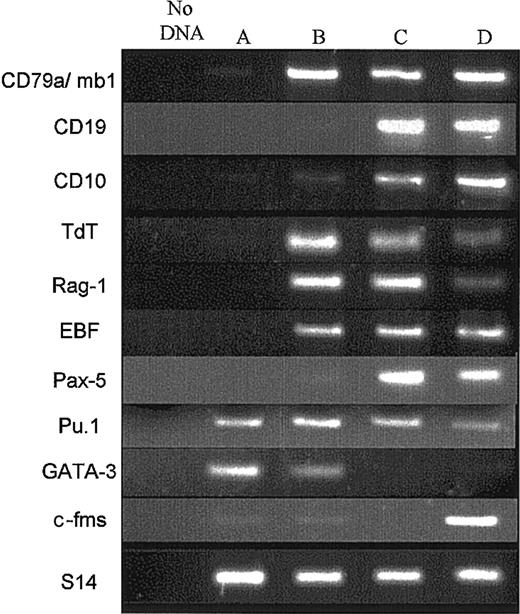
Analysis of B-cell transcripts by RT-PCR. Next, we studied in 3 experiments the profile of gene expression of CDw127+-CD11b–CD36–CD19– cells. Because this fraction contained more than 85% CD79a+ cells, it will be designed thereafter as CD79a+-enriched CDw127+ cells. CD79a+CD19+ cells sorted at days 14 or 21 (Figure 3) were treated simultaneously. cDNAs from equal numbers of cells (500 cells) of each population were subjected to PCR. Compared with the purified CD34+CD10–CD19– cells sorted at day 0 before the culture and the CD79a+CD19+ populations sorted either at days 14 or 21, the pattern of gene expression of CD79a+-enriched CDw127+ cells suggested that these cells represented an early stage of B-cell differentiation. Indeed, CD34+CD10–CD19– cells used to initiate the culture expressed neither CD10, CD19, and mb1/CD79a B-cell transcripts nor EBF or Pax-5 (Figure 3, lane A). Consistent with the phenotype described earlier, CD79a+-enriched CDw127+ cells sorted at day 14 lacked CD19 transcripts; CD10 mRNA was faintly expressed, whereas high amounts of mb-1 (CD79a) transcripts were present (Figure 3, lane B). The expression of Tdt and Rag-1 was higher in these cells than in CD79a+CD19+ cells generated at days 14 or 21 in the same culture conditions. As expected, EBF was detectable in CD79a+-enriched CDw127+ cells as well as in all samples of CD79a+CD19+ B cells. However, and interestingly enough, mRNA for Pax-5 was either undetectable in CD79a+-enriched CDw127+ cells (1 of 3 experiments) or only faintly expressed (2 of 3 experiments), which explained the lack of CD19. As expected, Pax-5 was consistently highly expressed in other CD19+ populations studied (Figure 3, lane C). Two other key genes for lymphoid cell differentiation, Pu-1 and Gata-3, were transcribed in CD79a+-enriched CDw127+ cells, the latter only faintly (n = 2). As expected, CD19+ cells also expressed Pu-1, whereas Gata-3 was completely down-regulated.
Expression of B-cell transcripts in CD79a+-enriched CDw127+CD19– cells. Four populations of flow cytometry–sorted cells were analyzed by RT-PCR. Lane A: fresh CD34+CD10–CD19– input cord blood cells; lane B, cultured CDw127+CD11b–CD36–CD19– cells, including 85% CD79a+, sorted twice at day 14; lane C, cultured CD19+ B cells sorted at day 14; lane D, cultured CD19+ B cells sorted at day 21. Cells used in lanes A-D were double-sorted to ensure more than 98% purity. The preparation of cDNAs and procedure used for the PCR reaction are described in “Materials and methods.” The cDNA input for each population was normalized to obtain equivalent signals with the S14 gene. All lanes were run in the same gel. Data shown are from 1 of 3 similar experiments with similar results.
Expression of B-cell transcripts in CD79a+-enriched CDw127+CD19– cells. Four populations of flow cytometry–sorted cells were analyzed by RT-PCR. Lane A: fresh CD34+CD10–CD19– input cord blood cells; lane B, cultured CDw127+CD11b–CD36–CD19– cells, including 85% CD79a+, sorted twice at day 14; lane C, cultured CD19+ B cells sorted at day 14; lane D, cultured CD19+ B cells sorted at day 21. Cells used in lanes A-D were double-sorted to ensure more than 98% purity. The preparation of cDNAs and procedure used for the PCR reaction are described in “Materials and methods.” The cDNA input for each population was normalized to obtain equivalent signals with the S14 gene. All lanes were run in the same gel. Data shown are from 1 of 3 similar experiments with similar results.
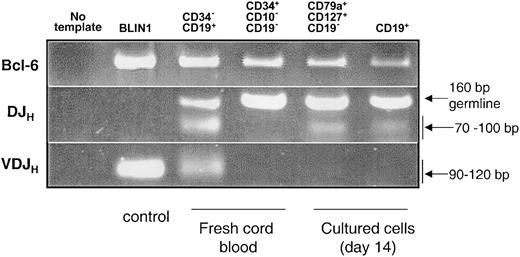
DJHrearrangement was detected in CD79a+-enriched CDw127+cells. DNA was isolated from 20 000 CD79a+-enriched CDw127+ cells (85% CD79a+) and CD79a+CD19+ cells sorted at day 14 of the culture. Mature CD34–CD19+ B cells sorted from fresh CB and the pre–B-cell line BLIN135 were used as positive controls. DNA integrity was shown by amplification of the control gene BCL-6 (Figure 4). A PCR assay was designed to detect DJH rearrangements of the immunoglobulin locus. For this, a mixture of 5′ primers specific for the 7 main DH gene families and a 3′ consensus JH primer were used. These primers were designed to overlap the upstream recombination sequence and the 5′ portion of the DH gene to avoid the PCR amplification of complete VDJH rearrangement. As shown in Figure 4, amplification of the germ line DNA segment located between the most 3′ DH gene (DHQ52) and the most 5′ JH segment (JH1) gave a 160-bp product. As expected, no DJH rearrangements were detected in BLIN1 and CD34+CD19–CD10– cells sorted at day 0, whereas both signals of DJH and VDJH rearrangements were present in fresh cord blood CD34–CD19+ B cells. A ladder of different-sized products ranging from 70 bp to 100 bp depending on the length of the specific rearrangement DJH rearrangements was observed in both CD79a+-enriched CDw127+ cells and CD79a+CD19+ cells sorted at day 14 of culture, but no VDJH bands were detectable in these populations (Figure 4).
PCR analysis of DJH and VDJH gene rearrangements on DNA from different cell subsets. BLIN1, a pre–B-cell line that had completely rearranged VDJH, and CD34–CD19+ cells sorted from fresh cord blood were used as controls. Purified CD34+CD10–CD19– cord blood cells sorted at day 0 to initiate the cultures, CD79a+-enriched CDw127+CD11b–CD36–CD19– cells and CD19+ cells (both subsets sorted twice at day 14 from the cultures). PCR was performed on 20 000 cells, and Bcl-6 was used to monitor the integrity of DNA.
PCR analysis of DJH and VDJH gene rearrangements on DNA from different cell subsets. BLIN1, a pre–B-cell line that had completely rearranged VDJH, and CD34–CD19+ cells sorted from fresh cord blood were used as controls. Purified CD34+CD10–CD19– cord blood cells sorted at day 0 to initiate the cultures, CD79a+-enriched CDw127+CD11b–CD36–CD19– cells and CD19+ cells (both subsets sorted twice at day 14 from the cultures). PCR was performed on 20 000 cells, and Bcl-6 was used to monitor the integrity of DNA.
On the basis of both phenotypic and molecular data, the CD79a+-enriched CDw127+ population generated in vitro from CD34+CD10–CD19– cord blood progenitor cells had activated a B-cell program and had initiated DJH rearrangement of the IgH locus. However, their lack (or faint expression) of Pax-5 and CD19 transcripts suggested that they represent an early B-cell progenitor, whose B-cell fate might not be irreversibly fixed.
CD79a+-enriched CDw127+ cells are not restricted to a B-cell fate and can differentiate into macrophages, NK cells, and T cells
Analysis of the differentiation potential of CD79a+-enriched CDw127+cells. CD79a+-enriched CDw127+ pro-B cells were reminiscent of the recently described pro-B cells isolated from Pax–/– mice that had undergone partial DJH rearrangement and retain the capacity to differentiate into non-B lineages.10 To determine whether CD79a+-enriched CDw127+ cells would express such a broad differentiating capacity, these cells were cultured during an additional 14 days (equal to a total of 28 days from day 0) in conditions known to favor macrophage, natural killer cell, T-, and B-cell differentiation. For this, B- and NK cell differentiation of CD79a+-enriched CDw127+ cells was assessed on MS-5 feeders in lymphoid (n = 3) or macrophage differentiation conditions (n = 4). T-cell differentiation was assessed once in FTOC cultures.
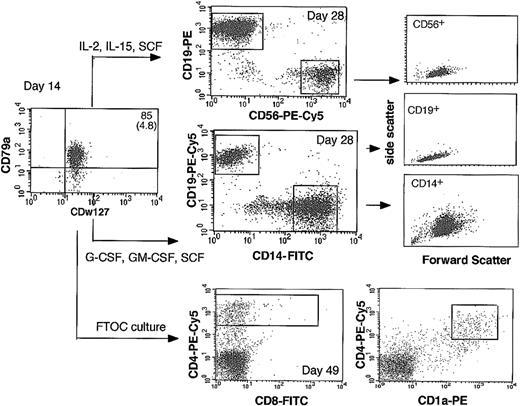
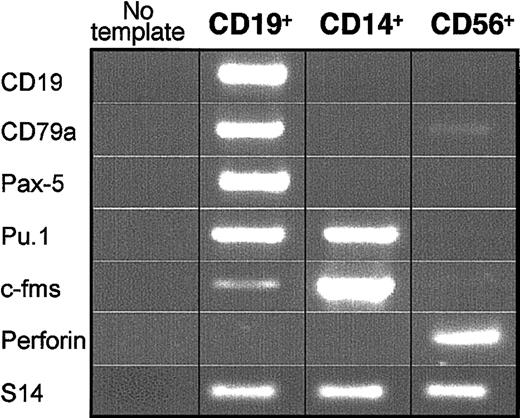
In all MS-5–based cultures, cells proliferated actively and differentiated completely (Figure 5). Thus, 55% to 83% (n = 4) of cells exposed to rhu-SCF, rhu-GM-CSF, and rhu-M-CSF expressed high levels of CD14 at day 28; coexpressed CD11b, c-fms (M-CSF-R), and CD64 but lacked CD19 and CD79a markers (not shown); and exhibited an FSC/SSC profile typical of macrophages (Figure 5). The remaining 10% to 15% of cells produced in these conditions were CD79a+CD19+ B cells with a typical lymphoid profile (Figure 5). Examination of May-Grünwald-Giemsa (MGG)–stained slides of sorted CD14+ cells confirmed their typical cytologic features (not shown). In each of 3 experiments, RT-PCR analysis performed on CD14+ cells sorted twice (99.8% pure, n = 3) showed high amounts of mRNAs for Pu-1 and c-fms, whereas CD19, mb1, and Pax-5 B-cell–specific transcripts were not detected (Figure 6).
Immunophenotype of differentiated cells generated by CD79a-enriched CDw127+ cells. CD79a+-enriched CDw127+CD11b–CD36–CD19– cells generated by CD34+CD10–CD19– cord blood cells were sorted at day 14 and incubated 14 days on MS-5 stromal cells in culture conditions that favor B and NK lymphoid differentiation (middle column, top panel) or macrophage differentiation (middle column, middle panel). After 14 additional days in these conditions, cells were stained with lineage-specific antibodies and analyzed by flow cytometry as illustrated. FSC/SSC profiles of CD19+, CD56+, and CD14+ cells are indicated on the right panels. CD79a+-enriched CDw127+CD11b–CD36–CD19– cells were also grown for 35 days in FTOC organotypic culture (middle column, bottom panel). After the culture, cells collected by mechanical dissociation were labeled with MoAbs against CD4, CD8, and CD1a.
Immunophenotype of differentiated cells generated by CD79a-enriched CDw127+ cells. CD79a+-enriched CDw127+CD11b–CD36–CD19– cells generated by CD34+CD10–CD19– cord blood cells were sorted at day 14 and incubated 14 days on MS-5 stromal cells in culture conditions that favor B and NK lymphoid differentiation (middle column, top panel) or macrophage differentiation (middle column, middle panel). After 14 additional days in these conditions, cells were stained with lineage-specific antibodies and analyzed by flow cytometry as illustrated. FSC/SSC profiles of CD19+, CD56+, and CD14+ cells are indicated on the right panels. CD79a+-enriched CDw127+CD11b–CD36–CD19– cells were also grown for 35 days in FTOC organotypic culture (middle column, bottom panel). After the culture, cells collected by mechanical dissociation were labeled with MoAbs against CD4, CD8, and CD1a.
Expression of lineage-specific transcripts by differentiated CD19+, CD56+, and CD14+ cells generated by CD79a+-enriched CDw127+-CD11b–CD36–CD19– cells. PCR was performed on cDNAs derived from 10 000 CD19+, CD14+, and CD56+ cells generated by cultured CD79a+-enriched CDw127+CD19– cells. Results are shown for 1 of 3 similar experiments with similar results.
Expression of lineage-specific transcripts by differentiated CD19+, CD56+, and CD14+ cells generated by CD79a+-enriched CDw127+-CD11b–CD36–CD19– cells. PCR was performed on cDNAs derived from 10 000 CD19+, CD14+, and CD56+ cells generated by cultured CD79a+-enriched CDw127+CD19– cells. Results are shown for 1 of 3 similar experiments with similar results.
CD79a+-enriched CDw127+ cells incubated in lymphoid conditions (n = 3) generated 10% to 21% CD56highCD19– NK cells and 70% to 90% CD19+CD56– B cells (Figure 5). The very high levels of CD56 antigen expressed by these cells, their typical cytologic appearance of large granular lymphocytes on MGG-stained slides, the high amount of perforin transcripts, and the lack of B-cell–specific transcripts in sorted CD56+ cells proved that they were natural killer cells (Figure 6). On the contrary, CD19+ cells sorted at day 28 expressed CD19, mb-1, Pax-5, Pu-1, and faintly c-fms transcripts but not perforin (Figure 6).
In one experiment, CD79a+-enriched CDw127+ cells were also incubated with E14 NOD-SCID thymic lobes (10 000 cells/lobe) for 35 days in FTOC conditions. As a negative control, 5 lobes were incubated with 10 000 IL-7Rα+CD19+ cells and as a positive control 10 lobes with 20 000 unfractionated day 14 cultured cells. Analysis of human cells collected from these thymic lobes showed that a high number of CD4high human cells were recovered in lobes seeded with unfractionated cells (not shown) or CD79a+-enriched CDw127+ cells. A few (5%) CD4high cells were double-positive (DP) CD4+CD8+ cells (Figure 5), which, as shown previously,33 indicates T-cell differentiation; we cannot exclude, however, the contribution of non-T cells to the CD4+ fraction. The low number of human cells collected from the thymic lobes precluded any extensive phenotyping and/or molecular analysis of T-cell receptor (TCR) rearrangement. In contrast, no CD4+ cells were recovered from lobes seeded with CDw127+CD19+ cells (data not shown).
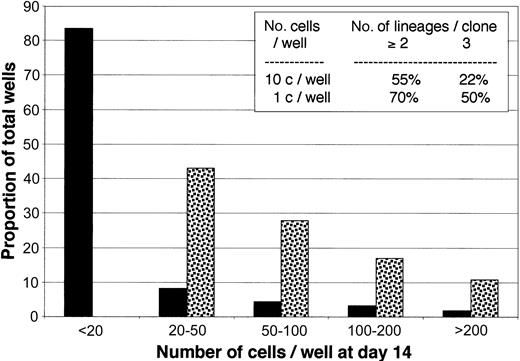
To unambiguously demonstrate that single CD79a+CDw127+ cells were endowed with multiple potentials, in one experiment, cells were seeded at 1 (n = 288) or 10 (n = 192) cells per well on MS-5 in 96-well plates with SCF, IL-2, and IL-15. After 14 days wells were individually examined, and the number of cells were counted under the microscope. As shown in Figure 7, 100% wells seeded with 10 cells and 18% of those seeded with single cells contained at least 20 cells. FACS analysis after 3-color labeling with MoAbs against CD19, CD56, and CD14+CD15 showed that 70% of the 26 clones generated by single cells contained cells belonging to at least 2 lineages and 50% to 3 lineages (Figure 7, insert). Analysis of 36 clones seeded with 10 cells confirmed results of single cell experiment.
Proliferation and differentiation of CD79a+-enriched CDw127+CD11b–CD36–CD19– cells cultured at limiting dilution. Plates (96 wells) precoated with MS-5 cells were seeded with either single cells (n = 288 wells, black bars) or 10 cells (n = 192 wells, dotted bars) in lymphoid conditions (see “Materials and methods” for details). Plates were grown for 14 days, and the number of nucleated cells in each was estimated by microscopic examination. Bars show the proportion of total wells containing the estimated number of nucleated cells indicated on the x-axis. The inset table shows the proportion of wells that contained cells from 2 or more lineages. Data are from 36 wells seeded with single cells and 26 wells seeded with 10 cells.
Proliferation and differentiation of CD79a+-enriched CDw127+CD11b–CD36–CD19– cells cultured at limiting dilution. Plates (96 wells) precoated with MS-5 cells were seeded with either single cells (n = 288 wells, black bars) or 10 cells (n = 192 wells, dotted bars) in lymphoid conditions (see “Materials and methods” for details). Plates were grown for 14 days, and the number of nucleated cells in each was estimated by microscopic examination. Bars show the proportion of total wells containing the estimated number of nucleated cells indicated on the x-axis. The inset table shows the proportion of wells that contained cells from 2 or more lineages. Data are from 36 wells seeded with single cells and 26 wells seeded with 10 cells.
Altogether, these results indicate that among CD79a+-enriched CDw127+ early B-cell progenitors, some were not yet irreversibly committed to a B-cell fate and retained the ability to differentiate into macrophages, NK cells, and T cells. Both phenotypic and molecular profiles of these mature cells demonstrated that engagement of CD79a+-enriched CDw127+ precursors into one of these pathways was coupled to the complete down-regulation of the B-cell program, ruling out the aberrant expression of myeloid, T, or NK markers on a committed B-cell progenitor.
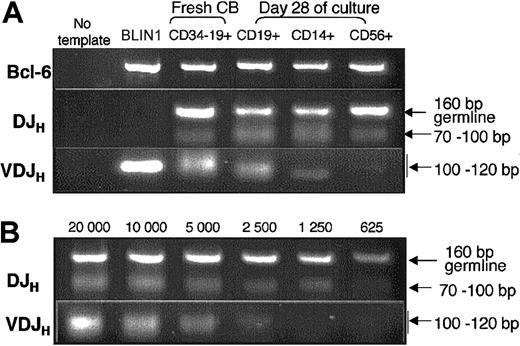
IgH gene rearrangement analysis confirmed the B-cell origin of CD14+macrophages and CD56+NK cells generated from CD79a+-enriched CDw127+CD11b–CD36–CD19–cells. To definitely prove that CD14+ macrophages and CD56+ NK cells originated from CD79a+CDw127+ cells that had activated the B-cell program, we analyzed in 3 separate experiments the status of the IgH locus in twice-sorted CD14+ macrophages, CD56+ NK and CD19+ B cells (> 98% purity) generated at day 28 from CD79a+-enriched CDw127+CD19– B cells. Figure 8 shows that a DJH band was detected as expected in CD19+ B cells, but also in sorted CD14+ macrophages and CD56+ NK cells generated in vitro from CD79a+-enriched CDw127+CD11b–CD36–CD19– cells. In these experiments, cross contamination between CD14+ cells or CD56+ cells and CD79a+CD19+ B cells generated simultaneously from CD79a+-enriched CDw127+CD19– pro-B cells was ruled out by examination of the threshold of detection of DJH rearrangements. In our PCR assay the presence of at least 25% of contaminating CD79a+CD19+ DJH rearranged B cells would be necessary to produce a detectable signal (Figure 8B), confirming the specificity of DJH rearrangements detected in CD14+ macrophages and CD56+ NK cells.
PCR analysis of DJH and VDJH gene rearrangements on DNA from differentiated CD19+, CD56+, and CD14+ cells generated by CD79a+-enriched CDw127+CD11b–CD36–CD19– cells. (A) BLIN1 and CD34–CD19+ were used as controls as described in the legend of Figure 4. CD19+ B cells, CD56+ NK cells, and CD14+ macrophages were obtained from cultured CD79a+-enriched CDw127+CD11b–CD36–CD19– cells and sorted twice at day 28 (> 98% purity). DNA was extracted from 20 000 cells, and PCR was performed as described in “Materials and methods.” (B) Results of PCR performed on serial dilutions of DNA extracted from CD19+ cells purified at day 28 as described in panel A.
PCR analysis of DJH and VDJH gene rearrangements on DNA from differentiated CD19+, CD56+, and CD14+ cells generated by CD79a+-enriched CDw127+CD11b–CD36–CD19– cells. (A) BLIN1 and CD34–CD19+ were used as controls as described in the legend of Figure 4. CD19+ B cells, CD56+ NK cells, and CD14+ macrophages were obtained from cultured CD79a+-enriched CDw127+CD11b–CD36–CD19– cells and sorted twice at day 28 (> 98% purity). DNA was extracted from 20 000 cells, and PCR was performed as described in “Materials and methods.” (B) Results of PCR performed on serial dilutions of DNA extracted from CD19+ cells purified at day 28 as described in panel A.
To further prove that DJH recombinations had occurred, we sequenced PCR products detected in CD79a+CD19+ and CD14+ cells generated in secondary cultures. All sequences were different and contained D segments with additional nucleotides at the segment junction with JH genes. Ten of 24 sequences from CD79a+-enriched CDw127+ cells were functional (ie, sequences did not contained stop codon and were in frame).
As expected, we found a detectable VDJH band in CD19+ B cells produced by CD79a+-enriched CDw127+CD19– pro-B cells purified at day 28, indicating normal progression of the B-cell differentiation program of these precursors. In contrast, complete VDJH rearrangements were detectable neither in CD14+ nor in CD56+ cells, except in one experiment in which a faint VDJH band was detectable in sorted CD14+ cells (Figure 8). This finding, together with the lack of expression of B-cell–specific transcripts in these non-B cells, indicated that the switch to a non–B-lymphoid fate occurred soon after the seeding of CD79a+-enriched CDw127+CD19– cells in secondary cultures.
Discussion
In this study we identified for the first time in healthy humans an early population of B-cell progenitors, which retain the ability to generate in vitro NK cells, macrophages, and T cells. These human early B cells lacked the pan-B CD19 antigen and mRNA, and they expressed 2 markers highly specific of the B-cell lineage, the BCR-associated CD79a molecule36, 37, 38 and incomplete rearrangement of the IgH locus.4,39 This latter finding is in accordance with the expression of Tdt and Rag-1 transcripts, indicating that genes necessary for recombination are transcribed at the onset of human B-cell differentiation. Half of these CD79a+CD19– early B cells expressed the IL-7Rα and were sorted according to this criteria. These CD79a+CDw127+ cells completed their maturation along the B-lymphoid lineage but, when exposed to appropriate cytokines, also differentiated into CD56+ NK cells,32 CD14+ macrophages, and, in one FTOC experiment CD4high precursor T cells, and this was true at the single cell level for B, NK, and macrophage lineages. To our knowledge, this is the fist demonstration that healthy primary human early B-cell precursors spontaneously exhibit such a broad lymphoid and myeloid potential. Retention of the IgH locus rearrangement in these non-B cells definitively proved that both macrophages and NK cells originated CD79a+-enriched CDw127+CD19– precursors that had initiated a B-cell program. This DNA rearrangement combined with the observation that both macrophages and NK cells issued from such CD79a+-enriched CDw127+CD19– early B cells repressed B-cell–specific genes ruled out contamination by B lymphocytes.
On the basis of their phenotype and functional properties, it is tempting to compare our novel population with the “multipotent” B220+c-Kit+ murine pro-B cells identified in the marrow of Pax5–/– mice.8,10 Indeed, Pax5–/– pro-B cells were CD19–, expressed c-fms, have initiated recombination of the IgH locus,40 and retained the capacity to generate cells derived from the monocytic lineage, including osteoclasts and dendritic cells, but also granulocytes, NK cells, and T cells in vivo.10,40 Interestingly, thymocyte populations produced by these Pax-5–/– cells retained the DHJH rearrangement present in the pro-B cells.40 IL-7Rα+ common lymphoid progenitor (CLP) identified by King et al41 can similarly convert to a myeloid fate when transfected with the IL-2Rβ and exposed to IL-2.
Populations with similar broad potential have not been described in wild-type mice, and the normal counterparts of Pax-5–/– pro-B cells had not been previously isolated in humans. Activation of the IgH locus rearrangement and the expression of CD79a in human bone marrow CD34+ cells before the expression of CD19 have both been described,3,5 but neither the functional properties nor the status of Pax5 expression was investigated in these studies. In vivo, CD10 precedes CD19 and identifies a lymphoid-restricted precursor among CD34+CD19– adult human bone marrow cells.27 These data have been recently extended by the identification among CD34+CD38–CD19– CB cells of a CD7+CD10+ subset, lacking Pax-5, TdT, and IL-7Rα transcripts. These cells differentiated in vitro in B, NK, and dendritic cells, but neither in myeloid nor erythroid lineages.42 T-cell potential was not investigated. In contrast, Ryan et al43 reported the expression of IL-7Rα on fresh CD34+lin– human bone marrow cells that transcribed Pax-5 and TdT, were depleted of myeloid potential but enriched in clonogenic B cells. All these studies were performed on fresh ex vivo samples, whereas we worked with cultured cells. This was justified by the remarkable reproducibility in the kinetics of emergence and phenotype of this CD79a+-enriched CDw127+CD19– population and in the numbers of cells that we could sort at day 14. However, in vitro conditions introduced some changes in the cell phenotype: for example, in vitro, in our hands, CD10 was never expressed prior to CD19, and pro-B cells that coexpress CD34 and CD19 were very rare.28,44
The complete down-regulation of B-cell–specific transcripts, the expression of lineage-specific genes, and the typical cytologic features observed in cultured CD14+ macrophages and CD56+ NK cells derived from CDw127+-enriched CD79a+CD19– early pro-B cells ruled out lineage infidelity and provided clear evidence for a complete program switch at the molecular level in CDw127+-enriched CD79a+CD19– cells. As expected, these early pro-B cells could also mature in vitro in typical CD19+ B cells with a complete IgH gene rearrangement and up-regulated Pax-5, whereas c-fms followed an opposite down-regulation.
The promiscuity between lymphoid and macrophage, and as more recently described, osteoclastic lineages, in healthy or manipulated mice models has been demonstrated in earlier reports. Several immortalized pre–B-cell lines could be converted into macrophages in vitro.45, 46, 47 This is also true for primary cells: a progenitor restricted to B cells and macrophages has been isolated from normal mouse fetal liver,15 although there was no indication that this progenitor had engaged a B-cell program. More recently, the possibility for CD19+ precursor B cell from postnatal bone marrow to differentiate into macrophages,16 dendritic cells,48 or osteoclast-related markers49 when grown with various combinations of cytokines has been reported. Acquisition of T or NK cell markers was not observed.
Key controls of such a plastic fate are the transcription factors Pax 58 and Pu-1.18,50 When highly expressed, Pax5 leads to the down-regulation of non–B-lineage differentiation gene expression, in part, through the control of receptors for non–B-lineage growth factors such as M-CSF (M-CSF-R).10 However, graded expression of the Pu-1 factor seems essential to specify the differentiation of macrophages versus B lymphocytes by controlling the expression of the M-CSF-R gene.50,51 Interestingly, we found that CD79a+-enriched CDw127+ pro-B cells expressed transcripts for Pu-1 and c-fms, which may render these cells permissive to macrophage differentiation when switched into cultures with M-CSF. According to their differentiation pattern, macrophage and NK cells generated from CD79a+-enriched CDw127+ B-cell precursors repressed the expression B-cell–specific markers in agreement with the absence of Pax5 expression and up-regulated M-CSF-R or perforin expression, respectively.52
Whether or not such flexibility in the fate of early B-cell precursors has a physiologic significance is unknown. A small fraction (0.4%) of freshly purified CD34+CD10–CD19– CB cells were CD79a+CD19– pro-B, but their potential is difficult to assess. The absence of detection of IgH gene rearrangement in ex vivo–purified CB monocytes (data not shown) would suggest that the terminal differentiation of multipotent pro-B cells along the macrophage lineage does not occur in vivo or is such a rare event that it fell below the threshold of detection of rearranged IgH cells. Finally, in physiologic conditions environmental factors may keep the balance between B and non-B differentiation of these CD79a+CD19– cells in favor of the B-cell pathway, a situation that is disrupted in vitro.
Our data raise the possibility that some human acute and chronic leukemias, such as hairy cell leukemia, which coexpressed B-cell and macrophage characteristics,53,54 represent the malignant counterparts of these normal precursors.55 Lineage infidelity associated with the aberrant malignant process has been proposed to explain the coexistence of markers from different lineages both in humans56 and in some murine B-cell lymphomas.57,58 A plausible explanation could also be that bipotent or multipotent B-cell progenitor cells as those described here might also be the targets of the leukemic event. Therefore, our observations in healthy cells described in this study will offer an invaluable tool to further investigate the molecular basis of the plasticity of early human B cells, with particular relevance to its induction by cytokines and stromal-derived signals, as well as defects associated with their leukemic transformation.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-07-2085.
Supported by INSERM, Association pour la recherche contre le cancer (LC 5461), Fondation pour la Recherche Médicale, Fondation de France (comité Leucémies), and Association contre les myopathies, as well as fellowships from La Ligue Nationale contre le Cancer (D.R.) and the French Ministry of Research (N.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. C. Gendron (Institut Jacques Monod, Paris), I. Bouchaert, and N. Lebrun (Institut Cochin, Paris) for their expert technical assistance in cell sorting and B. Izac for her help in the FTOC assay. We thank P. Ardouin for providing the pregnant NOD-SCID mice and Mrs Van Nifderick for cord blood samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal