Abstract
In diffuse large B-cell lymphoma (DLBCL), the combination of rituximab and CHOP (cyclophosphamide, doxorubicine, vincristine, prednisone; R-CHOP) has been shown to be more effective than CHOP for the treatment of elderly patients. Bcl-2 protein expression has been associated with poor prognosis in patients with DLBCL. To establish whether or not rituximab reduces bcl-2—associated treatment failure, we studied bcl-2 protein expression and clinical outcome in patients included in the Groupe d'Etude des Lymphomes de l'Adulte LNH-98-5 trial. Patients between 60 and 80 years of age were randomized to receive 8 cycles of either CHOP or R-CHOP every 3 weeks. Of the 399 patients included, 292 with histologically proven DLBCL had material available for bcl-2 study. Tumors were considered positive when at least 50% of tumor cells expressed bcl-2 protein. There were 193 (66%) bcl-2+ patients and 99 (34%) bcl-2– patients. The response rates for R-CHOP and CHOP were, respectively, 78% and 60% (P = .01) in bcl-2+ patients and 76% and 73% (P = .7) in bcl-2– patients. At a median of 2 years of follow-up, R-CHOP was significantly associated with a better overall survival than CHOP in bcl-2+ patients (67% ± 9% versus 48% ± 11%, P = .004). In bcl-2– patients there was no statistically significant difference (72% ± 12% versus 67% ± 14%, P = .6). In addition, R-CHOP was associated with significantly better event-free survival than CHOP in bcl-2+ patients (58% ± 10% versus 32% ± 10%, P < .001) but not in bcl-2– patients (60% ± 13% versus 40% ± 15%, P = .13). Multivariate analysis confirmed the significant benefit for survival and event-free survival of R-CHOP in bcl-2+ patients. These results suggest that rituximab is able to prevent chemotherapy failure in patients with bcl-2 protein overexpression.
Introduction
Diffuse large B-cell lymphomas (DLBCLs) are the most common lymphoid neoplasms and account for 30% to 40% of adult non-Hodgkin lymphomas (NHLs).1 Although patients with DLBCL can be cured with current chemotherapy regimens, 50% die of their disease. More than half the patients with DLBCL are over 60 years of age and the treatment of these elderly patients is difficult. The CHOP (cyclophosphamide, doxorubicine, vincristine, prednisone) regimen was the standard treatment for both younger and older patients with DLBCL, but it induced complete responses in only 40% to 50% of elderly patients, with 3-year overall survival rates of 35% to 40%.2 Intensified chemotherapy regimens may improve the outcome in young patients, but are poorly tolerated by the elderly.3,4 The Eastern Cooperative Oncology Group (ECOG) performance status (PS), tumor stage, level of lactate dehydrogenase (LDH), and number of sites of extranodal disease have prognostic value in DLBCL, and they are included in the International Prognostic Index (IPI).5 However, the clinical factors included in the IPI are likely to be surrogate markers of the intrinsic molecular heterogeneity of this disease. Therefore, it is not surprising that the patients with DLBCL who are unlikely to be cured by standard therapy cannot all be identified from the IPI.6,7
At present, the main focus is on the proteins that regulate apoptosis as well as on IPI factors for the identification of patients at increased risk of standard treatment failure, for whom experimental therapy would be appropriate.8,9 Bcl-2 is a member of the bcl-2 family of proteins that regulate programmed cell death.10 Bcl-2 is the best characterized member of this family, which has been shown to provide protection from apoptotic stimuli, including drug cytotoxicity.11 Overexpression of bcl-2 protein in NHL cells, which is not correlated to chromosome 14;18 translocation, has been incriminated in their resistance to chemotherapy both in vitro and in vivo.8,12, 13, 14
The Groupe d'Etude des Lymphomes de l'Adulte (GELA) study LNH-98-5 recently demonstrated that the combination of rituximab and CHOP (R-CHOP) was more effective than CHOP alone for the treatment of elderly patients with DLBCL.15 To establish in vivo whether or not rituximab reduces bcl-2—associated treatment failure, we compared bcl-2 expression to clinical outcome in patients from the LNH-98-5 trial.
Patients and methods
The patients studied for the expression of bcl-2 at the protein level were a subset of the 399 patients entered into the LNH-98-5 trial, a prospective multicentric trial conducted by the GELA, between July 1998 and March 2000.15 Approval was obtained from the Centre Hospitalier Lyon Sud institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
The LNH-98-5 protocol
Details regarding the design and data management of the trial have been published.15 Briefly, patients were eligible if they were 60 to 80 years of age and had untreated DLBCL.1 They were required to have stage II, III, or IV disease and a PS of 0 to 2 according to the ECOG criteria. Patients were not eligible if they had T-cell lymphoma, a history of indolent lymphoma, central nervous system involvement, active cancer, or any serious active concomitant disease, or if their general status did not permit the administration of 8 courses of CHOP. Patients were also excluded if they had a cardiac contraindication to doxorubicin therapy. Lastly, patients with positive serology for the HIV or unresolved hepatitis B virus infection were also excluded.
Eligible patients were randomly assigned by the coordinating center of the study to treatment with CHOP or R-CHOP. They were stratified according to center and age-adjusted IPI (aa-IPI). Patients treated with CHOP received the combination of 750 mg cyclophosphamide/m2 body surface area on day 1; 50 mg doxorubicin/m2 on day 1; 1.4 mg vincristine/m2, up to a maximal dose of 2 mg, on day 1; and 40 mg prednisone/m2/d for 5 days. They had 8 cycles of CHOP, one every 3 weeks. Patients treated with R-CHOP were also given 375 mg/m2 rituximab, on day 1 of each CHOP cycle. Patients who had severe neutropenia or febrile neutropenia after any cycle of chemotherapy were given granulocyte colony-stimulating factor. Tumor responses were assessed after the 8 cycles or at the end of treatment and were classified as complete response (CR), unconfirmed complete response (CRu), partial response, stable disease, or progressive disease according to the International Workshop criteria.16 These classifications were defined as follows: CR, the disappearance of all lesions and of radiologic or biologic abnormalities observed at diagnosis and the absence of new lesions; CRu, persistence for 4 months of palpable node or mass on computed tomography that had regressed in size by at least 75% but not disappeared, normal bone marrow, normal PS, no symptoms, and disappearance of initial biologic abnormalities; partial response, the regression of all measurable lesions by more than 50%, the disappearance of nonmeasurable lesions, and the absence of new lesions; stable disease, the regression of measurable lesions by 50% or less, or no change for the nonmeasurable lesions, and no growth of existing lesions or appearance of new lesions; progressive disease, the appearance of new lesions, growth of the initial lesions by more than 25%, or growth of measurable lesions that had regressed during treatment by more than 50% of their smallest dimensions.
Patient selection
Bcl-2 protein expression was assessed in patients given CHOP or R-CHOP whose diagnosis of DLBCL had been histologically confirmed and who could provide material for bcl-2 staining. In particular, cases of histologic transformation of indolent NHL into BDLCL were not included. A panel of at least 3 hematopathologists conducted a central pathology review, without knowledge of the patients' outcome, to confirm the diagnosis of CD20+ DLBCL.
Bcl-2 staining
Staining for bcl-2 was performed on paraffin-embedded sections using an indirect immunoperoxidase method and a specific monoclonal antibody (bcl-2 124; Dako, Glostrup, Denmark) with an automated immunostainer (Ventana Medical Systems, Tucson, AZ) according to the instructions of the manufacturer. Optimum labeling was obtained by microwave pretreatment (3 cycles of 5 minutes in 0.01 M citrate buffer, pH 7.3). Scoring for bcl-2 was independently performed by 2 of us (J.B. and P.G.) without knowledge of the clinical data. Consensus for discordant cases was reached using a 2-head microscope. Tumors were considered positive when at least 50% of tumor cells expressed bcl-2 protein.
Statistical analysis
All analyses were performed on an intention-to-treat basis. To allow direct comparison with the data given in our previous report, the stopping date of the present analysis was also set at June 30, 2001.15
Patient characteristics and complete remission rates were compared by the χ2 and Fisher exact tests. Event-free survival (EFS) was measured from the date of randomization to that of disease progression, relapse, or death from any cause or to the stopping date. Overall survival (OS) was measured from the date of randomization to either death from any cause or the stopping date. When the stopping date was not reached, the data were censored at the date of the last follow-up evaluation. Survival functions were estimated by the Kaplan-Meier method and compared by log-rank test.17 Differences between the results of comparative tests were considered significant at a 2-sided P < .05.
Because the LNH-98-5 trial was not stratified on bcl-2 expression, we controlled for the effects of prognostic factors on outcome due to sampling fluctuation in the treatment groups using multivariate analysis of survival. The potential prognostic factors for survival or relapse were the risk factors in the aa-IPI. A Cox model regression was fitted including these risk factors: (LDH > normal versus ≤ normal, PS 2-3 versus 0-1, stage III-IV versus I-II), bcl-2 expression, and treatment as explanatory variables.18 The interactions between risk factors and treatment were also included in the model. Model parameters were then used to estimate CHOP versus R-CHOP relative risk, according to various sets of explanatory variables.
All statistical analyses were performed using SAS 8.2 (SAS Institute, Cary, NC) and Splus 2000 (MathSoft, Cambridge, MA) software.
Results
Bcl-2 expression and baseline characteristics
In all, 399 patients were enrolled in the LNH-98-5 trial; the histologic material from 385 was reviewed: 49 did not have DLBCL, 4 had histologic transformation of a follicular lymphoma, and 332 had confirmed DLBCL. Forty more cases were excluded because of the absence of suitable slides (n = 35) or inadequate immunostaining (n = 5), leaving a total of 292 patients studied.
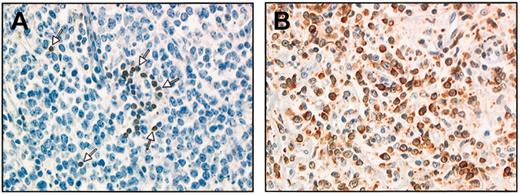
Of these, 193 (66%) exhibited high bcl-2 protein expression in more than 50% of tumor cells, and in most cases, this protein was expressed in the majority of most tumor cells. In addition, bcl-2 staining of tumor cells was usually strong resulting in clear-cut positive patterns. In the remaining 99 cases, either positive tumor cells were absent or staining was heterogeneous, with less than 50% positive tumor cells, so that these cases were scored as negative. Staining patterns are illustrated in Figure 1.
The bcl-2 staining patterns. The bcl-2 staining patterns for a nodal biopsy specimen (immunoperoxidase method and monoclonal antibody bcl-2). (A) A few positive T cells as positive control (arrows). Tumor cells × 537) are bcl-2–. (B) Almost all tumor cells × 537) are strongly positive for bcl-2 protein expression.
The bcl-2 staining patterns. The bcl-2 staining patterns for a nodal biopsy specimen (immunoperoxidase method and monoclonal antibody bcl-2). (A) A few positive T cells as positive control (arrows). Tumor cells × 537) are bcl-2–. (B) Almost all tumor cells × 537) are strongly positive for bcl-2 protein expression.
The 292 patients included 138 men and 154 women. Their median age was 69 years (range, 60-80 years). There was no significant difference between bcl-2+ and bcl-2– patients as regards aa-IPI prognostic factors; thus, PS was more than 1 in 19% versus 23% (P = .43), LDH more than normal in 30% versus 36% (P = .27), stage III to IV in 78% versus 81% (P = .38), and the score for aa-IPI factors equal 2 to 3 in 51% versus 50% (P = .9).
A total of 155 patients (53%) were given R-CHOP, and 137 (47%), CHOP. Clinical features according to bcl-2 staining and treatment are given in Table 1. The groups did not differ as regards the distribution of clinical prognostic factors. Among the bcl-2+ patients, more women than men were treated by R-CHOP (55% versus 46%), but the difference was not significant (P = .17).
Patient characteristics according to bcl-2 expression and treatment
. | LNH-98-5 . | Study population . | . | bcl-2+ . | . | bcl-2- . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | n = 399 . | CHOP n = 137 . | R-CHOP n = 155 . | CHOP n = 92 . | R-CHOP n = 101 . | CHOP n = 45 . | R-CHOP n = 54 . | |||
| Sex | ||||||||||
| Male (%) | 199 (50) | 70 (51) | 68 (44) | 50 (54) | 45 (45) | 20 (44) | 23 (43) | |||
| Female (%) | 200 (50) | 67 (49) | 87 (56) | 42 (46) | 56 (55) | 25 (56) | 31 (57) | |||
| Median age, y (range) | 69 (60-80) | 68 (60-80) | 69 (60-80) | 69 (60-80) | 70 (60-80) | 66 (60-80) | 67 (60-79) | |||
| LDH | ||||||||||
| Equal to or less than N (%) | 136 (34) | 42 (31) | 52 (34) | 28 (30) | 30 (30) | 14 (31) | 22 (41) | |||
| Greater than N (%) | 263 (66) | 95 (60) | 103 (67) | 64 (70%) | 71 (70) | 31 (69) | 32 (59) | |||
| Stage | ||||||||||
| I-II (%) | 81 (20) | 28 (20) | 33 (21) | 21 (23) | 21 (21) | 7 (16) | 12 (22) | |||
| III-IV (%) | 318 (80) | 109 (80) | 122 (79) | 71 (77) | 80 (79) | 38 (84) | 42 (78) | |||
| PS | ||||||||||
| 0-1 (%) | 321 (80) | 112 (82) | 120 (78) | 78 (85) | 78 (77) | 34 (76) | 42 (78) | |||
| 2-4 (%) | 78 (20) | 25 (18) | 35 (22) | 14 (15) | 23 (23) | 11 (24) | 12 (22) | |||
| Extra nodal sites | ||||||||||
| 0-1 (%) | 287 (72) | 100 (73) | 110 (71) | 69 (75) | 77 (75) | 31 (70) | 33 (62) | |||
| 2 or more (%) | 112 (28) | 37 (27) | 45 (29) | 23 (25) | 24 (25) | 14 (30) | 21 (38) | |||
| aa-IPI | ||||||||||
| 0 (%) | 41 (10) | 13 (9) | 15 (9) | 12 (13) | 7 (7) | 1 (2) | 8 (15) | |||
| 1 (%) | 117 (29) | 39 (29) | 48 (31) | 23 (25) | 34 (34) | 16 (36) | 14 (26) | |||
| 2 (%) | 181 (45) | 65 (47) | 64 (41) | 45 (49) | 40 (40) | 20 (44) | 24 (44) | |||
| 3 (%) | 60 (16) | 20 (18) | 28 (18) | 12 (13) | 20 (20) | 8 (18) | 8 (15) | |||
| β2-microglobulin | ||||||||||
| Equal to or less than N (%) | 147 (42) | 50 (41) | 58 (44) | 35 (42) | 37 (43) | 15 (41) | 21 (48) | |||
| Greater than N (%) | 201 (58) | 71 (59) | 72 (56) | 49 (58) | 50 (57) | 22 (59) | 22 (52) | |||
| Bone marrow | ||||||||||
| Normal (%) | 285 (73) | 103 (76) | 117 (76) | 72 (79) | 77 (77) | 31 (71) | 40 (74) | |||
| Involved (%) | 107 (27) | 32 (24) | 37 (24) | 19 (21) | 23 (23) | 13 (29) | 14 (26) | |||
. | LNH-98-5 . | Study population . | . | bcl-2+ . | . | bcl-2- . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | n = 399 . | CHOP n = 137 . | R-CHOP n = 155 . | CHOP n = 92 . | R-CHOP n = 101 . | CHOP n = 45 . | R-CHOP n = 54 . | |||
| Sex | ||||||||||
| Male (%) | 199 (50) | 70 (51) | 68 (44) | 50 (54) | 45 (45) | 20 (44) | 23 (43) | |||
| Female (%) | 200 (50) | 67 (49) | 87 (56) | 42 (46) | 56 (55) | 25 (56) | 31 (57) | |||
| Median age, y (range) | 69 (60-80) | 68 (60-80) | 69 (60-80) | 69 (60-80) | 70 (60-80) | 66 (60-80) | 67 (60-79) | |||
| LDH | ||||||||||
| Equal to or less than N (%) | 136 (34) | 42 (31) | 52 (34) | 28 (30) | 30 (30) | 14 (31) | 22 (41) | |||
| Greater than N (%) | 263 (66) | 95 (60) | 103 (67) | 64 (70%) | 71 (70) | 31 (69) | 32 (59) | |||
| Stage | ||||||||||
| I-II (%) | 81 (20) | 28 (20) | 33 (21) | 21 (23) | 21 (21) | 7 (16) | 12 (22) | |||
| III-IV (%) | 318 (80) | 109 (80) | 122 (79) | 71 (77) | 80 (79) | 38 (84) | 42 (78) | |||
| PS | ||||||||||
| 0-1 (%) | 321 (80) | 112 (82) | 120 (78) | 78 (85) | 78 (77) | 34 (76) | 42 (78) | |||
| 2-4 (%) | 78 (20) | 25 (18) | 35 (22) | 14 (15) | 23 (23) | 11 (24) | 12 (22) | |||
| Extra nodal sites | ||||||||||
| 0-1 (%) | 287 (72) | 100 (73) | 110 (71) | 69 (75) | 77 (75) | 31 (70) | 33 (62) | |||
| 2 or more (%) | 112 (28) | 37 (27) | 45 (29) | 23 (25) | 24 (25) | 14 (30) | 21 (38) | |||
| aa-IPI | ||||||||||
| 0 (%) | 41 (10) | 13 (9) | 15 (9) | 12 (13) | 7 (7) | 1 (2) | 8 (15) | |||
| 1 (%) | 117 (29) | 39 (29) | 48 (31) | 23 (25) | 34 (34) | 16 (36) | 14 (26) | |||
| 2 (%) | 181 (45) | 65 (47) | 64 (41) | 45 (49) | 40 (40) | 20 (44) | 24 (44) | |||
| 3 (%) | 60 (16) | 20 (18) | 28 (18) | 12 (13) | 20 (20) | 8 (18) | 8 (15) | |||
| β2-microglobulin | ||||||||||
| Equal to or less than N (%) | 147 (42) | 50 (41) | 58 (44) | 35 (42) | 37 (43) | 15 (41) | 21 (48) | |||
| Greater than N (%) | 201 (58) | 71 (59) | 72 (56) | 49 (58) | 50 (57) | 22 (59) | 22 (52) | |||
| Bone marrow | ||||||||||
| Normal (%) | 285 (73) | 103 (76) | 117 (76) | 72 (79) | 77 (77) | 31 (71) | 40 (74) | |||
| Involved (%) | 107 (27) | 32 (24) | 37 (24) | 19 (21) | 23 (23) | 13 (29) | 14 (26) | |||
N indicates normal.
Bcl-2 expression and clinical outcome
With a median follow-up of 2 years, 2-year survival estimates for all patients were 47% ± 6% for EFS and 62% ± 6% for OS. CR or CRu was achieved by 71% of patients. Two-year EFS and OS were significantly longer for patients treated with R-CHOP than for those treated with CHOP alone (58% ± 8% versus 35% ± 8%, P < .0001, for 2-year EFS, and 70% ± 7% versus 54% ± 9%, P < .009, for 2-year OS). The CR plus CRu rate was 77% for patients treated with R-CHOP, as compared with 65% for those treated with CHOP alone (P = .018). Prolongation of the disease-free survival (DFS) for CR + CRu patients in the R-CHOP arm was of the same magnitude as the prolongation of EFS, that is, 2-year DFS 71% ± 9% versus 49% ± 11% (P = .005).
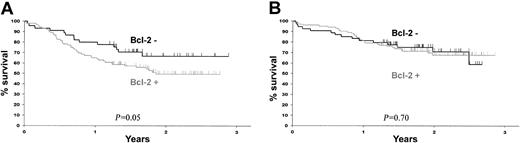
Clinical outcomes according to bcl-2 staining for each treatment are given in Table 2. The results of univariate analysis confirmed the prognostic effect of bcl-2. Among patients who received the standard CHOP treatment, the CR plus CRu rate was lower for bcl-2+ patients than for bcl-2– patients (60% versus 73%, P = .1). Figure 2A shows that bcl-2 protein also had a prognostic value for survival with a 2-year OS of 48% ± 11% for bcl-2+ patients and 67% ± 14% for bcl-2– patients (P = .05). However, neither positive nor negative bcl-2 had any prognostic value for patients treated with R-CHOP (Figure 2B).
Univariate analysis of clinical outcome for the study population (n = 292) according to bcl-2 expression and treatment
. | CHOP . | . | . | R-CHOP . | . | . | ||
|---|---|---|---|---|---|---|---|---|
. | Bcl-2+ . | bcl-2- . | P . | Bcl-2+ . | bcl-2- . | P . | ||
| Response (%) | ||||||||
| CR/CRu | 56 (60) | 33 (73) | .1 | 79 (78) | 41 (76) | .8 | ||
| Non-CR | 36 (40) | 12 (27) | 22 (22) | 13 (24) | ||||
| 2-y survival, % | ||||||||
| OS | 48 ± 11 | 67 ± 14 | .05 | 67 ± 9 | 72 ± 12 | .7 | ||
| EFS | 32 ± 9 | 40 ± 14 | .14 | 58 ± 9 | 60 ± 12 | .8 | ||
. | CHOP . | . | . | R-CHOP . | . | . | ||
|---|---|---|---|---|---|---|---|---|
. | Bcl-2+ . | bcl-2- . | P . | Bcl-2+ . | bcl-2- . | P . | ||
| Response (%) | ||||||||
| CR/CRu | 56 (60) | 33 (73) | .1 | 79 (78) | 41 (76) | .8 | ||
| Non-CR | 36 (40) | 12 (27) | 22 (22) | 13 (24) | ||||
| 2-y survival, % | ||||||||
| OS | 48 ± 11 | 67 ± 14 | .05 | 67 ± 9 | 72 ± 12 | .7 | ||
| EFS | 32 ± 9 | 40 ± 14 | .14 | 58 ± 9 | 60 ± 12 | .8 | ||
OS according to treatment and bcl-2 protein expression. (A) OS for patients treated with CHOP. (B) OS for patients treated with R-CHOP. Black lines indicate bcl-2– patients and gray lines indicate bcl-2+ patients.
OS according to treatment and bcl-2 protein expression. (A) OS for patients treated with CHOP. (B) OS for patients treated with R-CHOP. Black lines indicate bcl-2– patients and gray lines indicate bcl-2+ patients.
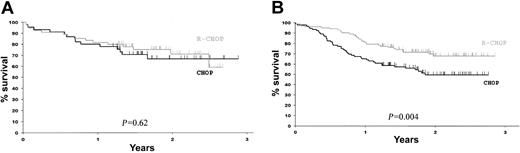
To investigate the impact of R-CHOP on the predictive value of bcl-2, we studied clinical outcome according to treatment in each bcl-2 group. The CR plus CRu rates for R-CHOP and CHOP were 78% and 60% (P = .01) in the bcl-2+ group and 76% and 73% (P = .7) in the bcl-2– group, respectively. The OS curves in Figure 3 show that, at median follow-up of 2 years, R-CHOP was significantly associated with a better OS than CHOP in bcl-2+ patients (67% ± 9% versus 48% ± 11%, P = .004), whereas in bcl-2– patients there was no statistically significant difference (72% ± 12% versus 67% ± 14%, P = .6). The 2-year EFS for bcl-2– patients was estimated to be 60% ± 12% for those treated with R-CHOP versus 40% ± 14% for those treated with CHOP (P = .13). Taken together, these results suggest that rituximab prevents bcl-2—associated chemotherapy failure in bcl-2+ patients, whereas the effect on the smaller group of bcl-2– patients was limited and did not result in statistically different OS and EFS.
OS according to bcl-2 protein expression and treatment. (A) bcl-2– patients. (B) bcl-2+ patients. Black lines indicate patients treated with CHOP and gray lines indicate patients treated with R-CHOP.
OS according to bcl-2 protein expression and treatment. (A) bcl-2– patients. (B) bcl-2+ patients. Black lines indicate patients treated with CHOP and gray lines indicate patients treated with R-CHOP.
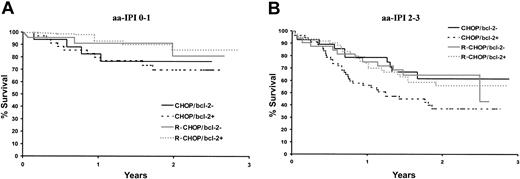
The OS curves according to aa-IPI factors in Figure 4 show that R-CHOP was more beneficial than CHOP whatever the severity of the disease. However, the LNH-98-5 trial was stratified on the basis of aa-IPI factors but not of bcl-2 protein expression. Therefore, the apparent effect of prognostic factors on outcome may have been due to sampling fluctuations with a limited number of bcl-2– patients in the treatment groups. To counteract this drawback, the impact of R-CHOP on patient outcome was evaluated here by multivariate analysis controlling for the effects of bcl-2 protein expression and aa-IPI. Interactions between bcl-2 staining and treatment were significant (OS P = .02, EFS P < .001) indicating that treatment efficacy varies according to the pattern of risk factors. Bcl-2 protein expression displayed a quantitative interaction with treatment that can be summarized by a gain in survival with R-CHOP, which was all the greater as the patient was bcl-2+. The multivariate analysis in Table 3 confirmed the independent prognostic impact of the aa-IPI (ie, 0-1 versus 2-3 factors). After adjustment for aa-IPI, R-CHOP was significantly more beneficial than CHOP in bcl-2+ patients as regards both OS and EFS, for example, the risk of death was nearly twice (RR = 1.84, P = .00015) when bcl-2+ patients were treated with CHOP and not significant (RR = 1.14, P = .7) for bcl-2– patients.
Relative risk estimates for CHOP versus R-CHOP according to aa-IPI score and bcl-2 expression
. | Relative risk . | CI (95%) . | P . |
|---|---|---|---|
| OS | |||
| aa-IPI 2-3 vs 0-1 | 2.75 | 1.7; 4.45 | .00002 |
| CHOP vs R-CHOP | |||
| bcl-2+ | 1.84 | 1.15; 2.93 | .00015 |
| bcl-2- | 1.14 | 0.41; 2.4 | .7 |
| EFS | |||
| aa IPI 2-3 vs 0-1 | 1.57 | 1.15; 2.22 | .009 |
| CHOP vs R-CHOP | |||
| bcl-2+ | 2.15 | 1.44; 3.21 | .00013 |
| bcl-2- | 1.5 | 0.84; 2.66 | .16 |
. | Relative risk . | CI (95%) . | P . |
|---|---|---|---|
| OS | |||
| aa-IPI 2-3 vs 0-1 | 2.75 | 1.7; 4.45 | .00002 |
| CHOP vs R-CHOP | |||
| bcl-2+ | 1.84 | 1.15; 2.93 | .00015 |
| bcl-2- | 1.14 | 0.41; 2.4 | .7 |
| EFS | |||
| aa IPI 2-3 vs 0-1 | 1.57 | 1.15; 2.22 | .009 |
| CHOP vs R-CHOP | |||
| bcl-2+ | 2.15 | 1.44; 3.21 | .00013 |
| bcl-2- | 1.5 | 0.84; 2.66 | .16 |
Discussion
Until recently, treatment of DLBCL was based on chemotherapy with the CHOP regimen. Several attempts were made to improve its efficacy mostly by using more dose-intensive regimens.3,7,19 However, such regimens are associated with increased toxicity and are not possible for the elderly. The probability of being cured by the initial treatment depends in first approach on the presence or absence of adverse prognostic factors such as those included in the IPI, and the general feeling among clinicians was that in the absence of new drugs, further significant progress could not be made in the treatment of DLBCL.5 Rituximab alone is active in most B-cell lymphomas and the authors of several phase 2 studies indicated that its combination with chemotherapy produced an additive effect.20 This was clearly demonstrated by a significant improvement in the survival and response rates in the early report of the randomized trial of CHOP versus R-CHOP in elderly patients with DLBCL.15 Because it constitutes one of the main advances over the past 20 years, several questions arise concerning the spectrum of R-CHOP efficacy in different situations. Further analyses of the trial just cited show that there was still a significant difference between the outcome for patients with adverse IPI prognostic factors and those without and analyses of the effect of age show that the same difference applied to the youngest patients in the trial, aged 60 to 65 years.
In the present study, we compared the efficacy of rituximab combined with CHOP with that of CHOP alone in elderly patients with DLBCL according to the overexpression of the antiapoptotic bcl-2 protein. Our aim was to establish, in vivo, whether or not rituximab reduces bcl-2—associated treatment failure caused by deficient apoptosis. In our analysis of the results of the LNH-98-5 trial, bcl-2 protein was overexpressed in 66% of patients, which is higher than the 45% found on the results of the LNH-87 trial.12 This higher rate of bcl-2 overexpression in DLBCL is likely to be due to the use of appropriate antigen retrieval by microwave pretreatment, which is known to enhance immunoreactivity of a number of antibodies, and appears similar to that found in other recent reports using similar antigen retrieval procedure. In the present study, tumors were considered positive when at least 50% of tumor cells expressed bcl-2 protein. Although a similar cut-off was used in other studies,8,12 some authors quantified the number of T cells or used a lower cut-off.21, 22, 23 A comparative sensitivity analysis by various bcl-2 staining methods might be useful to confirm our findings, but due to the retrospective design of our study, we cannot provide such an analysis here. In the present study, we confirmed the previously reported adverse prognostic value of bcl-2 protein expression on survival in patients on chemotherapy without rituximab.8,12,14
In the present study, bcl-2+ patients had higher response rates and improved survival when treated with R-CHOP rather than CHOP alone. Data concerning patients' clinical presentation and outcomes indicate that the 292 patients bcl-2 study population was representative of the 399 patients population entered on the LNH-98-5 trial, and therefore the probability of a selection bias seems low. In a retrospective analysis of a smaller sample of 78 patients, Wilson and colleagues reported a significant improvement in the DFS of bcl-2+ patients treated by the addition of rituximab to EPOCH (etoposide, prednisone, Oncovin [vincristine], cyclophosphamide, doxorubicine) chemotherapy.24 In our study, the median follow-up is 2 years and therefore patient follow-up is ongoing to confirm our findings. However, the particular pattern of aggressive NHL in which relapses happen early in the first 2 years and late death is attributed to age-associated illness makes sensitivity to variation in follow-up rather low.6 Bcl-2– patients in the present study did not show the same magnitude of the beneficial effect of rituximab as bcl-2+ patients although their number representing 34% of the study population cannot exclude a benefit of R-CHOP.
The mechanisms by which rituximab induces antitumor activity are not fully understood.25 Like other monoclonal antibodies, it has been shown to exert antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity, to inhibit cell proliferation, and more recently to induce apoptosis.26, 27, 28, 29 Different mechanisms by which bcl-2 suppresses apoptosis have been reported. Among them are the ability to act as an antioxidant, block caspase activity, and regulate Ca++ flux.30, 31, 32, 33, 34 In addition, interleukin 10 (IL-10) is a known promoter of bcl-2 expression in hematopoietic cells, and in vitro studies have shown that the rituximab induces down-regulation of IL-10 expression and consequently of bcl-2 protein expression.35,36 Taken together, these features common to bcl-2 function, chemotherapeutic drugs, and CD20 signaling suggest mechanisms that might be involved in the reversal of resistance to antitumoral drugs.29,36 Thus, through the rituximab-mediated depletion of bcl-2, the block of apoptosis by DNA-damaging drugs may be circumvented. Mechanisms other than bcl-2 resistance to apoptosis might be involved in resistance to chemotherapy by bcl-2– patients.25
In conclusion, in elderly patients with newly diagnosed DLBCL, we confirmed in this study the prognostic value of bcl-2 overexpression and demonstrated that this prognostic factor may be overcome by adding rituximab to standard CHOP chemotherapy. A future study evaluating the molecular characteristics of bcl-2 might provide further information regarding this observation.
Appendix
LNH-98-5 trial pathologic review committee: J. Brière, P. Gaulard, J. Bosq, J. F. Emile, B. Fabiani, and T. Petrella; statistics: E. Lepage and N. Nio; pharmacist: I. Madelaine.
The following clinicians actively participated in the LNH-98-5 trial: B. Coiffier, G. Salles, R. Herbrecht, H. Tilly, P. Solal, Celigny, R. Bouabdallah, P. Lederlin, C. Sebban, J. N. Munck, C. Fermé, P. Morel, F. Reyes, C. Haioun, M. Blanc, B. Christian, B. Quesnel, A. van Hoof, C. Gisselbrecht, M. Attal, D. Bordessoule, A. Bosly, M. Macro, G. Marit, J. Gabarre, S. Castaigne, E. Jourdan, E. Lepeu, B. Audhuy, A. Thyss, N. Albin, E. Baumelou, A. Delmer, S. Lefort, H. Orfeuvre, I. Plantier, G. Tertian, B. Varet, F. Boué, O. Casasnovas, D. Caillot, E. Van Den Neste, D. Decaudin, P. Brice, H. Dombret, J. P. Fermand, J. M. Zini, G. Fillet, M. Flesch, Y. Kerneis, C. Martin, P. Y. Péaud, P. Rodon, C. Rose, P. Travade, B. Velay, F. Bauduer, K. Bouabdallah, J. C. Eisenmann, C.Kulekci, S. Lampertz, G. Nedellec, A. M. Peny, V. Pulik, B. Salles, X. Vallantin, P. Agranat, J. P. Cassuto, J. Collignon, B. de Prijck, A. Delannoy, A. Devidas, J. F. Dor, F. Dreyfus, C. Dubois, G. Dupont, M. Fabbro, C. Fruchart, J.P. Gaillard, B. Grosbois, M. Janvier, N. Ketterer, C. Leroy, M. Maerevoet, F. Marechal, P. Mineur, P. Pierre, G. Sebahoun, C. Soussain, and C. Traulle.
The following pathologists actively participated in the LNH-98-5 trial: R. Angonin, I. Abd Alsamad, A. C. Baglin, F. Berger, N. Brousse, J.-P. Broulland, P. Brousset, D. Canioni, O. Casiraghi, D. Cazals-Hatem, A. M. Chesneau, F. Charlotte, M.-P. Chenard-Neu, C. Copie-Bergman, M.-C. Copin, M. Delos, C. Duval, G. Delsol, J. Diebold, C. Fromentin, F. Galateau, B. Gasser, B. Gosselin, C. Guettier, J. Hamels, N. Horschowski, R. Jeandel, J. P. Knopf, E. Labouyrie, C. Legendre, A. Letourneau, A. de Mascarel, V. Meignin, T. Molina, M. Parrens, B. Petit, M. Raphaèl, M. C. Raymond-Gelle, P. Straub, Y. Theate, S. Thiebaut, M. Tulliez, and L. Xerri.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-11-3442.
Supported in part by research funding from Hoffmann-LaRoche to the GELA.
A complete list of the members of the GELA appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Mathilde Dreyfus for English editing and Sylvie Corre for secretarial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal