Abstract
Venous thromboembolic events (VTEs) in children are associated with central venous lines (CVLs). The study objective was to assess whether CVL location and insertion technique are associated with the incidence of VTE in children. We hypothesized that VTE would be more frequent with (1) CVL location on the left body side, (2) CVL location in the subclavian vein rather than the jugular vein, and (3) CVL insertion by percutaneous technique rather than venous cut-down. This was a prospective, multicenter cohort study in children with acute lymphoblastic leukemia who had a CVL placed in the upper venous system during induction chemotherapy. Characteristics of CVL were documented prospectively. All children had outcome assessment for VTE by objective radiographic tests, including bilateral venography, ultrasound, echocardiography, and cranial magnetic resonance imaging. Among 85 children, 29 (34%) had VTE; 28 VTEs appeared in the upper venous system, and 1 was sinovenous thrombosis. Left-sided CVL (odds ratio [OR], 2.5; 95% confidence interval, 1.0-6.4; P = .048), subclavian CVL (OR, 3.1; 95% CI, 1.2-8.5; P = .025), and percutaneous CVL insertion (OR, 3.5; 95% CI, 1.3-9.2; P = .011) were associated with an increased incidence of VTE. Interaction occurred between CVL vein location and insertion technique. Subclavian vein CVL inserted percutaneously had an increased incidence (54%) of VTE compared with any other combination (P = .07). For CVL in the upper venous system, CVL placement on the right side and in the jugular vein may reduce the risk for CVL-related VTE. If subclavian vein placement is necessary, CVL insertion by venous cut-down appears preferable over percutaneous insertion.
Introduction
Venous thromboembolic events (VTEs) in children occur predominantly as secondary complications of severe underlying diseases, such as cancer, congenital heart disease, prematurity, infection, and others.1, 2, 3, 4 The major risk factor, accounting for more than two thirds of VTE in children, is the presence of a central venous line (CVL) frequently required for the treatment of primary disease.5,6 VTEs in children predominantly affect the upper body venous system, reflecting the preferred location of CVL placement. Pathogenic mechanisms of CVL-related VTEs include the intravascular presence of a foreign surface, obstruction of venous flow, trauma to the venous wall at CVL insertion, and endothelial irritation by the line or by the infusate.7, 8, 9 A biologic rationale suggests that the location and the insertion technique of the CVL are associated with different levels of obstruction of flow and venous trauma. Therefore, we hypothesized that CVL location and insertion technique would be associated with the risk for VTE. We hypothesized that VTEs would be more frequent with CVL location on the left body side than the right and with CVL location in the subclavian vein rather than the jugular vein. Based on the anatomy of the upper venous system (Figure 1), venous access is more difficult and there is an increased potential for obstruction to flow for a CVL located on the left side and in the subclavian vein. We also hypothesized that, in the subclavian vein, VTE would be more frequent with percutaneous CVL insertion than with surgical venous cut-down because the former may be associated with an increased risk for venous trauma.
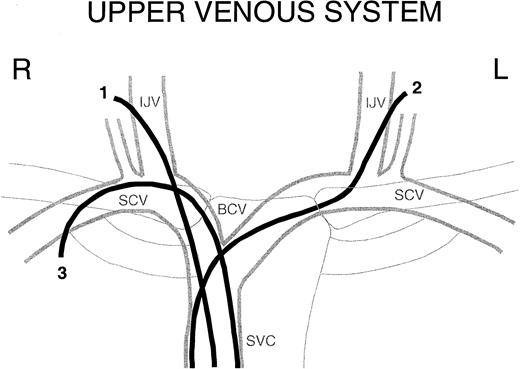
Upper venous system. Schematic of the central upper venous system with examples of a right internal jugular vein CVL (1), a left internal jugular vein CVL (2), and a right subclavian CVL (3). R indicates right; L, left; IJV, internal jugular vein; SCV, subclavian vein; BCV, brachiocephalic vein; and SVC, superior vena cava.
Upper venous system. Schematic of the central upper venous system with examples of a right internal jugular vein CVL (1), a left internal jugular vein CVL (2), and a right subclavian CVL (3). R indicates right; L, left; IJV, internal jugular vein; SCV, subclavian vein; BCV, brachiocephalic vein; and SVC, superior vena cava.
Few studies have provided data on the association between CVL body side,8,10, 11, 12, 13, 14, 15 CVL vein location,14,16, 17, 18, 19, 20 or CVL insertion technique15,21 with the incidence of VTE. Results from these studies are inconsistent or even contradictory, probably because of differences in study design, selection of study populations, and outcome assessment. Particularly, outcome assessment differed between studies that variably used clinical endpoints, ultrasound, or venography to detect VTE. Good evidence indicates that CVL-related VTEs frequently remain clinically undetected; therefore, clinical endpoints alone are inappropriate for such a study.12,14,22 Recent evidence also shows that ultrasound is insensitive for VTE in the subclavian vein and that venography is insensitive for VTE in the jugular vein.23,24 Hence, when screening for VTE in the 2 locations, both venography and ultrasound are required.
To date, no study has prospectively evaluated the association of CVL side, CVL vein location, and CVL insertion technique with the incidence of VTE using ultrasound and venography. The Prophylactic Antithrombin Replacement in Kids with ALL treated with Asparaginase (PARKAA) study was a multicenter clinical trial evaluating the incidence of VTE in children with acute lymphoblastic leukemia (ALL) during treatment with asparaginase. By inclusion criteria, each patient had a CVL placed in the upper venous system, and for outcome assessment, each patient underwent screening for VTE by a panel of objective radiographic tests. The PAARKA study presented a unique opportunity to perform a substudy designed to provide data on the association of CVL location and CVL insertion technique with the risk for VTE. Because these factors are controllable, information from this study could potentially aid in reducing CVL-related thrombotic complications.
Patients, materials, and methods
Study design
The design was a prospective cohort study executed in 9 centers in North America. The study itself was a substudy of PARKAA, which was an open-label, randomized, extended phase 2 study designed to determine the incidence of VTE in children with ALL who received asparaginase as part of their induction chemotherapy and to explore the potential of antithrombin concentrates in preventing VTE in these children. Results from the PARKAA study are reported elsewhere.25,26 The primary objective of the study presented here was to determine whether there is an association between CVL location and CVL insertion technique with the incidence of VTE. Central venous line–related variables were collected prospectively according to a standardized case report form. For outcome assessment, all patients were screened for VTE using a panel of objective radiographic tests after 4 weeks of induction chemotherapy.
Patient population
Children with ALL were enrolled between July 1997 and May 1999. The study was performed in 9 pediatric tertiary care centers in Canada (Calgary, Edmonton, London, Ottawa, Vancouver) and the United States (Atlanta, Houston, Palo Alto, Syracuse). Patients were eligible for the study if they were between 6 months and 18 years of age, were receiving induction chemotherapy including Escherichia coli asparaginase, and had a functioning indwelling CVL placed within 2 weeks of the initiation of chemotherapy. Patients were excluded if any of the following conditions existed: previously administered asparaginase, had known hypersensitivity to any of the ingredients in the antithrombin concentrate, had another medical condition that could interfere with participation in the study, administered other investigational drugs within 30 days of enrollment, or required therapeutic anticoagulation.
Patients were classified as having high-risk ALL if they were 10 to 18 years of age, had white blood counts higher than 50 × 109/L, or had testicular leukemia or central nervous system leukemia. Standard-risk ALL was defined by the absence of these criteria. The institutional review boards of all participating centers reviewed the study protocol, and informed consent was obtained from all patients' guardians and from children of appropriate age.
Central venous line characteristics
Central venous line placement was performed according to local standard of care at study centers. The body side and vein location of the CVL were the choice of the attending physician. Central venous lines were externalized, tunneled silastic catheters (Broviac or Hickman) or subcutaneously implanted port systems. Catheter sizes were chosen appropriate for age (6- to 10-French diameter). All CVLs were inserted under general anesthesia in the operating room. All CVLs were placed in the upper venous system, either in the subclavian vein by infraclavicular approach or in the jugular vein. Catheters were inserted either by percutaneous technique or by surgical venous cut-down. Percutaneous CVL insertion was achieved through the use of anatomic landmarks or ultrasound guidance. The vein was accessed by the Seldinger technique and a guidewire passed into the vein; after skin incision, the CVL was threaded into the vessel.27 For venous cut-down, the vein was accessed by surgical preparation, and the CVL was inserted through direct venotomy. The CVL tip was confirmed radiographically to lie in the superior vena cava or the right atrium. Finally, the CVL was tunneled through the subcutaneous tissue for some distance, where it was externalized or the subcutaneous port was placed. Minimum platelet counts of 50 × 109/L were generally required for CVL insertion, but the decision whether to give platelet transfusion was left to the investigator. Central venous line characteristics were documented prospectively using a standardized case report form. These data included the body side of CVL location (right, left), the vein used for CVL access (subclavian, jugular vein), location of the CVL tip (superior vena cava, right atrium), and CVL insertion technique (percutaneous, venous cut-down).
As per study protocol, patients did not receive therapeutic doses of heparin or warfarin. Patients did receive small amounts of unfractionated heparin for prophylaxis of CVL blockage either by continuous infusion (1-3 U/mL) or intermittent flushes (50-100 U/mL up to 4 times per day) according to local standard of care.
Laboratory prothrombotic markers tested in PARKAA included the factor V Leiden, the prothrombin gene Gly20210Ala mutation, plasma antithrombin levels, and antiphospholipid antibodies (lupus anticoagulant and anticardiolipin antibodies immunoglobulin G [IgG] and IgM). Details of the assays applied have been described in the primary study report.25
Outcome assessment
The primary study outcome was a VTE in any location manifesting with clinical symptoms, or it was an asymptomatic VTE identified at exit screening. During the study period, patients were closely monitored for clinical symptoms of VTE. No definitions were stipulated for clinical presentation of VTE, which was left to the judgment of the attending physician. There was one exception to this rule: if there was loss of CVL patency and local thrombolysis was unable to restore patency, or the decision was made to remove the CVL, objective testing for VTE had to be performed. Clinically suspected VTE was confirmed by objective radiographic test consisting of color Doppler ultrasound, bilateral venography of the upper venous system (conventional or magnetic resonance venography), echocardiography, and magnetic resonance imaging of the head. Bilateral venography was performed to prevent the washout of contrast media from the contralateral brachiocephalic vein. Patients who did not have symptoms of VTE were screened for asymptomatic VTE with each of the 4 radiographic tests after completing 4 weeks of induction chemotherapy.
Protocols for the performance and interpretation of radiographic tests were defined a priori. An independent Central Adjudication Committee evaluated and interpreted all radiographic tests by reviewing radiographic films and video documentation. The committee consisted of 2 physicians with appropriate expertise who were blinded to patient identity and treatment allocation and who had no other involvement with the study patients.
Statistics
Study patients were included in the primary analysis on a per-protocol basis. Reasons for exclusion were no exit venogram and premature withdrawal from the study for any reason other than VTE. Frequencies of CVL characteristics were analyzed in relation to frequencies of patients with or without VTE using 2 × 2 contingency tables. Associations between CVL characteristics and the occurrence of VTE were analyzed using χ2 analysis or Fisher exact test. In addition, the exact odds ratio (OR) and 95% confidence interval (95% CI) were calculated. Multivariable analyses to test for several factors simultaneously (CVL body side, vein location, insertion type, study center) and their interactions in association with the absolute risk for VTE were performed. A generalized linear model with an identity link and underlying binomial structure was fit using the GMBO module of the Epicure 2.10 software. All tests were 2-sided.
Results
Patient population
One hundred nine patients were enrolled in the PARKAA study and were randomized in a 1:2 ratio to antithrombin or no antithrombin treatment. Twenty-four patients were excluded because of premature withdrawal from the study (n = 9) or missing or inadequate exit venography (n = 15). Eighty-five (78%) patients satisfied the criteria for the per-protocol analysis. Demographic information for the patients included in the study is provided in Table 1. The median number of patients per study center was 7, and the range was 2 to 16. The 24 excluded patients did not differ from the study cohort with respect to age, height, weight, sex, and ALL risk category.
Patient demographics
Demographic variable . | No thrombosis . | Thrombosis . |
|---|---|---|
| No. patients | 56 | 29 |
| Age, y (range) | 4.8 (1.6-16.6) | 7.3 (1.9-17.2) |
| Height, cm (range) | 106 (82-177) | 127 (85-183) |
| Weight, kg (range) | 19 (10-83) | 23 (11-71) |
| Female sex (%) | 22 (39) | 16 (55) |
| Race (%) | ||
| White | 40 (71) | 23 (79) |
| Black | 5 (9) | 2 (7) |
| Asian | 2 (4) | 2 (3) |
| Other | 9 (16) | 3 (11) |
| ALL risk category, high (%) | 20 (36) | 13 (45) |
| Platelet counts, × 109/L (range) | ||
| Day 1 | 61 (7-507) | 82 (12-372) |
| Day 15 | 102 (5-438) | 104 (20-476) |
| Day 28 | 295 (24-740) | 245 (101-543) |
Demographic variable . | No thrombosis . | Thrombosis . |
|---|---|---|
| No. patients | 56 | 29 |
| Age, y (range) | 4.8 (1.6-16.6) | 7.3 (1.9-17.2) |
| Height, cm (range) | 106 (82-177) | 127 (85-183) |
| Weight, kg (range) | 19 (10-83) | 23 (11-71) |
| Female sex (%) | 22 (39) | 16 (55) |
| Race (%) | ||
| White | 40 (71) | 23 (79) |
| Black | 5 (9) | 2 (7) |
| Asian | 2 (4) | 2 (3) |
| Other | 9 (16) | 3 (11) |
| ALL risk category, high (%) | 20 (36) | 13 (45) |
| Platelet counts, × 109/L (range) | ||
| Day 1 | 61 (7-507) | 82 (12-372) |
| Day 15 | 102 (5-438) | 104 (20-476) |
| Day 28 | 295 (24-740) | 245 (101-543) |
None of the variables listed were significantly associated with the incidence of VTE as tested by logistic regression.
Central venous line characteristics
Central venous lines were located on the left side of the body in 42 (49%) patients and on the right side in 43 (51%) patients. One patient had a left-sided subclavian CVL replaced by a right-sided subclavian CVL on study day 10 because of CVL infection. All other patients had only one CVL during the study period. Catheters were located in the subclavian veins in 50 (59%) patients and in the jugular veins in 35 (41%) patients—26 (30%) in the external jugular vein and 9 (11%) in the internal jugular vein. Tips of CVL were located in the superior vena cava in 45 (53%) patients, in the right atrium in 31 (36%) patients, and other locations in 9 (11%) patients. Insertion of CVL was by percutaneous technique in 45 (53%) patients and by venous cut-down in 40 (47%) patients. These CVL attributes did not differ in relation to patient characteristics. One exception to this was that female patients more frequently (56%) had subclavian than jugular CVL (29%; P = .012), which might have been for cosmetic reasons because subclavian CVL are less visible beneath the clothing. There was considerable variation in CVL characteristics across study centers, with left-sided CVL location ranging from 14% to 100% and subclavian CVL location and percutaneous CVL insertion varying from 0% to 100%.
Incidence and location of thrombosis
Venous thromboembolic events occurred in 29 of 85 (34%) patients. Four of 29 (14%) patients had clinical symptoms leading to the diagnosis of VTE. Symptoms included limb swelling, pain, subcutaneous collateral veins, headache, and eye movement abnormalities. The remaining 25 patients were clinically asymptomatic, and VTEs were identified through radiographic exit screening.
Twenty-eight of 29 (97%) children with VTE had VTE in the central upper venous system, and 1 patient had sinovenous thrombosis in combination with internal jugular vein thrombosis. The subclavian vein was the most frequently (90%) affected vein, with approximately half these thrombi extending into more centrally located venous segments. Three thrombi extended into the right atrium. Twenty-one (72%) VTEs were located on the left side. Venous occlusion was classified as 100% in 3 (13%), 75% to 100% in 9 (39%), 50% to 75% in 3 (13%), and 25% to 50% in 5 (22%) of the 23 VTEs detected by venography. Collateral veins were present in 14 (61%) patients and were graded as major in 9 (39%) and minor in 5 (22%) VTEs identified by venography. Severe venous occlusion (more than 75%) was significantly associated with the presence of collaterals (P = .04).
There were no significant differences in the incidence of VTE in relation to age, height, weight, sex, ALL risk category, and study center as tested by logistic regression (Table 1). Comparing patients with symptomatic VTE and patients with asymptomatic VTE, median age was 10.7 years (range, 2.0-16.2 years) and 6.5 years (range, 1.9-17.2 years), height was 141 cm (range, 88-166 cm) and 120 cm (range, 85-183 cm), weight was 43 kg (range, 15-49 kg) and 22 kg (range, 11-71 kg), and 3 of 4 (75%) and 13 of 25 (52%) were girls, respectively. There were no significant differences in patient demographics between patients with symptomatic and asymptomatic VTE.
Association of CVL characteristics with thrombosis
There was a close relationship between CVL location and VTE location. Almost all VTEs were located on the same side as the CVL, but in 2 patients CVL was located on the right side and VTE was located on the left. The patient with an initial left-sided CVL replaced by a right-sided CVL was found on exit venography to have bilateral thrombotic occlusion. Twenty-four (83%) VTEs were located in the venous segment, where the CVL accessed the venous system. The patient with sinovenous thrombosis had a jugular vein CVL located on the same side. In 5 patients, VTE was located not at the CVL insertion site but more centrally, along the course of the CVL.
Association of CVL body side with thrombosis
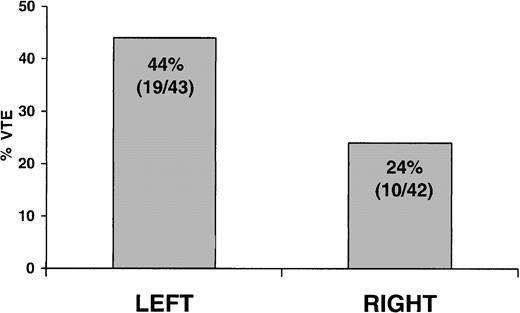
Patients with CVL on the left side of the body had a 44% (19/43) incidence of VTE compared with a 24% (10 of 42) incidence in patients with CVL on the right side (OR, 2.5; 95% CI, 1.0-6.4; P = .048) (Figure 2). In the patient with a second CVL, only the right-sided CVL present at the time of exit screening was included in the analysis. There was no association between CVL side and severity of thrombotic occlusion.
Association of CVL body side with thrombosis. Incidence of VTE in patients with CVL on the left compared with the right side of the body (OR, 2.5; 95% CI 1.0-6.4; P = .048).
Association of CVL body side with thrombosis. Incidence of VTE in patients with CVL on the left compared with the right side of the body (OR, 2.5; 95% CI 1.0-6.4; P = .048).
Association of CVL vein location with thrombosis
Patients with CVL in the subclavian vein had a 44% (22 of 50) incidence of VTE compared with a 20% (7 of 35) incidence in patients with jugular vein CVL (OR, 3.1; 95% CI, 1.2-8.5; P = .025). There was no significant difference in the incidence of VTE between CVL located in the external or internal jugular vein. Among patients with subclavian CVL, a trend was seen for more severe thrombotic occlusion (50%, 11 of 22 with more 75% occlusion) compared with patients with jugular CVL (14%, 1 of 7; P = .095). The location of CVL tips did not show an association with the incidence of VTE.
Association of CVL insertion technique with thrombosis
Patients with percutaneously inserted CVLs had a 47% (21 of 45) incidence of VTE compared with 20% (8 of 40) VTEs with CVLs inserted by venous cut-down (OR, 3.5; 95% CI, 1.3-9.2; P = .011). No association was observed between CVL insertion technique and severity of thrombotic occlusion.
Association of CVL location and CVL insertion technique with thrombosis
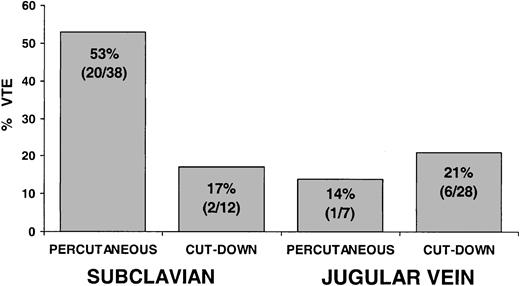
Analyzing the combined effect of CVL vein location and CVL insertion technique on the incidence of VTE by multivariable analysis revealed a strong interaction between the 2 factors on an additive linear scale (P = .07) (Figure 3). Among patients whose CVLs were inserted percutaneously in the subclavian vein, 53% (20 of 38) had VTEs compared with 17% (2 of 12) who had VTEs with subclavian CVLs inserted by venous cut-down, 14% (1 of 7) had VTEs with percutaneously inserted jugular CVLs, and 21% (6 of 28) had VTEs with jugular CVLs inserted by cut-down. These results suggest that subclavian CVL inserted percutaneously was associated with an increased risk for VTE compared with any other combination of location and insertion technique.
Association of CVL vein location and CVL insertion technique with thrombosis. The combination of CVL located in the subclavian vein and inserted percutaneously was associated with an increased incidence of VTE compared with any other combinations (test for interaction, P = .07).
Association of CVL vein location and CVL insertion technique with thrombosis. The combination of CVL located in the subclavian vein and inserted percutaneously was associated with an increased incidence of VTE compared with any other combinations (test for interaction, P = .07).
There was no interaction of CVL body side with either CVL vein location or CVL insertion technique or with both in association with VTE. Similarly, there was no interaction of patient characteristics with CVL side, vein location, or insertion technique in association with VTE.
Other potential risk factors for thrombosis
Platelet counts at study entry, day 15, and study end are summarized in Table 1. There was no association between platelet count and the incidence of VTE (P = .87). The factor V Leiden mutation was present in 4 of 77 (5%) patients, 2 of whom had asymptomatic VTE (P = .29). The prothrombin gene Gly20210Ala mutation was present in 1 of 74 (1%) patients who did not have VTE, and no patient had antithrombin deficiency. Low-titer antiphospholipid antibodies were transiently positive in 3 of 85 (4%) patients, 2 of whom had asymptomatic VTE (P = .23).25 No interaction occurred between antithrombin treatment group and CVL characteristics in association with VTE.26
Discussion
Venous thrombosis is a serious secondary complication in children and is frequently associated with the use of CVLs. However, CVLs are essential for the successful treatment of children with life-threatening diseases. Therefore, the identification of risk factors for CVL-related VTE is important, particularly factors that can be modified without compromising clinical care. The present study, designed to identify such factors, was a prospective cohort study of children in whom CVLs were placed in the upper venous system and who were screened for VTE using objective radiographic tests. Study results show that CVLs on the left body side, in the subclavian vein, or inserted by percutaneous technique are associated with an increased risk for VTE. An increased risk for VTE was observed with subclavian CVL inserted percutaneously compared with any other combination of location and insertion technique.
The rationale for studying CVL location in relation to the risk for VTE is based on the anatomy of the upper body venous system (Figure 1). In comparison with the right side, the left brachiocephalic vein is longer and has a more horizontal course, leading to a sharper angle into the superior vena cava. A CVL located in the right jugular vein represents the shortest and most direct access to the heart. In contrast, a CVL in the left jugular vein has a greater distance to the heart and passes 2 angles in the venous system, increasing the potential for flow obstruction and venous wall adherence and causing endothelial damage. Compared with jugular CVL, subclavian CVLs follow an even sharper curve into the central venous system, resulting in wall adherence.16 The CVL enters where the vein passes between the clavicle and the first rib, which may cause vein compression and kinking of the CVL.
Inconsistent information has been published on the association of CVL body side with VTE. Four studies report left-sided CVL to be associated with an increased incidence for VTE.10, 11, 12, 13 However, a similar number of studies report no significant influence of CVL side on VTE.8,14,15,20 It is unclear whether interactions from other CVL-related factors could have influenced these different findings. In the present study, left-sided CVL was associated with an increased frequency for VTE independent of CVL vein location and insertion technique.
Several studies in the literature directly compare CVL location in the subclavian and jugular veins, but findings were contradictory. Two studies in adult patients report incidences of VTE in 42% to 50% of patients with subclavian CVL compared with 0% to 10% with jugular vein CVL.16,17 In contrast, Timsit et al14 observed 42% VTE with jugular CVL compared to 10% VTE with subclavian CVL. These discrepancies may be related to differing outcome assessments, which were by venography in the first 2 studies and by ultrasound in the latter study. Venography is not sensitive for the detection of VTE in the jugular veins, and ultrasound is not sensitive for VTE in the subclavian veins.23,24 The present study, which demonstrates a significantly increased incidence of VTE with subclavian (44%) compared with jugular (20%) CVL, provides the most objective information because venography and ultrasound were both used to screen for VTE.
In the present study, most (83%) VTEs were located close to the CVL entry site rather than the CVL tip. Moreover, there was no association between various CVL tip locations and the incidence of VTE. These findings support the concept that endothelial disruption at CVL insertion is an important risk factor for the development of VTE and may be more relevant than endothelial irritation at the CVL tip.
Insertion technique may be associated with the development of VTE because of the relative trauma to the venous wall and the perivascular tissue.7 A study on percutaneous CVL reports that the number of punctures required to correctly place CVL was signifi-cantly correlated with the incidence of VTE, providing indirect evidence that venous trauma influences the development of VTE.8 Two studies directly comparing percutaneous CVL insertion with venous cut-down in patients with cancer had contradictory results. One pediatric study observed CVL failure—that is, loss of patency—to be less likely with percutaneous CVL than with surgically inserted CVL.21 Loss of CVL patency, however, does not necessarily correlate with large vessel thrombosis. In contrast, a recent study in adults observed an increased frequency of VTE with CVL inserted by radiologists—that is, percutaneously—than with CVL inserted by surgical cut-down.15
In the present study, percutaneous CVL insertion was the factor most strongly associated with an increased risk for VTE. It is important to emphasize that the increased risk for VTE was only seen with subclavian CVL inserted percutaneously compared with subclavian venous cut-down and jugular CVL inserted by either technique. Several factors may be responsible for this interaction. First, percutaneous CVL insertion into the subclavian vein is considered technically more difficult than into the jugular vein. Second, ultrasound guidance of CVL insertion into the subclavian vein is hampered by the presence of the clavicle. Third, because the subclavian vein takes a sharp curve at the site of CVL entry, endothelial damage at the opposite wall of the vein may occur when introducing the dilatator or catheter sheath. In contrast, the jugular vein is more easily accessible for puncture and ultrasound guidance, and, because of the vein's straight course, is less susceptible to trauma. Consistent with these considerations, we observed no significant difference between percutaneous CVL insertion and venous cut-down in the jugular vein.
A limitation of the present study was that the various modes of CVL placement were the choice of attending physicians and were not compared in a randomized fashion. Therefore, the results must be considered preliminary and must be confirmed in future randomized clinical trials. Other limitations of the study were that a number of CVL-related factors with potential influence on the incidence of VTE were not assessed, such as the experience of the physician inserting the CVL, the time required for CVL insertion and immediate complications, type of CVL, and the number of CVL lumen. Previous reports have suggested the operator's experience,28 CVL insertion time,15 and complications8 to be associated with VTE, though a recent well-designed study did not observe any of these associations.14 No association was reported between CVL type12,15,29 or number of CVL lumen8,14,16 and the incidence of VTE. In addition, the time of CVL insertion was not recorded, which restricted the ability to assess whether the duration of CVL placement was associated with VTE. Although the study period was fairly uniform, the duration of CVL placement might have varied from approximately 2 to 4 weeks because CVLs could be inserted up to 2 weeks after study entry. However, based on previous studies, most VTEs occur early, and the duration of CVL placement is not associated with the incidence of VTE.12,19 Finally, the present study was not designed to assess the effect of heparin prophylaxis on CVL-associated VTE because there was no standardization of dose and mode of heparin prophylaxis.
The issue of the risk for CVL-related VTE in relation to congenital prothrombotic markers is controversial.30,31 In the present study, factor V Leiden mutation was present in 4 (5%) patients, 2 of whom had asymptomatic VTE, the prothrombin gene Gly20210Ala mutation was present in 1 (1%) patient who did not have VTE, and no patient had antithrombin deficiency. Compared with the effect of CVL-related factors on the risk for VTE, congenital prothrombotic disorders had minor, if any, influence on the incidence of VTE. However, the present study was not powered to assess whether there was a statistically significant association between prothrombotic conditions and CVL-related VTE.
A definition for the clinical presentation of VTE was not stipulated because clinical symptoms are insensitive and nonspecific. This lack of definition might have influenced the proportion of symptomatic VTE among all VTEs detected in this study. However, outcome screening using multiple objective tests guaranteed that no VTEs were missed. Asymptomatic VTEs were included as outcomes because there is good evidence that most CVL-related VTEs remain clinically undetected.12,14,22 The development of CVL-related DVT is usually gradual, permitting collaterals to form, thereby minimizing typical symptoms of acute VTE. Subtle symptoms of DVT are frequently not recognized in children with severe underlying disease. The asymptomatic DVT observed in PAARKA were clinically significant—two thirds occluded more than 50% of the vessel and had collateral veins. Short-term complications of CVL-related DVT, symptomatic and asymptomatic, are pulmonary embolism,32,33 chylothorax,34 embolic stroke through intracardiac right-to-left shunting,35 CVL-related sepsis,36,37 and repeated loss of CVL patency requiring local thrombolytic therapy or CVL replacement.36 A limitation of the present study is that follow-up lasted only a few weeks; therefore, the long-term outcome of VTE is unknown. Reported long-term consequences of CVL-related DVT in children are postthrombotic syndrome, recurrent DVT, and loss of future venous access.6,38
In conclusion, the risk for CVL-related VTE was significantly increased with CVL on the left side, in the subclavian vein, and inserted percutaneously. Subclavian CVLs inserted percutaneously were associated with the highest risk for VTE. The study results suggest that CVL in the upper venous system should be placed on the right side and in the jugular vein to minimize the risk for CVL-related VTE. If subclavian vein placement is necessary, CVL insertion by venous cut-down appears preferable over the percutaneous approach.
Appendix
The investigators and institutions participating in the PAARKA study were: Ron Anderson, Alberta Children's Hospital, Calgary, AB, Canada; Sunil Desai, MacKenzie Health Sciences Centre, Edmonton, AB, Canada; Jacqueline Halton, Children's Hospital of Eastern Ontario, Ottawa, ON, Canada; Patricia McCusker, Children's Hospital of Western Ontario, London, ON, Canada; John Wu, BC Children's Hospital, Vancouver, BC, Canada; Thomas Abshire, Emory University School of Medicine, Atlanta, GA, USA; Irene Cherrick, University Hospital, Syracuse, NY, USA; Gary Dahl, Stanford University School of Medicine, Palo Alto, CA, USA; Bridget Freedman-Nord, Nemours Children's Clinic, Jacksonville, FL, USA; and Donald Mahoney, Texas Children's Hospital, Houston, TX, USA.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-09-2731.
Supported by grant PA14283 from the Canadian Institutes of Health Research and by Bayer Inc. C.M. is a scholar of the Austrian Science Fund. M.A. was a career scientist with the Heart and Stroke Foundation of Ontario. L.M. is a research scholar of the Canadian Institutes of Health Research.
The PAARKA Investigators are listed in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are all deeply saddened by the loss of our colleague and friend, Dr Maureen Andrew, who passed away suddenly on August 28, 2001. Dr Andrew was instrumental in the work reported here, and we respectfully dedicate this paper to her memory.
We thank the study coordinators Patsy Vegh, Monica Adams, Karen Bilynsky, Alex Blay, Tracy Corr, Elaine Dollard, Christine McDonald, Julie Nichols, Judy Powers, Chris Tremblay, and Hanna Zaire.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal