Abstract
L-selectin mediates leukocyte tethering and rolling, the first step in a sequential process of leukocyte adhesion and migration. Additionally, L-selectin has important signaling roles perhaps contributing to leukocyte activation and integrin-mediated adhesion. Because chemokines are critically involved in leukocyte activation, we questioned whether L-selectin signaling affects chemokine receptor expression and function. We observed that whereas only 5% to 15% of freshly isolated lymphocytes expressed CXCR4 on the cell surface, intracellular CXCR4 was detectable in all cells. Engagement of L-selectin by antibody cross-linking or the L-selectin ligands fucoidan or sulfatide mobilized intracellular CXCR4 to significantly increase surface CXCR4 expression but did not affect CCR5, CCR7, or β2-integrin expression. L-selectin stimulation also inhibited stromal-derived factor 1 (SDF-1)–induced CXCR4 internalization. The combined effects of L-selectin on CXCR4 trafficking are likely important in markedly enhancing cell activation by SDF-1. Blockade of SDF-1–induced CXCR4 internalization resulted in enhanced actin polymerization on subsequent exposure to SDF-1. Physiologically more important, L-selectin stimulation increased SDF-1–induced lymphocyte adhesion and transendothelial migration, which were inhibited by anti–leukocyte function-associated antigen 1 antibodies, tyrosine kinase inhibitors, and pertussis toxin. To further corroborate the additive stimulating effects, L-selectin signaling and SDF-1 increased β2-integrin activation. Taken together, L-selectin–mediated signals specifically enhance CXCR4 expression and function, suggesting a novel mechanism for the modulation of lymphocyte activation during cell adhesion and transmigration.
Introduction
Effective immune responses require the recruitment of distinct leukocyte subsets to appropriate tissue sites. This recruitment process is orchestrated by both chemokines and cell adhesion molecules.1, 2, 3 Chemokines provide a directional guide for leukocyte migration but are also active in the pathogenesis of HIV-1 infection, hematopoiesis, angiogenesis, embryonic development, and tumor metastasis.4, 5, 6, 7, 8 Stromal-derived factor 1 (SDF-1, CXCL12) is a potent chemotactic factor for a variety of cells including lymphocytes, monocytes, dendritic cells, and hematopoietic stem cells. SDF-1, binding to its specific receptor CXCR4, can activate integrins and initiate firm adhesion of rolling leukocytes.9,10 CXCR4 plays a pivotal role in lymphocyte homing11 and recruitment into inflammatory sites.12, 13, 14
Cell adhesion molecules participate in leukocyte recruitment by direct interaction with their respective ligands on endothelial cells. Recruitment of selective subtypes of leukocytes can be controlled by differential expression of adhesion molecules and chemokine receptors. Adhesion molecules are also capable of inducing intracellular signaling cascades related to leukocyte activation and migration. L-selectin engagement via antibody cross-linking or ligation with ligands such as GlyCAM-1 or sulfatide has been demonstrated to trigger calcium flux, tyrosine phosphorylation, mitogen-activated protein (MAP) kinase activation, and p21ras oncoprotein (Ras) activation.15, 16, 17 L-selectin cross-linking increased expression of a β2-integrin activation–dependent epitope18 and induced neutrophil adhesion and transmigration across interleukin 1 (IL-1)–stimulated human umbilical vein endothelial cell (HUVEC) monolayers.19
Experimental evidence suggests that L-selectin, in particular, may play an important role in leukocyte migration through its signaling properties.19 Human subjects with a defect in endothelial ligands for L-selectin exhibit a profound defect in neutrophil chemotaxis in vivo.20 In L-selectin knockout mice, leukocyte migration to the CXC chemokine KC is impaired.21 This important observation suggests that chemokines and adhesion molecules may function cooperatively in regulating leukocyte adhesion and migration. In exploring this possibility, we discovered that L-selectin stimulation of peripheral blood lymphocytes could modulate CXCR4 expression through receptor mobilization and inhibition of internalization, resulting in enhanced lymphocyte F-actin polymerization, integrin activation, adhesion, and transendothelial migration in response to SDF-1.
Materials and methods
Materials
Fucoidan, sulfatide, paraformaldehyde (PFA), collagenase type IV, bovine serum albumin (BSA) fraction V, herbimycin A, genistein, pertussis toxin (PTX), mouse IgG1, IgG2a, and fluorescein isothiocyanate (FITC)– and phycoerythrin (PE)–conjugated sheep antimouse antibodies were from Sigma (St Louis, MO). Recombinant human SDF-1 and RANTES were obtained from Peprotech (Rocky Hill, NJ). 125I-SDF-1 was purchased from Perkin Elmer Life Sciences (Boston, MA). Anti-L-selectin antibodies LAM1-14 and LAM1-3, binding to lectin domain and short concensus repeat of L-selectin molecules, respectively, were kind gifts from Dr Scott I. Simon (University of California, Davis). LAM1-116 recognizing lectin domain of L-selectin was from Dr Douglas A. Steeber (Duke University Medical Center, Durham, NC). MAb24 recognizing an activation-associated epitope in β2 integrin was a kind gift from Dr Nancy Hogg (Imperial Cancer Research Fund, London, United Kingdom). Anti-LFA-1β monoclonal antibody (mAb) 60.3 was kindly provided by Dr J. M. Harlan (University of Washington, Seattle). Anti-very late antigen 4 (VLA-4; HP2/1) and antibody to an activation epitope of β1 integrin (HUTS-21) were generous gifts from Dr F. Sanchez-Madrid (Universidad Autonoma de Madrid, Spain). Anti-CD45RA (G1-15) and anti-CD45RO (UCHL-1) were obtained from Immunotech (Westbrook, ME). Goat antimouse IgG was from Jackson Immunoresearch Laboratories (West Grove, PA). Anti-CXCR4 (12G5) anti-CCR5 (2D7), anti-CCR7 (2H4), and PE-conjugated anti-CXCR4 (12G5) were purchased from BD Pharmingen (Missisauga, ON, Canada). Dextran T500 and Ficoll-Paque were obtained from Pharmacia Biotech (Piscataway, NJ). NBD (N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl))–phallicidin and calcein-AM were from Molecular Probes (Eugene, OR).
PBMC isolation
Venous blood was collected from healthy, adult donors with 10 U/mL heparin. Erythrocytes were removed by dextran sedimentation at room temperature for 30 minutes. The leukocyte-rich plasma was collected, transferred to a Ficoll-Paque gradient (density 1.077 g/mL), and centrifuged at 1400 rpm for 20 minutes. Peripheral blood mononuclear cells (PBMCs) sitting at the interface between Ficoll and plasma were collected. To remove platelets, cells were washed 3 times with calcium- and magnesium-free phosphate-buffered saline (PBS) supplemented with 0.1% BSA.
Lymphocyte preparation
The isolated PBMCs were resuspended in RPMI 1640 with 10% human platelet-poor plasma (PPP) and applied to a nylon-wool column. After 60 minutes of incubation, the unbound leukocytes were eluted, washed, resuspended in fresh RPMI medium plus 10% PPP, and cultured overnight in tissue-culture flasks. Cells recovered the next morning contained more than 96% T cells, less than 4% B cells, and less than 1% monocytes by immunofluorescence staining and were more than 98% viable by trypan blue exclusion.
T lymphocytes were resuspended at 5 × 107 cells/mL in Hanks balanced salt solution (HBSS) plus 15 mM HEPES (N-2-hydroxyethylpiperazine-N′2-ethanesulfonic acid) plus 10% fetal bovine serum (FBS) and labeled by incubating with 50 μCi/mL (1.85 MBq)
Immunofluorescence staining and flow cytometric analysis
Cells were incubated with primary antibodies in PBS plus 0.2% BSA plus 0.1% sodium azide (NaN3) at 4°C for 30 minutes. Then cells were washed and incubated with PE-conjugated sheep antimouse antibody at 4°C for 30 minutes. The cells were finally washed and resuspended in 1% PFA. Flow cytometric data were collected using Epics XL Expo32 ADC software (Coulter, Hialeah, FL) or FACScan flow cytometry (Becton Dickinson, San Jose, CA). Lymphocytes and monocytes were differentiated by characteristic side and forward light scatter properties and confirmed by CD3 and CD14 staining. The threshold level was based on the maximum staining of a matched isotypic antibody with irrelevant specificity used in the same concentration. Isotype-control antibody bound to less than 1% of cells.
To detect both surface and intracellular CXCR4, cells were first fixed by resuspending in 1 mL 4% PFA at room temperature for 10 minutes, pelleted, and permeabilized with 0.05% saponin plus 0.05% NaN3. Cells were then pelleted and resuspended with 20 μg/mL mouse IgG2a in 0.05% saponin buffer containing 0.2% gelatin. PE-conjugated anti-CXCR4 (12G5) was added and cells were allowed to incubate at 4°C for 30 minutes. Finally, cells were washed and analyzed by flow cytometry.
To monitor changes in the cell surface expression of CXCR4 after SDF-1 treatment, cells were incubated with 500 ng/mL SDF-1 at 37°C for 60 minutes. This concentration was higher than that we used for lymphocyte transendothelial migration assay22 but lower than 1 to 2 μg/mL that has been used in previous reports for signal transduction and receptor internalization experiments.23, 24, 25, 26 Cells treated with SDF-1 were diluted and washed with ice-cold washing buffer (PBS plus 0.2% BSA plus 0.1% NaN3). CXCR4 expression was examined as described. To exclude the possibility that SDF-1 may hinder CXCR4 antibody binding, we used acidic glycine buffer (50 mM glycine, 100 mM NaCl, pH 2.5) to briefly treat cells and remove SDF-1 bound to the cell surface before CXCR4 staining.27 No significant differences in anti-CXCR4 binding were observed after washing with PBS or acidic glycine buffer. Therefore, only PBS washing buffer was used in subsequent experiments. To investigate the effect of L-selectin engagement on CXCR4 expression, cells were pretreated with anti-L-selectin mAb on ice and washed. Then the primary antibodies were cross-linked by goat antimouse antibody at 37°C. After stimulation, cells were washed and redundant antibody-binding sites were blocked with 50 μg/mL mouse IgG before CXCR4 expression was examined by PE-conjugated anti-CXCR4 mAb.
Measurement of CXCR4 internalization
Cell surface CXCR4 expression is the net result of several processes including receptor internalization, recycling, and re-expression. To examine if fucoidan and sulfatide modify CXCR4 internalization, 2 different methods were used with Jurkat cell line (T lymphoma), which consistently expresses a high level of surface CXCR4. First, cell surface CXCR4 was labeled with anti-CXCR4 mAb at 5 μg/mL. Then cells were treated with SDF-1 at 500 ng/mL in the presence or absence of the L-selectin ligands fucoidan or sulfatide. Recycled CXCR4 and CXCR4 freshly mobilized from intracellular stores to the cell surface will not have bound any anti-CXCR4 antibody. Therefore, PE-conjugated sheep antimouse antibody will only detect remaining CXCR4 originally expressed on the cell surface. Second, as a more direct measure, Jurkat cells at 107/mL in RPMI with 10 mM HEPES and 1% BSA were labeled with 125I-SDF-1 (0.19 nM) on ice for 40 minutes. The cells were washed; then the internalization of surface bound 125I-SDF-1 was induced by incubating at 37°C for 30 minutes. Finally, 125I-SDF-1 remaining at the cell surface was removed by incubation with acidic glycine buffer at 4°C for 5 minutes. The acid-resistant radioactivity, representing intracellular 125I-SDF-1, was measured in a gamma counter.
Endothelial cell culture
HUVECs were isolated by collagenase digestion as described by Jaffe et al.28 Briefly, human umbilical veins were flushed with Ringer lactate, then incubated with 0.5 mg/mL collagenase type II (Sigma Chemical) at 37°C for 30 minutes. Detached endothelial cells were collected, washed, and then cultured in gelatin-coated flasks (Nunc, Naperville, IL) in RPMI 1640 medium (Sigma) containing 20% FBS (Hyclone Laboratories, Logan, UT), 25 μg/mL endothelial cell growth supplement (Collaborative Research, BD Biosciences, Bedford, MA), 45 μg/mL heparin, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Cell adhesion assay
HUVECs were cultured to confluence in gelatin-coated 96-well tissue-culture plates and were washed once with RPMI plus 10% FCS. 51Cr-labeled T lymphocytes (2 × 105 cells in 100 μL) were added to each well with or without SDF-1 (100 ng/mL) in quadruplicate. The adhesion assay was allowed to proceed for 60 minutes. Then the plates were washed 4 times with warm RPMI medium. The bound T cells were lysed by the addition of 100 μL 1 N NaOH and the cell lysates were collected into tubes. The radioactivity in each of these samples was determined by gamma counting. The percentage of cell adhesion was calculated by dividing the radioactivity of bound cells by the radioactivity of the total cells added to the wells. For some experiments, L-selectin engagement by the ligand was achieved by preincubation of T cells with fucoidan (50 μg/mL) at 37°C for 30 minutes. For L-selectin cross-linking, T cells were first treated with 20 μg/mL LAM1-116 at room temperature for 20 minutes, washed once, then treated with 20 μg/mL goat antimouse IgG at 37°C for 30 minutes.
To define the adhesion molecules required for lymphocyte adhesion to the endothelium, T cells were treated with anti-LFA-1β (60.3) or anti-VLA-4 (HP2/1) for 20 minutes at room temperature. To examine the signal transduction pathways involved in fucoidan and SDF-1–induced T-cell adhesion to the endothelium, T cells were pretreated with herbimycin A (5 μg/mL), genistein (50 μg/mL), or PTX (100 ng/mL) before use in the adhesion assay.
Transendothelial migration assay
HUVECs were plated onto polycarbonate Transwell filters of 6.5-mm diameter and 5-μm pore size (Costar Corning, Cambridge, MA). The Transwell filters were prepared by coating with 0.01% gelatin (37°C overnight) followed by another coating with 3 μg human fibronectin (Gibco, Grand Island, NY) at 37°C for 3 hours. One hundred microliters of endothelial cells (1.2 × 105/mL) was seeded onto each filter and 600 μLof the culture medium was added to each lower chamber beneath the filter. After 6 days of culture, the integrity of confluent endothelial monolayers was assessed as described previously.22,29
The endothelial monolayers in the Transwell inserts were rinsed once with RPMI, then 100 μL 51Cr-labeled T cells (2 × 106 cells/mL) was placed in the upper chamber and the inserts were transferred to new wells (lower chambers) containing 600 μL fresh RPMI medium with 5 mg/mL HSA. SDF-1 at 100 ng/mL was added to the lower chambers for chemotaxis assays. The Transwell chambers were then incubated at 37°C in 5% CO2. After 4 hours, T cells that had migrated across the endothelial cell monolayer into the lower chamber were recovered. In addition, the T cells that had migrated through the monolayers but still remained adherent to the bottom surface of the filters were recovered by using a cotton swab. The radioactivity in each of these samples was determined by gamma counting. These results were combined to give a measure of the total migrated cells. The percentage of cell migration was calculated by dividing the radioactivity of the migrated cells by the radioactivity of the total cells added to the upper chamber. In some experiments, lymphocytes binding to the endothelial monolayer in the upper chamber after repeated washing with RPMI medium were recovered at the end of migration assay to calculate the percentage of lymphocyte adhesion. Spontaneous release of 51Cr from the labeled T cells during the 4-hour migration assay was less than 3%.
Actin polymerization
PBMCs were preincubated with SDF-1 (200 ng/mL), SDF-1 (200 ng/mL) plus fucoidan (100 μg/mL), or SDF-1 plus sulfatide (100 μg/mL) for 1 hour at 37°C. Then cells were washed and restimulated with SDF-1 (200 ng/mL) for 1 minute at 37°C followed immediately by fixation and permeabilization by adding 10 μL 18% formaldehyde with 1 mg/mL lysophosphatidylcholine in PBS. Cells were incubated for 5 minutes at 37°C before 5 μL NBD-phallacidin (evaporated down from methanol and resuspended in HBSS at 3.3 × 10–6 M) was added. Cells were incubated for another 10 minutes at 37°C; then cells were washed and examined on a flow cytometer (Coulter). Values are expressed as mean fluorescence intensity (MFI). This method has been shown to correlate with biochemical measurement of F-actin.30
Statistical analysis
All values are given as means ± SEM. Analysis of variance (ANOVA) was used to compare the differences among means and the Tukey method of multiple comparisons was applied by using GraphPad software (San Diego, CA) if a statistically significant difference was detected.
Results
L-selectin cross-linking increases CXCR4 expression on peripheral blood lymphocytes
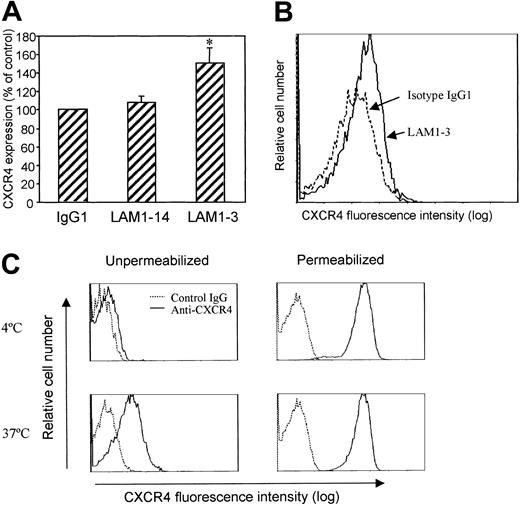
Altered CXCR4 expression may affect lymphocyte responses to SDF-1 and determine whether lymphocytes remain within the circulation or emigrate out of the vessels. It has been previously reported that L-selectin–mediated signals greatly enhanced neutrophil migration to the chemokine KC.21 Using freshly isolated human blood lymphocytes, we examined whether L-selectin cross-linking altered cell surface CXCR4 expression in vitro. We observed that L-selectin cross-linking by anti-L-selectin mAb and goat antimouse secondary antibody significantly increased CXCR4 surface expression on lymphocytes (Figure 1A). The effect was seen after treatment with LAM1-3, a neutralizing antibody whose binding site is located in the ligand-binding domain (lectin domain) of L-selectin. Increased CXCR4 expression appeared to affect the entire population of lymphocytes because there was no separate peak of higher CXCR4-expressing cells (Figure 1B). LAM1-14, a nonneutralizing antibody that binds to the consensus repeat domain of L-selectin, did not affect CXCR4 expression (Figure 1A).
Surface expression of CXCR4. (A) L-selectin cross-linking increased lymphocyte surface expression of CXCR4. PBMCs were preincubated with isotype control antibody (IgG1), LAM1-14, or LAM1-3 at 10 μg/mL on ice for 30 minutes, washed, then followed by cross-linking with goat antimouse antibody (20 μg/mL) at 37°C for 1 hour. CXCR4 expression was measured by staining cells with PE-conjugated anti-CXCR4 (12G5) after blocking nonspecific binding sites with mouse IgG (50 μg/mL). Means ± SEM of relative expression (% of control) from 3 separate experiments are shown. *P < .05 compared to IgG1. (B) Representative histograms demonstrating CXCR4 expression after cross-linking using isotype control IgG1 (dotted line) or LAM1-3 (solid line). (C) Lymphocyte surface CXCR4 was increased after in vitro incubation. Freshly isolated human PBMCs were incubated in vitro for 1 hour at 4°C or 37°C. Intact or saponin-permeabilized cells were assessed for CXCR4 expression by immunofluorescence staining and flow cytometric analysis. Lymphocytes were differentiated from monocytes by light scatter characteristics.
Surface expression of CXCR4. (A) L-selectin cross-linking increased lymphocyte surface expression of CXCR4. PBMCs were preincubated with isotype control antibody (IgG1), LAM1-14, or LAM1-3 at 10 μg/mL on ice for 30 minutes, washed, then followed by cross-linking with goat antimouse antibody (20 μg/mL) at 37°C for 1 hour. CXCR4 expression was measured by staining cells with PE-conjugated anti-CXCR4 (12G5) after blocking nonspecific binding sites with mouse IgG (50 μg/mL). Means ± SEM of relative expression (% of control) from 3 separate experiments are shown. *P < .05 compared to IgG1. (B) Representative histograms demonstrating CXCR4 expression after cross-linking using isotype control IgG1 (dotted line) or LAM1-3 (solid line). (C) Lymphocyte surface CXCR4 was increased after in vitro incubation. Freshly isolated human PBMCs were incubated in vitro for 1 hour at 4°C or 37°C. Intact or saponin-permeabilized cells were assessed for CXCR4 expression by immunofluorescence staining and flow cytometric analysis. Lymphocytes were differentiated from monocytes by light scatter characteristics.
CXCR4 is found in intracellular stores
The level of CXCR4 expression on lymphocytes reported in the literature has been quite variable. The factors affecting this expression are not well understood.23, 24, 25, 26 Using immunofluorescence staining and flow cytometry, we observed that approximately 5% to 15% of freshly isolated lymphocytes were positive for cell surface CXCR4 expression (Figure 1C). To detect intracellular stores of CXCR4, lymphocytes were fixed and permeabilized, then stained with PE-conjugated anti-CXCR4. Isotype-matched control IgG2a was added in excess together with anti-CXCR4 to block nonspecific binding sites. After permeabilization, fluorescence intensity reflects both cell surface and intracellular CXCR4 (Figure 1C, right panel). Almost 100% of freshly isolated peripheral blood lymphocytes were positive for intracellular CXCR4 staining. Simple in vitro culture of these lymphocytes at 37°C for 1 hour in RPMI medium greatly increased the percentage of cells expressing surface CXCR4 but did not change the fluorescence intensity of permeabilized cells. This observation may explain the variable level of CXCR4 expression previously reported.
L-selectin engagement by ligands increases CXCR4 expression
Fucoidan (polymer of sulfated L-fucose) and sulfatide (3-sulfated galactosylceramide), 2 putative ligands for L-selectin, also significantly increased cell surface expression of CXCR4 (Figure 2A). Fucoidan increased the level of surface CXCR4 to 1.4-fold, sulfatide to 1.7-fold of the control level. The time course of fucoidan and sulfatide stimulation demonstrated that as little as 10 minutes of exposure significantly increased CXCR4 expression in lymphocytes (P < .05). Longer stimulation further increased CXCR4 expression (Figure 2B). At the same time, even without fucoidan or sulfatide stimulation, cell surface CXCR4 spontaneously increased, but at a slower rate compared with the cells not stimulated with fucoidan or sulfatide.
L-selectin engagement by ligands increased lymphocyte surface expression of CXCR4. (A) PBMCs were incubated with 100 μg/mL fucoidan or sulfatide at 37°C for 1 hour. CXCR4 expression was examined by staining cells with PE-conjugated anti-CXCR4. Means ± SEM of relative expressions (% of control) from 11 experiments are shown. *P < .05, **P < .01 compared to untreated. (B) Time-dependent stimulation of CXCR4 expression (n = 4). ○ indicates control; □, fucoidan; and ▵, sulfatide. (C) Fucoidan and sulfatide did not alter β2-integrin, CCR5, and CCR7 expression on lymphocytes. PBMCs were incubated with fucoidan or sulfatide each at 100 μg/mL for 1 hour at 37°C. Cell surface expression of β2 integrin, CCR5, and CCR7 was analyzed by immunofluorescence staining with antibodies IB4, 2D7, and 2H4, respectively. Means ± SEM of relative fluorescence intensity (% of control) from 3 separate experiments are presented. (D) HeLa cells were treated with 100 μg/mL fucoidan or sulfatide at 37°C for 1 hour. CXCR4 expression was examined by anti-CXCR4 and flow cytometry. Representative histograms from 1 of 3 similar experiments are shown. Thick lines indicate anti-CXCR4; thin lines, control IgG.
L-selectin engagement by ligands increased lymphocyte surface expression of CXCR4. (A) PBMCs were incubated with 100 μg/mL fucoidan or sulfatide at 37°C for 1 hour. CXCR4 expression was examined by staining cells with PE-conjugated anti-CXCR4. Means ± SEM of relative expressions (% of control) from 11 experiments are shown. *P < .05, **P < .01 compared to untreated. (B) Time-dependent stimulation of CXCR4 expression (n = 4). ○ indicates control; □, fucoidan; and ▵, sulfatide. (C) Fucoidan and sulfatide did not alter β2-integrin, CCR5, and CCR7 expression on lymphocytes. PBMCs were incubated with fucoidan or sulfatide each at 100 μg/mL for 1 hour at 37°C. Cell surface expression of β2 integrin, CCR5, and CCR7 was analyzed by immunofluorescence staining with antibodies IB4, 2D7, and 2H4, respectively. Means ± SEM of relative fluorescence intensity (% of control) from 3 separate experiments are presented. (D) HeLa cells were treated with 100 μg/mL fucoidan or sulfatide at 37°C for 1 hour. CXCR4 expression was examined by anti-CXCR4 and flow cytometry. Representative histograms from 1 of 3 similar experiments are shown. Thick lines indicate anti-CXCR4; thin lines, control IgG.
To clarify whether the effect of fucoidan and sulfatide on CXCR4 expression was selective and specific, other surface molecules, β2 integrin, CCR5, and CCR7 were examined. These molecules remained unchanged under similar conditions of fucoidan or sulfatide stimulation (Figure 2C). The requirement for L-selectin in fucoidan- and sulfatide-induced up-regulation of CXCR4 expression was corroborated in HeLa cells (human ovarian epithelial cell line). HeLa cells expressed CXCR4 but did not express L-selectin. Neither fucoidan nor sulfatide significantly increased CXCR4 expression by HeLa cells (Figure 2D).
L-selectin engagement blocks CXCR4 down-regulation induced by SDF-1
In addition to the up-regulation of lymphocyte surface CXCR4, which was most likely through translocation of intracellular CXCR4 to the cell surface, the effect of fucoidan and sulfatide on SDF-1–induced CXCR4 down-regulation was also investigated. For these studies, we used Jurkat cells that have higher constitutive levels of surface CXCR4. Treatment of Jurkat cells with 500 ng/mL SDF-1 at 37°C for 60 minutes decreased CXCR4 expression to 10% to 20% of the control level (Figure 3A-B). The presence of fucoidan, sulfatide, or L-selectin cross-linking by LAM1-3 markedly maintained the surface expression of CXCR4. The efficiency in blocking CXCR4 down-regulation induced by SDF-1 was dependent on the concentrations of fucoidan and sulfatide (Figure 3B). At 10 μg/mL, fucoidan or sulfatide had similar potency as cross-linking of L-selectin molecules by LAM1-3, maintaining CXCR4 expression at 54% to 61% of the control level. However, both fucoidan and sulfatide at 100 μg/mL demonstrated the strongest effect, maintaining CXCR4 expression at 84% and 108% of the control level, respectively. LAM1-14 was not effective in blocking CXCR4 internalization, similar to its lack of effect on CXCR4 up-regulation in primary lymphocytes (Figure 1A). A similar observation that L-selectin stimulation blocks SDF-1–induced CXCR4 internalization was also reproduced in peripheral blood lymphocytes cultured overnight where the levels of surface CXCR4 were highly induced (data not shown).
L-selectin stimulation blocked CXCR4 down-regulation induced by SDF-1 on Jurkat cells. Jurkat cells were incubated with SDF-1 (500 ng/mL) in the presence or absence of fucoidan, sulfatide, or L-selectin cross-linking at 37°C for 1 hour. Representative histograms (A) and means ± SEM of relative fluorescence intensity of CXCR4 (% of control) from 3 experiments (B) are shown.
L-selectin stimulation blocked CXCR4 down-regulation induced by SDF-1 on Jurkat cells. Jurkat cells were incubated with SDF-1 (500 ng/mL) in the presence or absence of fucoidan, sulfatide, or L-selectin cross-linking at 37°C for 1 hour. Representative histograms (A) and means ± SEM of relative fluorescence intensity of CXCR4 (% of control) from 3 experiments (B) are shown.
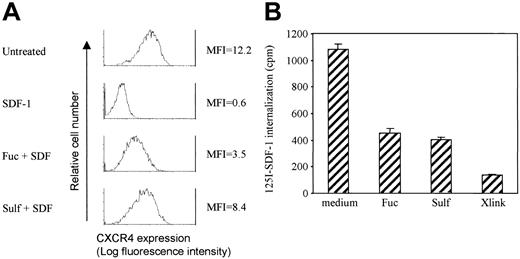
The maintenance of CXCR4 expression in the presence of SDF-1 by fucoidan or sulfatide may be due to several potential mechanisms. One is the inhibition of CXCR4 internalization induced by SDF-1. Another is facilitation of recycling and reexpression of internalized CXCR4. A third potential mechanism is translocation of intracellular stores of CXCR4. To investigate whether fucoidan and sulfatide modulate SDF-1–induced CXCR4 internalization, 2 different methods were used. First, Jurkat cells were prelabeled with anti-CXCR4 mAb. The cells were washed to remove unbound antibody, followed by stimulation with SDF-1 (500 ng/mL) in the presence or absence of fucoidan and sulfatide. Surface CXCR4 remaining on the cell surface should still have anti-CXCR4 antibody detectable by PE-conjugated goat antimouse antibody, whereas recycled CXCR4 and CXCR4 mobilized from cytoplasmic stores do not have anti-CXCR4 antibody and therefore will not be recognized by PE-conjugated secondary antibody. By these criteria, fucoidan and sulfatide significantly blocked SDF-1–induced CXCR4 internalization (Figure 4A).
Fucoidan and sulfatide inhibited SDF-1–induced CXCR4 internalization. (A) Jurkat cells were prelabeled with anti-CXCR4 antibody. Cells were then washed and incubated with SDF-1 (500 ng/mL) plus fucoidan or sulfatide (100 μg/mL) for 1 hour at 37°C. CXCR4 that was originally expressed and still remaining on cell surface was detected by PE-conjugated sheep antimouse antibody and flow cytometry. Representative histograms from 1 of 3 similar experiments are shown. (B) Jurkat cells were prelabeled with 125I-SDF-1. CXCR4 internalization was induced by incubation at 37°C with or without L-selectin stimulation and quantitated by a gamma counter.
Fucoidan and sulfatide inhibited SDF-1–induced CXCR4 internalization. (A) Jurkat cells were prelabeled with anti-CXCR4 antibody. Cells were then washed and incubated with SDF-1 (500 ng/mL) plus fucoidan or sulfatide (100 μg/mL) for 1 hour at 37°C. CXCR4 that was originally expressed and still remaining on cell surface was detected by PE-conjugated sheep antimouse antibody and flow cytometry. Representative histograms from 1 of 3 similar experiments are shown. (B) Jurkat cells were prelabeled with 125I-SDF-1. CXCR4 internalization was induced by incubation at 37°C with or without L-selectin stimulation and quantitated by a gamma counter.
Second, as a more direct measure, Jurkat cells were labeled with 125I-SDF-1 on ice and internalization of CXCR4 was induced by incubation at 37°C. Cell surface 125I-SDF-1 was removed by incubation with acid glycine buffer. The presence of fucoidan, sulfatide, or L-selectin cross-linking by LAM1-3 significantly decreased the internalization of 125I-SDF-1 (Figure 4B).
L-selectin stimulation selectively inhibits CXCR4 internalization
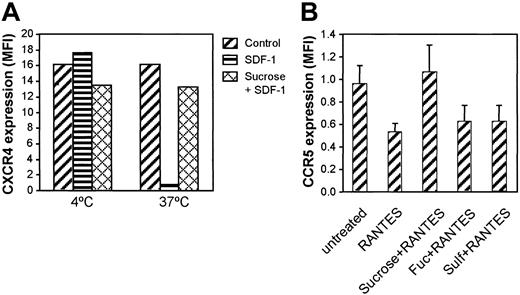
Clathrin is involved in the internalization of a variety of cell surface receptors including chemokine receptors. It is possible that fucoidan or sulfatide inhibited clathrin-mediated receptor internalization in a nonspecific manner. We attempted to determine whether SDF-1–induced CXCR4 down-regulation was mediated through clathrin and whether fucoidan and sulfatide affected other clathrin-related chemokine receptor internalization. As shown in Figure 5A, SDF-1 induced CXCR4 down-regulation at 37°C but not at 4°C. CXCR4 down-regulation was blocked by preincubation of Jurkat cells with 0.6 M sucrose, a high osmotic solution that inhibits clathrin-mediated receptor internalization.31 CCR5 was internalized by RANTES stimulation, which was inhibited by 0.6 M sucrose (Figure 5B). However, fucoidan and sulfatide did not block CCR5 internalization, suggesting that fucoidan and sulfatide selectively inhibit CXCR4 internalization, not all clathrin-dependent events.
Effect of clathrin on CXCR4 and CCR5 internalization. (A) Jurkat cells were incubated with SDF-1 (500 ng/mL) or SDF-1 plus 0.6 M sucrose in RPMI for 1 hour at 4°C or 37°C. Then CXCR4 expression was examined by immunofluorescence staining. (B) PBMCs were incubated with RANTES (500 ng/mL) in the presence of sucrose, fucoidan, or sulfatide for 1 hour at 37°C. Then CCR5 expression was examined by immunofluorescence staining and flow cytometry.
Effect of clathrin on CXCR4 and CCR5 internalization. (A) Jurkat cells were incubated with SDF-1 (500 ng/mL) or SDF-1 plus 0.6 M sucrose in RPMI for 1 hour at 4°C or 37°C. Then CXCR4 expression was examined by immunofluorescence staining. (B) PBMCs were incubated with RANTES (500 ng/mL) in the presence of sucrose, fucoidan, or sulfatide for 1 hour at 37°C. Then CCR5 expression was examined by immunofluorescence staining and flow cytometry.
L-selectin–mediated persistence of CXCR4 increases actin polymerization in response to SDF-1
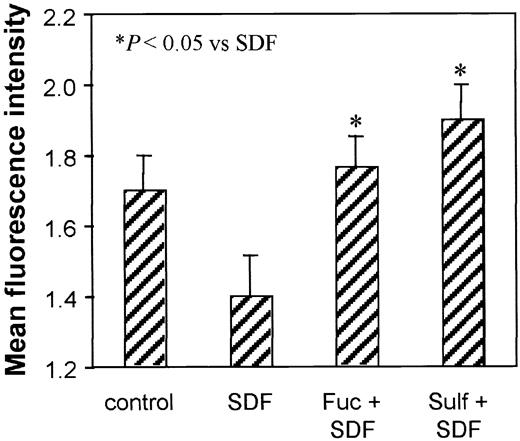
To further examine whether the persistent CXCR4 expression caused by L-selectin stimulation had functional consequences in these peripheral blood lymphocytes, cells were pretreated with SDF-1, SDF-1 plus fucoidan, or SDF-1 plus sulfatide at 37°C for 60 minutes. The cells were then washed, and the function of CXCR4 remaining on the cell surface was examined by a second stimulation with SDF-1 at 37°C for 1 minute. NBD-phallacidin binding was used to measure actin polymerization. SDF-1 induced a rapid and transient actin polymerization in nonpretreated lymphocytes (MFI = 1.7 ± 0.10 versus control cells without SDF-1 treatment, MFI = 0.5; data not shown). Pretreatment with SDF-1 resulted in decreased actin polymerization on re-exposure to a second stimulus with SDF-1 (MFI = 1.4 ± 0.12, P < .05; Figure 6). This observation is consistent with decreased CXCR4 levels (Figure 3). For the lymphocytes treated with either fucoidan or sulfatide at the time of the first SDF-1 exposure, the level of actin polymerization in response to a second SDF-1 stimulation was maintained or even increased (MFI = 1.8 ± 0.09 and 1.9 ± 0.10, respectively).
Effect of persistent CXCR4 expression on lymphocyte actin polymerization. PBMCs were either untreated or pretreated with SDF-1 (200 ng/mL), SDF-1 plus fucoidan (100 μg/mL), or SDF-1 plus sulfatide (100 μg/mL) for 1 hour at 37°C. Then all cells were washed and restimulated with SDF-1 (200 ng/mL) for 1 minute. Actin polymerization was measured by NBD-phallacidin staining and flow cytometry. Means ± SEM of the fluorescence intensity from 3 experiments are presented. *P < .05 compared with SDF-1 pretreated group.
Effect of persistent CXCR4 expression on lymphocyte actin polymerization. PBMCs were either untreated or pretreated with SDF-1 (200 ng/mL), SDF-1 plus fucoidan (100 μg/mL), or SDF-1 plus sulfatide (100 μg/mL) for 1 hour at 37°C. Then all cells were washed and restimulated with SDF-1 (200 ng/mL) for 1 minute. Actin polymerization was measured by NBD-phallacidin staining and flow cytometry. Means ± SEM of the fluorescence intensity from 3 experiments are presented. *P < .05 compared with SDF-1 pretreated group.
L-selectin engagement stimulates SDF-1–induced lymphocyte adhesion to endothelial cells
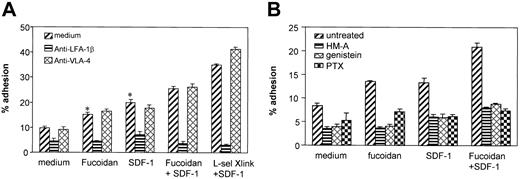
L-selectin stimulation and chemokine activation are both assumed to activate lymphocyte integrins and facilitate lymphocyte adhesion to endothelial cells. The combined stimulation of the 2 molecules on T-cell adhesion to endothelial cells and the adhesion molecules involved were examined. Human T lymphocytes were left untreated or pretreated with fucoidan and antibody cross-linking of L-selectin at 37°C for 30 minutes. SDF-1 was added to the T-cell suspension at the time of starting the cell adhesion assay. Fucoidan significantly increased T-cell adhesion to 15.1% (P < .01) compared with the background T-cell adhesion of 9.8% (Figure 7A). SDF-1 at 100 ng/mL significantly increased T-cell adhesion to 19.8% (P < .01 compared with the background). The combined stimulation of SDF-1 with fucoidan induced 25.6% of T cells to adhere. SDF-1 plus L-selectin cross-linking induced 35% T-cell adhesion. Adhesion in response to RANTES was not augmented by fucoidan (data not shown). Lymphocyte adhesion to endothelial cells in the presence or absence of the above stimulants were all significantly inhibited by an antibody against LFA-1β integrin but not by anti-VLA-4, suggesting that the adhesion is predominantly mediated by LFA-1.
Effect of L-selectin stimulation and SDF-1 on lymphocyte adhesion to the endothelium. Chromium-labeled T lymphocytes were added to confluent monolayers of HUVECs in 96-well plates for 1 hour at 37°C. Fucoidan, anti-L-selectin cross-linking, and SDF-1 either alone or in combinations were used for stimulation. Nonadherent cells were removed by repeated washing. The adherent cells were lysed by 1 N NaOH and radioactivity was measured by gamma counting. (A) L-selectin and SDF-1–induced lymphocyte adhesion to endothelial cells was blocked by anti-LFA-1 but not by anti-VLA-4. (B) Tyrosine kinase inhibitors and PTX inhibited lymphocyte adhesion to endothelial cells. *P ≤ .01 compared with medium alone (selected pairwise comparisons selected a priori).
Effect of L-selectin stimulation and SDF-1 on lymphocyte adhesion to the endothelium. Chromium-labeled T lymphocytes were added to confluent monolayers of HUVECs in 96-well plates for 1 hour at 37°C. Fucoidan, anti-L-selectin cross-linking, and SDF-1 either alone or in combinations were used for stimulation. Nonadherent cells were removed by repeated washing. The adherent cells were lysed by 1 N NaOH and radioactivity was measured by gamma counting. (A) L-selectin and SDF-1–induced lymphocyte adhesion to endothelial cells was blocked by anti-LFA-1 but not by anti-VLA-4. (B) Tyrosine kinase inhibitors and PTX inhibited lymphocyte adhesion to endothelial cells. *P ≤ .01 compared with medium alone (selected pairwise comparisons selected a priori).
Signaling pathways involved in lymphocyte adhesion to endothelial cells were also examined. Protein tyrosine kinase inhibitors herbimycin A, genistein, and G protein inhibitor PTX were all effective in blocking baseline and inducible adhesion of lymphocytes to endothelial cells (Figure 7B).
L-selectin engagement enhances lymphocyte transendothelial migration to SDF-1
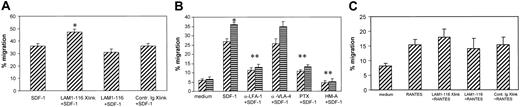
SDF-1 is a potent CXC chemokine for lymphocyte activation and transmigration. In a Transwell migration model, SDF-1 at 100 ng/mL in the lower chamber induced an average of 35.8% of lymphocytes to migrate across HUVEC monolayers in a 4-hour migration assay (Figure 8A). Cross-linking of L-selectin by LAM1-116 and goat-antimouse IgG significantly enhanced T-cell migration to SDF-1 (P < .05), whereas lymphocyte pretreatment with LAM1-116 but without cross-linking slightly decreased cell migration to 31%. Isotype control antibody plus cross-linking did not alter lymphocyte migration to SDF-1.
Molecular mechanisms involved in lymphocyte transendothelial migration induced by L-selectin stimulation and SDF-1. HUVECs were cultured in Transwells. Chromium-labeled lymphocytes were added to the upper chamber and SDF-1 was added to the lower chamber. As indicated, lymphocytes were pretreated with fucoidan, L-selectin cross-linking, antiadhesion molecules, or chemical inhibitors. (A) L-selectin cross-linking increased lymphocyte migration to SDF-1. (B) Fucoidan-stimulated lymphocyte migration to SDF-1 was inhibited by anti-LFA-1, PTX, and herbimycin A. (C) L-selectin cross-linking did not affect migration induced by RANTES. ▨. indicates no fucoidan; ▤, with fucoidan. *P < .05 compared with SDF-1 alone; **P ≤ .05 compared with SDF-1 + fucoidan.
Molecular mechanisms involved in lymphocyte transendothelial migration induced by L-selectin stimulation and SDF-1. HUVECs were cultured in Transwells. Chromium-labeled lymphocytes were added to the upper chamber and SDF-1 was added to the lower chamber. As indicated, lymphocytes were pretreated with fucoidan, L-selectin cross-linking, antiadhesion molecules, or chemical inhibitors. (A) L-selectin cross-linking increased lymphocyte migration to SDF-1. (B) Fucoidan-stimulated lymphocyte migration to SDF-1 was inhibited by anti-LFA-1, PTX, and herbimycin A. (C) L-selectin cross-linking did not affect migration induced by RANTES. ▨. indicates no fucoidan; ▤, with fucoidan. *P < .05 compared with SDF-1 alone; **P ≤ .05 compared with SDF-1 + fucoidan.
Prestimulation of lymphocytes with fucoidan alone did not increase lymphocyte transendothelial migration (Figure 8B). However, in the presence of SDF-1, fucoidan significantly increased lymphocyte transendothelial migration (P < .05). Under microscopy, we also noticed that fucoidan-treated lymphocytes migrating through HUVEC monolayers were in small aggregates, whereas lymphocytes migrating to SDF-1 only were individually dispersed, suggesting that T cells that have migrated to fucoidan and SDF-1 are more activated, resulting in aggregation. Addition of fucoidan into the lower chamber did not induce significant lymphocyte migration as compared to the control medium.
Lymphocyte transendothelial migration in response to SDF-1 has been demonstrated to be mediated mainly by LFA-1. We examined whether LFA-1 is also the major adhesion molecule involved in lymphocyte transendothelial migration induced by the dual stimulation of L-selectin and CXCR4. Blocking LFA-1 significantly decreased T-cell migration to SDF-1 (P < .01) for cells pretreated with fucoidan and untreated T cells (Figure 8B). Blocking VLA-4 had no effect. Similar to what was observed in the adhesion assay, PTX and herbimycin A significantly inhibited transendothelial migration of T cells with or without prior treatment with fucoidan.
Fucoidan and SDF-1 induce expression of the activation-associated epitope of LFA-1
T-cell adhesion and transendothelial migration induced by L-selectin engagement and SDF-1 are mediated through LFA-1.29 Next, we examined if β1 and β2 integrins are activated to express activation-associated epitopes by using specific antibodies HUTS21 and mAb24. The total expression of β1 and β2 integrins was not altered by fucoidan and SDF-1 stimulation (Table 1). Fucoidan slightly enhanced mAb24 antigen expression. SDF-1 had a much greater effect. The combination of fucoidan and SDF-1 induced the highest expression of the active epitope of β2 integrin. In contrast, fucoidan and SDF-1 either alone or in combination did not significantly alter the expression of β1 integrin or the active epitope mapped by HUTS21. L-selectin was not markedly decreased by either fucoidan or SDF-1 alone. However, it was partially but significantly decreased by dual stimulation.
Adhesion molecule expression on lymphocytes treated with fucoidan and SDF-1
. | Control . | Fucoidan . | SDF-1 . | Fucoidan + SDF-1 . |
|---|---|---|---|---|
| β2 integrin | 42.5 ± 3.48 | 42.9 ± 3.43 | 46.4 ± 3.27 | 50.2 ± 1.15 |
| Active β2 integrin | 7.2 ± 0.69 | 8.5 ± 0.96 | 14.9 ± 2.67* | 18.5 ± 4.18† |
| β1 integrin | 28.9 ± 2.22 | 28.5 ± 2.80 | 29.3 ± 4.07 | 30.1 ± 4.62 |
| Active β1 integrin | 4.5 ± 0.76 | 4.6 ± 0.69 | 6.3 ± 1.26 | 5.5 ± 0.68 |
| L-selectin | 108.4 ± 7.14 | 94.1 ± 12.12 | 98.7 ± 7.42 | 71.1 ± 8.20* |
. | Control . | Fucoidan . | SDF-1 . | Fucoidan + SDF-1 . |
|---|---|---|---|---|
| β2 integrin | 42.5 ± 3.48 | 42.9 ± 3.43 | 46.4 ± 3.27 | 50.2 ± 1.15 |
| Active β2 integrin | 7.2 ± 0.69 | 8.5 ± 0.96 | 14.9 ± 2.67* | 18.5 ± 4.18† |
| β1 integrin | 28.9 ± 2.22 | 28.5 ± 2.80 | 29.3 ± 4.07 | 30.1 ± 4.62 |
| Active β1 integrin | 4.5 ± 0.76 | 4.6 ± 0.69 | 6.3 ± 1.26 | 5.5 ± 0.68 |
| L-selectin | 108.4 ± 7.14 | 94.1 ± 12.12 | 98.7 ± 7.42 | 71.1 ± 8.20* |
Results (MFI) are given as mean ± SEM. Human T lymphocytes were stimulated with fucoidan, SDF-1, or both. Cell surface β2 integrin and activation-associated epitope were detected by antibodies 60.3 and mAb24, respectively, using flow cytometry. Surface β1 integrin and activation-associated epitope were detected by antibodies HP2/1 and HUTS-21, respectively. L-selectin was detected by LAM1-116.
P < .05 compared with control.
P < .01 compared with control.
Discussion
Regulation of CXCR4 expression may be an important mechanism controlling lymphocyte migration, especially during inflammation and immune responses. We have demonstrated for the first time that L-selectin stimulation by antibody cross-linking or by ligands (fucoidan or sulfatide) significantly increased lymphocyte surface CXCR4 expression (Figures 1A and 2A). This increase of CXCR4 is likely from intracellular stores of preformed CXCR4 (Figure 1B). L-selectin stimulation also strongly inhibited CXCR4 internalization induced by SDF-1 (Figures 3, 4). The CXCR4 remaining at the cell surface was functional inasmuch as it led to enhanced lymphocyte responses to a second SDF-1 stimulation, inducing stronger actin polymerization (Figure 6). L-selectin and SDF-1 stimulation together also induced increased expression of β2 integrin (Table 1) and increased lymphocyte adhesion and transendothelial migration, which were inhibited by anti-LFA-1 antibody, tyrosine kinase inhibitors, and PTX (Figures 7, 8). It is therefore suggested that L-selectin may enhance lymphocyte surface expression of CXCR4 and functional responses to SDF-1.
This study demonstrated that L-selectin stimulation specifically up-regulated CXCR4 but not CCR5 or CCR7 (Figure 2C). Additionally, CXCR1, CXCR2, and CXCR4 expression by neutrophils were also not altered (data not shown). Therefore, it is suggested that L-selectin signaling may preferentially facilitate cell responses to certain chemokines resulting in selective migration of cells expressing appropriate chemokine receptors. However, it is not clear whether L-selectin selectively enhances CXCR4 expression in certain subsets of circulating lymphocytes and facilitates these cells to respond to SDF-1 stimulation. We did double immunofluorescence staining to analyze β2 integrin activation in naive and memory T cells stimulated by fucoidan and SDF-1, but did not find a striking difference (data not shown). More experiments about targeted cell subtypes of the additive stimulation of L-selectin signaling and SDF-1 are planned.
L-selectin–induced expression of CXCR4 is from intracellular stores because most of the CXCR4 in freshly prepared lymphocytes is inside the cell (Figure 1C) and it is induced to mobilize to the surface within 10 minutes (Figure 2B). Because leukocytes in vivo become arrested within seconds after L-selectin–mediated rolling, we speculate that L-selectin–mediated up-regulation of CXCR4 may be more important in driving firmly adherent cells to migration rather than switching rolling cells to firm adhesion. Recently, SDF-1 has been demonstrated to be critical in lymphocyte migration across high endothelial monolayers, which are typical structures in lymphoid tissues.11 L-selectin is also important in lymphocyte rolling and homing to lymphoid tissues. Here we demonstrated for the first time that L-selectin signaling not only increased SDF-1–induced lymphocyte adhesion to endothelial cells but also facilitated lymphocyte transendothelial migration. However, the in vivo significance of this cooperative effect requires further investigation. We have already confirmed in vitro that L-selectin stimulation can increase surface expression of CXCR4 in murine lymphocyte cell lines. Experiments using in vivo animal models are currently in progress.
In addition to mobilization of intracellular stores, L-selectin signaling also inhibited CXCR4 internalization. A similar phenomenon has also been observed with cyclic adenosine monophosphate (cAMP), which induces CXCR4 mobilization as well as inhibits internalization.32 Internalization is apparently not required for signal transduction. Deletion of the intracellular C-terminal tail of CXCR4 reduced SDF-1–induced CXCR4 internalization but cell responses to SDF-1 were increased.33,34 In addition, the truncated form of CXCR4 showed normal function as a coreceptor for HIV entry and was equally capable of Ca2+ signaling.27 Therefore, CXCR4 internalization likely functions more as a desensitization mechanism for controlling the strength and duration of cellular responses. Mechanisms of CXCR4 internalization have not been well characterized. It has been suggested that SDF-1 induces phosphorylation of the cytoplasmic domain of CXCR4, which recruits arrestins and dynamins and initiates clathrin-dependent endocytosis. CXCR4 internalization is not inhibitable by PTX, although receptor signaling, cell activation, and migration are all inhibitable.27 The current study clearly demonstrates that fucoidan and sulfatide do not generally inhibit clathrin-mediated chemokine receptor internalization (Figure 5B). Their effect on arrestins and dynamins, however, merits further investigation. To ensure that the effects observed were indeed mediated by L-selectin, several approaches were used. Two different primary antibodies against L-selectin were used to cross-link L-selectin. Only the antibody recognizing the ligand-binding domain (LAM1-3) increased CXCR4 expression and blocked CXCR4 internalization induced by SDF-1. The other antibody, LAM 1-14, that recognizes a region outside the ligand-binding domain had no effect. This was consistent with a previous observation that engagement of the ligand-binding domain was required for L-selectin signaling.35 Two structurally distinct chemical ligands for L-selectin were also used. Both sulfatide and fucoidan induced up-regulation of CXCR4 expression and blocked CXCR4 internalization, similar to LAM 1-3. As an additional control, HeLa cells, which constitutively express CXCR4 but not L-selectin, demonstrated no response to sulfatide or fucoidan.
It is also noteworthy that SDF-1 stimulation did not induce L-selectin shedding as is frequently observed after leukocyte activation (Table 1). We speculate that during lymphocyte migration, under the directional guidance of SDF-1, L-selectin may continuously signal the cells to express a high level of CXCR4 and prevent cells from being desensitized by blocking CXCR4 internalization after continuous SDF-1 stimulation. This might be important because receptor down-regulation rapidly desensitizes cell response to chemokine ligands.36 The presence of extravascular ligands for L-selectin37,38 suggests that L-selectin may play a role in coordinating lymphocyte activation and movement in the extravascular space. However, the precise physiologic significance of L-selectin–induced modulation of CXCR4 expression needs to be further studied.
In the current study, the novel observation that L-selectin signaling selectively up-regulates CXCR4 expression and function may reveal a new mechanism involved in the selective and tissue-specific lymphocyte migration and accumulation in various inflammatory reactions. It has been hypothesized that the coordinated interactions of adhesion molecules and chemokines dominate the sequential process of cell migration.1,2 However, attention was previously directed to the effect of chemokines on adhesion molecule activation. The current data suggest an alternative possibility—that adhesion molecules may trigger signals for enhanced chemokine receptor expression and function.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-06-1782.
T.K.W. is a recipient of the Second Alfred Blalock Research Scholarship from the American Association for Thoracic Surgery. Z.D. is supported by a postdoctoral fellowship from Canadian Institutes of Health Research (CIHR). G.P.D. is the recipient of a Canada Research Chair from the CIHR and currently holds the R. Fraser Elliott Chair in Transplantation Research at the Toronto General Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal