Abstract
Glycoprotein (GP) VI is an essential collagen receptor on platelets and may serve as an attractive target for antithrombotic therapy. We have previously shown that a monoclonal antibody (mAb) against the major collagen-binding site on mouse GPVI (JAQ1) induces irreversible down-regulation of the receptor and, consequently, long-term antithrombotic protection in vivo. To determine whether this unique in vivo effect of JAQ1 is based on its interaction with the ligand-binding site on GPVI, we generated new mAbs against different epitopes on GPVI (JAQ2, JAQ3) and tested their in vitro and in vivo activity. We show that none of the mAbs inhibited platelet activation by collagen or the collagen-related peptide in vitro. Unexpectedly, however, injection of either antibody induced depletion of GPVI with the same efficacy and kinetics as JAQ1. Importantly, this effect was also seen with monovalent F(ab) fragments of JAQ2 and JAQ3, excluding the involvement of the Fc part or the dimeric form of anti-GPVI antibodies in this process. This indicates that anti-GPVI agents, irrespective of their binding site may generally induce down-regulation of the receptor in vivo.
Introduction
Coronary artery thrombosis is often initiated by platelet adhesion and aggregation on subendothelial collagens exposed on the surface of the ruptured atherosclerotic plaque.1-4Platelet-collagen interactions are complex and involve a large number of receptors that directly or indirectly interact with the matrix protein, most importantly GPIb-V-IX,5 integrins α2β16 and αIIbβ3,5 and glycoprotein (GP) VI.7 Among these, GPVI plays a central role as it mediates the activation of integrins α2β1 and αIIbβ3, which is a prerequisite for firm platelet adhesion and thrombus growth.8 GPVI (62 kDa) belongs to the immunoglobulin superfamily9,10 and is noncovalently associated with the Fc receptor (FcR)γ chain.11,12 GPVI-deficient patients suffer from a mild bleeding diathesis and their platelets show severely impaired responses to collagen.7,13,14 Similarly, platelets from FcRγ chain–deficient mice, which lack GPVI,15 are unresponsive to collagen16 but no major bleeding has been observed in those mice, making GPVI an interesting target for safe antithrombotic therapy.
The first direct proof for effective antithrombotic protection by anti-GPVI treatment came from studies in mice using the anti-GPVI monoclonal antibody (mAb), JAQ1. JAQ1 is directed against the major collagen-binding site on GPVI and inhibits platelet activation by collagen and the collagen-related peptide (CRP).15,17 In vivo treatment of mice with JAQ1 or monovalent F(ab) fragments of the antibody induces the internalization and proteolytic degradation of GPVI in circulating platelets resulting in a prolonged GPVI-knock-out–like phenotype.18 Similar mechanisms of GPVI down-regulation appear to exist in humans because one GPVI-deficient patient had developed highly specific antibodies against the apparently absent receptor suggesting that she suffered from an acquired GPVI deficiency based on antibody-induced clearing of the receptor from her platelets.13 F(ab) fragments of the antibodies isolated from this patient inhibited collagen-induced activation of normal human platelets.7,13 Together, these observations led to the hypothesis that targeting of the collagen-binding site on GPVI may elicit signals that finally result in down-regulation of the receptor in vivo.18
To develop effective anti-GPVI agents and to predict their mode of action in vivo, a better understanding of the mechanisms underlying GPVI down-regulation is required. To test the hypothesis that targeting of the ligand-binding site on the receptor is essential for this process to occur, we generated mAbs against other epitopes on mouse GPVI and examined their in vivo activity. Unexpectedly, we found that the in vivo depletion of GPVI was induced by all anti-GPVI mAbs or F(ab) fragments thereof, irrespective of their binding site on the receptor.
Materials and methods
Animals
Specific pathogen-free mice (NMRI, C57Bl/6), 6 to 10 weeks of age, were obtained from Charles River (Sulzfeld, Germany). C57Bl/6 mice deficient in the FcRγ chain were obtained from Taconics (Germantown, NY).
Antibodies
The mAbs against mouse GPVI (JAQ1), GPIIIa (EDL1), and GPV (DOM2, IgG2a) have been described previously.15phycoerythrin (PE)–labeled JON/A, which preferentially binds to activated GPIIb/IIIa, has been reported previously.19JAQ2 and JAQ3 were produced as described.15 F(ab) fragments were prepared as described.18 Briefly, mAbs were incubated for 6 to 8 hours with immobilized papain (Pierce, Rockford, IL), and the preparations were then applied to an immobilized protein A column followed by an immobilized protein G column (both Pharmacia, Uppsala, Sweden) to remove Fc fragments and undigested IgG. Purity of F(ab) fragments was tested by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Goat antirat IgG (Dianova, Hamburg, Germany), streptavidin–horseradish peroxidase (HRP), and rabbit antirat-HRP (both Dako, Hamburg, Germany) were purchased.
Platelet preparation
Mice were bled under ether anesthesia from the retro-orbital plexus. Blood was collected in a tube containing 10 U/mL heparin, and platelet-rich plasma (prp) was obtained by centrifugation at 300g for 10 minutes at room temperature (RT). For washed platelets, prp was centrifuged at 1000g for 8 minutes and the pellet was resuspended twice in modified Tyrode-HEPES (N-2-hydroxyethylpiperazine-N′2-ethanesulfonic acid) buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 0.35% bovine serum albumin, pH 6.6) in the presence of prostacyclin (0.1 μg/mL) and apyrase (0.02 U/mL). Platelets were then resuspended in the same buffer (pH 7.0, 0.02 U/mL apyrase) and incubated at 37°C for at least 30 minutes before analysis.
Immunoblotting and immunoprecipitation
Immunoprecipitation was performed as described previously.15 Briefly, 108 washed platelets were surface labeled with EZ-Link sulfo-NHS-LC-biotin (Pierce, 100 μg/mL in phosphate-buffered saline [PBS]) and subsequently solubilized in 1 mL lysis buffer (Tris-buffered saline containing 20 mM Tris [tris(hydroxymethyl)aminomethane])/HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM phenylmethylsulfonyl fluoride, and 1% Nonidet P-40). Cell debris was removed by centrifugation (15 000g, 10 minutes). Following preclearing (8 hours), 10 μg mAb was added together with 25 μL protein G-Sepharose (Pharmacia) and precipitation took place overnight with rotation at 4°C. Samples were separated by 12% SDS-PAGE along with a molecular weight marker and transferred onto a polyvinylidene difluoride membrane. The membrane was incubated with streptavidin-HRP (1 μg/mL) for 1 hour after blocking. After extensive washing, biotinylated proteins were visualized by enhanced chemiluminescence (ECL; Amersham, Freiburg, Germany). For immunoblotting, platelets were not surface labeled. After lysis, whole cell extract was run on an SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. The membrane was first incubated with 5 μg/mL JAQ1 followed by HRP-labeled rabbit antirat immunoglobulin and proteins were visualized by ECL.
Flow cytometry
Washed platelets (2 × 106) or whole blood samples were incubated with fluorophore-conjugated mAbs at saturating concentrations for 15 minutes at RT and directly analyzed on a FACScalibur (Becton Dickinson, Heidelberg, Germany). Platelets were gated by forward scatter/side scatter (FSC/SSC) characteristics. For competition studies, platelets were preincubated with unlabeled antibodies (50 μg/mL, 30 minutes), washed with PBS, and then incubated with fluorescein isothiocyanate (FITC)–labeled JAQ1, JAQ2, or JAQ3.
Aggregometry
To determine platelet aggregation, light transmission was measured using prp (200 μL with 0.5 × 106platelets/μL). Transmission was recorded in a Fibrintimer 4 channel aggregometer (APACT Laborgeräte und Analysensysteme, Hamburg, Germany) over 10 minutes and was expressed as arbitrary units with 100% transmission adjusted with plasma. Platelet aggregation was induced by addition of CRP (0.25-5 μg/mL), collagen (1-20 μg/mL), adenosine diphosphate (ADP; 5 μM), or cross-linking of bound antibodies by antirat IgG. For inhibition studies, prp was preincubated with various concentrations of JAQ1, JAQ2, JAQ3, or control IgG followed by addition of CRP or collagen.
Adhesion under flow conditions
Mouse blood (1 vol) was collected into 0.5 vol HEPES buffer, pH 7.4, containing 20 U/mL heparin and Ca2+ (1 mM). Coverslips (24 × 60 mm) were coated with fibrillar (Horm) collagen (0.25 mg/mL, Nycomed, Munich, Germany), and blocked for 1 hour with 1% bovine serum albumin. Perfusion studies were then performed as described.8 Briefly, transparent flow chambers with a slit depth of 50 μm, equipped with the collagen-coated coverslips, were connected to a syringe filled with the anticoagulated blood. Perfusion was performed using a pulse-free pump under high shear stress equivalent to a wall shear rate of 1000 s−1 (4 minutes). Thereafter, chambers were rinsed by a 4-minute perfusion with HEPES buffer at the same shear stress, and phase-contrast images were recorded from at least 5 different microscope fields (× 63 objectives).
Results
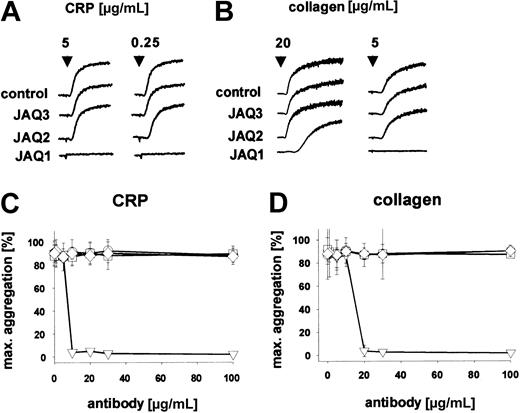
To determine whether the in vivo depletion of GPVI is dependent on targeting of the ligand-binding site on the receptor, we generated new anti-GPVI mAbs recognizing different epitopes on the receptor and tested their in vitro and in vivo activity. Both antibodies (JAQ2, JAQ3, rat IgG2a) precipitated a single-chain protein of an apparent molecular weight of approximately 60 kDa (Figure1A, IP). The identity of the precipitated protein with GPVI was verified by immunoblotting with JAQ1 (not shown). JAQ2 and JAQ3 also recognized GPVI in Western blot analysis under nonreducing conditions (Figure 1A, WB). JAQ2 and JAQ3 did not bind to platelets from FcRγ chain–deficient mice that are known to lack GPVI15 (not shown). Similar to JAQ1,15 JAQ2 and JAQ3 alone did not induce platelet aggregation, whereas strong and irreversible aggregation occurred on cross-linking of the bound antibodies by antirat immunoglobulin antibodies (Figure 1B), confirming that multivalent clustering of GPVI is required to induce platelet activation.20 Flow cytometric preincubation studies demonstrated that JAQ1, JAQ2, and JAQ3 do not block each other's binding, suggesting that they recognize different epitopes on GPVI (Figure 1C). Consistent with this, JAQ2 and JAQ3 (up to 100 μg/mL) did not inhibit CRP-induced platelet aggregation, whereas this was completely blocked by JAQ1 (Figure 2A,C). Similarly, collagen-induced aggregation was largely inhibited by JAQ1 in a dose-dependent manner, whereas it was not affected by JAQ2 or JAQ3 (Figure 2B,D). Together, these results demonstrated that JAQ2 and JAQ3 bind to epitopes on mouse GPVI that are different from the collagen-binding site on the receptor.
JAQ1, JAQ2, and JAQ3 bind to different epitopes on mouse GPVI.
(A) Whole platelet proteins were separated by SDS-PAGE under nonreducing conditions and immunoblotted with the indicated antibodies. Bound mAb was detected by HRP-labeled rabbit antirat Ig and ECL (upper, WB). Surface biotinylated platelets were lysed and immunoprecipitation was performed with the indicated antibodies. Precipitated proteins were detected with streptavidin-HRP and ECL (lower, IP). (B) Heparinized prp was incubated with JAQ1, JAQ2, JAQ3, or irrelevant IgG2a (all 20 μg/mL) followed by addition of polyclonal rabbit antirat immunoglobulin antibody (10 μg/mL) and light transmission was recorded on a Fibrintimer 4 channel aggregometer. (C) Platelets were preincubated with the indicated antibodies (20 μg/mL, 30 minutes) and washed; binding of FITC-labeled JAQ1, JAQ2, or JAQ3 was detected by flow cytometry.
JAQ1, JAQ2, and JAQ3 bind to different epitopes on mouse GPVI.
(A) Whole platelet proteins were separated by SDS-PAGE under nonreducing conditions and immunoblotted with the indicated antibodies. Bound mAb was detected by HRP-labeled rabbit antirat Ig and ECL (upper, WB). Surface biotinylated platelets were lysed and immunoprecipitation was performed with the indicated antibodies. Precipitated proteins were detected with streptavidin-HRP and ECL (lower, IP). (B) Heparinized prp was incubated with JAQ1, JAQ2, JAQ3, or irrelevant IgG2a (all 20 μg/mL) followed by addition of polyclonal rabbit antirat immunoglobulin antibody (10 μg/mL) and light transmission was recorded on a Fibrintimer 4 channel aggregometer. (C) Platelets were preincubated with the indicated antibodies (20 μg/mL, 30 minutes) and washed; binding of FITC-labeled JAQ1, JAQ2, or JAQ3 was detected by flow cytometry.
JAQ2 and JAQ3 do not inhibit platelet aggregation induced by CRP and collagen.
(A-B) Heparinized prp was stimulated with CRP (A) or collagen (B) and aggregation was recorded. The results shown are representative of 6 individual experiments. (C-D) Heparinized prp was incubated with different concentrations of IgG2a (○), JAQ1 (▿), JAQ2 (■), or JAQ3 (⋄) for 5 minutes before the addition of CRP (0.25 μg/mL, C) or collagen (5 μg/mL, D) and aggregation was recorded. Results are expressed as mean ± SD of 6 mice/group.
JAQ2 and JAQ3 do not inhibit platelet aggregation induced by CRP and collagen.
(A-B) Heparinized prp was stimulated with CRP (A) or collagen (B) and aggregation was recorded. The results shown are representative of 6 individual experiments. (C-D) Heparinized prp was incubated with different concentrations of IgG2a (○), JAQ1 (▿), JAQ2 (■), or JAQ3 (⋄) for 5 minutes before the addition of CRP (0.25 μg/mL, C) or collagen (5 μg/mL, D) and aggregation was recorded. Results are expressed as mean ± SD of 6 mice/group.
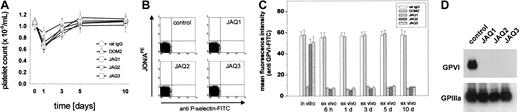
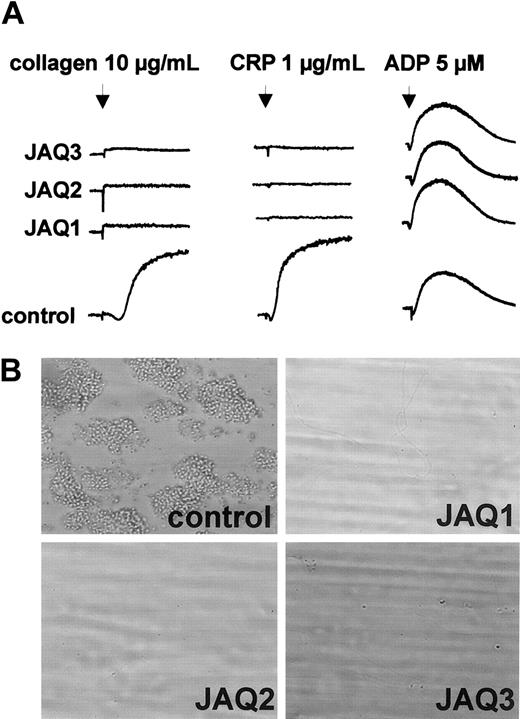
To test the effects of JAQ2 and JAQ3 on the in vivo expression of GPVI, mice received 100 μg of the respective antibodies and platelets were monitored for 10 days. As controls, mice received 100 μg nonspecific rat immunoglobulin or DOM2, a rat IgG2a directed against mouse GPV. Similar to JAQ1,18 JAQ2, JAQ3, and DOM2 had a mild and transient effect on platelet counts with a maximum drop of about 40% on day 1 and a return to almost normal after 48 to 72 hours (Figure3A). Circulating platelets in JAQ-treated mice were not activated at any time point after injection as shown by flow cytometric analysis of P-selectin expression and integrin activation19 (Figure 3B). GPVI expression on the platelet surface was analyzed by flow cytometry at different time points after antibody injection using JAQ1FITC. Very unexpectedly, as soon as 6 hours after antibody injection GPVI was undetectable on platelets from JAQ2- or JAQ3-treated mice and this remained unchanged for 10 days (Figure 3C). In contrast, GPVI levels on circulating platelets were not altered significantly at any time in DOM2-treated mice. These findings suggested that GPVI had been specifically depleted by JAQ2 and JAQ3 in vivo. This was confirmed when separate groups of mice received the antibodies (100 μg/mouse) and platelets were tested on day 5 for the presence of GPVI in Western blot analysis. As shown in Figure 3D, GPVI was undetectable in platelets from mice treated with JAQ1, JAQ2, or JAQ3, whereas normal amounts of GPIIIa were found in all platelets. Importantly, the expression of other receptors including GPIb-IX, GPV, and α2β1 was not altered in any group of mice as confirmed by flow cytometry (not shown). In agreement with the specific absence of GPVI, these platelets were completely resistant to collagen- and CRP-induced aggregation but reacted normally to ADP (5 μM; Figure 4A). Finally, adhesion of those platelets to collagen was tested in whole blood perfusion experiments (1000 s−1). Whereas marked platelet adhesion and aggregate formation was observed with blood from control mice, adhesion was completely abolished with blood from JAQ1-, JAQ2-, or JAQ3-treated mice, confirming the crucial role of GPVI in this process8 21 (Figure 4B).
JAQ1, JAQ2, and JAQ3 deplete GPVI in vivo.
(A) Platelet counts from antibody-treated mice were monitored over 10 days after injection of the indicated antibodies (all 100 μg). Results are expressed as mean ± SD of 9 mice/group. (B) Flow cytometric analysis of P-selectin expression and GPIIb/IIIa activation on platelets from JAQ1-, JAQ2-, JAQ3-treated mice or control mice on day 3 after injection. The results shown are representative of 6 mice/group. Similar results were obtained at all other time points tested. (C) Diluted blood collected at the indicated time points after antibody injection was incubated with FITC-labeled JAQ1 (5 μg/mL) for 15 minutes at RT and analyzed directly. Results are presented as mean ± SD of 9 mice/group. (D) Whole platelet proteins were separated by SDS-PAGE under nonreducing conditions and immunoblotted with HRP-labeled JAQ1 (anti-GPVI) or EDL1 (anti-GPIIIa) followed by ECL.
JAQ1, JAQ2, and JAQ3 deplete GPVI in vivo.
(A) Platelet counts from antibody-treated mice were monitored over 10 days after injection of the indicated antibodies (all 100 μg). Results are expressed as mean ± SD of 9 mice/group. (B) Flow cytometric analysis of P-selectin expression and GPIIb/IIIa activation on platelets from JAQ1-, JAQ2-, JAQ3-treated mice or control mice on day 3 after injection. The results shown are representative of 6 mice/group. Similar results were obtained at all other time points tested. (C) Diluted blood collected at the indicated time points after antibody injection was incubated with FITC-labeled JAQ1 (5 μg/mL) for 15 minutes at RT and analyzed directly. Results are presented as mean ± SD of 9 mice/group. (D) Whole platelet proteins were separated by SDS-PAGE under nonreducing conditions and immunoblotted with HRP-labeled JAQ1 (anti-GPVI) or EDL1 (anti-GPIIIa) followed by ECL.
In vitro analysis of GPVI-depleted platelets.
(A) Heparinized prp from mice on day 5 after treatment with irrelevant IgG2a or the indicated anti-GPVI mAb was stimulated with collagen, CRP, or ADP and light transmission was recorded. The results shown are representative of 6 mice/group. (B) Whole blood from mice treated with irrelevant IgG2a, JAQ1, JAQ2, or JAQ3 was perfused at wall shear rates of 1000 s−1 (4 minutes) over a collagen-coated surface and representative phase-contrast microscope images were taken (n = 3-6). Original magnification, × 630.
In vitro analysis of GPVI-depleted platelets.
(A) Heparinized prp from mice on day 5 after treatment with irrelevant IgG2a or the indicated anti-GPVI mAb was stimulated with collagen, CRP, or ADP and light transmission was recorded. The results shown are representative of 6 mice/group. (B) Whole blood from mice treated with irrelevant IgG2a, JAQ1, JAQ2, or JAQ3 was perfused at wall shear rates of 1000 s−1 (4 minutes) over a collagen-coated surface and representative phase-contrast microscope images were taken (n = 3-6). Original magnification, × 630.
To address the question whether the Fc part or the dimeric form of anti-GPVI antibodies plays a role in depletion of the receptor, we produced monovalent F(ab) fragments of JAQ1, JAQ2, and JAQ3 and tested their in vivo effects. After injection of F(ab) fragments (100 μg), platelet counts and GPVI expression were monitored for 10 days. Interestingly, F(ab) fragments of JAQ2 or JAQ3 induced a similar transient decrease in platelet counts as the intact IgG (Figure5A), demonstrating that this effect was not mediated by antibody-induced Fc receptor or complement activation. Strikingly, both F(ab) fragments of JAQ2 and JAQ3 induced the depletion of GPVI (Figure 5B). However, the duration of F(ab)–induced GPVI depletion was significantly reduced as compared to intact IgG. As shown in Figure 5B-C, very low levels of GPVI were detected in platelets on day 3 by flow cytometry and Western blot analysis, respectively, and this increased to almost normal on day 10. Consequently, during the first 3 days, platelets from these mice were resistant to activation with CRP (Figure 5D) and did not adhere to collagen in whole blood perfusion experiments (not shown).
In vivo depletion of GPVI by monovalent F(ab) fragments of JAQ1, JAQ2, and JAQ3.
(A) Platelet counts were monitored for 10 days after injection of the indicated F(ab) fragments or control IgG (all 100 μg). Results are expressed as mean ± SD of 6 mice/group. (B) Flow cytometric analysis of GPVI expression on platelets from mice treated with JAQ1, JAQ2, JAQ3 F(ab) fragments or control IgG. Diluted blood collected at the indicated time points was incubated with FITC-labeled JAQ1 (5 μg/mL) for 15 minutes at RT and analyzed directly. Results are presented as mean ± SD of 6 mice/group. (C) Western blot analysis of GPVI expression on day 3 after injection of anti-GPVI F(ab) fragments. Whole platelet proteins were separated by SDS-PAGE under nonreducing conditions and immunoblotted with HRP-labeled JAQ1 (anti-GPVI) or EDL1 (anti-GPIIIa) followed by ECL. (D) Platelets from F(ab) fragment-treated or control mice were stimulated with CRP (5 μg/mL) and analyzed for P-selectin expression and GPIIb/IIIa activation by flow cytometry. The results shown are representative of 6 mice/group.
In vivo depletion of GPVI by monovalent F(ab) fragments of JAQ1, JAQ2, and JAQ3.
(A) Platelet counts were monitored for 10 days after injection of the indicated F(ab) fragments or control IgG (all 100 μg). Results are expressed as mean ± SD of 6 mice/group. (B) Flow cytometric analysis of GPVI expression on platelets from mice treated with JAQ1, JAQ2, JAQ3 F(ab) fragments or control IgG. Diluted blood collected at the indicated time points was incubated with FITC-labeled JAQ1 (5 μg/mL) for 15 minutes at RT and analyzed directly. Results are presented as mean ± SD of 6 mice/group. (C) Western blot analysis of GPVI expression on day 3 after injection of anti-GPVI F(ab) fragments. Whole platelet proteins were separated by SDS-PAGE under nonreducing conditions and immunoblotted with HRP-labeled JAQ1 (anti-GPVI) or EDL1 (anti-GPIIIa) followed by ECL. (D) Platelets from F(ab) fragment-treated or control mice were stimulated with CRP (5 μg/mL) and analyzed for P-selectin expression and GPIIb/IIIa activation by flow cytometry. The results shown are representative of 6 mice/group.
Discussion
Our findings show that neither the Fc part nor the dimeric form of anti-GPVI antibodies is responsible for the observed transient thrombocytopenia or the depletion of the receptor. However, the significantly shorter absence of GPVI in F(ab) versus IgG-treated mice strongly suggests that in vivo stability and avidity of anti-GPVI agents may have a major influence on their potential to induce long-term antithrombotic protection. At present, the mechanisms underlying anti-GPVI–induced transient thrombocytopenia are not clear, but it may be based on weak activation of GPIIb/IIIa leading to the formation of loose aggregates and their temporary sequestration to the spleen where the actual loss of GPVI may occur. This assumption may be supported by the observation that GPVI down-regulation cannot be induced by antibodies in vitro, suggesting that a second signal is required for the induction of this process and that this signal is provided by other cells in vivo.18
In summary, our results demonstrate that the antibody-induced depletion of GPVI from circulating platelets in vivo is neither dependent on the exact binding epitope recognized by the antibody nor its Fc part or dimeric form. This unexpected finding strongly suggests that anti-GPVI agents that do not directly activate the platelet may generally induce the down-regulation of the receptor in vivo, resulting in long-term antithrombotic protection. This may have important implications for the development of anti-GPVI– based therapeutics for the prevention of ischemic cardiovascular diseases.
We would like to thank M. Koch and S. Hartmann for excellent technical assistance and U. Barnfred for constant support throughout the study.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-10-3242.
Supported by grant Ni556/4-1 (B.N.) from the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bernhard Nieswandt, Rudolf Virchow Center for Experimental Biomedicine, University of Würzburg, Versbacher Str 9, 97078 Würzburg, Germany; e-mail:bernhard.nieswandt@virchow.uni-wuerzburg.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal