Abstract
A controlled study has been carried out to assess the efficacy of rituximab, a chimeric antibody that binds to the B-cell surface antigen CD20, in 20 patients with mixed cryoglobulinemia (MC) and hepatitis C virus (HCV)–positive chronic active liver disease, resistant to interferon α (IFN-α) therapy. They received an intravenous infusion of 375 mg/m2 rituximab once a week for 4 consecutive weeks. Infusion of rituximab had a good safety profile and no severe side effects were reported. Sixteen patients (80%) showed a complete response (CR), characterized by rapid improvement of clinical signs (disappearance of purpura and weakness arthralgia and improvement of peripheral neuropathy), and decline of cryocrit. CR was associated with a significant reduction of rheumatoid factor (RF) activity and anti-HCV antibody titers. Decline of IgG anti-HCV titers in the cryoprecipitates was usually associated with a favorable response (r = 0.81; P < .005). No differences in the dynamics of B-cell depletion and recovery were found between responders and nonresponders. Molecular monitoring of the B-cell response revealed disappearance/deletion of peripheral clones in the responders and great stability in the nonresponders. Rituximab had a deep impact on hepatitis C viremia; HCV RNA increased approximately twice the baseline levels in the responders, whereas it remained much the same in the nonresponders. Twelve (75%) of 16 responders remained in remission throughout the follow-up. The results indicate that rituximab has clinical and biologic activity in patients with HCV+ MC. However, in view of the increased viremia in the responders, additional modes of application and combination of rituximab with other agents need to be investigated.
Introduction
Mixed cryoglobulinemia (MC) is a chronic immune complex–mediated disease.1 Cryoglobulins are cold precipitable immunoglobulins that are accounted for by 2 or more immunoglobulin isotypes, with (type II) or without (type III) a monoclonal component.2 MC is frequently associated with the development of vascular, renal, and neurologic lesions.3 Oligoclonal or monoclonal B-cell expansions are significant molecular features of bone marrow4 and liver.5 It is estimated that almost 10% of patients with MC progress to frank B-cell non-Hodgkin lymphoma (NHL).6
The association between hepatitis C virus (HCV) and MC has been rendered evident since the recognition of serologic markers of HCV infection.7-9 In Italy, more than 80% of MC patients are infected with HCV.10 The primary role of HCV in the mechanism of cryoprecipitation is mainly suggested by its selective concentration in cryoglobulins.11 12
Based on the close correlation between HCV infection and MC, treatment with interferon α (IFN-α) has been strongly advocated.13 IFN-α produces significant clinical improvement in 40% to 70% of MC patients.14-17 Its efficacy is closely associated with inhibition of HCV replication. Reduction of HCV RNA in responsive patients usually precedes decline of cryocrit. However, the improvement is generally short-lived and more than 80% of responders have a relapse within 6 months.17In addition, in patients with cryoglobulinemic neuritic or nephropathic complications or active skin ulcers, IFN-α frequently precipitates neuropathies or aggravates renal failure and delays ulcer healing.18
This has led us to search for innovative therapeutic strategies with the aim of reducing or depleting the B-cell clonal expansion that sustains production of IgM rheumatoid factor (RF) molecules. One such approach involves the use of monoclonal antibodies directed to CD20 antigen, a transmembrane protein expressed on pre-B lymphocytes and mature lymphocytes.19 Rituximab, a humanized murine monoclonal antibody of this kind, is highly effective for in vivo B-cell depletion.20 Peripheral blood B lymphocytes become undetectable after a single infusion and recover 6 to 9 months after discontinuation of treatment.21 Rituximab was originally approved for the treatment of low-grade B-cell non-Hodgkin lymphoma (NHL).22 It has since become a promising therapeutic approach for diffuse large B-cell lymphomas, mantle cell lymphoma,23 hairy cell leukemia,20 and chronic lymphocytic leukemia.24 It has also been used in several other hematologic disorders including pure red cell aplasia and hemolytic anemia,25 primary cold agglutinin disease,26 posttransplantation B-lymphoproliferative disorders,27 Waldenström macroglobulinemia,28 and idiopathic thrombocytopenic purpura.29
The present report summarizes the results of a 4-dose rituximab course in HCV+ MC patients who failed to respond to IFN-α therapy. Clinical observations were accompanied by monitoring of immunophenotypic profile of lymphocytes, molecular B-cell response, specific anti-HCV response, and HCV viremia.
Patients, materials, and methods
Patients
Twenty MC patients with histologically proven chronic active liver disease entered the study. They had detectable serum cryoglobulins associated with the triad of purpura-weakness-arthralgia, and were positive for antibodies to HCV and HCV RNA and negative for hepatitis B surface antigen (HBsAg) and anti-HIV antibodies. All but one had chronic active hepatitis without cirrhosis. Twelve showed a histologic activity index below 4. One patient presented with frank cirrhosis coupled with a high activity index. All patients were treated with IFN-α therapy for 1 year or longer. Twelve were considered nonresponders, whereas the remaining 8 achieved a partial remission, but had a relapse within 6 months after IFN-α discontinuation. Most patients were previously given low to moderate doses of corticosteroids for several weeks or months, but none of them had received any treatment for at least 6 months before the enrollment into this study.
Exclusion criteria were: (1) pregnancy; (2) concomitant serious illness likely to preclude completion of the study; (3) hepatic failure characterized by history of ascites, bleeding, esophageal varices, hepatic encephalopathy, bilirubin level more than 3 mg/dL, serum albumin level less than 3 g/dL, and prothrombin time 3 seconds longer than that of controls; (4) leukocyte count less than 3 × 109/L or hemoglobin level less than 10 g/dL; and (5) positive test for antibodies to HIV.
The study was approved by the Institutional Ethical Committee and written informed consent was obtained from all patients. Rituximab was diluted in normal saline up to a maximum concentration of 1 mg/mL. Patients were scheduled to receive 4 intravenous infusions of 375 mg/m2 once a week over a period of 1 month. Premedication with acetaminophen and diphenhydramine as well as sufficient hydration was recommended. Infusion was started at 50 mg/h during the first hour and increased to a final 400 mg/h if well tolerated. The use of concomitant corticosteroids was limited to the treatment of severe allergic reactions.
Baseline evaluation included disease history and stage, actual signs and symptoms, and previous medication. Physical examination, laboratory values, adverse events, and formulation of stereotyped questions30 were recorded weekly throughout the treatment. Hematologic and physical examinations were then performed monthly up to 12 months. Laboratory evaluation at baseline included complete hemogram, serum chemistry profile, direct and indirect Coombs test, and serologic tests for hepatitis B virus (HBV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), and HIV. Immunology included C3 and C4 fractions of complement, RF activity, and immunoglobulin concentration. Neurologic assessment included strength evaluation,31electromyography, motor and sensory nerve conduction velocity, and short-latency somatosensory-evoked potentials.
Mononuclear cells were isolated from fresh blood by Lymphoprep separation (Nycomed Pharma, Oslo, Norway), washed and stained with fluorochrome-conjugated antibodies against CD19, CD20, CD3, CD4, CD8 (fluorescein isothiocyanate [FITC]–labeled; Becton-Dickinson, San Diego, CA), CD5 and HLA-DR (phycoerythrin [PE]–conjugated; Immunotech, Marseille, France). Samples were analyzed in single or double immunofluorescence on a FACScan instrument (Becton-Dickinson) before and after each rituximab infusion and then monthly up to 12 months.
The primary efficacy was the objective response rate, that is, the proportion of patients achieving a complete response (CR) at any time. CR was based on previously established criteria17 and defined as reduction of cryocrit to less than 75% of the initial value associated with at least 2 of the following: disappearance of purpura, arthralgia, and weakness and improvement of neuropathy. Relapse from CR was defined as an increase of the cryocrit to more than 50% of the decrement value, along with deterioration of one or more clinical parameters.
An adverse event was defined as any adverse change from the patient's baseline condition, whether related to the treatment or not. Each event after rituximab infusion was graded according to the National Cancer Institute Common Toxicity Criteria grading system.32Grades 3 and 4 events plus grade 2 infections were recorded in detail, grade 1 and 2 events were not.
The results were expressed as means ± SD. Differences between dichotomous variables were analyzed by Fisher exact test. Continuous variables were compared by Student t test. Multivariate logistic regression was used to analyze the response to treatment. Rate of remission was calculated with life-table analysis.
Cryoprecipitate preparation was carried out as specified elsewhere.13 Cryoprecipitates diluted in 0.5 M NaCl were fractionated by high-resolution gel electrophoresis to type cryoglobulins. Individual monoclonal bands were identified by immunofixation after electrophoresis using a cellulose acetate strip impregnated with antibodies specific for heavy and light chains (Dako, Copenaghen, Denmark).
HCV RNA in unfractionated sera, supernatants, and corresponding cryoprecipitates was determined using a reverse transcription-polymerase chain reaction (RT-PCR) test (Amplicor HCV; Roche Diagnostic Systems, Branchburg, NY) and quantified with the Quantiplex HCV RNA kit (Chiron, Emeryville, CA) based on the quantitative branched DNA signal amplification assay.33
HCV genotypes were determined with the line probe assay (Inno-Lipa HCV; Innogenetics, Zwijnaarde, Belgium). This identifies HCV types and subtypes. Measures to prevent contamination were used at all times.
Anti-HCV antibodies directed to structural and nonstructural proteins were detected in unfractionated serum samples, cryoprecipitates and corresponding supernatants using a microparticle enzyme immunoassay (AxSYM HCV 3.0; Abbott, Rome, Italy). Results were calculated as optical density (OD) sample/OD cutoff and were considered positive when the ratio was greater than 1.1.
Molecular analysis of B-cell clonality
DNA was purified from circulating lymphocytes by phenol-chloroform extraction according to standard protocols. Samples from heparinized blood were immediately used for mononuclear cell isolation by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) gradient centrifugation. Recovered mononuclear cells (2 × 106cells) were processed for DNA preparation according to the method previously described.34 Spectrophotometry with a Genequant DNA/RNA calculator (Pharmacia) was used to quantitate DNA.
For each sample 0.5 to 1 μg DNA was processed in the PCR analysis for B-cell clonal expansion using a seminested protocol of amplification, according to well-established procedures.35 The upstream primer was complementary to the third framework V region (Fr3, 5′-ACACGGC[C/T][G/C]TGTATTACTGT-3′) of the IgH gene; the downstream primer was directed to an outer conserved region of the IgH J (5′-TGAGGAGACGGTGACC-3′) in the first round of amplification and to an inner conserved sequence of the same J region in the second round (5′-GTGACCAGGGTNCCTTGGCCCCAG-3′). DNA was amplified for 30 cycles in the first round and for 20 cycles in the second. Each cycle consisted of 94°C for 45 seconds, 50°C for 45 seconds, and 72°C for 30 seconds, with an additional extension interval of 5 minutes at 72°C after the last cycle. The sensitivity of the technique was checked by the amplification of serial dilutions of DNA from clonal B cells admixed with DNA from polyclonal B cells. The detection threshold was estimated to be about 0.5% to 1%.36
For each experiment, a control was included using primers for the β-actin gene, which resulted in a single band of 300 base pair (bp). In all cases, a β-actin PCR product was identified by gel electrophoresis to check quality, quantity, and the presence of potential contaminants.
PCR products were analyzed by electrophoresis in 5% agarose gel (Seaken LE, FMC Bioproduct, Rockland, ME) in TBE buffer (100 mM Tris [tris(hydroxymethyl)aminomethane], 100 mM boric acid, 2 mM EDTA [ethylenediaminetetraacetic acid]), stained with ethidium bromide, and optically evaluated by UV transillumination. Clonal expansion was indicated by one or more dominant bands within the predicted size and a fully polyclonal pattern by a smear with no specific dominant bands.
A monoclonal B-cell expansion was defined as 1 or 2 (if both alleles were rearranged) discrete narrow band(s) within the predicted size. Distinction of biclonal from monoclonal biallelic rearrangements was based on the results of subsequent sequence analyses, in that a nonfunctional rearrangement of 1 of the 2 alleles (ie, one dominant band) was detected in the case of a monoclonal disorder, whereas both dominant bands were representative of a functional IgH rearrangement in biclonal disorders. An oligoclonal picture was represented by 3 or more discrete bands.
Cloning and sequencing analyses
PCR products amplified from lymphocyte DNA were run on agarose gel in Tris-borate-EDTA buffer and stained with ethidium bromide, according to standard procedures. Individual bands were excised from the gel, cloned, and sequenced. DNA was purified using the QIAEX II Gel extraction kit (Qiagen, Hilden, Germany), ligated into a pGem-T cloning vector (Promega, Madison, WI), and transfected into Escherichia coli DH5α-competent cells. Transfected cells were plated onto Luria-Bertani (LB)–ampicillin agar plates containing X-gal and isopropylthiogalactose. Uncolored colonies were selected at random and cultured. Plasmid DNA was purified with the Wizard Plus Minipreps DNA Purification System (Promega).
Sequence reactions were carried out on an ABI Prism 310 Genetic Analyzer (Perkin Elmer, Foster City, CA). All sequences were confirmed by sequencing in both directions with primers T7 and SP6. Ten or more clones were sequenced for each dominant band. Sequences were considered to be related if they shared the same CDRH3 regions, but had differences in the number of point mutations. Where a sequence showed similarity to more than one DH region, all possible DH regions in the CDRH3 were identified and those that could be assigned without overlap, and with the smallest number of nucleotides in between, were used.
Results
The main initial laboratory features and histologic findings of the 4 men and 16 women enrolled in this study are summarized in Table1. Cryoglobulins were classified as mixed type II in 13 (65%) and type III in 7 (35%).
Laboratory, virologic, and histologic findings in 20 patients with MC at entry into the study
| Laboratory findings | |
| Cryocrit, %, mean ± SD | 9.45 ± 7.15 |
| Cryoglobulin immunochemical types, no. of patients (%) | |
| II | 13 (65) |
| III | 7 (35) |
| γ-globulin level (normal, 0.7-1.6 g/dL) | 1.2 ± 0.59 g/dL |
| IgM value (normal, 40-230 mg/dL, mean ± SD) | 472.87 ± 308.9 mg/dL |
| RF activity (normal, 20 IU/mL or less, mean ± SD) | 528.31 ± 1095.7 IU/mL |
| Complement fractions | |
| C3 (normal, 80-140 mg/dL) | 113.93 ± 31.2 mg/dL |
| C4 (normal, 15-48 mg/dL) | 5.43 ± 3.8 mg/dL |
| Virologic findings | |
| Anti-HCV antibodies | |
| Positive, no. of patients (%) | 20 (100) |
| Titer, OD sample/OD cutoff | 80.55 ± 47.8 |
| HCV RNA | |
| Positive, no. of patients (%) | 20 (100) |
| Titer, IU/mL | 542 718 ± 354 439 IU/mL |
| HCV genotype, no. of patients (%) | |
| Type 1b | 11 (55) |
| Type 2a/2c | 9 (45) |
| Histologic findings, no. of patients (%) | |
| Chronic active hepatitis | 19 (95) |
| Cirrhosis | 1 (5) |
| Laboratory findings | |
| Cryocrit, %, mean ± SD | 9.45 ± 7.15 |
| Cryoglobulin immunochemical types, no. of patients (%) | |
| II | 13 (65) |
| III | 7 (35) |
| γ-globulin level (normal, 0.7-1.6 g/dL) | 1.2 ± 0.59 g/dL |
| IgM value (normal, 40-230 mg/dL, mean ± SD) | 472.87 ± 308.9 mg/dL |
| RF activity (normal, 20 IU/mL or less, mean ± SD) | 528.31 ± 1095.7 IU/mL |
| Complement fractions | |
| C3 (normal, 80-140 mg/dL) | 113.93 ± 31.2 mg/dL |
| C4 (normal, 15-48 mg/dL) | 5.43 ± 3.8 mg/dL |
| Virologic findings | |
| Anti-HCV antibodies | |
| Positive, no. of patients (%) | 20 (100) |
| Titer, OD sample/OD cutoff | 80.55 ± 47.8 |
| HCV RNA | |
| Positive, no. of patients (%) | 20 (100) |
| Titer, IU/mL | 542 718 ± 354 439 IU/mL |
| HCV genotype, no. of patients (%) | |
| Type 1b | 11 (55) |
| Type 2a/2c | 9 (45) |
| Histologic findings, no. of patients (%) | |
| Chronic active hepatitis | 19 (95) |
| Cirrhosis | 1 (5) |
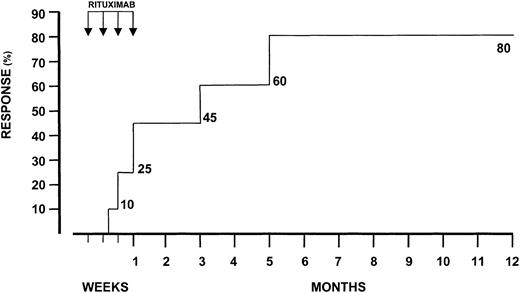
CR was achieved by 16 (80%) patients. Mean length of disease, HCV genotype 1b distribution, and mean age prior to treatment were not different between responders and nonresponders (12.4 ± 7.7 versus 10.3 ± 6.9 years; 56.2% versus 50%; 56.8 ± 13.6 versus 59.4 ± 12.8 years). Average cryocrit values were also similar (8.6% ± 6.4% versus 10.3% ± 7.9%). The mean cryocrit value regressed to 2.1% ± 2.6% in the responders. In 3 patients (18.7%), cryoglobulins stably disappeared throughout the observation period. As depicted in Figure1, the probability of CR 2 months after therapy was 62.5% (10 of 16). The overall response rate occurred within the next 5 months.
Probability of complete response during treatment with rituximab.
Arrows indicate the number of infusions.
Probability of complete response during treatment with rituximab.
Arrows indicate the number of infusions.
Frequency of organ-related signs and symptoms before and after rituximab treatment is reported in Table2. Purpura was present in 14 of the 16 responders and rapidly disappeared in 12 (87.5%). Rapid disappearance was also noted for cutaneous urticarial manifestations, whereas features and extension of livedo reticularis remained unchanged. Interestingly, long-lasting leg ulcers showed a consistent reduction followed by dramatic healing in 3 of 5 patients. Peripheral neuropathy, occurring in 12 patients (75%) at the time of enrollment, improved in 6 (50%). Positive changes in motor conduction were recorded, associated with improvement of motor and sensory nerve conduction. No changes of muscular strength were noticed. Weakness and arthralgia were no longer recorded in 7 and 8 patients, respectively. A dramatic improvement of muscular pain and morning stiffness was reported. On the contrary, no significant improvement of renal functional parameters was detected in the only patient with kidney involvement. Finally, both frequency and size of hepatomegaly and mean serum transaminase levels were substantially unchanged.
Clinical assessment of responsive and nonresponsive patients at baseline and 12 months after rituximab treatment
| Organ-related signs . | Responders (n = 16) . | Nonresponders (n = 4) . | ||
|---|---|---|---|---|
| Start (%) . | End (%) . | Start (%) . | End (%) . | |
| Skin | ||||
| Purpura | 14 (87.5) | 2 (12.5) | 2 (50) | 2 (50) |
| Urticarial lesions | 3 (18.7) | 0 | 0 | 0 |
| Livedo reticularis | 4 (25) | 4 (25) | 1 (25) | 1 (25) |
| Leg ulcers | 5 (31.2) | 2 (12.5) | 2 (50) | 2 (50) |
| Peripheral nerves | ||||
| Muscular strength deficiency | 6 (37.5) | 6 (37.5) | 1 (25) | 1 (25) |
| Impairment of nerve conduction velocity | 12 (75) | 6 (37.5) | 2 (50) | 2 (50) |
| Motor | ||||
| Amplitude (normal, 10 ± 3.8 mV) | 6.6 ± 4.0 | 9.34 ± 3.1 | 5.9 ± 3.8 | 6.2 ± 5.4 |
| Distal latency (normal, 4.3 ± 0.8 m/s) | 2.1 ± 2.6 | 3.8 ± 1.1 | 1.9 ± 1.4 | 1.8 ± 2.3 |
| Maximal motor conduction (normal, 46.6 ± 6 m/s) | 29.4 ± 16.0 | 35.4 ± 7.7* | 25.5 ± 21.0 | 24.3 ± 19.0 |
| Sensory | ||||
| Amplitude (normal, 12.8 ± 6.7 μV) | 4.3 ± 3.1 | 5.9 ± 4.0 | 5.0 ± 6.6 | 4.4 ± 5.1 |
| Maximal conduction velocity (normal, 43.2 ± 2.2 m/s) | 27.6 ± 16.0 | 34.9 ± 11.4 | 25.5 ± 6.0 | 27.0 ± 10.3 |
| Symptomatology | ||||
| Parenthesia | 10 (62.5) | 6 (37.5) | 1 (25) | 1 (25) |
| Dysesthesia | 2 (12.5) | 0 | 1 (25) | 1 (25) |
| Musculoskeletal | ||||
| Weakness | 16 (100) | 9 (56.2) | 4 (100) | 4 (100) |
| Morning stiffness | 11 (68.7) | 5 (31.2) | 3 (75) | 3 (75) |
| Peripheral joint arthralgias | 16 (100) | 8 (50) | 4 (100) | 4 (100) |
| Muscle pain | 16 (100) | 4 (25) | 4 (100) | 3 (75) |
| Kidney | ||||
| Creatinine clearance reduction | 1 (6.2) | 1 (6.2) | 0 | 0 |
| Proteinuria (normal, 0.23 g/dL or less) | 1.2 | 1.6 | 0 | 0 |
| Liver | ||||
| Hepatomegaly | 9 (56) | 9 (56) | 2 (50) | 2 (50) |
| ALT serum levels (normal, less than 30 IU/mL) | 64.0 ± 67.7 | 71.0 ± 48.0 | 46.0 ± 23.0 | 64.0 ± 88.0 |
| Organ-related signs . | Responders (n = 16) . | Nonresponders (n = 4) . | ||
|---|---|---|---|---|
| Start (%) . | End (%) . | Start (%) . | End (%) . | |
| Skin | ||||
| Purpura | 14 (87.5) | 2 (12.5) | 2 (50) | 2 (50) |
| Urticarial lesions | 3 (18.7) | 0 | 0 | 0 |
| Livedo reticularis | 4 (25) | 4 (25) | 1 (25) | 1 (25) |
| Leg ulcers | 5 (31.2) | 2 (12.5) | 2 (50) | 2 (50) |
| Peripheral nerves | ||||
| Muscular strength deficiency | 6 (37.5) | 6 (37.5) | 1 (25) | 1 (25) |
| Impairment of nerve conduction velocity | 12 (75) | 6 (37.5) | 2 (50) | 2 (50) |
| Motor | ||||
| Amplitude (normal, 10 ± 3.8 mV) | 6.6 ± 4.0 | 9.34 ± 3.1 | 5.9 ± 3.8 | 6.2 ± 5.4 |
| Distal latency (normal, 4.3 ± 0.8 m/s) | 2.1 ± 2.6 | 3.8 ± 1.1 | 1.9 ± 1.4 | 1.8 ± 2.3 |
| Maximal motor conduction (normal, 46.6 ± 6 m/s) | 29.4 ± 16.0 | 35.4 ± 7.7* | 25.5 ± 21.0 | 24.3 ± 19.0 |
| Sensory | ||||
| Amplitude (normal, 12.8 ± 6.7 μV) | 4.3 ± 3.1 | 5.9 ± 4.0 | 5.0 ± 6.6 | 4.4 ± 5.1 |
| Maximal conduction velocity (normal, 43.2 ± 2.2 m/s) | 27.6 ± 16.0 | 34.9 ± 11.4 | 25.5 ± 6.0 | 27.0 ± 10.3 |
| Symptomatology | ||||
| Parenthesia | 10 (62.5) | 6 (37.5) | 1 (25) | 1 (25) |
| Dysesthesia | 2 (12.5) | 0 | 1 (25) | 1 (25) |
| Musculoskeletal | ||||
| Weakness | 16 (100) | 9 (56.2) | 4 (100) | 4 (100) |
| Morning stiffness | 11 (68.7) | 5 (31.2) | 3 (75) | 3 (75) |
| Peripheral joint arthralgias | 16 (100) | 8 (50) | 4 (100) | 4 (100) |
| Muscle pain | 16 (100) | 4 (25) | 4 (100) | 3 (75) |
| Kidney | ||||
| Creatinine clearance reduction | 1 (6.2) | 1 (6.2) | 0 | 0 |
| Proteinuria (normal, 0.23 g/dL or less) | 1.2 | 1.6 | 0 | 0 |
| Liver | ||||
| Hepatomegaly | 9 (56) | 9 (56) | 2 (50) | 2 (50) |
| ALT serum levels (normal, less than 30 IU/mL) | 64.0 ± 67.7 | 71.0 ± 48.0 | 46.0 ± 23.0 | 64.0 ± 88.0 |
ALT indicates alanine aminotransferase.
Statistically significant.
Rituximab dramatically reduced the number of peripheral blood cells expressing CD20 antigen. The immunophenotype of lymphocyte subsets was always determined during and up to 12 months after treatment. The number of CD20+ and CD19+ cells in addition to that of CD20+/CD5+ and CD3−HLA-DR+ decreased distinctly against the baseline values and remained low for 6 to 7 months. T-cell counts (CD3+, CD4+, CD8+) were not modified.
The dynamics of B-cell depletion and recovery was similar in the responders and nonresponders. The percentage of CD20+ cells dropped in both responders (13.2% ± 8.8%) and nonresponders (11.2% ± 3.3%) to less than 1% after the first infusion. Recovery of B-cell count began from 6 months in responders (4% ± 2.2%) and nonresponders (5.6% ± 1.4%).
CRs were correlated with patients' characteristics to identify factors associated with a favorable response. Immunologic parameters were serially determined in sera, cryoprecipitates, and supernatants (Table3). No significant differences between responders and nonresponders were found for serum baseline levels of IgM, RF activity, IgG, and C3 and C4 fractions.
Immunologic parameters at baseline and 12 months after rituximab treatment
| . | Start . | End . | ||||
|---|---|---|---|---|---|---|
| . | Serum . | Supernatant . | Cryoprecipitate . | Serum . | Supernatant . | Cryoprecipitate . |
| Responders (n = 16) | ||||||
| IgM, mg/dL | 566.0 ± 281.4 | 263.0 ± 139.2 | 256.0 ± 220.2 | 336.5 ± 2303-150 | 324.6 ± 179.8 | 194.0 ± 238.8 |
| RF, IU/mL | 669.0 ± 1437.9 | 424.6 ± 346.1 | 642.4 ± 332.13-150 | 449.9 ± 1037.8 | 424.5 ± 346.5 | 514.7 ± 339.53-150 |
| IgG, mg/dL | 1553.2 ± 683.13-150 | 1155.6 ± 372.33-150 | 108.0 ± 124.2 | 1395.2 ± 665.9 | 1258.0 ± 508.2 | 67.6 ± 93.23-150 |
| C3, mg/dL | 124.3 ± 27.2 | 154.25 ± 41.5 | 1.8 ± 2.13-150 | 124.7 ± 34.9 | 141.8 ± 43.1 | 1.6 ± 3.3 |
| C4, mg/dL | 6.8 ± 4.2 | 5.6 ± 7.2 | 0.16 ± 0.113-150 | 8.8 ± 6.6 | 9.7 ± 7.6 | 0.1 ± 0.3 |
| Nonresponders (n = 4) | ||||||
| IgM, mg/dL | 414.2 ± 165.2 | 61.5 ± 173-150 | 410.5 ± 403.73-150 | 435.2 ± 389.4 | 53.0 ± 29.73-150 | 794.0 ± 2053-150 |
| RF, IU/mL | 489.7 ± 532.83-150 | 88.5 ± 17 | 600.5 ± 282.13-150 | 116.2 ± 74.43-150 | 81.5 ± 19 | 898.5 ± 680.9 |
| IgG, mg/dL | 1336.0 ± 504.1 | 715.5 ± 64.33-150 | 257.5 ± 266.6 | 1102.5 ± 439.7 | 888.0 ± 415.8 | 275.5 ± 328.8 |
| C3, mg/dL | 102.0 ± 25.0 | 118.5 ± 47.4 | 9.5 ± 0.73-150 | 98.5 ± 7.5 | 125.5 ± 41.7 | 2.5 ± 0.8 |
| C4, mg/dL | 4.0 ± 2.3 | 3.5 ± 2.1 | 0.5 ± 0.73-150 | 4.5 ± 1.7 | 3.5 ± 3.5 | 0.5 ± 0.4 |
| . | Start . | End . | ||||
|---|---|---|---|---|---|---|
| . | Serum . | Supernatant . | Cryoprecipitate . | Serum . | Supernatant . | Cryoprecipitate . |
| Responders (n = 16) | ||||||
| IgM, mg/dL | 566.0 ± 281.4 | 263.0 ± 139.2 | 256.0 ± 220.2 | 336.5 ± 2303-150 | 324.6 ± 179.8 | 194.0 ± 238.8 |
| RF, IU/mL | 669.0 ± 1437.9 | 424.6 ± 346.1 | 642.4 ± 332.13-150 | 449.9 ± 1037.8 | 424.5 ± 346.5 | 514.7 ± 339.53-150 |
| IgG, mg/dL | 1553.2 ± 683.13-150 | 1155.6 ± 372.33-150 | 108.0 ± 124.2 | 1395.2 ± 665.9 | 1258.0 ± 508.2 | 67.6 ± 93.23-150 |
| C3, mg/dL | 124.3 ± 27.2 | 154.25 ± 41.5 | 1.8 ± 2.13-150 | 124.7 ± 34.9 | 141.8 ± 43.1 | 1.6 ± 3.3 |
| C4, mg/dL | 6.8 ± 4.2 | 5.6 ± 7.2 | 0.16 ± 0.113-150 | 8.8 ± 6.6 | 9.7 ± 7.6 | 0.1 ± 0.3 |
| Nonresponders (n = 4) | ||||||
| IgM, mg/dL | 414.2 ± 165.2 | 61.5 ± 173-150 | 410.5 ± 403.73-150 | 435.2 ± 389.4 | 53.0 ± 29.73-150 | 794.0 ± 2053-150 |
| RF, IU/mL | 489.7 ± 532.83-150 | 88.5 ± 17 | 600.5 ± 282.13-150 | 116.2 ± 74.43-150 | 81.5 ± 19 | 898.5 ± 680.9 |
| IgG, mg/dL | 1336.0 ± 504.1 | 715.5 ± 64.33-150 | 257.5 ± 266.6 | 1102.5 ± 439.7 | 888.0 ± 415.8 | 275.5 ± 328.8 |
| C3, mg/dL | 102.0 ± 25.0 | 118.5 ± 47.4 | 9.5 ± 0.73-150 | 98.5 ± 7.5 | 125.5 ± 41.7 | 2.5 ± 0.8 |
| C4, mg/dL | 4.0 ± 2.3 | 3.5 ± 2.1 | 0.5 ± 0.73-150 | 4.5 ± 1.7 | 3.5 ± 3.5 | 0.5 ± 0.4 |
Statistically significant.
After fractionation, as compared with the supernatants, almost half of IgM molecules were retained in the cryoprecipitates in the responders, whereas they were significantly more concentrated in the nonresponders (P = .03). In step with the decrement of cryocrit levels, responders showed a significant decline of mean serum IgM levels (P = .002), whereas no significant changes were noted in the cryoprecipitates, suggesting that reduction of IgM did not modify the amount of cryoprecipitating molecules. In contrast, in nonresponders mean serum IgM levels did not change and significant increment in the cryoprecipitates was documented (P = .02).
As compared with the supernatants, RF activity was significantly more concentrated in the cryoprecipitates in both responders (P = .04) and nonresponders (P = .003). A decrement was documented in the sera (P = .15) and in cryoprecipitates (P = .02) in the responders after rituximab treatment. In the nonresponders, a significant decrement of RF activity in the sera was accompanied by an increment in the cryoprecipitates (P = .006).
Mean serum IgG levels, on the other hand, were significantly enriched in the supernatants of the responders (P = .002) and nonresponders (P =.046). After rituximab therapy mean decrement of about 40% was noted in the cryoprecipitates (P = .20) in responders, whereas no changes occurred in the nonresponders. Complement-related factors were also considered. C3 and C4 protein mean levels were significantly less represented in the cryoprecipitates as compared with supernatants in both responders (P = .0002 and P = .0006, respectively) and nonresponders (P = .008 and P = .001, respectively). No significant variations were noticed at the different time points.
Influence of rituximab on circulating viral load and anti-HCV titers is reported in Table 4. Mean HCV RNA baseline levels were similar in the 2 groups. After fractionation, HCV RNA was significantly enriched in the cold-precipitated fractions as compared with corresponding supernatants in the responders (P = .02) and the nonresponders (P = .01). Surprisingly, despite substantial reduction of cryocrit levels, HCV RNA increased to a significant extent in the responders. At the end of follow-up, a sustained increment of HCV RNA was achieved in unfractionated sera (P = .02), supernatants (P = .04), and cryoprecipitates (P = .016). These results were similarly validated when HCV RNA was normalized to cryoprecipitating proteins. After rituximab treatment, HCV RNA concentration in the cryoprecipitates significantly differed from baseline values (808 311 ± 712 865 IU/mg versus 569 277 ± 495 250 IU/mg, P = .02) whereas no significant changes were noted in the nonresponders.
Virologic parameters at baseline and 12 months after rituximab treatment
| . | Start . | End . | ||||
|---|---|---|---|---|---|---|
| . | Serum . | Supernatant . | Cryoprecipitate . | Serum . | Supernatant . | Cryoprecipitate . |
| Responders (n = 16) | ||||||
| HCV RNA, IU/mL | 477 231 ± 323 144 | 170 216 ± 141 228 | 2 846 136 ± 2 476 2524-150 | 765 667 ± 261 058 | 374 628 ± 367 060 | 4 041 559 ± 3 564 3274-150 |
| Anti-HCV antibody titer, sample/cutoff ratio | 91.0 ± 30.0 | 64.5 ± 42.5 | 21.3 ± 17.54-150 | 65.8 ± 41.58 | 56.7 ± 38.6 | 5.3 ± 3.54-150 |
| Nonresponders (n = 4) | ||||||
| HCV RNA, IU/mL | 452 667 ± 365 761 | 560 233 ± 776 427 | 3 039 259 ± 4 029 6984-150 | 456 333 ± 345 104 | 451 873 ± 249 449 | 2 881 900 ± 3 900 952 |
| Anti-HCV antibody titer, sample/cutoff ratio | 83.6 ± 34.3 | 52.6 ± 44.6 | 21.0 ± 24.14-150 | 73.07 ± 53.63 | 47.5 ± 40.0 | 26.4 ± 17.7 |
| . | Start . | End . | ||||
|---|---|---|---|---|---|---|
| . | Serum . | Supernatant . | Cryoprecipitate . | Serum . | Supernatant . | Cryoprecipitate . |
| Responders (n = 16) | ||||||
| HCV RNA, IU/mL | 477 231 ± 323 144 | 170 216 ± 141 228 | 2 846 136 ± 2 476 2524-150 | 765 667 ± 261 058 | 374 628 ± 367 060 | 4 041 559 ± 3 564 3274-150 |
| Anti-HCV antibody titer, sample/cutoff ratio | 91.0 ± 30.0 | 64.5 ± 42.5 | 21.3 ± 17.54-150 | 65.8 ± 41.58 | 56.7 ± 38.6 | 5.3 ± 3.54-150 |
| Nonresponders (n = 4) | ||||||
| HCV RNA, IU/mL | 452 667 ± 365 761 | 560 233 ± 776 427 | 3 039 259 ± 4 029 6984-150 | 456 333 ± 345 104 | 451 873 ± 249 449 | 2 881 900 ± 3 900 952 |
| Anti-HCV antibody titer, sample/cutoff ratio | 83.6 ± 34.3 | 52.6 ± 44.6 | 21.0 ± 24.14-150 | 73.07 ± 53.63 | 47.5 ± 40.0 | 26.4 ± 17.7 |
Statistically significant.
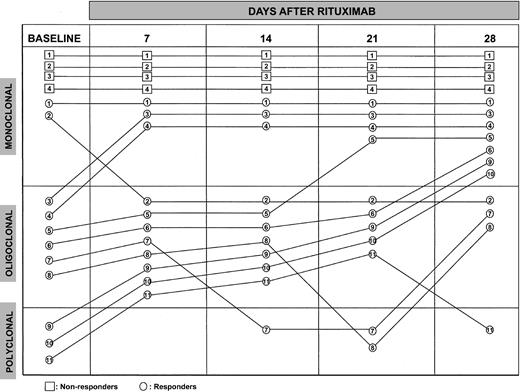
Anti-HCV reactivity titered semiquantitatively was initially similar in the 2 groups. After cold precipitation, the titers were less represented in the cryoglobulins compared with the supernatants in the responders (P = .019) and the nonresponders (P = .011). In the responders, they were significantly reduced in the supernatants by an average of 13% (P = .04) and more than 70% in the cryoprecipitates (P = .02). Regression analysis pointed to a highly significant correlation between decrement of anti-HCV titers in the cryoprecipitates and reduction of cryocrit levels (r = 0.81,P < .005). In sharp contrast, anti-HCV titers were virtually unchanged in the nonresponders. PCR directed toIgH VDJ genes on DNA extracted from peripheral blood cells was performed to analyze B-cell clonal restriction and determine the persistence or disappearance of originally expanded clones in 11 responders and the 4 nonresponders before and after each infusion (Figure 2). Gel agarose analysis showed that 2 and 6 responders had monoclonal (1 or 2 bands) and oligoclonal (≥ 3 bands) B-cell expansions, respectively, whereas polyclonality was found in the remaining patients. The 4 nonresponders displayed monoclonality at all times. Despite dramatic reduction of the number of circulating B cells, IgH VDJ PCR revealed persistence of B-cell clonotypes; one responder with a monoclonal picture changed to oligoclonal at day 7, the other remained unchanged. Four responders with initial oligoclonality displayed monoclonal restriction (2 at day 7, 1 at day 21, and 1 at day 28). The fifth responder presented a polyclonal picture at day 14 and became again oligoclonal at day 28; the sixth became polyclonal at day 21 and again oligoclonal at day 28. The 3 patients with polyclonal feature changed into oligoclonal at day 7; 2 became monoclonal at day 28, whereas in the other, transition to polyclonality occurred at day 28.
PCR analysis of peripheral B-cell clonal expansions before and during 4-dose rituximab.
PCR analysis of peripheral B-cell clonal expansions before and during 4-dose rituximab.
VH-CDR3 nucleotide sequences of dominant clonotypes in responders and nonresponders are reported in Table5. Sequence analyses were carried out in 5 responders and 3 nonresponders. In responders, initially expanded B-cell clones declined and disappeared, and new and distinct clonotypes appeared at varying time points, even in patients with a monoclonal pattern.
Nucleotide sequences of VDJ gene region of circulating B cell clones before and during rituximab treatment
| Patients . | Time-related therapy . | V-region . | N1 . | DHfamily . | D-region . | N2 . | J-region . | JH family . |
|---|---|---|---|---|---|---|---|---|
| Responsive | ||||||||
| 1 | Start | tgtgcaaaag | agtttgaccaggg | D4-17*01 | gagtacggtgctta | ggc | cttttgatatctgg | J3*02 |
| tgtgcgagag | atggtgcgccta | D3-22*01 | aatattatgatagtaggggata | ccccattagttatt | actactttgactactgg | J4*01 | ||
| tgtgcgaga | gcctt | D3-22*01 | tggttatgatagtagtgattattactac | t | actactgg | J4*01 | ||
| End | tgtgcgagagg | cgggcg | D6-19*01 | gagtataggagtgcctg | ccagt | tttgactactgg | J4*01 | |
| tgtgcggg | cagagag | D3-3*01 | cggcttggagtggttatta | gta | accctgactactgg | J4*03 | ||
| tgtgcgagacg | cagacagga | D4-23*01 | tgactccggtggtaa. . . . . | t | . .tggtttgactactgg | J4*03 | ||
| 1 mo follow-up | tgtgcaaaag | accgttcc | D1-26*01 | ggtataatgggagctactcc | cctcccacccccggtag | ttgacagctgg | J4*01 | |
| tgtgcg | D1-26*01 | gtgggggctgtga | aaagaat | gtttgactcctgg | J5*01 | |||
| tgtgcgaaag | ataatg | D3-3*01 | agtacggtttttggagtggctataa | atggggtcgtt | actactttgactcctgg | J4*01 | ||
| tgtgcgaaag | cccccg | D3-3*01 | aatatgagttttggagtggttat | cgggggaaatat | ttgactactgg | J4*01 | ||
| tgtgcgagag | attttatcc | D6-19*01 | gtcagagcactggctggt | ggttaa | ttgactactgg | J4*03 | ||
| 2 | Start | tgtgtgaaag | atct | D3-10*01 | tattataatggttccgggagctatt | ctccctaggg | actactactactacatggacgtctgg | J6*03 |
| tgtgcgaga | ggga | D6-19*01/in | gtcccgggcagtg | gc. . | tggattactgg | J4*01 | ||
| After first infusion | tgtgcgag | c | D6-19*01 | ttgggcagtggctggtac | gggggga | actactgg | J4*01 | |
| End | tgtgcgaga | ggggc | D3-3*01 | gtattacaattttttcggtg | attcttaccat | tacttggattactgg | J4*01 | |
| 1 mo follow-up | tgtgcgaga | atc | D5-18*01 | gtggatacagctgtaatt | ttatc | tgtttttgatttctgg | J3*02 | |
| 3 | Start | tgtgcgagag | aggggatacgag | D4-11*01/in | gttacagtaacc. . . . . . . . . . . . . . . . . . . . … | ct | . . . . . .tttgactactgg | J4*02 |
| tgtgtgagag. | D5-12*01 | . . . . . . .atatcggctatg. . . . . . | gccactttgactgctgg | J4*03 | ||||
| tgtgccaga | gagaggg | D6-19*01in | . . . . . . . . . . . . . . .cccgtacgagacc | ggcccctt | actactttgactactgg | J4*03 | ||
| End | tgtgcgagag | aggggatacgag | D4-11*01/in | gttacagtaacc. . . . . . . . . . . . . . . . . . . . … | ct | . . . . . .tttgactactgg | J4*01 | |
| 1 mo follow-up | tgtgcgaga | gaggggtttaa | D5-18*01 | . .ggatacagctatggttac | .cccctttgactactgg | |||
| 4 | Start | tgtgcgaaa | gaacagt | D4-23*01 | . .actacggtgacttc | ctcggt | . . . . . . . . . . . . . . . . . . . . .ggtatggacgtctgg | J6*02 |
| tgtgcaaca | gtgccc | D3-22*01 | .tcttactatgatagtagtggttatccata | .cccctttgactactgg | J4*03 | |||
| tgtgccaga | gagaggg | D6-19*01 | . . . . . . . . . . . . . . . .cccgtacgagacc. | ggcccctt | actactttgactactgg | J4*02 | ||
| tgtgcgaga | ga | D2-15*01 | tcgatattgtagtgct. . . . . . . . . . . . . . . . . | ggtagctgctttg | . .actggtacttcgatctctgg | J2*01 | ||
| tgtgcgaca | ttgtcacg | D4-17*01 | tgactacggtgactac | gtt | .tactactacaagggtatggacgtctgg | J6*02 | ||
| End | tgtgtgaga | gatgg | D3-10*01 | gtattattatggttcggggagttacccta. . | tg | . . . . . . . . . .gacgtctgg | J4*01 | |
| tgtgcgaga | gac | D3-10*01 | . .atcattatgattcggggaggtattata. . | . .aactggctcgacccctgg | J5*02 | |||
| tgtgcgaga | ggagggttagcc | D6-19*01 | gggtatagcagtggctggt. . | actactttgactactgg | J4*01 | |||
| tgtgcgaga | gatc | D2-15*01 | . .gatattggagtggtggtagctgct. . . . . . | ttg | . .actggtacttccatctctgg | J2*01 | ||
| 5 | Start | tgtgcgagag | atc | D1-26*01 | ggtatagtgggag. . . . . … | a | . .tactatgactactgg | J4*03 |
| tgtgcgaaag. | atag | D6-13*01 | ggacatagcagcagctgg… | cggg | . . . . . .tttgactactgg | J4*03 | ||
| tgtgcgaaag. | a | D5-24*01 | . . . . .taggagttttgg. . . . . . . . . . . . . . . … | ccgtct | agatgcttttgatatctgg | J3*02 | ||
| tgtgcgagag. | agggccggggcaa | D4-23*01 | tgacaaggggggtaaccc. | . . . . .ctttgattactgg | J4*01 | |||
| tgtgcgaaa | gaagtag | D5-12*01 | gtggatatagtggctacgccccc | cca | . . . . . . . . . . . . . . . . .tacggtatggacgtctgg | J6*02 | ||
| After first infusion | tgtgcgaga | gagggccggggcaa | D4-23*01 | tgacaagggaggta. . . . . . | acccctttgactactgg | J4*01 | ||
| tgtgcgaga | ggagcg | D5-18*01 | ggggagacagctatgg. . . . . | aagggttt | . . . . .tttgactgctgg | J4*03 | ||
| tgtgcgaga | gggaac | D6-13*01 | . .caccgcaactggtcc. . . . . . . . . . . . . . . . . | gaggggt | acaactggttcgacccctgg | J5*02 | ||
| tgtgcgaaa | gaagtag | D5-12*01 | gtggatacagtggctacg. . . . . . | ccccccca | . . . . . . . . . . . . . . . . .tacggtatggacgtctgg | J6*02 | ||
| tgtgcgag. | tc | D3-10*01 | . . . . . . . . . .aggcttcggggacttatt. . . . . . | ccggt | . . . . .tttgagttctgg | J4*03 | ||
| End | tgtgcgaga | gagggccggggcaa | D4-23*01 | tgacaagggaggta. . . . . . | acccctttgactactgg | J4*01 | ||
| 2 mo follow-up | tgtgcgag. | ggacc | D2-15*01 | . .gattttgtagtggtggtagctgcttc… | g | . .actggtacttcgatctctgg | J2*01 | |
| Patients | Time-related therapy | V-region | N1 | DHfamily | D-region | N2 | J-region | JH family |
| 3 mo follow-up | tgtgcgag. | ggacc | D2-15*01 | . .gattttgtagtggtggtagctgcttc… | g | . .actggtacttcgattactgg | J2*01 | |
| tgtgcgaga | gaggaccgagactgg | D5-18*01 | agattcagcttat… | . .tattttgactactgg | J4*03 | |||
| tgtgcgaaa | g | D5-12*01 | gtgagtatagtggttacg. . . . . . | ccccccca | . . . . . . . . . . . . . . . . .tacgggatggacgtctgg | J6*02 | ||
| gaagtag | ||||||||
| Unresponsive | ||||||||
| 6 | Start | tgtactag. | D2-8*02 | . . . . . . . . . . .tcctggtggctt. . . . . . . . . . . | taaa | . . . . . …gactactgg | J4*03 | |
| tgtgcgaga | ggtaaaac | D4-17*01 | …ctacggtgactac | gcc | .ggagcttttgatatctgg | J3*02 | ||
| End | tgtgcgaga | ggtaaaac | D4-17*01 | …ctacggtgactac | gcc | .ggagcttttgatatctgg | J3*02 | |
| 7 | Start | tgtgcgaga | D6-13*01 | . .gaaacgcagcagc. . . . . . . | c | gggtgcttttgatatctgg | J3*02 | |
| End | tgtgcgaga | D6-13*01 | . .gaaacgcagcagc. . . . . . . | c | gggtgcttttgatatctgg | J3*02 | ||
| 8 | Start | tgtgcgaga | gaatatgggaca | D3-22*01 | . . . . . .gatactagtggttattactac | t | . . . . . . . . . . .actactgg | J4*01 |
| End | tgtgcgaga | gaatatgggaca | D3-22*01 | . . . . . .gatactagtggttattactac | t | . . . . . . . . . . .actactgg | J4*01 |
| Patients . | Time-related therapy . | V-region . | N1 . | DHfamily . | D-region . | N2 . | J-region . | JH family . |
|---|---|---|---|---|---|---|---|---|
| Responsive | ||||||||
| 1 | Start | tgtgcaaaag | agtttgaccaggg | D4-17*01 | gagtacggtgctta | ggc | cttttgatatctgg | J3*02 |
| tgtgcgagag | atggtgcgccta | D3-22*01 | aatattatgatagtaggggata | ccccattagttatt | actactttgactactgg | J4*01 | ||
| tgtgcgaga | gcctt | D3-22*01 | tggttatgatagtagtgattattactac | t | actactgg | J4*01 | ||
| End | tgtgcgagagg | cgggcg | D6-19*01 | gagtataggagtgcctg | ccagt | tttgactactgg | J4*01 | |
| tgtgcggg | cagagag | D3-3*01 | cggcttggagtggttatta | gta | accctgactactgg | J4*03 | ||
| tgtgcgagacg | cagacagga | D4-23*01 | tgactccggtggtaa. . . . . | t | . .tggtttgactactgg | J4*03 | ||
| 1 mo follow-up | tgtgcaaaag | accgttcc | D1-26*01 | ggtataatgggagctactcc | cctcccacccccggtag | ttgacagctgg | J4*01 | |
| tgtgcg | D1-26*01 | gtgggggctgtga | aaagaat | gtttgactcctgg | J5*01 | |||
| tgtgcgaaag | ataatg | D3-3*01 | agtacggtttttggagtggctataa | atggggtcgtt | actactttgactcctgg | J4*01 | ||
| tgtgcgaaag | cccccg | D3-3*01 | aatatgagttttggagtggttat | cgggggaaatat | ttgactactgg | J4*01 | ||
| tgtgcgagag | attttatcc | D6-19*01 | gtcagagcactggctggt | ggttaa | ttgactactgg | J4*03 | ||
| 2 | Start | tgtgtgaaag | atct | D3-10*01 | tattataatggttccgggagctatt | ctccctaggg | actactactactacatggacgtctgg | J6*03 |
| tgtgcgaga | ggga | D6-19*01/in | gtcccgggcagtg | gc. . | tggattactgg | J4*01 | ||
| After first infusion | tgtgcgag | c | D6-19*01 | ttgggcagtggctggtac | gggggga | actactgg | J4*01 | |
| End | tgtgcgaga | ggggc | D3-3*01 | gtattacaattttttcggtg | attcttaccat | tacttggattactgg | J4*01 | |
| 1 mo follow-up | tgtgcgaga | atc | D5-18*01 | gtggatacagctgtaatt | ttatc | tgtttttgatttctgg | J3*02 | |
| 3 | Start | tgtgcgagag | aggggatacgag | D4-11*01/in | gttacagtaacc. . . . . . . . . . . . . . . . . . . . … | ct | . . . . . .tttgactactgg | J4*02 |
| tgtgtgagag. | D5-12*01 | . . . . . . .atatcggctatg. . . . . . | gccactttgactgctgg | J4*03 | ||||
| tgtgccaga | gagaggg | D6-19*01in | . . . . . . . . . . . . . . .cccgtacgagacc | ggcccctt | actactttgactactgg | J4*03 | ||
| End | tgtgcgagag | aggggatacgag | D4-11*01/in | gttacagtaacc. . . . . . . . . . . . . . . . . . . . … | ct | . . . . . .tttgactactgg | J4*01 | |
| 1 mo follow-up | tgtgcgaga | gaggggtttaa | D5-18*01 | . .ggatacagctatggttac | .cccctttgactactgg | |||
| 4 | Start | tgtgcgaaa | gaacagt | D4-23*01 | . .actacggtgacttc | ctcggt | . . . . . . . . . . . . . . . . . . . . .ggtatggacgtctgg | J6*02 |
| tgtgcaaca | gtgccc | D3-22*01 | .tcttactatgatagtagtggttatccata | .cccctttgactactgg | J4*03 | |||
| tgtgccaga | gagaggg | D6-19*01 | . . . . . . . . . . . . . . . .cccgtacgagacc. | ggcccctt | actactttgactactgg | J4*02 | ||
| tgtgcgaga | ga | D2-15*01 | tcgatattgtagtgct. . . . . . . . . . . . . . . . . | ggtagctgctttg | . .actggtacttcgatctctgg | J2*01 | ||
| tgtgcgaca | ttgtcacg | D4-17*01 | tgactacggtgactac | gtt | .tactactacaagggtatggacgtctgg | J6*02 | ||
| End | tgtgtgaga | gatgg | D3-10*01 | gtattattatggttcggggagttacccta. . | tg | . . . . . . . . . .gacgtctgg | J4*01 | |
| tgtgcgaga | gac | D3-10*01 | . .atcattatgattcggggaggtattata. . | . .aactggctcgacccctgg | J5*02 | |||
| tgtgcgaga | ggagggttagcc | D6-19*01 | gggtatagcagtggctggt. . | actactttgactactgg | J4*01 | |||
| tgtgcgaga | gatc | D2-15*01 | . .gatattggagtggtggtagctgct. . . . . . | ttg | . .actggtacttccatctctgg | J2*01 | ||
| 5 | Start | tgtgcgagag | atc | D1-26*01 | ggtatagtgggag. . . . . … | a | . .tactatgactactgg | J4*03 |
| tgtgcgaaag. | atag | D6-13*01 | ggacatagcagcagctgg… | cggg | . . . . . .tttgactactgg | J4*03 | ||
| tgtgcgaaag. | a | D5-24*01 | . . . . .taggagttttgg. . . . . . . . . . . . . . . … | ccgtct | agatgcttttgatatctgg | J3*02 | ||
| tgtgcgagag. | agggccggggcaa | D4-23*01 | tgacaaggggggtaaccc. | . . . . .ctttgattactgg | J4*01 | |||
| tgtgcgaaa | gaagtag | D5-12*01 | gtggatatagtggctacgccccc | cca | . . . . . . . . . . . . . . . . .tacggtatggacgtctgg | J6*02 | ||
| After first infusion | tgtgcgaga | gagggccggggcaa | D4-23*01 | tgacaagggaggta. . . . . . | acccctttgactactgg | J4*01 | ||
| tgtgcgaga | ggagcg | D5-18*01 | ggggagacagctatgg. . . . . | aagggttt | . . . . .tttgactgctgg | J4*03 | ||
| tgtgcgaga | gggaac | D6-13*01 | . .caccgcaactggtcc. . . . . . . . . . . . . . . . . | gaggggt | acaactggttcgacccctgg | J5*02 | ||
| tgtgcgaaa | gaagtag | D5-12*01 | gtggatacagtggctacg. . . . . . | ccccccca | . . . . . . . . . . . . . . . . .tacggtatggacgtctgg | J6*02 | ||
| tgtgcgag. | tc | D3-10*01 | . . . . . . . . . .aggcttcggggacttatt. . . . . . | ccggt | . . . . .tttgagttctgg | J4*03 | ||
| End | tgtgcgaga | gagggccggggcaa | D4-23*01 | tgacaagggaggta. . . . . . | acccctttgactactgg | J4*01 | ||
| 2 mo follow-up | tgtgcgag. | ggacc | D2-15*01 | . .gattttgtagtggtggtagctgcttc… | g | . .actggtacttcgatctctgg | J2*01 | |
| Patients | Time-related therapy | V-region | N1 | DHfamily | D-region | N2 | J-region | JH family |
| 3 mo follow-up | tgtgcgag. | ggacc | D2-15*01 | . .gattttgtagtggtggtagctgcttc… | g | . .actggtacttcgattactgg | J2*01 | |
| tgtgcgaga | gaggaccgagactgg | D5-18*01 | agattcagcttat… | . .tattttgactactgg | J4*03 | |||
| tgtgcgaaa | g | D5-12*01 | gtgagtatagtggttacg. . . . . . | ccccccca | . . . . . . . . . . . . . . . . .tacgggatggacgtctgg | J6*02 | ||
| gaagtag | ||||||||
| Unresponsive | ||||||||
| 6 | Start | tgtactag. | D2-8*02 | . . . . . . . . . . .tcctggtggctt. . . . . . . . . . . | taaa | . . . . . …gactactgg | J4*03 | |
| tgtgcgaga | ggtaaaac | D4-17*01 | …ctacggtgactac | gcc | .ggagcttttgatatctgg | J3*02 | ||
| End | tgtgcgaga | ggtaaaac | D4-17*01 | …ctacggtgactac | gcc | .ggagcttttgatatctgg | J3*02 | |
| 7 | Start | tgtgcgaga | D6-13*01 | . .gaaacgcagcagc. . . . . . . | c | gggtgcttttgatatctgg | J3*02 | |
| End | tgtgcgaga | D6-13*01 | . .gaaacgcagcagc. . . . . . . | c | gggtgcttttgatatctgg | J3*02 | ||
| 8 | Start | tgtgcgaga | gaatatgggaca | D3-22*01 | . . . . . .gatactagtggttattactac | t | . . . . . . . . . . .actactgg | J4*01 |
| End | tgtgcgaga | gaatatgggaca | D3-22*01 | . . . . . .gatactagtggttattactac | t | . . . . . . . . . . .actactgg | J4*01 |
Persistence of B-cell clonal expansions was demonstrated in the nonresponders. Their single B-cell clones exhibited identical rearrangement at different time points and were highly resistant and refractory to rituximab.
Amino acid length and composition determine the ability of CDRH3 to bind antigen with higher affinity. In this data set (data not reported) we noticed CDRH3 ranging from 13 to 25 amino acids in responder patients and from 14 to 17 in nonresponders. There was no difference in gene usage between the 2 groups. D2, D3, D4, D5, and D6 families were represented in the responders, and D3, D4, and D6 in the nonresponders. No selective use of JH fragment was noted (JH2-JH6 gene segments were used), although JH4 was the most represented.
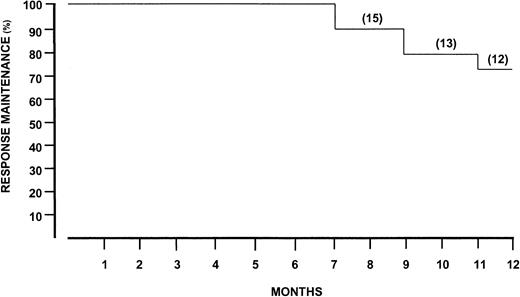
Twelve months after discontinuation of rituximab, the response maintenance rate was 75% (Figure 3). Four patients (25%) had a relapse, with the estimated time to relapse exceeding 7 months. Clinically, relapse was characterized by reappearance of purpura and recurrence of weakness and arthralgias. Simultaneous increment of cryocrit and IgM RF molecules were also demonstrated. Interestingly, anti-HCV antibody titers reached pretreatment levels in the cryoprecipitate.
Response maintenance after discontinuation of rituximab treatment.
Number of patients indicated in parentheses.
Response maintenance after discontinuation of rituximab treatment.
Number of patients indicated in parentheses.
Discussion
The present results indicate that rituximab reduces cryoglobulin levels and significantly improves cutaneous vasculitis and several clinical and immunologic parameters in patients who had been resistant to IFN-α. Tolerance was good in nearly all patients. Two patients experienced mild adverse reactions during the first infusion. In another patient septic fever, probably of bacterial origin, occurred after the third infusion.
The rituximab schedule was borrowed from earlier trials in which this antibody was used to treat relapsed or refractory low-grade or follicular B-cell NHLs.21 This study, the largest to date, confirms and extends recent limited observations in MC.37-39
We considered rituximab to be effective treatment of MC because 80% of patients were responders. More than 60% showed rapid improvement of general signs. Nonresponders, on the contrary, did not improve at all and this suggests that an early clinical response may predict its efficacy. Modality of the response was mainly related to changes in the cryoprecipitates modifying the biologic characteristics of cold-precipitable material. We monitored components of cryoglobulins before and after rituximab and demonstrated that their precipitation was strictly related to a number of factors, namely, decrease of Ig concentration, decline of RF activity, and reduction of anti-HCV titers.
The negative relation between cryocrit levels and circulating HCV load raises some important issues about the mechanisms of cryoglobulin precipitation. Cold-dependent insolubility of HCV particles and proteins seems the result of IgM RF activity, which acts as incomplete cryoglobulin, in that it precipitates at low temperature in the presence of IgG with specific anti-HCV reactivity. The dynamics of cold-dependent insolubility demonstrates that the addition of an irrelevant IgG to an IgM RF/HCV proteins mixture was unable to precipitate the protein. This implies that a potentially functional RF repertoire may be positively selected by IgG anti-HCV antibodies.
At variance from the immediate and dramatic decline in viral load after rituximab in EBV-infected patients,40 our data are consistent with a sustaining effect of anti-CD20 antibody on HCV virogenesis. In responders, in step with reduction of cryocrit levels, increment of viral load has been documented. Sequential measurements of HCV RNA levels, quantified with branched DNA signal amplification assay, showed that they progressively increased after rituximab. Twelve months later, they increased by approximately twice the baselines. Importantly, these results deeply differed from those obtained in nonresponders, in which HCV RNA levels were unchanged over sequential time points.
Because B lymphocytes are thought to harbor HCV,41 it could be argued that the HCV RNA increment reflects virus shedding through rituximab-induced B-cell cytotoxicity. However, this is not a plausible explanation because, despite the B-cell depletion in the nonresponders, no significant variations of HCV RNA levels were noted. Thus, it can be envisaged that the impact of rituximab on immunoglobulin levels, that is, IgG anti-HCV antibodies, is an effective mechanism capable of modulating and interfering with HCV replication. Decline of anti-HCV titers leads HCV to avoid immune pressure and favors its replication. This points to the presence of immunoglobulin with neutralizing properties in immune response in HCV chronic carriers.42 However, it must be emphasized that selective increments of HCV RNA did not match significant changes of hepatocytolytic activity or deterioration of liver function, as defined by sequential measurements of serum transaminases and the clinical findings. This further supports the concept that HCV is not cytopathic for the cells it infects and that immune response plays the major role in the hepatocyte damage.43
PCR has been increasingly used for the rapid detection of gene rearrangements during B-lymphocyte development.44 It can be assumed that the consensus primers currently used amplify the CDRH3 of most B cells, but the products of a small clonal population may be obscured by the smear of polyclonal B cells in the sample. Indeed, we found B-cell polyclonality in a minority of patients at baseline, indicating that B-cell response is mainly not polyclonal and that selected antigens are preferentially recognized. Sequential IgH VDJ analyses demonstrated disappearance/deletion of individual B-cell clones in patients who responded to rituximab. On the contrary, persistence and stability of initially expanded clonotypes were found in nonresponders, suggesting that their B-cell clones are less sensitive to rituximab. Clonal susceptibility to rituximab may depend on other mechanisms and additional regulatory factors expressed on B cells, for example, levels of CD20 antigen45 and expression of complement inhibitors,46 failure of Fc receptor-dependent mechanism,47 or disturbed signals leading to apoptosis.48
Disappearance/deletion of B-cell clones and appearance of different clones in responders demonstrate that selected antigens are preferentially recognized as part of a limited host response to a pathogen capable of undergoing spontaneous long-term mutations that heavily contribute to the viral burden.49 Clonal disappearance/deletion never occurred in nonresponders and their HCV RNA concentration was stable at different time points.
Sequencing of VH genes illustrated the role of antigens prior to or during rituximab treatment. Analysis of CDRH3 segments pointed to positive selection in responders, who displayed ongoing somatic mutations. The absence of selection in nonresponders suggests that clonal persistence may be antigen-independent. Alternatively, it can be envisaged that affinity of antibody toward the antigens is high enough to avoid a positive ongoing mutation, or else superantigens that bind immunoglobulin receptor via framework regions provide negative selection pressure on these regions without positive selection pressure on CDRH3 regions.50
Compared with the general population, the occurrence of B-cell NHL remains higher in MC patients, even in those who clear HCV-RNA following IFN-α therapy.13 This indicates that IFN-α provides low protection against NHL and that factors other than HCV are potentially involved in the pathogenesis of B-cell malignant transformation. The impact of rituximab in declining B-cell clonal expansion possibly provides effective changes in the risk of developing NHL in MC patients. These data support the need for further large-sized studies exploring the optimal duration and dosage of rituximab administration.
Enhanced viremia occurring after this treatment, however, is a potentially harmful outcome, although no significant variations of serum transaminases or deterioration of liver disease were noted in our series. To reduce HCV replication, association of rituximab with effective antiviral agents, including IFN-α, may thus be a more rational approach. The same conclusions are also supported by the clinical picture of patients partially responsive to IFN-α therapy; because rituximab achieved a prompt clinical response in all of them, it can be assumed that its combination with IFN-α may result in a synergistic therapeutic effect.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-10-3162.
Supported in part by Associazione Italiana per la Ricerca sul Cancro (AIRC; Milan, Italy) and by a grant from the Italian Ministry of University and Scientific and Technological Research, National Project “Chronic liver damage induced by hepatitis C virus.” F.A.T. is recipient of a Fondazione Italiana per la Ricerca Sul Cancro (FIRC) fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Franco Dammacco, Department of Biomedical Sciences and Human Oncology, Section of Internal Medicine and Clinical Oncology, University of Bari Medical School, Policlinico, Piazza G. Cesare 11, 70124 Bari, Italy; e-mail:dimoclin@cimedoc.uniba.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal