Abstract
Complement has recently been implicated in developmental pathways and noninflammatory processes. The expression of various complement components and receptors has been shown in a wide range of circulating myeloid and lymphoid cells, but their role in normal hematopoiesis and stem cell homing has not yet been investigated. We report that normal human CD34+ cells and lineage-differentiated hematopoietic progenitors express the complement anaphylatoxin C3a receptor (C3aR) and respond to C3a. Moreover, C3a, but not the biologically inactive desArg-C3a, induces calcium flux in these cells. Furthermore, we found that C3 is secreted by bone marrow stroma and that, although C3a does not influence directly the proliferation/survival of hematopoietic progenitors, it (1) potentiates the stromal cell–derived factor 1 (SDF-1)–dependent chemotaxis of human CD34+ cells and lineage-committed myeloid, erythroid, and megakaryocytic progenitors; (2) primes SDF-1–dependent trans-Matrigel migration; and (3) stimulates matrix metalloproteinase-9 secretion and very late antigen 4 (VLA-4)–mediated adhesion to vascular cell adhesion molecule 1 (VCAM-1). Furthermore, we found that murine Sca-1+ cells primed by C3a engrafted faster in lethally irradiated animals. These results indicate that normal human hematopoietic stem and progenitor cells express functional C3aR and that the C3aR-C3a axis sensitizes the responses of these cells to SDF-1 and thus may be involved in promoting their homing into the bone marrow via cross talk with the SDF–CXC chemokine receptor-4 (CXCR4) signaling axis. C3a is the first positive regulator of this axis to be identified.
Introduction
The complement system consists of a complex group of serum proteins and glycoproteins as well as soluble or membrane-bound receptors, which mediate critical functions in host defense and inflammation.1-4 Complement, a phylogenetically conserved arm of innate immunity, functions together with the adaptive immune response as a downstream effector of antigen-antibody interactions. It also provides an interface between the innate and adaptive immune systems by providing costimulatory signals to B cells and enhancing the humoral response against specific antigens.3
Complement is activated in a cascadelike fashion through the classical, alternative, or lectin pathways. Its activation leads to the generation of small bioactive peptides with potent proinflammatory properties, termed anaphylatoxins. The C3a anaphylatoxin is a 78-amino-acid peptide derived from the proteolytic cleavage of the complement protein C3 that mediates various proinflammatory and immunoregulatory functions through its binding to C3a receptor (C3aR), a G-protein–coupled, 7 transmembrane-spanning receptor.5 C3aR is predominantly expressed on the surface of human mast cells, eosinophils, and monocytes and is also found on activated T lymphocytes.6-9It mediates eosinophil and mast cell migration, mast cell degranulation, histamine release, and superoxide anion production.6 Enzymatic removal of arginine from the C-terminus of C3a yields a desArg-C3a molecule that is unable to signal though the C3aR.10
Complement has long been perceived as an effector arm of innate immunity that mediates important immunoregulatory and inflammatory functions. Recent studies, however, suggest that complement may assume noninflammatory roles in certain biologic settings.11 For example, complement components appear to be involved in early bone development by promoting osteoclast differentiation and potentiating the growth factor–dependent proliferation of progenitor cells along the macrophage-monocyte lineage.12 Furthermore, complement components have also recently been shown to induce cell activation, DNA synthesis, and proliferation in various biologic situations.13,14 In particular, C3 has been shown to support the growth of the lymphoma Raji cell line.14
In seeking a better understanding of how complement affects noninflammatory and developmental processes, we investigated the potential role of complement in normal human hematopoiesis. In adults, hematopoiesis takes place predominantly in the bone marrow (BM) and requires the synergistic interaction of a wide array of chemokine-, growth factor–, and cytokine-dependent signaling pathways.15 16 Since the immune and hematopoietic systems occupy similar compartments and the bone marrow is an immunologically privileged tissue, we hypothesized that complement may act in the bone marrow to modulate critical cell-cell interactions or signaling responses that regulate hematopoiesis. In particular, we investigated the role of the complement anaphylatoxin C3a and its receptor C3aR in the homing/trafficking of early hematopoietic progenitors and their involvement in hematopoietic engraftment.
In this study we have shown that while normal human CD34+cells enriched in hematopoietic stem/progenitor cells, as well as purified human myeloblasts, erythroblasts, and megakaryoblasts, express the complement C3a anaphylatoxin receptor C3aR, C3 is secreted by bone marrow stroma. Thus, we have assessed the biologic responses of early hematopoietic cells to C3a stimulation. Furthermore, we compared the biologic responses of these early hematopoietic cells to C3a with their responses to the Α-chemokine stromal cell-derived factor 1 (SDF-1), which also binds to a G-protein–coupled, 7 transmembrane-spanning CXC chemokine receptor-4 (CXCR4) and regulates the trafficking/homing of early hematopoietic cells.17-23 Our study demonstrates that the presence of functional C3aR on human CD34+ cells and early myeloid, erythroid, and megakaryocytic progenitors is associated with their ability to modulate various homing activities in response to SDF-1. Furthermore, pretreatment of murine hematopoietic stem/progenitor cells with C3a enhanced their engraftment into irradiated mice further emphasizing that C3aR plays an essential role in promoting the trafficking/homing of these progenitors to the bone marrow through its synergistic interaction with the SDF-1–CXCR4 signaling axis.
Materials and methods
Cell lines
All cell lines used in these studies (THP-1, Jurkat, and PB-1) were purchased from American Type Culture Collection (Rockville, MD) and maintained in RPMI 1640 medium (GibcoBRL, Long Island, NY) supplemented with 10% bovine calf serum (BCS; Hyclone, Logan, UT).
Human CD34+ cells
Light-density bone marrow (BM), mobilized peripheral blood (PB), and cord blood (CB) cells were obtained from healthy volunteer donors who had given informed consent; the protocols used were approved by the institutional review board of the University of Louisville. Light-density cells were depleted of adherent cells and T lymphocytes (A−T−mononuclear cells [MNCs]) and enriched for CD34+ cells by immunoaffinity selection with MiniMACS paramagnetic beads (Miltenyi Biotec, Auburn, CA) as described previously.24 The purity of isolated BM CD34+ cells was higher than 95% as determined by fluorescence-activated cell sorter (FACS) analysis using a FACscan (Becton Dickinson, San Jose, CA). Mobilized PB cells from patients diagnosed with non-Hodgkin lymphoma were obtained by leukapheresis using the Cobe Spectra Apheresis System (COBE, Lakewood, CO) after mobilization with granulocyte colony-stimulating factor (G-CSF; Filgastrim, Amgen, Thousand Oaks, CA). CB cells were obtained as described.25 Cell samples were enriched for CD34+ cells using immunoaffinity MiniMACS paramagnetic beads as described previously.25 The purity of the isolated PB CD34+ cells was higher than 97%.
Human myeloid, erythroid, and megakaryocytic precursor cells
CD34+ cells were cloned in serum-free methylcellulose cultures as previously described.25 In brief, CD34+ A−T− MNCs (104/mL) were suspended in Iscoves Dulbecco modified Eagle medium (DMEM; Gibco BRL) supplemented with 25% artificial serum. Growth of colony-forming unit-granulocyte/macrophage (CFU-GM) was stimulated with recombinant human (rh) interleukin-3 (IL-3, 10 ng/mL) and rh granulocyte/macrophage colony-stimulating factor (GM-CSF, 5 ng/mL); growth of burst-forming unit-erythroid (BFU-E), with rh erythropoietin (EPO, 2 U/mL) and rh kit ligand (KL, 10 ng/mL); and growth of colony-forming unit-megakaryocyte (CFU-Meg), with rh thrombopoietin (TPO, 50 ng/mL). All rh cytokines and growth factors were obtained from R & D Systems (Minneapolis, MN). Cultures were incubated at 37°C in a fully humidified atmosphere supplemented with 5% CO2. CFU-GM–, BFU-E–, and CFU-Meg–derived cells were used in our chemotaxis experiments. In some of the experiments, suboptimal (1/4 or 1/10 of optimal dose) doses of growth factors were used for stimulation. Colonies were counted under an inverted microscope as described previously.25
Complement reagents
Recombinant human C3a was obtained from Sigma (St Louis, MO). The specific C3aR antagonist (SB 290157) was kindly provided initially by Dr R. S. Ames (Glaxo-SmithKline, King of Prussia, PA); in subsequent studies it was synthetically produced in our laboratory, according to a previously described method.26
Enzyme-linked immunosorbent assay (ELISA) on bone marrow–derived fibroblasts
Bone marrow–derived fibroblasts were expanded (in DMEM supplemented with 12.5% BCS + 12.5% horse serum) from a population of adherent marrow cells and purified from macrophages by prolonged trypsinization during 2 first passages. For the ELISA experiments, cells were cultured for 24 hours in DMEM supplemented with 0.5% bovine serum albumin (BSA). Microtiter plates were coated with a rabbit antirat immunoglobulin G (IgG) Fc antibody (2.3 μg/mL dilution in phosphate-buffered saline [PBS], pH 7.4; ICN Pharmaceuticals, Santa Ana, CA) for 2 hours. Nonspecific binding to the wells was prevented by incubation with blocking buffer (PBS containing 1% BSA). A monoclonal rat antimouse C3 antibody (1:160 dilution of hybridoma supernatant [clone 2/16] in blocking buffer) was added to each well and incubated for one hour. Serial dilutions of mouse serum (1:500 starting dilution in blocking buffer) were added and incubated for one hour. Subsequently, a polyclonal goat antimouse C3 (horseradish-peroxidase [HRP] conjugated, 3.2 μg/mL in blocking buffer; ICN Pharmaceuticals) was added and incubated for one hour. C3-containing complexes were detected by the addition of substrate solution (0.05% 2,2′-azino-di-(3 ethylbenzthiazoline sulfonic acid)) (ABTS; Roche, Indianapolis, IN) and 0.01% H2O2 in 0.1 M NaCitrate buffer, pH 4.2), and the optical density was measured in an ELISA reader at 405 nm.
Detection of C3 in murine bone marrow by immunoblotting
Total bone marrow extracts were reduced with 0.4% sodium dodecyl sulfate (SDS)–containing loading dye and boiled at 100°C for 5 minutes. Protein extracts were then analyzed by SDS–polyacrylamide gel electrophoresis (PAGE) and blotted onto a polyvinylidene fluoride membrane. Membrane containing blotted proteins was blocked with 10% milk/PBS for one hour and incubated with a rabbit antimouse C3c antiserum (1:5000, in PBS/10% milk) for one hour. After 2 washings with PBS 0.01% and Tween20, the membrane was incubated with HRP-conjugated goat antirabbit IgG (1:2000 dilution in 10%milk/PBS) for 30 minutes. After addition of a developing solution, chemiluminescence corresponding to immunoreactive bands was visualized by exposure of the membrane to an x-ray film (Eastman Kodak, Rochester, NY).
FACS analysis
The expression of C3aR was evaluated by FACS as previously described.27 C3aR was detected with an anti-C3aR monoclonal antibody (mAb), clone no. 218, and a phycoerythrin (PE)–labeled goat antimouse antibody. Subsequently, the cells were stained with fluorescein isothiocyanate (FITC)–labeled anti-CD34 mAb (Becton Dickinson, San Jose, CA). The cells were stained in PBS (Ca2+- and Mg2+-free) supplemented with 5% BCS (Hyclone). After the final wash, cells were fixed in 1% paraformaldehyde and subjected to FACS analysis using a FACScan analyser (Becton Dickinson, San Jose, CA).
Adhesion to vascular cell adhesion molecule 1 (VCAM-1)
CD34+ cells were metabolically labeled by S35-methionine (by overnight culture), washed twice, and incubated for 30 minutes with C3a or control desArg-C3a.21Subsequently, cell suspensions (1 × 105 cells/100 μL) were applied to the 96-well microtiter plates covered with VCAM-1 (10 μg/mL) and incubated for 3 hours at 37°C. After incubation, the plates were washed 4 times and the number of adherent cells was estimated in a scintillation counter as described.28 In blocking studies, cells were incubated with 10 mg/mL anti–very late antigen 4 (VLA-4) integrin mAb (clone P1H4, Chemicon, Temecula, CA) for 30 minutes prior to the adhesion assay. The optimal concentration of the antibody was determined in preliminary experiments.
Isolation of mRNA and reverse transcriptase–polymerase chain reaction (RT-PCR) for detection of C3aR
Cells (1 × 106) were lysed in 200 μL RNAzol (Biotecx Labs, Houston, TX) plus 22 μL chloroform as previously described.24 The aqueous phase was collected and mixed with 1 volume isopropanol (Sigma, St Louis, MO). RNA was precipitated overnight at −20°C. The RNA pellet was washed in 75% ethanol and resuspended in 3-times autoclaved H2O. mRNA (0.5 μg) was reverse-transcribed with 500 U Moloney murine leukemia virus reverse transcriptase and 50 pmol of an oligodeoxynucleotide primer complementary to the 3′ end of the reported cDNA sequence of C3aR (5′-AGA AAG ACA GCC ACC ACC ACG-3′).29 The resulting cDNA fragments were amplified using 5 U Thermus aquaticus (Taq) polymerase and primers specific for the 5′ end of the C3aR sequence (5′-GCC GCC TGG AGA AAT GAA TGA TAG G-3′).29 Amplified products (10 μL) were electrophoresed on a 2% agarose gel, transferred to a nylon filter, and further documented photographically. The specificity of the amplified products was further confirmed by Southern blotting.
Calcium flux assay
Briefly, cells were incubated for 30 minutes at 30°C with 1 to 2 μM Fura-2/am (Molecular Probes, Eugene, OR). After incubation, the cells were washed once; resuspended in loading buffer without BCS; stimulated with C3a, desArg-C3a, or SDF-1β (500 ng/mL); and analyzed within one hour as previously described.21
Chemotaxis assay
Cells were resuspended in RPMI with 0.5% BSA and equilibrated for 10 minutes at 37°C. Prewarmed serum-free medium containing either no chemoattractant or C3a, SDF-1, or C3a plus SDF-1 was added to the lower chambers of a Costar Transwell 24-well plate (Costar Corning, Cambridge, MA). We used 6.5-mm diameter plates with a 5-μm pore filter for CD34+ and MNCs and an 8 μm-pore filter for cell lines. Aliquots of the cell suspension (1 × 105cells/100 μL) were loaded onto the upper chambers, and the cultures were incubated (at 37°C, 95% humidity, 5% CO2) for 3 hours, after which the cells in the lower chamber were scored using FACS analysis. The results are presented as a chemotactic index (the ratio of the number of cells that migrated toward the medium containing C3a and/or SDF-1 to the number of cells that migrated toward the medium alone). In some experiments, CD34+ cells recovered from the lower chambers were plated in semisolid methylcellulose cultures supplemented with artificial serum and stimulated to grow CFU-Mix, CFU-GM, BFU-E, and CFU-Meg colonies as described.25 27
Zymography
Matrix metalloproteinases (MMPs), which degrade extracellular matrix components, facilitate the migration of cells across the subendothelial basement membrane barrier. To evaluate MMP-2 and MMP-9 activities, CD34+ cells (2 × 106/mL) were incubated for 48 hours at 37°C, and 5% CO2 in serum-free media in the absence (control) or presence of C3a (1 μg/mL) and the cell-conditioned media were collected and analyzed by zymography as previously described.21 30 The intensity of the bands in the zymograms was quantitated using the Kodak Digital Science 1D Image Analysis software (Eastman Kodak) and the fold stimulation of MMP-9 secretion was calculated relative to the control.
Trans-Matrigel migration assay
BM and PB CD34+ cells were evaluated using the in vitro trans-Matrigel assay as described previously31,32 after stimulation by C3a. Briefly, polycarbonate filters (13-mm diameter, 8-μm pore size) were coated with 25 μg Matrigel and placed between the upper and lower compartments of modified Boyden chambers. BM or PB CD34+cells that were preincubated either with (1 μg/mL) or without C3a were loaded onto the upper compartments (3 × 105cells/chamber) and incubated for 3 hours at 37°C and 5% CO2. Cells that invaded the Matrigel barrier toward media alone (Iscoves modified Dulbecco medium with 0.1% BSA) or toward an SDF-1 gradient (20 or 300 ng/mL) were recovered from the lower compartments and counted using a Neubauer hemocytometer. To examine the role of MMPs in the trans-Matrigel migration of CD34+ cells, the inhibitors of MMPs, recombinant human tissue inhibitor of metalloproteinases-1 (rhTIMP-1, provided by Dr Dylan Edwards, University of East Anglia, Norfolk, United Kingdom), anti–MMP-9 monoclonal antibody (Ab-1, Oncogene Research Products, Cambridge, MA), and o-phenanthroline (Sigma, Oakville, ON, Canada) were used. For these experiments the cells were preincubated at 37°C and 5% CO2 as we have previously described33 in the presence of C3a (1 μg/mL) with 10 μg/mL each of TIMP-1 or anti–MMP-9 antibody for 2 hours, or with 0.5 mM o-phenanthroline for 30 minutes, before being loaded into the upper compartments of the Boyden chambers; the Matrigel assay was performed as above. The trans-Matrigel migration index was calculated from the ratio of the number of cells invading the Matrigel toward an SDF-1 gradient to the number of cells migrating toward media alone. Each experiment was performed using at least 4 chambers for each condition, and counts were performed in duplicate.
Phosphorylation of intracellular pathway proteins
Western blot analysis was performed on extracts prepared from cell lines and CD34+ cells, which were kept in RPMI medium containing low levels of BSA (0.5%) to render the cells quiescent. The cells were then stimulated with 1 μg/mL C3a for 10 minutes at 37°C and then lysed for 10 minutes on ice in M-Per lysing buffer (Pierce, Rockford, IL) containing protease and phosphatase inhibitors (Sigma, St Louis, MO). The extracted proteins were separated on 10% SDS-PAGE gel and transferred to nitrocellulose membrane (Hybond enhanced chemiluminescence [ECL], Amersham Life Science, Little Chalfont, United Kingdom). Phosphorylation of AKT, 44/42 mitogen-activated protein kinase (MAPK), and Stat 1, 3, 5, and 6 proteins was detected by protein immunoblotting using mouse monoclonal 44/42 phospho-specific MAPK antibody, and rabbit phosphospecific polyclonal antibodies (all from New England Biolabs, Beverly, MA) for each of the remaining proteins, with horseradish peroxidase–conjugated goat antimouse IgG or goat antirabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) used as secondary antibodies, as described.21 33 Equal loading in the lanes was evaluated by stripping the blots and reprobing them with the appropriate monoclonal or polyclonal antibodies: p42/44 anti-MAPK antibody clone 9102, anti-AKT antibody clone 9272, anti–Stat 3 clone 9132 (New England Biolabs), anti–Stat 1 sc-464 (Santa Cruz Biotechnology) and anti–Stat 5 clone 89 (Transduction Laboratories, Lexington, KY). The membranes were developed with an ECL reagent (Amersham Life Science), dried, and exposed to film (HyperFilm, Amersham Life Science).
Bone marrow transplantation in lethally irradiated mice
Female BalbC mice (4-6 weeks old) were obtained from Jackson Laboratory (Bar Harbor, ME). For the transplantation experiments mice were irradiated with a lethal dose of γ-irradiation (900 cGy). After 24 hours the mice received transplants (by tail-vein injection) of 105 BM MNC Sca-1+ cells that had been obtained from the murine donor marrow cells and isolated using immunomagnetic beads (Miltenyi Biotec). Mice that underwent transplantation were bled at various intervals (days 0, 5, 7, 12, 16, and 21) from the retro-orbital plexus to obtain samples for leukocyte, platelet, and hematocrit counts using Unopette Microcollection (Becton Dickinson, Rutherford, NJ) and heparinized microhematocrit capillary tubes (Oxford Labware, St Louis, MO).
Evaluation of engraftment
Colony-forming unit-spleen (CFU-S) assay.
For the CFU-S assays, BalbC mice received transplants of 5 × 104 Sca-1+ marrow cells. At day 12, spleens were removed and fixed in Tellysyniczky fixative and CFU-S colonies were counted on the surface of the spleen using a magnifying glass as previously described.22
Number of cells/femur.
Femora of mice that underwent transplantation were flushed with PBS at day 16 after transplantation, and the number of mononuclear cells was counted manually in cell suspensions as described earlier.22
Clonogenic assays.
Sca-1+ cells were isolated using immunomagnetic beads (Miltenyi Biotec) from animals at day 16 after transplantation. Cells were plated in serum-free methylcellulose cultures and stimulated to grow CFU-GM colonies by murine recombinant GM-CSF (5 ng/mL) + IL-3 (10 ng/mL) (R & D Systems).
Statistical anaysis
Arithmetic means and standard deviations were calculated on a Macintosh computer powerbase 180, using Instat 1.14 (Graphpad, San Diego, CA) software. Statistical significance was defined as aP value of less than .01. Data were analyzed using the Student t test for unpaired samples.
Results
C3a increases chemotaxis of hematopoietic cells to SDF-1
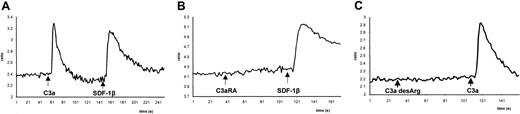
Although the C3a anaphylatoxin receptor, a member of the G-protein–coupled, 7 transmembrane-spanning receptor family, is known to be expressed by human T lymphocytes, basophils, eosinophils, and monocytes,6-9 the role of the C3a-C3aR axis in human hematopoiesis has not been previously addressed. To gain further insight into the function of C3a during hematopoiesis, we selected as an experimental model the T-lymphoid cell lines Jurkat and PB-1 and the monocytic cell line THP-1, all of which express functional C3aR. These cell lines showed calcium flux in response to stimulation by C3a as they did to the α-chemokine SDF-1, which we used as a positive control based on our previous studies.21 22 As demonstrated in the case of the monocytic cell line THP-1 (Figure1), stimulation of calcium flux by C3a was found to be C3aR specific, with the response to C3a, but not to SDF-1, inhibited by preincubation of the cells with a specific C3aR antagonist (Figure 1B). Moreover, C3a, but not its derivative, desArg-C3a, induced calcium flux in these cells (Figure 1C), further supporting the specificity of the C3a-C3aR interaction.
Calcium flux studies of Fura-2–loaded THP-1 cells.
C3a (1 μg/mL) or SDF-1β (300 ng/mL) was added at indicated time points, and calcium flux was evaluated by spectrophotofluorimetry. (A) Response to C3a followed by SDF-1β. (B) Cells were preincubated for 30 minutes with the C3aR antagonist (C3aRA) SB290157 (5 μM). (C) Cells were stimulated first by desArg-C3a (1 μg/mL), followed by C3a (1 μg/mL). Data presented are from a representative experiment, which was repeated 3 times with similar results.
Calcium flux studies of Fura-2–loaded THP-1 cells.
C3a (1 μg/mL) or SDF-1β (300 ng/mL) was added at indicated time points, and calcium flux was evaluated by spectrophotofluorimetry. (A) Response to C3a followed by SDF-1β. (B) Cells were preincubated for 30 minutes with the C3aR antagonist (C3aRA) SB290157 (5 μM). (C) Cells were stimulated first by desArg-C3a (1 μg/mL), followed by C3a (1 μg/mL). Data presented are from a representative experiment, which was repeated 3 times with similar results.
Although all of these cell lines expressed functional C3aR, we did not observe any effect of C3a stimulation on either proliferation of these cells under serum-free conditions or their survival (data not shown). Hence, since C3a was recently found to possess chemotactic activity,6 we focused on its role in inducing chemotaxis. We found that even though all 3 cell lines showed strong chemotaxis to SDF-1, none of them responded to C3a alone (Table1). To our surprise, however, when we used SDF-1 and C3a together, the latter was able to increase their chemotactic response to low doses of SDF-1. Moreover, a similar effect was observed when cells were prestimulated with C3a before the assay. These findings suggest that stimulation of C3aR enhances CXCR4-dependent responses in hematopoietic cells and that C3a and SDF-1 may regulate migration of human hematopoietic cells in a synergistic manner. Furthermore, C3a may be a hitherto unidentified modulator of the CXCR4–SDF-1 axis as well as a factor that allows cells to better “sense” the SDF-1 gradient.
Chemotactic responses of human lympho/hematopoietic cell lines to C3a and SDF-1
| . | Chemotactic index* . | |||
|---|---|---|---|---|
| Cell type . | C3a . | SDF-1, low dose . | C3a + SDF-1, low dose . | SDF-1, high dose . |
| THP-1 | 1.15 ± 0.51 | 1.79 ± 0.20 | 11.03 ± 2.39 | 15.59 ± 5.75 |
| Jurkat | 0.80 ± 0.07 | 11.65 ± 7.49 | 43.02 ± 26.67 | 58.95 ± 25.50 |
| PB-1 | 0.89 ± 0.09 | 9.97 ± 2.43 | 79.65 ± 27.24 | 82.95 ± 26.28 |
| . | Chemotactic index* . | |||
|---|---|---|---|---|
| Cell type . | C3a . | SDF-1, low dose . | C3a + SDF-1, low dose . | SDF-1, high dose . |
| THP-1 | 1.15 ± 0.51 | 1.79 ± 0.20 | 11.03 ± 2.39 | 15.59 ± 5.75 |
| Jurkat | 0.80 ± 0.07 | 11.65 ± 7.49 | 43.02 ± 26.67 | 58.95 ± 25.50 |
| PB-1 | 0.89 ± 0.09 | 9.97 ± 2.43 | 79.65 ± 27.24 | 82.95 ± 26.28 |
Chemotactic index to medium alone is 1.0.
Normal human CD34+ cells express C3aR, and C3 is a physiologic constituent of the hematopoietic microenvironment
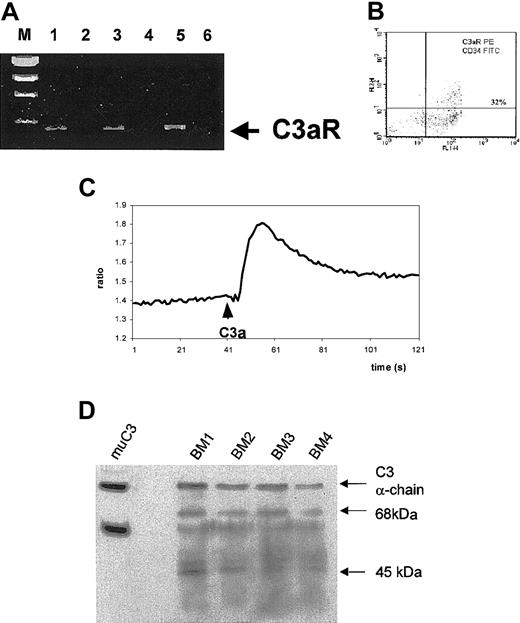
To investigate whether C3aR acts in the same way in normal human hematopoiesis as it does in the cell lines tested, we first evaluated whether normal primary hematopoietic cells express this receptor. Using RT-PCR we observed that highly purified normal human CD34+cells obtained from BM, mobilized (mPB), or cord blood express mRNA for CXCR4 (Figure 2A). Also, CFU-GM–derived myeloblasts, BFU-E–derived erythroblasts, and CFU-Meg–derived megakaryoblasts express mRNA for C3aR (data not shown). More significantly, C3aR protein expression was detected by FACS on bone marrow–derived CD34+ cells (Figure 2B). Expression of C3aR was also confirmed at the protein level on purified myeloblasts, erythroblasts, and megakaryoblasts derived from the expansion cultures (not shown).
Expression of C3aR by human CD34+ cells and C3 in bone marrow extracts.
(A) RT-PCR analysis of C3aR expression in normal human BM- (lane 1), PB- (lane 3), and CB-derived (lane 5) CD34+ cells. Lanes 2, 4, and 6 show negative control (H2O instead of mRNA) RT-PCR reactions. (B) FACS analysis of C3aR expression on human CD34+ cells sorted by magnetic beads. Data presented are from a representative experiment, which was repeated 3 times with similar results. We found that 34 ± 11% of CD34+ cells express C3aR. (C) Calcium flux studies of Fura-2–loaded normal mobilized PB CD34+ cells. C3a (1 μg/mL) was added at the indicated time points, and calcium flux was evaluated by spectrophotofluorimetry. Data are from a representative experiment, which was repeated 3 times with similar results. (D) Western blot analysis of expression of C3 and its cleaveage products in bone marrow extracts from 4 different mice. The α- and β-chains of murine C3 are shown in lane 1 (muC3). BM1 to BM4 analysis of C3 and its cleavage products (68-kDa and 45-kDa fragments of α-chain) in murine bone marrow extracts. The presence of a 68-kDa fragment suggests generation of C3a.
Expression of C3aR by human CD34+ cells and C3 in bone marrow extracts.
(A) RT-PCR analysis of C3aR expression in normal human BM- (lane 1), PB- (lane 3), and CB-derived (lane 5) CD34+ cells. Lanes 2, 4, and 6 show negative control (H2O instead of mRNA) RT-PCR reactions. (B) FACS analysis of C3aR expression on human CD34+ cells sorted by magnetic beads. Data presented are from a representative experiment, which was repeated 3 times with similar results. We found that 34 ± 11% of CD34+ cells express C3aR. (C) Calcium flux studies of Fura-2–loaded normal mobilized PB CD34+ cells. C3a (1 μg/mL) was added at the indicated time points, and calcium flux was evaluated by spectrophotofluorimetry. Data are from a representative experiment, which was repeated 3 times with similar results. (D) Western blot analysis of expression of C3 and its cleaveage products in bone marrow extracts from 4 different mice. The α- and β-chains of murine C3 are shown in lane 1 (muC3). BM1 to BM4 analysis of C3 and its cleavage products (68-kDa and 45-kDa fragments of α-chain) in murine bone marrow extracts. The presence of a 68-kDa fragment suggests generation of C3a.
Furthermore, we found that C3 mRNA was expressed by expanded ex vivo human bone marrow stromal fibroblasts. Using a sensitive ELISA assay, we determined that 1 × 106 human stromal fibroblasts secreted 0.6 ± 0.03 μg/mL of C3 after a 24-hour incubation at 37°C. Expression of C3 was also confirmed by ELISA assay and Western blot analysis performed on adherent cells isolated from murine bone marrow (Figure 2D). These findings support the concept that C3 is a physiologic constituent of the bone marrow environment and that the C3a-C3aR axis may regulate certain aspects of the biology of early hematopoietic cells, locally, within the bone marrow environment.
Functional C3aR is present on normal human CD34+ cells, myeloblasts, megakaryoblasts, and erythroblasts and regulates their motility but not their proliferation or survival
The finding that C3aR is expressed on normal human CD34+ cells and their progenitors and that C3 is secreted by bone marrow–derived stromal cells suggests a physiologic role for the C3a-C3aR axis in regulating normal human hematopoiesis. Hence, we sought to determine whether C3aR is functional on normal human CD34+ cells. Highly purified CD34+ cells were exposed to C3a, and their calcium flux and chemotactic responses were measured. DesArg-C3a, which does not activate C3aR,10 was used as a negative control for the calcium flux studies. C3a was found to activate calcium flux in normal human mPB CD34+ cells (Figure 2C), but the latter did not respond to inactive C3a desArg (data not shown). Similar results were obtained with bone marrow–derived CD34+ cells. Furthermore, calcium flux did not occur when the cells were pretreated with SB 290157, a selective C3aR antagonist.26 At the same time this antagonist did not inhibit calcium flux in CD34+ cells stimulated by the α-chemokine SDF-1, which activates CXCR4, another G-protein–coupled, 7 transmembrane-spanning receptor (data not shown). Using a chemotaxis assay we evaluated the functionality of C3aR and found that although C3a alone did not produce a chemotactic response in normal human CD34+ cells, it did increase their response to a low, “threshold” dose of SDF-1 (10 ng/mL) (Figure3). This effect, however, was not visible when SDF-1 was used at high dose (300 ng/mL).
Influence of C3a on chemotaxis of CD34+cells.
Chemotaxis of normal BM CD34+ cells was evaluated toward medium alone (control), C3a, SDF-1β, or C3a plus SDF-1β. Data are pooled from quadruplicate samples from 3 independent experiments. *P < .00001 compared with SDF-1β alone at low dose (10 ng/mL).
Influence of C3a on chemotaxis of CD34+cells.
Chemotaxis of normal BM CD34+ cells was evaluated toward medium alone (control), C3a, SDF-1β, or C3a plus SDF-1β. Data are pooled from quadruplicate samples from 3 independent experiments. *P < .00001 compared with SDF-1β alone at low dose (10 ng/mL).
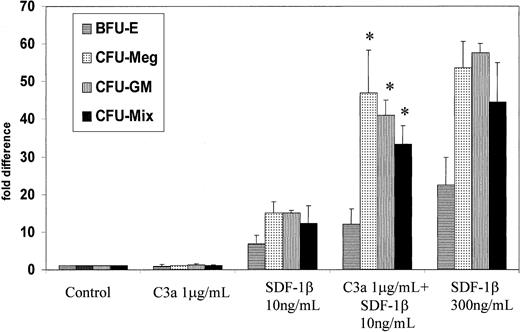
Since the population of CD34+ cells is heterogeneous, and stem/progenitor cells account for only about 5% of these cells,34 35 we investigated whether C3a could specifically stimulate chemotaxis of the stem/progenitor cell fraction only. Following the chemotaxis assay, CD34+ cells were collected from the lower chambers, plated in methylcellulose cultures, and stimulated to develop into colonies of the various hematopoietic lineages (CFU-Mix, BFU-E, CFU-GM, and CFU-Meg). Figure4 shows the relative number of colonies (as a percentage of the control) that were formed by CD34+cells showing chemotaxis to C3a and/or SDF-1. We found that C3a increases the SDF-1–directed chemotaxis not only of CD34+cells (Figure 3) but also of CD34+ clonogenic progenitors committed along all of the different hematopoietic lineages (Figure 4).
Influence of C3a on chemotaxis of clonogenic CD34+ cells.
The CD34+ cells showing chemotaxis in response to medium alone (control), C3a (1 μg/mL), low-dose SDF-1β (10 ng/mL), C3a (1 μg/mL) plus low-dose SDF-1β (10 ng/mL), and high-dose SDF-1β (300 ng/mL) were collected after chemotaxis assay from the lower chambers of transwells and plated in methylcellulose. The number of colonies formed by cells harvested from the lower chambers of transwell plates is shown as fold increase compared with control values. Data are pooled from quadruplicate samples from 3 independent experiments. * P < .00001.
Influence of C3a on chemotaxis of clonogenic CD34+ cells.
The CD34+ cells showing chemotaxis in response to medium alone (control), C3a (1 μg/mL), low-dose SDF-1β (10 ng/mL), C3a (1 μg/mL) plus low-dose SDF-1β (10 ng/mL), and high-dose SDF-1β (300 ng/mL) were collected after chemotaxis assay from the lower chambers of transwells and plated in methylcellulose. The number of colonies formed by cells harvested from the lower chambers of transwell plates is shown as fold increase compared with control values. Data are pooled from quadruplicate samples from 3 independent experiments. * P < .00001.
Moreover, C3a combined with a low dose of SDF-1 (10 ng/mL) restored the chemotaxis of CD34+ cells to 52 ± 3% of their maximal response as observed with the optimal dose of SDF-1 (300 ng/mL) (Figure3). In the case of the CD34+ clonogenic progenitors, C3a combined with a low dose of SDF-1 (10 ng/mL) restored the chemotaxis of CD34+ cells to almost approximately 80% of their maximal response to the optimal dose of SDF-1 (300 ng/mL). This suggests that C3a acting on CD34+ cells selectively enhances the chemotaxis of CD34+ clonogenic progenitors and has a weaker effect on nonclonogenic CD34+ cells.
Next, since CD34+ progenitors express functional C3aR we investigated whether C3a could influence the proliferation/survival of normal human early hematopoietic cells. Highly purified CD34+ cells were cultured in serum-free methylcellulose cloning medium with various doses of C3a (0.1-10 μg/mL) and stimulated to grow CFU-Mix, CFU-GM, BFU-E, and CFU-Meg colonies with suboptimal and optimal doses of lineage-specific growth factors/cytokines. In another set of experiments we incubated CD34+ cells for 7 days in serum-free medium in the presence or absence of C3a and estimated in secondary clonogenic assays the number of clonogenic progenitors that survived this period of time. In all of these experiments, however, no effect of C3a on the proliferation/survival of CD34+ cells was observed (data not shown). Consistently, purified C3a did not influence the clonogenic growth of CD34+ progenitors or affect their survival in serum-free medium. Thus, in agreement with what we have previously reported for SDF-1,21 33 C3a does not affect the proliferation/survival of hematopoietic cells and is primarily involved in the regulation of cell migration/trafficking.
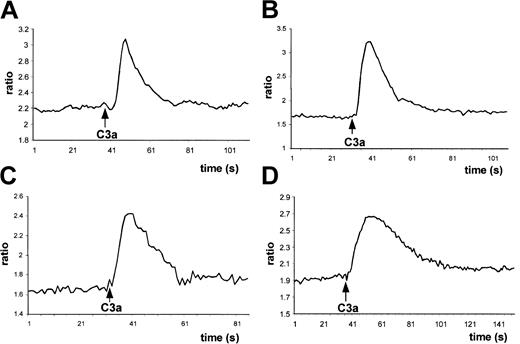
Next, having observed that normal human myeloblasts, megakaryoblasts, and erythroblasts express C3aR, using both calcium flux and chemotaxis as functional assays we examined whether C3aR is functional on these cells also. We found that C3a induced calcium flux not only in normal BM-derived MNCs (Figure 5A) but also in all the CD34+ cell-expanded lineages (Figure 5B-D). At the same time, no effect was observed when we applied inactive desArg-C3a (not shown).
C3a induces calcium flux in lineage-expanded hematopoietic cells.
Normal human BM Fura-2–loaded MNCs (A), myeloblasts (B), erythroblasts (C), and megakaryoblasts (D) were stimulated by C3a (1 μg/mL) added at the indicated time points, and calcium flux was evaluated by spectrophotofluorimetry. Data presented are from a representative experiment, which was repeated 5 times for BM cells and 3 times for myeloblasts, erythroblasts, and megakaryoblasts with similar results.
C3a induces calcium flux in lineage-expanded hematopoietic cells.
Normal human BM Fura-2–loaded MNCs (A), myeloblasts (B), erythroblasts (C), and megakaryoblasts (D) were stimulated by C3a (1 μg/mL) added at the indicated time points, and calcium flux was evaluated by spectrophotofluorimetry. Data presented are from a representative experiment, which was repeated 5 times for BM cells and 3 times for myeloblasts, erythroblasts, and megakaryoblasts with similar results.
In the chemotaxis assays, C3a alone stimulated migration of normal myeloblasts (Figure 6A). To our surprise, however, C3a caused a synergistic increase in the chemotactic responses of normal human erythroblasts and megakaryoblasts to a low dose of SDF-1 (10 ng/mL) (Figure 6B-C), as it had for CD34+ cells. Moreover, addition of C3a to low-dose SDF-1 restored the maximal chemotactic responses of these cells to that of the optimal dose of SDF-1 (not shown). This suggests that C3a enhances the chemotactic response to SDF-1 of both early and more differentiated cells and may thus play an important role in the homing of these cells in the bone marrow.
C3a induces chemotaxis of lineage-expanded hematopoietic cells.
Normal human myeloblasts (A), erythroblasts (B), and megakaryoblasts (C) were exposed in transwell chambers to medium alone (control), C3a, SDF-1, and C3a plus SDF-1. Data are pooled from quadruplicate samples from 3 independent experiments. *P < .00001.
C3a induces chemotaxis of lineage-expanded hematopoietic cells.
Normal human myeloblasts (A), erythroblasts (B), and megakaryoblasts (C) were exposed in transwell chambers to medium alone (control), C3a, SDF-1, and C3a plus SDF-1. Data are pooled from quadruplicate samples from 3 independent experiments. *P < .00001.
As with CD34+ cells, we did not observe any effect of C3a (0.1-10 μg/mL) on the expansion rate of CFU-GM, CFU-Meg, and BFU-E progenitors in serum-free liquid cultures or on the survival of early myeloblasts, megakaryoblasts, and erythroblasts (data not shown).
C3a does not influence the expression of CXCR4 on human hematopoietic cells or SDF-1–dependent phosphorylation of MAPK p42/44 and AKT
Having observed that C3a enhances the response of early hematopoietic cells to SDF-1, we turned our attention to the potential molecular mechanism(s) that may be responsible for this effect. First, since the expression of CXCR4 on normal early human hematopoietic cells is modulated by several factors,27 35 we hypothesized that C3a may mediate its effect on CD34+ cells by up-regulating the expression of CXCR4 on these cells, which could in part explain the increased sensitivity of their response to SDF-1. Normal purified human CD34+ cells, as well as erythroblasts, myeloblasts, and megakaryoblasts (derived from expansion cultures), were exposed to C3a for up to 36 hours and subsequently CXCR4 expression was compared in cells stimulated and nonstimulated by C3a. Using sensitive real-time RT-PCR as well as FACS analysis we did not find any changes in the expression of CXCR4 at the mRNA or protein levels (data not shown). Thus, enhanced responsiveness of hematopoietic progenitors to SDF-1 after priming by C3a cannot simply be explained by up-regulation of CXCR4 expression on their surface. Moreover, we also found that C3a did not influence internalization and intracellular expression of CXCR4 (not shown).
We have previously demonstrated that SDF-1 induces phosphorylation of MAPK p42/44 and activates the phosphoinositide-3-kinase (PI-3K)–AKT axis in normal human CD34+ cells, and SDF-1–dependent migration is inhibited after exposure of the cells to the PI-3K inhibitor Ly240024.21 To determine whether similar intracellular effectors are recruited after C3a stimulation, we decided to investigate whether C3a can enhance the SDF-1–dependent activation of the MAPK p42/44 or PI-3K–AKT axes. Surprisingly, C3a did not influence activation of either pathway, which suggests the potential involvement of other mechanisms in the sensitization of CXCR4 by the C3a-C3aR axis (eg, modulation of expression/activity of regulators of G-protein signaling, focal adhesion kinase [FAK], or phosphatase and tensin homolog [PTEN] kinase).37-39 We are currently conducting further experiments to delineate these alternative pathways of CXCR4 activation.
C3a primes the SDF-1–dependent trans-Matrigel migration of human CD34+cells
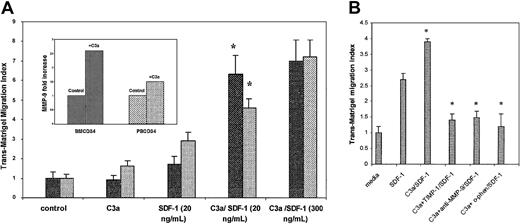
As the SDF-1–CXCR4 axis plays an essential role in the homing of hematopoietic cells, we decided to investigate whether C3a can modulate functions that are critical to the engraftment of human hematopoietic stem/progenitor cells. The ability of cells to cross the reconstituted basement membrane Matrigel has been used as a measure of their invasive or engraftment potential.31,32 We performed thetrans-Matrigel migration assay to ascertain whether C3a can promote migration of hematopoietic progenitors. We found that C3a (1 μg/mL) alone did not increase trans-Matrigel migration of BM and mobilized PB CD34+ cells toward media alone; however, it did significantly increase the BM and PB response (P = .008 and .004, respectively) to a low dose of SDF-1 (20 ng/mL), which nearly reached the level achieved with a high SDF-1 gradient (300 ng/mL) (Figure 7A). At the same time BM CD34+ cells stimulated by C3a secreted about 2.6-fold more MMP-9 into the conditioned media than nonstimulated cells, as measured by zymography30 (Figure 7A, inset). Moreover, Figure 7B shows that MMP-9 neutralizing antibodies or the presence of the MMP-9 inhibitor TIMP-1 or the nonspecific MMP inhibitoro-phenanthroline reduced significantly (by > 70%) thetrans-Matrigel migration toward SDF-1 (20 ng/mL) of CD34+ cells prestimulated with C3a (1 μg/mL), which suggests a major involvement of MMP-9 in this process. These results provide further evidence that C3a is involved in the migration/trafficking of hematopoietic stem/progenitor cells by modulating the release of matrix-degrading enzymes.
C3a enhances SDF-1–dependenttrans-Matrigel migration and secretion of MMP-9.
(A) Stimulation of SDF-1–dependent trans-Matrigel migration of human CD34+ cells by C3a. BM CD34+ (▪) and mobilized PB CD34+ cells (░) prestimulated or not with C3a (1 μg/mL) were loaded onto Boyden chambers and allowed to migrate across Matrigel toward media alone (control) or toward an SDF-1 gradient (20 or 300 ng/mL). Each experiment was performed using at least 4 chambers for each set of conditions and cell counts were done in duplicate. *P < .002. (Inset) Stimulation of MMP-9 secretion by C3a. Media conditioned by BM- or PB-derived CD34+ cells preincubated or not with C3a (1 μg/mL) were analyzed by zymography. The bands indicating MMP-9 activity were quantitated by densitometric analysis, and fold stimulation of MMP-9 secretion in the presence of C3a was calculated relative to control. The data shown are pooled from experiments using 3 to 4 samples of BM and PB CD34+ cells. (B) Inhibition of SDF-1–dependent trans-Matrigel migration by MMP inhibitors. PB CD34+ cells were preincubated with C3a (1 μg/mL) with or without 10 μg/mL each of rhTIMP-1 or anti–MMP-9 monoclonal antibody for 2 hours or with 0.5 mMo-phenanthroline for 30 minutes. The bottom chambers contained 20 ng/mL SDF-1 except for the control (media alone). Each experiment was performed using at least 4 chambers for each set of conditions and cell counts were done in duplicate. *P < .0001.
C3a enhances SDF-1–dependenttrans-Matrigel migration and secretion of MMP-9.
(A) Stimulation of SDF-1–dependent trans-Matrigel migration of human CD34+ cells by C3a. BM CD34+ (▪) and mobilized PB CD34+ cells (░) prestimulated or not with C3a (1 μg/mL) were loaded onto Boyden chambers and allowed to migrate across Matrigel toward media alone (control) or toward an SDF-1 gradient (20 or 300 ng/mL). Each experiment was performed using at least 4 chambers for each set of conditions and cell counts were done in duplicate. *P < .002. (Inset) Stimulation of MMP-9 secretion by C3a. Media conditioned by BM- or PB-derived CD34+ cells preincubated or not with C3a (1 μg/mL) were analyzed by zymography. The bands indicating MMP-9 activity were quantitated by densitometric analysis, and fold stimulation of MMP-9 secretion in the presence of C3a was calculated relative to control. The data shown are pooled from experiments using 3 to 4 samples of BM and PB CD34+ cells. (B) Inhibition of SDF-1–dependent trans-Matrigel migration by MMP inhibitors. PB CD34+ cells were preincubated with C3a (1 μg/mL) with or without 10 μg/mL each of rhTIMP-1 or anti–MMP-9 monoclonal antibody for 2 hours or with 0.5 mMo-phenanthroline for 30 minutes. The bottom chambers contained 20 ng/mL SDF-1 except for the control (media alone). Each experiment was performed using at least 4 chambers for each set of conditions and cell counts were done in duplicate. *P < .0001.
C3a primes the SDF-1–dependent adhesion of human CD34+ cells to VCAM-1
Several adhesion molecules may potentially play an essential role in the homing of hematopoietic stem cells in the bone marrow microenvironment.39-43 An interaction between VLA-4 and VCAM-1 has recently been reported to play a crucial role,41 and SDF-1 has been shown to modulate the affinity of VLA-4 for binding to VCAM-1.20 Hence we examined whether C3a can modulate the adhesion of normal human CD34+cells to VCAM-1 as well. Using FACS analysis we observed that C3a alone, while it did not influence the expression of VLA-4 on normal human CD34+ cells, increased by about 2-fold the SDF-1–dependent adhesion of CD34+ cells to VCAM-1 (Figure8). The specificity of the VCAM-1–VLA-4 interaction was demonstrated by anti–VLA-4 blocking antibodies (Figure 8).
C3a modulates SDF-1–dependent adhesion of CD34+ cells to VCAM-1.
Cells resuspended in the adhesion medium with or without C3a (1 μg/mL) were added to the wells and allowed to settle and were subsequently stimulated with 1 μM of recombinant human SDF-1β for 2 minutes. In blocking studies cells were incubated with 10 mg/mL anti–VLA-4 integrin mAb (clone P1H4, Chemicon) for 30 minutes prior to the adhesion assay. Data are pooled from quadruplicate samples from 4 independent experiments. *P < .01.
C3a modulates SDF-1–dependent adhesion of CD34+ cells to VCAM-1.
Cells resuspended in the adhesion medium with or without C3a (1 μg/mL) were added to the wells and allowed to settle and were subsequently stimulated with 1 μM of recombinant human SDF-1β for 2 minutes. In blocking studies cells were incubated with 10 mg/mL anti–VLA-4 integrin mAb (clone P1H4, Chemicon) for 30 minutes prior to the adhesion assay. Data are pooled from quadruplicate samples from 4 independent experiments. *P < .01.
Priming of murine BM Sca-1+ cells with C3a enhances their engraftment after transplantation into lethally irradiated mice
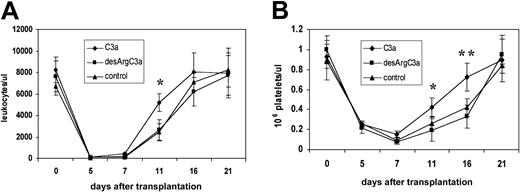
Prompted by our observation that C3a exerts a costimulatory effect on hematopoietic stem/progenitor cell chemotaxis and trans-Matrigel migration in vitro, we hypothesized that priming of hematopoietic stem/progenitor cells with C3a may enhance homing into the bone marrow after transplantation. Thus, we examined in an in vivo murine transplantation model the role of C3a in the engraftment of hematopoietic stem/progenitor cells into the bone marrow. In lethally irradiated mice, we transplanted Sca-1+ BM MNCs that were primed before transplantation with C3a or inactive desArg-C3a (as a control). We then assessed hematopoietic engraftment in mice that received transplants of primed and unprimed cells by counting the number of day-12 CFU-S colonies and examining the recovery of peripheral blood leukocytes and platelets. We found that mice that were primed with C3a before transplantation had about 60% more day-12 CFU-S colonies in the spleen compared with animals that received transplants of Sca-1+ cells primed with desArg-C3a or unprimed cells (Table 2). Similarly, we found that mice that received transplants of Sca-1+ cells primed with C3a showed accelerated recovery of leukocytes and platelets in their peripheral blood by 3 to 5 days (Figure9).
Day-12 CFU-S colony formation in lethally irradiated mice
| BM mononuclear Sca-1+cells . | Number of CFU-Ss,* Σ ± SD . |
|---|---|
| Mock prestimulated (−) | 12.2 ± 2.3 |
| Prestimulated by C3a, 1 μg/mL, 3 h | 21.7 ± 4.1† |
| Prestimulated by desArg C3a, 1 μg/mL, 3 h | 11.1 ± 2.7 |
| BM mononuclear Sca-1+cells . | Number of CFU-Ss,* Σ ± SD . |
|---|---|
| Mock prestimulated (−) | 12.2 ± 2.3 |
| Prestimulated by C3a, 1 μg/mL, 3 h | 21.7 ± 4.1† |
| Prestimulated by desArg C3a, 1 μg/mL, 3 h | 11.1 ± 2.7 |
Data shown are from 2 independent experiments (n = 15).
P < .00001 compared with mock prestimulated or desArg prestimulated Sca-1+ cells.
Priming of BM MN Sca-1+ cells with C3a before transplantation accelerates hematopoietic recovery and bone marrow engraftment.
(A) Leukocyte and (B) platelet recovery in the peripheral blood of lethally irradiated (900 cGy) mice that received transplants of 2 × 105 Sca-1+ cells (n = 10 mice/group). Triangles indicate mice that received transplants of untreated Sca-1+ cells; squares, mice that received transplants of Sca-1+ (1 μg/mL, 30 minutes) prestimulated with desArg-C3a (1 μg/mL, 30 minutes); diamonds, mice that received transplants of Sca-1+ cells prestimulated with C3a (1 μg/mL, 30 minutes). *P < .00001; **P < .01.
Priming of BM MN Sca-1+ cells with C3a before transplantation accelerates hematopoietic recovery and bone marrow engraftment.
(A) Leukocyte and (B) platelet recovery in the peripheral blood of lethally irradiated (900 cGy) mice that received transplants of 2 × 105 Sca-1+ cells (n = 10 mice/group). Triangles indicate mice that received transplants of untreated Sca-1+ cells; squares, mice that received transplants of Sca-1+ (1 μg/mL, 30 minutes) prestimulated with desArg-C3a (1 μg/mL, 30 minutes); diamonds, mice that received transplants of Sca-1+ cells prestimulated with C3a (1 μg/mL, 30 minutes). *P < .00001; **P < .01.
We also noticed that mice that received transplants of C3a primed cells compared with those that received transplants of nonprimed cells or cells preincubated with C3a antagonist had lower numbers of total MNC Sca-1+ clonogenic progenitors at day 16 after transplantation (Table 3).
Day-16 bone marrow reconstitution
| . | Number of CFU-GMs/femur,3-150 Σ ± SD . |
|---|---|
| Mock prestimulated (−) | 8 245 ± 2891 |
| Prestimulated by C3a, 1 μg/mL, 3 h | 17 902 ± 42113-151 |
| Prestimulated by desArg C3a, 1 μg/mL, 3 h | 7 807 ± 3201 |
| . | Number of CFU-GMs/femur,3-150 Σ ± SD . |
|---|---|
| Mock prestimulated (−) | 8 245 ± 2891 |
| Prestimulated by C3a, 1 μg/mL, 3 h | 17 902 ± 42113-151 |
| Prestimulated by desArg C3a, 1 μg/mL, 3 h | 7 807 ± 3201 |
Data shown are from 2 independent experiments (n = 12).
P < .00001 compared with mock prestimulated or desArg prestimulated Sca-1+ cells.
Discussion
In this study, we are presenting evidence for the first time that normal human CD34+ cells, as well as ex vivo–expanded myeloblasts, erythroblasts, and megakaryoblasts, express a functional receptor for the complement anaphylatoxin C3a. Although we found that C3a did not influence the proliferation/survival of the CD34+ hematopoietic progenitors, these cells responded to C3a stimulation by induction of an intracellular calcium flux. Since activation of calcium flux occurs in a number of biologic processes integral to cell movement/homing, this finding prompted us to investigate the role of C3a in the trafficking of early hematopoietic cells.
We found that although C3a on its own was a very weak chemoattractant for human hematopoietic cells, it significantly “sensitized” their chemotactic response to the α-chemokine SDF-1. Since SDF-1, secreted by bone marrow stromal cells, regulates the migration of hematopoietic cells and thereby plays a central role in anchoring stem cells to the bone marrow microenvironment,18-20,43,44 the “sensitization” of the SDF-1–CXCR4 axis by C3a that we identified could have an important role in the homing of early hematopoietic cells in the bone marrow. Additional evidence supporting this notion came from experiments demonstrating that C3a also synergistically enhanced the SDF-1–dependent migration of bone marrow–derived CD34+ cells across a Matrigel barrier. Trans-Matrigel migration, which mimics the physiologic process of cell migration across the subendothelial basement membrane barrier, requires the release of matrix-degrading enzymes that facilitate penetration of migrating cells into tissues.39-41 These processes are integral to the trafficking and homing of hematopoietic and lymphoid stem cells/progenitors to the bone marrow and lymphoid organs, respectively. Consistent with this we observed that C3a up-regulated secretion of MMP-9 by normal human CD34+ cells.
Another aspect of trafficking is the hematopoietic progenitors' capacity to modulate their adhesion to vascular endothelial and bone marrow–stromal surfaces. This is achieved in part by the inducible activation/expression of specific integrins (eg, VLA-4) and their interaction with adhesion receptors, such as VCAM-1, present on target surfaces. SDF-1 was recently reported to play a role in modulating the adhesion of CD34+ cells to endothelial cells.20 In our studies, C3a was found to enhance the SDF-1–dependent adhesion of human CD34+ cells to VCAM-1, which suggests that a synergistic interaction between CXCR4 and C3aR may increase the propensity of CD34+ progenitors to attach to the vascular endothelium or bone marrow stroma and thus promote their homing into the bone marrow.
A putative role for the C3aR-C3a axis in regulating normal human hematopoiesis is further supported by our observation that C3, like SDF-1,17-20 is expressed and secreted by bone marrow–derived stromal cells. In view of this finding, it would be interesting to speculate that local synthesis of C3, cleavage, and C3a generation in the bone marrow might modulate SDF-1–dependent responses. The mechanism(s) by which C3 is degraded in the bone marrow and releases its C3a fragment remain to be elucidated; however, there are several lines of evidence that support the local generation of C3a in the bone marrow. For example, recent studies have shown that C3a-like fragments can be generated upon cleavage of C3 by noncomplement proteases (eg, tryptase, cathepsin G) secreted by neutrophils and mast cells.44,45 It has also been shown that during mobilization of stem cells to the peripheral blood, the bone marrow environment becomes highly enriched in proteolytic enzymes (such as matrix metalloproteinases, neutrophil-derived elastase, or cathepsin G) that promote the migration of stem cells into circulation.45 46 The possibility that these proteases cleave C3 and produce C3a locally in the bone marrow, under acute or “stress”-related conditions, cannot be ruled out.
The multiple mechanisms and factors that regulate anchorage of hematopoietic stem cells in the bone marrow are still not fully understood. Changes in gradient/availability of several factors including SDF-1, VEGF, and the stem cell factor kit-ligand (KL) have been suggested to contribute to this process.17-20,43,47,48 Accumulating evidence, however, indicates that SDF-1 plays a central role in anchoring stem cells in the bone marrow environment, and a decrease in its concentration in bone marrow after its degradation by locally secreted proteases facilitates the egress of stem cells into peripheral blood. In this context we have identified a novel pathway by which the SDF-1–CXCR4 axis might modulate the homing activity of hematopoietic stem cells, in addition to the known mechanisms such as degradation of SDF-148 or changes in expression of CXCR4 on the surface of early hematopoietic cells.36 We postulate here that modulation of the SDF-1–CXCR4 axis by signals derived from other G-protein–coupled receptors (eg, C3aR) could explain how the functions of this axis are affected by other factors involved in homing/mobilization of hematopoietic cells. Supporting this is the recent finding that the SDF-1–CXCR4 axis in CD34 cells is negatively regulated by β-chemokine macrophage inflammatory protein-1α (MIP-1α),49 which may explain at the molecular level how MIP-1α mobilizes hematopoietic stem cells into peripheral blood.51
Having shown that C3a enhances SDF-1–dependent homing activities of hematopoietic progenitors in vitro, including chemotaxis,trans-Matrigel migration, and adhesion of CD34+cells, we subsequently used an in vivo murine transplantation model to elucidate the role of C3a in homing and engraftment of progenitors after transplantation. Treatment of murine bone marrow stem/progenitor cells with C3a promoted their homing and engraftment in lethally irradiated animals. This finding further corroborates our in vitro data on the C3a-mediated effects on hematopoietic stem/progenitor cell migration and demonstrates that C3a is a mediator of hematopoietic stem/progenitor cell trafficking/homing in vivo. The more rapid engraftment of C3a-primed hematopoietic stem/progenitor cells in lethally irradiated mice, as evidenced by the (1) accelerated recovery of leukocytes and platelets in these animals, (2) number of day-11 CFU-S in the spleens, and (3) number of Sca-1+clonogenic progenitors present in marrow cavities at day 16 after transplantation, suggests that ex vivo priming of human CD34+ cells with C3a could similarly improve hematopoietic engraftment in clinical transplantation settings.
Furthermore, it is known that mobilized peripheral blood cells engraft faster after transplantation than bone marrow cells harvested from nonmobilized donors.51,52 The mechanisms underlying this phenomenon have, however, not yet been elucidated, although several have been proposed.22,33,53,54 Because the complement system is activated during mobilization and leukapheresis,55 mobilized stem/progenitor cells are exposed to C3a anaphylatoxin, whose priming effect could increase their homing after transplantation. In fact, we observed in these studies that murine Sca-1+ BM MNCs primed by C3a before transplantation engrafted faster.
It has also been reported that while SDF-1 as well as CXCR4 knock-out mice embryos have reduced numbers of hematopoietic cells in their bone marrow cavities,23 C3- and C3aR-deficient mice show normal hematopoietic development.56-58 Nevertheless, the fact that SDF-1 and CXCR4 knock-out embryos still have some hematopoietic progenitors in the bone marrow suggests that SDF-1 is not the only homing agent for these cells and that other factors may compensate for SDF-1 deficiency. We suggest that complement anaphylatoxins could play an important role here. Supporting this, preliminary studies by our group have shown that C3-deficient animals have worse hematopoietic engraftment than their normal littermates and are more sensitive to G-CSF–induced mobilization (M.Z.R., manuscript in preparation, December 2002).
The putative cross talk between C3aR and CXCR4 that we have identified in this study could have significant implications in several other biologic processes. For example, C3a and SDF-1 could synergistically regulate the functions of T lymphocytes, monocytes, basophils, and eosinophils, all of which are known to express both C3aR and CXCR4 receptors.7,27,33 Moreover, it has been reported that the SDF-1–CXCR4 axis plays an important role in the homing of lymphoid cells to the lymph nodes and thymus,21,23,34,53 and it has also been implicated in directing certain metastatic tumor cells to colonize the bone marrow, lungs, and lymph nodes.28,59Therefore, it would be intriguing to speculate that C3a may exert a novel modulatory role in these processes. Similarly, “sensitization” of the CXCR4 receptor by the C3a-C3aR axis could potentially modulate the susceptibility of cells to infection by T-tropic HIV strains.24 Finally, since both C3aR and CXCR4 are expressed by human megakaryocytes and platelets, C3a and SDF-1 could also modulate platelet formation or activation.21,53 60
In conclusion, we provide evidence for the first time that normal human hematopoietic stem/progenitor cells and lineage-expanded hematopoietic precursors express functional C3aR and that the activation of this receptor sensitizes their responses to SDF-1. Our study also indicates that C3aR-mediated signaling influences the homing of hematopoietic stem cells to the bone marrow by promoting their (1) chemotactic response to SDF-1, (2) SDF-1–dependent migration across subendothelial membranes and expression/secretion of MMP-9, and, finally, (3) accelerated engraftment after transplantation into lethally irradiated animals. The biologic implications of this newly identified synergy between C3a and SDF-1 warrant further research in order to elucidate its mechanistic aspects in various pathophysiologic settings and its contribution to normal hematopoietic development.
The technical assistance of Jacek Kijowski is greatly appreciated.
Prepublished online as Blood First Edition Paper, January 2, 2003; DOI 10.1182/blood-2002-10-3233.
Supported in part by National Institutes of Health (NIH) grant R01 HL61796-01 (M.Z.R.), and grants GM56698, AI 30040, and DK59422 (J.D.L.); and by Canadian Blood Services R & D and Anemia Institute for Research and Education grants (A.J.-W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mariusz Z. Ratajczak, Stem Cell Biology Program, James Graham Brown Cancer Center, University of Louisville, 529 South Jackson St, Louisville, KY 40202; e-mail:mzrata01@louisville.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal