Abstract
The Abl kinase inhibitor imatinib mesylate (STI571) has significant and rapid antileukemic activity in Philadelphia chromosome/Bcr-Abl–positive acute lymphoblastic leukemia (Ph+ ALL) but such activity is usually of short duration except for a small proportion of patients. To determine the prognostic significance of early Bcr-Abl levels and changes in peripheral blood (PB) and bone marrow (BM), serial samples of 56 patients with relapsed or refractory Ph+ ALL treated in phase 2 trials of imatinib were analyzed by quantitative polymerase chain reaction (PCR). Imatinib induced a complete hematologic response (CHR) or complete marrow response (marrow-CR) in 40 patients (good responders) and a partial (n = 2) or no (n = 14) remission in the remaining patients (poor responders). Compared with baseline, the median Bcr-Abl/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ratios decreased significantly in PB by 2.65, 2.64, and 3.11 log steps after 2 weeks, 4 weeks, and at the time of best response, respectively. In BM, the decline of median Bcr-Abl/GAPDH was 0.75, 1.37, and 2.78 logs, respectively. Thus, Bcr-Abl levels decreased more rapidly in PB than in BM (median time to best level 31 vs 39 days). Low Bcr-Abl/GAPDH ratios below 10−4 in PB and below 10−2 in BM after 2 weeks were significantly associated with good responses after 4 weeks. Moreover, Bcr-Abl levels (< 10−2) in BM of good responders after 4 weeks discriminated between 2 groups of patients with significantly different median time to progression (139 vs 22 days). The data show that Bcr-Abl levels in PB and BM after 2 weeks of imatinib treatment and in BM after 4 weeks have predictive relevance and may guide the application of additional therapies.
Introduction
In adult patients with newly diagnosed Philadelphia chromosome/Bcr-Abl–positive acute lymphoblastic leukemia (Ph+ ALL), current chemotherapy regimens induce complete remissions (CR) in 70% or more, but most patients relapse within the first year of treatment (median, 9 months).1 Disease-free survival with intensive chemotherapy alone ranges from 0% to 15%,2-4 and outcome in relapsed and refractory patients is even inferior. Allogeneic stem cell transplantation (SCT) is at present the only curative treatment option, although it is associated with substantial morbidity and mortality as well as high relapse rates ranging from 29% to 71%.5 Recently, the ABL protein tyrosine kinase inhibitor imatinib mesylate (also referred to as Glivec or STI571) has been demonstrated to induce a complete hematologic response (CHR) or complete marrow response (marrow-CR) in 60% to 70% of patients with relapsed or refractory acute Ph+ ALL, including patients previously undergoing transplantation. Unfortunately, the median time to progression is only 2 to 2.5 months overall6 and 3 months in CHR and marrow-CR patients, reflecting the development of resistance to imatinib.7,8 As a consequence, most patients with advanced Ph+ ALL in whom imatinib induces a complete remission will require additional treatment, of which allogeneic SCT is the preferred modality. Because results of allogeneic SCT correlate with disease status at the time of conditioning, additional treatments additive or synergistic to imatinib are frequently necessary9-11 to ensure CR at the time of SCT. A minority of patients remains in remission for longer than 4 to 6 months. Accordingly, it would be important to predict the duration of response to imatinib at an early time point to guide timing and aggressiveness of additional therapies.
Sensitive quantitative reverse transcription–polymerase chain reaction (qRT-PCR) techniques that enable detection of a single leukemia cell in a background of 104 to 106 normal cells have revealed that the level of residual leukemia reflects the probability of relapse. An inverse relationship between minimal residual disease (MRD) level after induction chemotherapy and length of remission has been documented in large cohorts of Ph− ALL patients for children12-14 and adults.15,16 Preliminary reports on MRD analysis in a large cohort of standard-risk adult ALL patients have demonstrated that certain thresholds at particular phases of the treatment protocol predict the clinical outcome.17High MRD levels typical of early relapse were associated with the presence of Philadelphia chromosome as a risk factor in childhood ALL.18 Quantification of Bcr-Abl transcripts in Ph+ ALL patients has been used to assess the effect of therapy and to detect increasing residual leukemia prior to relapse.19 One study suggested association of high Bcr-Abl levels with early relapse.20 In ALL patients undergoing allogeneic bone marrow transplantation (BMT), the pretransplantation level of MRD was found to correlate with outcome after transplantation,21 whereas any evidence of MRD after allogeneic BMT for ALL and particularly for Ph+ patients was a poor prognostic sign.22,23 It appears that specific criteria to interpret MRD results have to be established for different age groups, risk groups, therapy protocols, and treatment phases.24-26
To date, it is not known whether peripheral blood (PB) or bone marrow (BM) samples and whether absolute Bcr-Abl levels or the magnitude of decline are suitable predictive parameters for clinical outcome in Ph+ ALL, especially during imatinib therapy. It was therefore the major aim of this study to determine the prognostic relevance of Bcr-Abl expression and reduction in BM and PB samples after 2 and 4 weeks of imatinib treatment in regard to type and duration of response for Ph+ ALL patients.
Patients and methods
Patients and study design
A total of 68 patients were enrolled into 2 successive clinical phase 2 trials of imatinib (CSTI571 109 and CSTI571 114) designed to determine the safety and efficacy of imatinib in patients with relapsed or refractory Ph+ ALL.27 Approval was obtained from the institutional review board of Goethe University Hospital for these studies. Informed consent was provided according to the Declaration of Helsinki. It was required that patients undergoing transplantation had undergone hematopoetic reconstitution prior to relapse. Sequential PB and BM samples were obtained from 56 of these 68 patients eligible for the present MRD study.
Imatinib was started as a single agent at a daily oral dose of 400 mg or 600 mg. Prednisone was permitted in patients who previously underwent transplantation if it was needed to control graft-versus-host disease (GVHD). Imatinib administration was continued as long as a clinical benefit was evident unless adverse events or toxicity as defined by the protocol necessitated interruption or termination of treatment.
A CHR was defined as a reduction of marrow blasts to less than 5% with no blasts in peripheral blood, hematopoietic recovery with absolute neutrophil counts (ANCs) and platelet counts at least 1.5 × 109/L and at least 100 × 109/L, respectively, and no evidence of extramedullary disease. A Marrow-CR was defined as a reduction of marrow blasts to less than 5% with no blasts in peripheral blood, no evidence of extramedullary involvement, but incomplete hematopoietic recovery. Partial response (PR) was defined as reduction of bone marrow blasts to 6% to 25%. Relapse was defined as disease recurrence with bone marrow blasts exceeding 5% or reappearance of PB blasts in a patient who had achieved a CHR or marrow-CR. Patients were considered refractory to imatinib if there was no elimination of PB blasts or of extramedullary disease and/or a failure to reduce marrow blasts to below 25%.
For the purpose of this MRD study, to establish an early predictive parameter a good response was defined by CHR or marrow-CR and a poor response by PR or refractory disease.
Molecular remission was defined by negative quantitative PCR reactions at good sensitivity (> 105 glyceraldehyde-3-phosphate dehydrogenase [GAPDH] copies) in both BM and PB for at least 1 month.
All patients had received chemotherapy, 18 patients allogeneic SCT, and 4 autologous SCT prior to imatinib. At start of imatinib, 1 patient was in marrow-CR, 2 patients in PR, 22 in first relapse, 10 in second relapse, 1 in fourth relapse, and 20 refractory (19 in primary chemotherapy). Nine patients in first relapse (additional autologous SCT: n = 1), 8 patients in second relapse (SCT: 3 in CR1; 5 in CR2), and 1 in fourth relapse (2 autologous, 3 allogeneic SCT) had undergone prior allogeneic SCT. One patient in first relapse received an autologous SCT in addition to allogeneic SCT, another patient in second relapse received 3 autologous SCT, and another patient 1 in CR1.
Four patients received intrathecal chemotherapy (15 mg methotrexate, 40 mg cytarabine, 10 mg prednisone) during imatinib therapy because of central nervous (CNS) system relapse (n = 3) or as a prophylaxis (n = 1). One additional patient had CNS relapse concomittant with marrow relapse. Immunosuppression had been withdrawn prior to imatinib therapy in 15 of 18 patients having undergone transplantation. Only in 4 of these 18 patients did GVHD occur during imatinib treatment. Thirteen patients who were taken off the study to receive an allogeneic SCT discontinued imatinib 2 days prior to myeloablative therapy and were censored for our present analysis of time to progression at this time point: 10 patients in marrow-CR or CHR achieved by imatinib (28, 38, 41, 43, 49, 55, 57, 66, 66, and 67 days after starting imatinib), 1 patient after reaching marrow-CR with imatinib and relapsing (discontinued after 31 days), 1 patient with partial remission (53 days), and 1 patient with cytoreduction (58 days).
Thirty-nine patients expressed minor Bcr-Abl (e1a2), 10 patients major Bcr-Abl (b2a2), and 7 patients major Bcr-Abl (b3a2).
Cell samples and real-time PCR
Bone marrow aspirates (2 to 5 mL) and peripheral blood samples (10 mL) were taken in EDTA (ethylenediaminetetraacetic acid) just before starting imatinib therapy, 2 weeks, 4 weeks, and subsequently monthly thereafter. Mononuclear bone marrow and peripheral blood cells were separated by Ficoll-Hypaque density gradient centrifugation, and aliquots of viable cells were cryopreserved in liquid nitrogen. RNA was extracted by Ambion's total RNA extraction kit (Austin, TX) per the supplier's instructions. Complementary DNA was synthesized from 1 to 5 μg RNA according to standard conditions.28 Plasmid standard titrations with defined copy numbers for Bcr-Abl and GAPDH (housekeeping gene) were analyzed simultaneously with patient samples. TaqMan PCR was conducted in duplicate reactions employing ABI PRISM 7700 (Applied Biosystems, Weiterstadt, Germany) with standard conditions (50°C for 2 minutes, 95°C for 10 minutes, 45 cycles at 95°C for 15 seconds, and 60°C for 1 minute). To amplify minor–Bcr-Abl (e1a2), 5 μL of template was used in 50 μL reaction mixtures consisting of the primer a2-F (CAGACCCTGAGGCTCAAAGTC) at 200 nM and the primer rALL-TB (GCAAGACCGGGCAGATCT) at 200 nM and the breakpoint-specific probe ALL12-FAM (CCGCTGAAGGGCTTCTGCGTCTCC) labeled with FAM at the 5′ end and TAMRA at the 3′ end at 200 nM final concentration. MgCl2was used at 5 mM, and other reagents were added per the supplier's instructions (Core Reagents Kit; Applied Biosystems, Weiterstadt, Germany). To amplify major–Bcr-Abl (b2a2) primers, a2-F and b2-1R (GCATTCCGCTGACCATCAA) were used in combination with breakpoint-specific probe CML22-FAM (CCGCTGAAGGGCTTCTTCCTTATTG) and 6 mM MgCl2concentration. Major–Bcr-Abl (b3a2) was amplified by primers a2b3-F (GAGTTCCAACGAGCGGCTT) and b3-1R (TCATCGTCCACTCAGCCACT) and breakpoint-specific probe CML32-FAM (CCGCTGAAGGGCTTTTGAACTCTG) at 4.5 mM MgCl2. For normalization, GAPDH housekeeping gene expression was analyzed using a predeveloped assay by Applied Biosystems. In addition, sensitivity was determined using defined numbers of SD1 (e1a2) or BV173 (b2a2) or K562 (b3a2) cells diluted in Bcr-Abl− cells (Nalm6) as well as patient samples. The sensitivity in titrations of examplary patients' leukemic blasts in normal BM or PB mononuclear cells amounted to 10−5 for e1a2 and b2a2 as well as 10−6 for b3a2. It was required to have at least 105 GAPDH plasmid equivalents in a sample to consider a negative PCR result valid.
Statistical analysis
Statistical analysis of differences in Bcr-Abl levels and changes between good and poor responders was performed by nonparametric Mann-Whitney U test. The Fisher exact probability test was employed to detect whether Bcr-Abl levels below a set threshold were significantly associated with good responses versus Bcr-Abl levels above this threshold with poor responses. Time to progression and overall survival were evaluated by Kaplan-Meier plots and log-rank tests. For Kaplan-Meier curves, time to progression (TTP) or survival was calculated from the time the particular PB or BM specimen was taken to relapse, death, or censoring, respectively. Logarithmic changes of Bcr-Abl levels with negative PCR results were calculated by substituting the value of the particular sensitivity of the PCR reaction for the negative result. These values were depicted as empty symbols in diagrams.
Results
Hematologic response during imatinib treatment
Forty of 56 patients achieved a good response after at least 4 weeks of imatinib. Sixteen patients displayed a poor response due to persisting PB blasts (n = 4) or more than 5% blasts in BM. At the time of analysis, 24 of 40 patients with good response had relapsed after a median time of 104.5 days (range, 45 to 441 days) of imatinib therapy. Continuous CR was observed in 4 patients at the time of analysis, with a duration of follow-up at 228, 257, 524, and 746 days, respectively. Additionally, one death due to pneumonia and septicemia occurred in a well-responding patient with a history of allogeneic stem cell transplantation. Thirteen patients were transferred to SCT early at a median of 49 days (range, 26 to 67 days) after starting imatinib and censored at the start of conditioning. Median time to progression of poor responders was 43.5 days (range, 19 to 62 days).
Correlation of Bcr-Abl levels in PB and BM with clinical response
To determine the degree with which Bcr-Abl levels and kinetics corresponded to clinical response, we analyzed PB and BM samples collected serially throughout imatinib treatment. The median Bcr-Abl/GAPDH ratio prior to study did not differ between patients with subsequent good or poor response (Figure1). Good responders showed a rapid significant decline of Bcr-Abl levels in PB, with the major reduction already seen after 2 weeks (2.65 logs). Although a further decline of Bcr-Abl level was observed in a small number of patients after 4 weeks, turning undetectable in some, the median Bcr-Abl level did not change between 2 and 4 weeks (again, 2.64 logs lower than at start). The median Bcr-Abl level at the time of best response was 3.11 logs below the median at the beginning of treatment. Conspicuously, the range of Bcr-Abl levels spanned several log steps (about 7) in good responders even at the time of best response (Figure 1).
Diagram showing absolute Bcr-Abl levels in PB and BM samples.
The ratios of Bcr-Abl/GAPDH at start, after 2 weeks (2W), 4 weeks (4W), and at the time of best response (best) during imatinib therapy are depicted on a logarithmic scale for good responders (●) and poor responders (▴) separately. The medians are represented by lines, and negative PCR results are shown (○) at the level of the particular sensitivity of the PCR reaction.
Diagram showing absolute Bcr-Abl levels in PB and BM samples.
The ratios of Bcr-Abl/GAPDH at start, after 2 weeks (2W), 4 weeks (4W), and at the time of best response (best) during imatinib therapy are depicted on a logarithmic scale for good responders (●) and poor responders (▴) separately. The medians are represented by lines, and negative PCR results are shown (○) at the level of the particular sensitivity of the PCR reaction.
In BM, a similar pattern of Bcr-Abl levels was observed and the median level at the time of best response was close to that of PB samples at best (Figure 1). However, the kinetics of significantly decreasing Bcr-Abl levels in BM of good responders were much slower and had not even by far reached the best level at 4 weeks (Figure 1). The median Bcr-Abl/GAPDH ratio decreased by 0.75 logs, 1.37 logs, and 2.78 logs after 2 and 4 weeks and at best response, respectively, compared with levels prior to imatinib therapy. Good responders reached best Bcr-Abl levels 31 days (range, 13 to 140 days) in PB and 39 days (range, 12 to 166 days) in BM after starting imatinib.
As expected, poor responders (PR or failure) showed no decrease but rather an increase of Bcr-Abl expression in BM. However, even poor responders showed some reduction of median Bcr-Abl levels in PB at 2 and 4 weeks, indicating a low degree effect of imatinib in this compartment (Figure 1).
Relation between individual Bcr-Abl changes in PB and BM and subsequent remission
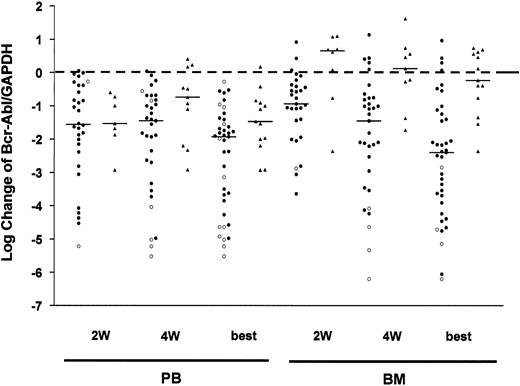
After displaying Bcr-Abl levels (Figure 1) to analyze the kinetics of MRD response collectively in clinical response groups, we also evaluated the kinetics of leukemic blasts by Bcr-Abl expression in individual patients in relation to clinical outcome. Therefore, we determined the change of Bcr-Abl/GAPDH ratio for each patient at 2 and 4 weeks and at best compared with start (Figure2). Individual Bcr-Abl changes basically confirmed the pattern described for Bcr-Abl levels. There were gradually increasing significant reductions of Bcr-Abl/GAPDH in BM of good responders by median −0.94, −1.45, and −2.40 logs at 2 and 4 weeks and at best, respectively. Compared with BM, reductions of Bcr-Abl in PB of good responders are much more pronounced after 2 weeks (−1.56 logs) without further change after 4 weeks (−1.45 logs) almost reaching the maximum degree (−1.93 logs). Interestingly, the median of changes shows a stronger best response in BM than in PB (−2.40 vs −1.93 logs). Despite Bcr-Abl increases by median in BM of poor responders, those patients showed some early Bcr-Abl decreases (−1.53 logs) after 2 weeks in PB (Figure 2). Therefore, imatinib appears to exert a faster or stronger effect on leukemic blasts in PB.
Diagram showing logarithmic changes of Bcr-Abl levels in PB and BM samples.
Log changes after 2 weeks (2W), 4 weeks (4W), and at the time of best response (best) during imatinib therapy are depicted for good responders (●) and poor responders (▴) separately. The medians are represented by lines. The changes of Bcr-Abl turning into a negative PCR result were calculated by using the particular sensitivity of the PCR reaction instead. These values are depicted by ○.
Diagram showing logarithmic changes of Bcr-Abl levels in PB and BM samples.
Log changes after 2 weeks (2W), 4 weeks (4W), and at the time of best response (best) during imatinib therapy are depicted for good responders (●) and poor responders (▴) separately. The medians are represented by lines. The changes of Bcr-Abl turning into a negative PCR result were calculated by using the particular sensitivity of the PCR reaction instead. These values are depicted by ○.
Predictive potential of Bcr-Abl levels at 2 weeks in terms of response
In view of the highly significant differences between Bcr-Abl levels in good and poor responders after 2 and 4 weeks, we examined whether early Bcr-Abl quantification could be used to predict subsequent quality of remission. Because the time to identify good and poor responders was 4 weeks in general, Bcr-Abl determinations only before this time (eg, after 2 weeks of imatinib treatment) appeared to be useful in predicting the type of response. Bcr-Abl/GAPDH ratios below 10−4 in PB samples after 2 weeks of imatinib treatment were significantly (P = .0003) associated with good responses at 4 weeks opposed to higher levels and poor responses (Table 1). Although low PB levels (< 10−4) were found only in good responders (n = 19), 8 of 17 patients with high PB levels (≥ 10−4) finally attained a good response. Thus, very early low Bcr-Abl levels (< 10−4) in PB are a strongly prognostic indicator of subsequent good response, whereas high levels are only a weak indicator of subsequent poor response.
Frequency of patients with low and high Bcr-Abl levels in PB (2 weeks) and subsequent good and poor clinical responses (4 weeks)
| Bcr-Abl/GAPDH level . | Good clinical response . | Poor clinical response . |
|---|---|---|
| Below 10−4 | 19 | 0 |
| 10−4 and above | 8 | 9 |
| Bcr-Abl/GAPDH level . | Good clinical response . | Poor clinical response . |
|---|---|---|
| Below 10−4 | 19 | 0 |
| 10−4 and above | 8 | 9 |
P = .0003.
Statistical analysis revealed that Bcr-Abl levels in BM below 10−2 after 2 weeks were significantly (P < .0001) associated with good responses and vice versa (Table 2). All patients with Bcr-Abl below 10−2 in BM after 2 weeks (n = 23) became good responders. However, 5 of 16 patients with high Bcr-Abl levels in BM finally turned into good responders. Thus, a very early (2 weeks) Bcr-Abl level below 10−2 in BM predicts a good response, whereas a level above 10−2 does not necessarily predict a poor response.
Frequency of patients with low and high Bcr-Abl levels in BM (2 weeks) and subsequent good and poor clinical responses (4 weeks)
| Bcr-Abl/GAPDH level . | Good clinical response . | Poor clinical response . |
|---|---|---|
| Below 10−2 | 23 | 0 |
| 10−2 and above | 5 | 11 |
| Bcr-Abl/GAPDH level . | Good clinical response . | Poor clinical response . |
|---|---|---|
| Below 10−2 | 23 | 0 |
| 10−2 and above | 5 | 11 |
P < .0001.
In contrast, Bcr-Abl changes in PB after 2 weeks did not and changes in BM (< −0.5 logs) did only weakly predict good and poor responders.
Prognostic role of Bcr-Abl levels regarding response duration and survival
We wondered whether qRT-PCR of Bcr-Abl could be used to predict response duration during imatinib therapy. This issue is important in regard to practical implications concerning whether allogeneic stem cell transplantation, donor lymphocyte infusions, or other treatment options have to be initiated. Bcr-Abl levels after 4 weeks of imatinib were evaluated for their predictive potential because preliminary data had shown that this time point was superior to that at 2 weeks. On the other hand, later time points after 4 weeks could not be considered to be predictive due to frequent early relapses. Kaplan-Meier plots of good responders with low Bcr-Abl levels at 4 weeks versus those with high levels revealed a significant difference regarding time to progression (Figure 3). A Bcr-Abl/GAPDH ratio of 10−2 was the significantly discriminatory threshold between early and late progressors (P = .0002). Whereas median time to progression in patients with Bcr-Abl levels below 10−2 amounted to 139 days, it was only 22 days in patients with Bcr-Abl levels 10−2 or more.
Kaplan-Meier plots showing the time to progression dependent on early Bcr-Abl levels.
Ph+ ALL patients treated with imatinib (only good responders) were allocated to either of 2 groups dependent upon their Bcr-Abl/GAPDH ratio in BM above or below 10−2 after 4 weeks of therapy. The difference was significant (P = .0002).
Kaplan-Meier plots showing the time to progression dependent on early Bcr-Abl levels.
Ph+ ALL patients treated with imatinib (only good responders) were allocated to either of 2 groups dependent upon their Bcr-Abl/GAPDH ratio in BM above or below 10−2 after 4 weeks of therapy. The difference was significant (P = .0002).
Bcr-Abl reductions in BM samples after 4 weeks did not discriminate prognostic groups significantly.
Neither Bcr-Abl levels nor changes in PB samples of good responders at 4 weeks were associated with significant differences in time to progression for any threshold.
A further important prognostic criterion is overall survival. However, neither assignment by Bcr-Abl level nor by Bcr-Abl change in PB or BM at 4 weeks, respectively, generated significantly different patient groups in regard to overall survival.
Relevance of negative qRT-PCR findings
Negative quantitative TaqMan PCR results were considered only if the sensitivity of the particular PCR reaction was adequate by sufficient GAPDH copy numbers (> 105). By these criteria, 14 patients exhibited at least 1 negative PCR result either in PB (13 patients; 42 samples) or in BM (3 patients; 13 samples). There were 10 simultaneously taken paired samples from PB and BM (8 patients) of which 9 were PB−BM+ and 1 was PB+BM−. Thus, PCR results from BM samples are more sensitive.
Nine paired PCR results simultaneously negative in both PB and BM were found in 2 patients. One of these with an allogeneic stem cell graft became Bcr-Abl− after 3 months of imatinib treatment, now continuing for 21 months. Another patient after allogeneic SCT became Bcr-Abl− (1 month after starting imatinib) for 1 month in BM and PB but relapsed 3 months thereafter.
Overall, 10 of the 14 patients in whom imatinib induced Bcr-Abl negativity in either PB or BM relapsed except for the patient in long-term molecular remission (see previous paragraph), 1 good responder dying due to septicemia, and 2 patients who were subjected to SCT early. The time interval between most recently negative PCR and relapse was 112 and 66 days for BM and by a median of 81 days (range, 24 to 286 days) for PB samples (9 patients).
Discussion
Imatinib induces hematologic responses in 60% to 70% of patients with relapsed or refractory Ph+ ALL, but remission quality is usually poor as indicated by a median response duration of only 2 to 3 months.6 27 The ability to identify early the subgroup of patients who will fail to achieve a hematologic response and to differentiate between patients with a high probability of early relapse and those likely to have a more sustained response could considerably assist patient management. To determine whether quantitative real-time PCR analysis of Bcr-Abl transcripts could be used to monitor the efficacy of imatinib and predict response and response duration, we examined serial PB and BM samples from 56 patients with relapsed or refractory Ph+ ALL treated with imatinib. In good responders, Bcr-Abl levels decreased more rapidly in PB than in BM. Median Bcr-Abl levels were consistently lower in PB than in BM after 2 and 4 weeks and at the time of best molecular response, although at the latter time point the median log reduction was greater in BM than in PB. Moreover, an early decrease of Bcr-Abl/GAPDH ratios to below 10−4 in PB and below 10−2 in BM samples after 2 weeks of imatinib therapy was significantly associated with achievement of a subsequent CHR or marrow-CR. Conversely, high levels do not necessarily predict poor responses. Furthermore, we demonstrated that Bcr-Abl levels in BM below 10−2 at 4 weeks predicted a significantly longer interval to progression but not longer overall survival. Whether a threshold of 10−2 Bcr-Abl/GAPDH in BM at 4 weeks is the optimal choice for clinical purposes requires further validation in prospective studies. Nevertheless, the Bcr-Abl level measured in BM at 4 weeks of treatment is a useful prognostic parameter to assess response duration.
It has been shown that analysis of PB samples from patients with ALL might cause an underestimation of MRD levels compared with BM.19 Our results are similar to data by Brisco et al, which showed that the median MRD level in PB was approximately 12 times lower than the median in BM at the end of induction therapy of children with Bcr-Abl− ALL.29 Similarly, Martin et al have found that Bcr-Abl levels were about 35-fold lower in PB than in BM of 6 patients with Ph+ ALL.30 However, others observed similar levels in PB and in BM.31 32Potential causes of the difference in MRD levels and kinetics between PB and BM that are associated with imatinib include redistribution of the blasts and different susceptibility to this agent (eg, due to distinct proliferative properties of leukemic blast cells in these compartments). Overall, these data indicate that in patients with Ph+ ALL undergoing treatment with imatinib, analysis of BM is more sensitive than PB with regard to assessment of MRD.
MRD analysis was also applied to characterize the pharmacodynamic efficacy of imatinib. Bcr-Abl quantitative MRD results have not been published for imatinib-treated Ph+ ALL patients elsewhere, but there are data on Bcr-Abl levels under chemotherapy19that may serve for comparison. MRD levels of minor Bcr-Abl decreased in responding patients by a median of −1.8 logs (−0.5 to −2.9) in PB and BM and major Bcr-Abl levels by −1.5 logs (−0.9 to −2.4) in PB and by −1 log (−0.05 to −2.3) in BM under conventional primary chemotherapy. Imatinib-treated patients in our study (predominantly minor Bcr-Abl) who reached at least marrow-CR showed a decrease of Bcr-Abl by a median of −1.93 logs (−0.27 to −5.52) in PB and −2.40 logs (0.97 to −6.20) in BM, respectively. However, Ph+ ALL patients after salvage chemotherapy, who were more similar to our cohort, had a smaller reduction of −0.4 logs in PB and −0.85 logs in BM19 compared with an overall median decrease of −1.89 in PB and −2.11 logs in BM samples found in the present study. The relatively high efficacy of imatinib compared with a polychemotherapy may be ascribed to the high specificity of this compound.
It is noteworthy that negative PCR results occur in BM or PB only sporadically and even in case of good sensitivity have no significance in terms of long-term outcome. BM analysis proved to be more sensitive than PB, and simultaneous PCR negativity in both compartments was exceedingly rare. Such a complete molecular response was sustained for at least 4 weeks in only 2 patients, 1 of whom nevertheless relapsed 3 months later. Only one of the patients analyzed in this study remains in ongoing continuous complete molecular and hematologic remission, as assessed in parallel PB and BM samples, more than 24 months after initiating imatinib for relapsed Ph+ ALL. Interestingly, one patient previously undergoing transplantation is in ongoing hematologic remission at 524 days despite evidence of MRD with Bcr-Abl expression constant at a low level and no evidence of GVHD. This observation is reminiscent of findings by Radich et al,22 who showed that detection of Bcr-Abl transcripts by RT-PCR after allogeneic stem cell transplantation was highly predictive of eventual relapse in patients with p185 (e1a2) Ph+ ALL but not necessarily in patients with p210 (b3a2)–positive ALL. Possibly, these differences reflect that less proliferative capacity is mediated by p210 (b3a2) compared with p185 (e1a2) as shown in a mouse model.33 Taken together, our data emphasize that most patients with relapsed or refractory Ph+ ALL who are treated with imatinib as a single agent eventually relapse even if a complete molecular response of several weeks' duration was achieved. More prolonged PCR negativity of several months' duration confirmed at a good level of sensitivity in both PB and BM may indicate long-term remission, but criteria on which to base safe discontinuation of imatinib remain to be established. Although occasional patients may experience prolonged hematologic remissions despite persistent MRD positivity, continued detection of Bcr-Abl transcripts after the first few weeks of imatinib therapy should prompt additional therapy.
Our observation that the level of Bcr-Abl transcripts determined 4 weeks after starting imatinib is predictive of remission duration but not of overall survival may be due to confounding effects of subsequent therapy (eg, allogeneic SCT or salvage therapy). An alternative explanation is that the mechanisms underlying secondary resistance to imatinib differ from those responsible for up-front refractoriness to this kinase inhibitor; this would preclude detection by techniques that primarily assess the initial blast population prior to or during early stages of treatment (eg, by gene expression profiling34 or PCR-based MRD analysis). In accordance with this hypothesis, point mutations in the adenosine triphosphate (ATP)–binding domain of Abl have been identified as a frequent cause of secondary resistance7 but have so far not been reported prior to imatinib treatment. Given the often rapid kinetics of relapse in Ph+ ALL, it is therefore plausible that the outgrowth of a mutant ALL clone due to the selective pressure of imatinib cannot be anticipated by assessing the initially dominant population of leukemic blasts and its early response to treatment.
In summary, determination of Bcr-Abl levels by quantitative real-time RT-PCR was able to discriminate different prognostic groups among refractory or relapsed Ph+ ALL treated with imatinib. Low Bcr-Abl levels in PB and BM after 2 weeks of imatinib were predictive of achieving a CHR or marrow-CR. Moreover, low Bcr-Abl levels in BM after 4 weeks of treatment were prognostic to identify patients with prolonged responses. Although these results do not obviate the need for employing alternative therapies in conjunction with imatinib (eg, allogeneic SCT or combination therapy), the results of MRD analysis performed at small intervals during initial treatment with imatinib may guide treatment decisions regarding the timing and/or intensity of additional therapeutic modalities. In this respect, the optimal cutoff has yet to be validated. Serial determination of Bcr-Abl transcripts by quantitative RT-PCR may also be useful for assessing the efficacy of combination strategies incorporating imatinib, some of which are being evaluated in ongoing clinical studies.
We are indebted to S. Kriener for the pathological review of marrow histologies, Anja Binckebank for coordinating the study, and Holger Thüringer, Anja Goodwin, Heike Nürnberger, Martine Pape, and Sandra Wagner for their excellent technical assistance.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-02-0360.
Supported by grants from the Adolf Messer Stiftung, Germany; Kompetenznetzwerk akute und chronische Leukämien, Mannheim, Germany; and Novartis AG, Nürnberg, Germany.
H.G. is employed by Novartis AG, which has developed Glivec.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Urban J. Scheuring, Medizinische Klinik III, Abteilung für Hämatologie und Onkologie der Johann Wolfgang Goethe-Universität, Theodor-Stern-Kai 7, D-6090 Frankfurt, Germany; e-mail: scheuring@em.uni-frankfurt.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal