Abstract
Thrombocytopenia is a relatively common side effect observed during glycoprotein (GP) IIb/IIIa antagonist therapy. With the oral antagonist roxifiban, we observed thrombocytopenia, defined as 50% reduction of platelets over predose values or below 90 000/μL (9 × 1010/L), with a frequency of 2% (8 of 386). Thrombocytopenia occurred either early (days 2 to 4) or delayed (days 11 to 16). No additional cases were observed with up to 6 months of treatment. Retrospective analysis provided evidence for drug-dependent antibodies (DDABs) to GP IIb/IIIa in 5 of 6 subjects, suggestive of an immune etiology of thrombocytopenia. The hypothesis that excluding patients based on positive DDAB reaction would reduce the frequency of thrombocytopenia was tested. Patients were screened for DDABs during the study qualification period and, overall, 3.9% of the patients were excluded based on pre-existing DDAB concentrations above a statistically defined medical decision limit. An additional 2.6% were excluded based on therapy-related antibody production during the first 2 weeks. With antibody testing, 0.2% of patients (2 of 1044) developed immune-mediated thrombocytopenia. One case developed a rapidly increasing antibody concentration and presented with thrombocytopenia despite discontinuation of roxifiban therapy. The second case was related to a false-negative test result. The frequency of thrombocytopenia was statistically significantly reduced from 2% to 0.2% (P = .0007) comparing nonscreened and screened patients. Testing for DDABs can reduce the frequency of thrombocytopenia in patients treated with roxifiban and, by analogy, other GP IIb/IIIa antagonists. Thus, DDAB testing may be employed to increase the safety of GP IIb/IIIa antagonists.
Introduction
Thrombocytopenia has been observed with all currently approved glycoprotein (GP) IIb/IIIa antagonists (intravenous formulations) and a number of developmental candidates. Thrombocytopenic patients experience more bleeding and ischemic events.1,2 In the case of approved intravenous agents, this side effect has been observed with a frequency of up to 5.6%.1-9 In addition, a number of oral GP IIb/IIIa antagonists have undergone clinical testing, and treatment, for example, with RPR 109891 was associated with a thrombocytopenia frequency of up to 13%.10 Lower frequencies have been observed for the other oral agents in clinical development. For sibrafiban, the thrombocytopenia frequency was not different compared with placebo,11 whereas in the case of orbofiban, increased frequencies were observed in the treatment arms.12 We recently reported 2 patients with drug-induced thrombocytopenia associated with roxifiban treatment.13The sample size was too small to obtain accurate estimates as to the frequency of thrombocytopenia or to generalize as to the prevailing pathomechanism in the patient population treated with roxifiban.
Some general risk factors for thrombocytopenia induced by GP IIb/IIIa antagonist therapy have been identified, including advanced age, lower body weight, and lower baseline platelet count.1,2Retrospective studies in humans and preclinical animal models implicated drug-dependent antibodies (DDABs) to GP IIb/IIIa in the etiology of thrombocytopenia. For example, a chimpanzee presented with acute thrombocytopenia upon treatment with GP IIb/IIIa antagonists.14 Platelet fluorescence-activated cell sorter (FACS) analysis provided evidence for the presence of GP IIb/IIIa antagonist-dependent antiplatelet antibodies to GP IIb/IIIa antagonist that elicited thrombocytopenia but not to antagonists that were well tolerated in the same chimpanzee.14 Support for the immune nature of thrombocytopenia in humans is derived from 2 patients treated with roxifiban, an orally bioavailable GP IIb/IIIa antagonist. Both patients underwent conversion to a highly positive drug-dependent antibody status temporally associated with thrombocytopenia.13Previous studies are consistent with the hypothesis that the antibodies bind to platelets in a roxifiban-dependent manner via their ability to recognize roxifiban-induced conformational changes in GP IIb/IIIa.13
Here, we report that thrombocytopenia is a relatively common side effect during treatment with roxifiban and occurs with a frequency of about 2%. Thrombocytopenia was observed either early (days 2 to 4) or delayed (days 11 to 16). No additional cases occurred beyond this susceptible time period. Most cases presented with drug-dependent antibodies to GP IIb/IIIa. Thus, we tested the hypothesis that prospective testing for pre-existing or increasing DDAB concentrations would reduce the incidence of thrombocytopenia in patients treated with roxifiban. Patients were tested for the presence of pre-existing DDABs prior to enrollment and during the first 2 weeks of therapy. We provide evidence that controlling patient enrollment based on DDAB status significantly reduces the incidence of GP IIb/IIIa antagonist-induced thrombocytopenia, further supporting the immune etiology of GP IIb/IIIa antagonist-induced thrombocytopenia.
Patients, materials, and methods
Study populations
Specimens for DDAB testing were collected during phase 2 clinical trials of roxifiban. DMP 754-010 (parts C and D) and DMP 754-017 were randomized, double-blind, multicenter, multidose studies of the GP IIb/IIIa antagonist roxifiban. Inclusion criteria included documented coronary artery disease (CAD) (myocardial infarction, percutaneous transluminal coronary angioplasty [PTCA], angiography, unstable angina, coronary artery bypass graft [CABG]) within 3 months or documented CAD plus an additional risk factor (aged 60 years and older, diabetes, smoking). Estimates as to the frequency of thrombocytopenia in the absence of DDAB testing were derived from DMP 754-010 (parts A and B; CAD population), DMP 754-008 (CAD population), and DMP 754-302. The latter study was conducted using patients with advanced peripheral artery disease (ankle-to-brachial index 0.6 or toe-to-brachial index 0.4). The retrospective testing for DDABs in study DMP 754-010, parts A and B has been reported.13 Thrombocytopenia is defined as reduction of the platelet number to below 90 000/μL (9 × 1010/L) or below 50% compared with predose values. In general, clinical samples were obtained at least 12 hours after the last treatment with roxifiban. Roxifiban has a high affinity for resting platelets, and the free concentration of roxifiban in plasma is less than 1% of total drug, avoiding interference with the differential enzyme-linked immunosorbent assay (ELISA; see “Quantification of free DDABs in plasma”).
Quantification of free DDABs in plasma
Blood from patients in DMP 754-010 studies was collected into 1:10 volume of 3.2% sodium citrate. Platelet-poor plasma (PPP) was prepared by centrifugation at 1500g for 15 minutes and stored at less than −15°C or less than −60°C. Plasma was analyzed by a differential ELISA using GP IIb/IIIa passively absorbed to microtiter wells.13 Briefly, plasma diluted 1/20 was analyzed in the absence or presence of roxifiban, and bound immunoglobulin G (IgG) was detected with horseradish peroxidase (HRP)–labeled polyclonal goat antihuman IgG (Kirkegaard and Perry, Gaithersburg, MD) antibodies followed by 3,3′,5,5′-tetramethylbenzidine (Pierce, Rockford, IL). The detecting antibody reacts with human IgG 1 to 4 but not with human IgM (not shown). Plates were washed between the incubation steps with an automated multireagent plate washer (model AM60; Dynex, Chantilly, VA).
Quantification of platelet-bound and free DDABs
DDABs to roxifiban bind to conformationally sensitive epitopes in GP IIb/IIIa.13 EDTA (ethylenediaminetetraacetic acid) treatment of platelets at 37°C results in the dissociation of the GP IIb/IIIa complex on intact platelets,15-18 leading to the release of bound antibodies that recognize complex-specific determinants induced by roxifiban.13 After removal of platelets by centrifugation, the resulting plasma is expected to contain free and previously platelet-bound conformationally sensitive DDABs. To test whether quantification of the platelet-bound and free pool of DDABs increases the sensitivity of the DDAB ELISA, blood from patients enrolled in DMP 754-017 was collected into vacutainers containing 15% EDTA, heat-treated at 37°C for 1 hour with end-over-end rocking, and PPP prepared as above. A parallel sample was also collected in sodium citrate and archived.
EDTA treatment also dissociates roxifiban from the platelet surface, precluding the direct analysis of the plasma by the differential ELISA (antibody binding in the presence and absence of roxifiban). A roxifiban removal step with C18 (Orochem), a hydrophobic matrix with high affinity for roxifiban, was included. Briefly, 50 mg of the hydrophobic matrix was activated with 100% methanol and then equilibrated with buffer. EDTA PPP (500 μL containing 100 nM DuP 714 [final concentration], an in-house thrombin inhibitor, and 0.32% sodium citrate) was incubated with the matrix for 15 minutes. PPP was recovered by centrifugation at 2500g for 5 minutes. Control experiments (not shown) revealed that the drug absorption procedure reduced the roxifiban concentrations to a level not interfering with the differential ELISA. In addition, DDABs are not absorbed from plasma specimens.
The following changes were included compared with the “quantification of free DDABs in plasma” method, including use of less diluted plasma (1/10), and procedures aimed at reducing the background. The latter consisted of IgG depletion of purified GP IIb/IIIa13 and GP IIb/IIIa antagonist addition in the “no-drug” control wells during the detection step with the secondary antibody. The latter procedure reduced the background readings of some plasmas, thereby increasing the signal-to-noise ratio of the assay. Although the mechanism of background reduction by roxifiban has not been completely elucidated, it may be related to the dissociation of conformationally sensitive, non–drug-dependent antibodies from the ELISA plate.
Result reporting and quality assurance
The prospective use of the assay required rapid turnaround of results (24 to 48 hours) for specimens shipped on dry ice from the various clinical sites. Analysis was performed under good laboratory practice (GLP) guidelines at Covance (Vienna, VA). The assay format incorporated both positive and negative controls on all plates. Plate acceptance and rejection criteria were established initially using citrate plasma from a previously described DDAB-positive thrombocytopenic patient (patient 330 described by Billheimer et al13). Briefly, this patient experienced a thrombocytopenic episode in a previous trial with roxifiban. This quality control material was replaced during the first clinical study with a renewable source of antibodies (a recombinant chimeric DDAB [rJK094]). Briefly, the antibody was derived using standard hybridoma technology after immunization of mice with purified GP IIb/IIIa/roxifiban complexes. Resulting hybridomas were screened in the differential ELISA, and one clone with differential reactivity was isolated, cloned, and the constant region replaced with human IgG4 (K. O'Neil et al, unpublished data, 2002). This antibody was used as a positive control after cross-validation with plasma from patient 330. Changes in absorbance after substrate addition were determined kinetically for 5 minutes at 650 nm or until the change in absorbance reached 0.5 optical density (OD) units. The kinetic reading is insensitive to minor variations in the time of substrate addition to individual wells. In addition, specimens with different background readings (signal in the absence of roxifiban addition) can be accommodated on the same ELISA plate.13 Results are expressed as delta ([Vmax + compound] − [Vmax − compound]). Assays were performed with triplicate determinations, and a coefficient of variation around the cutoff of less than 15% was observed. Limited stability studies revealed that the DDAB concentration was unchanged in samples stored for up to 2 years. The ELISA results from 108 nonthrombocytopenic subjects treated with roxifiban (predose samples from a previous phase 2 roxifiban study13) were used to derive a statistical cutoff value (medical decision level) with a positive rate of 5% (delta = 12 for “quantification of free DDABs” and a 1/20 sample dilution, and delta = 35 for “quantification of platelet-bound and free DDABs” and 1/10 sample dilution; 95th percentile of nonparametric rank order test).
Miscellaneous
Results
Frequency of thrombocytopenia in patients treated with roxifiban in the absence of DDAB testing
A total of 386 patients with CAD or peripheral artery disease (PAD) were treated with roxifiban without DDAB testing (retrospective testing in Table 1). Eight cases of thrombocytopenia were observed (Table 1). The frequency of thrombocytopenia was not different between CAD (4 of 203) and PAD (4 of 175) clinical trials (Table 1). The statistical power of the clinical studies was insufficient to determine whether thrombocytopenic patients differed by age, sex, weight, or morbidity and mortality. The overall frequency of thrombocytopenia in the patient group was 2.1%. Half of the cases occurred early (days 2 to 4), whereas the other half presented with delayed (days 11 to 16) thrombocytopenia. The vulnerable period for thrombocytopenia appears to be the first 3 weeks of treatment. Additional cases were not observed in patients treated for up to 6 months. The extent of thrombocytopenia varied from moderate to severe (platelets < 20 000/μL [2 × 1010/L]). In all cases, treatment with roxifiban was discontinued, and patient safety was ensured by close clinical and laboratory monitoring and by hospitalization and platelet infusions if required. None of the thrombocytopenic patients experienced major bleeding episodes at the time of thrombocytopenia, and all patients recovered without additional sequelae. Based on the availability of specimens, retrospective testing for DDABs was performed as indicated. Five of 6 patients tested positive for DDABs. Some patients were already antibody positive prior to treatment, whereas others developed an increase in antibody titer temporally associated with the thrombocytopenic events. These observations suggest that the prevailing pathomechanism for thrombocytopenia in this patient population is immune-mediated platelet clearance and/or destruction.
Thrombocytopenia and DDAB status in clinical trials of roxifiban
| Subject no. . | Nadir platelet count, ×103/μL . | Day of thrombocytopenia . | DDABs prior to treatment . | DDABs at time of thrombocytopenia . |
|---|---|---|---|---|
| Retrospective testing for DDABs | ||||
| 304 | 15 | 4 | No sample | No sample |
| 503 | 122 | 3 | No sample | No sample |
| 10 205* | 47 | 3 | No | No |
| 10 403* | 79 | 2 | Yes | Yes |
| 307† | BQL | 11 | Yes | Yes |
| 330† | 59 | 16 | No | Yes |
| 55 202* | 35 | 13 | Yes | Yes |
| 58 204* | 5 | 14 | Yes | Yes |
| Prospective testing for DDABs | ||||
| 99016 | 2 | 14 | Yes | Yes |
| 21013 | 8 | 11 | No | Yes |
| Subject no. . | Nadir platelet count, ×103/μL . | Day of thrombocytopenia . | DDABs prior to treatment . | DDABs at time of thrombocytopenia . |
|---|---|---|---|---|
| Retrospective testing for DDABs | ||||
| 304 | 15 | 4 | No sample | No sample |
| 503 | 122 | 3 | No sample | No sample |
| 10 205* | 47 | 3 | No | No |
| 10 403* | 79 | 2 | Yes | Yes |
| 307† | BQL | 11 | Yes | Yes |
| 330† | 59 | 16 | No | Yes |
| 55 202* | 35 | 13 | Yes | Yes |
| 58 204* | 5 | 14 | Yes | Yes |
| Prospective testing for DDABs | ||||
| 99016 | 2 | 14 | Yes | Yes |
| 21013 | 8 | 11 | No | Yes |
Thrombocytopenia is defined as platelet number below 90 000/μL or reduction by more than 50% compared with the predose specimen.
Nadir indicates platelet count per microliter during thrombocytopenia; day, earliest time point thrombocytopenia definition was met; no, DDAB concentration below the medical decision limit; yes, DDAB concentration above the medical decision limit; and BQL, below quantifiable limits.
Thrombocytopenia cases during a PAD clinical trial. The remainder of the cases were derived from CAD clinical trials.
These patients have been described previously.13
Implementation of testing for free DDABs in a phase 2 study with a drug holiday
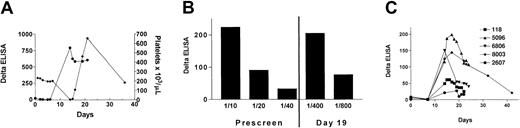
With the observation that thrombocytopenia is a relatively common side effect of roxifiban, we tested the hypothesis that prospective testing for DDAB reduces the frequency of thrombocytopenia. Patients negative by ELISA entered into a qualification period and were treated with roxifiban on 5 consecutive days, followed by a drug holiday for 9 days. Additional antibody testing was performed on days 7 and 14. A confirmed positive DDAB result was used as an exclusion criterion. The remaining patients entered into a 6-month treatment phase. Platelet counts were taken to further ensure patient safety. A total of 4.3% of patients (57 of 1332) tested positive at prescreen, 0.5% (4 of 823) tested positive for DDAB at day 7-8, and an additional 2.2% (18 or 821) tested positive on day 14. Note that some of the subjects were not enrolled because other study enrollment criteria were not met. During this study, one patient (99016) tested negative for the presence of DDABs at prescreen and developed thrombocytopenia on day 14, 9 days after discontinuation of drug treatment. The temporal association between increase in antibody concentration and reduction in platelet count is illustrated in Figure1A. A strong positive DDAB response was detected in the day 14 plasma (Figure 1A). Notably, platelet GP IIb/IIIa serves as a reservoir for roxifiban, and blood concentrations are estimated to be 1 to 2 nM 9 days after discontinuation of treatment. This drug level is sufficient for a maximal response in the DDAB ELISA13 and predicts an occupancy of 5% of GP IIb/IIIa binding sites in vivo. Thus, these data do not argue against DDABs being causative for thrombocytopenia during a drug holiday. The relationship between the antibody present in plasma and the pathogenetic antibody is suggested by the following observations. Platelet absorption studies revealed that the ELISA-positive DDAB bound to gel-purified platelets in the presence but not absence of roxifiban. Moreover, antibodies could be dissociated from the platelet surface by EDTA treatment, a condition previously reported to disrupt the GP IIb/IIIa complex (not shown).
Time course of DDAB titer development and platelet count.
Patients with a negative DDAB prescreen using citrated plasma were treated with roxifiban for 5 days (qualification period), followed by a drug holiday for 10 days. During the drug holiday, antibody development was monitored on days 7 and 14. (A) The prescreen citrated plasma for patient 99016 was negative for DDABs to roxifiban. Antibody binding, denoted as delta, is determined by the difference in IgG bound to purified GP IIb/IIIa in the presence of roxifiban to that in the absence of roxifiban. The patient was treated with roxifiban for 5 days and developed severe thrombocytopenia on day 14. The time course of DDAB reactivity (●) and decrease in platelet count (♦) is indicated. (B) Prescreen citrated plasma samples of patient 99016, as well as peak DDAB samples, were reanalyzed by the DDAB ELISA. The washing steps with an automated plate washer were replaced by gentle “hand washing” using a multichannel pipettor. Note that the prescreen of patient 99016 is clearly positive using these assay modifications. (C) An increase in antibody concentration was observed in 5 additional patients (identified by ID numbers). Patients were excluded from the study based on a confirmed positive test result and participated in follow-up antibody testing. The antibody development (Delta ELISA) as a function of time is indicated (day 1 equals start of qualification period). The platelet counts did not change significantly from baseline values.
Time course of DDAB titer development and platelet count.
Patients with a negative DDAB prescreen using citrated plasma were treated with roxifiban for 5 days (qualification period), followed by a drug holiday for 10 days. During the drug holiday, antibody development was monitored on days 7 and 14. (A) The prescreen citrated plasma for patient 99016 was negative for DDABs to roxifiban. Antibody binding, denoted as delta, is determined by the difference in IgG bound to purified GP IIb/IIIa in the presence of roxifiban to that in the absence of roxifiban. The patient was treated with roxifiban for 5 days and developed severe thrombocytopenia on day 14. The time course of DDAB reactivity (●) and decrease in platelet count (♦) is indicated. (B) Prescreen citrated plasma samples of patient 99016, as well as peak DDAB samples, were reanalyzed by the DDAB ELISA. The washing steps with an automated plate washer were replaced by gentle “hand washing” using a multichannel pipettor. Note that the prescreen of patient 99016 is clearly positive using these assay modifications. (C) An increase in antibody concentration was observed in 5 additional patients (identified by ID numbers). Patients were excluded from the study based on a confirmed positive test result and participated in follow-up antibody testing. The antibody development (Delta ELISA) as a function of time is indicated (day 1 equals start of qualification period). The platelet counts did not change significantly from baseline values.
Reanalysis of the predose sample of patient 99016 provided evidence for an inconsistently detectable antibody concentration (3 of 7 determinations were positive). Additional studies suggested a low-affinity DDAB with binding being sensitive to the washing conditions of the ELISA plate. Replacing the automated plate washer with a “hand wash” enabled consistent detection of this antibody (Figure 1B). Comparison of serial dilutions of the prescreen versus day 19 specimen provided evidence for a 20-fold increase in antibody concentration (Figure 1B). Additional plasma samples were obtained from 7 patients who were negative upon prescreen but were excluded from the clinical trial based on a positive antibody concentration (Figure 1C). No significant change in platelet counts was observed in these patients.
Implementation of testing for free and platelet-bound DDABs in a phase 2 study without drug holiday
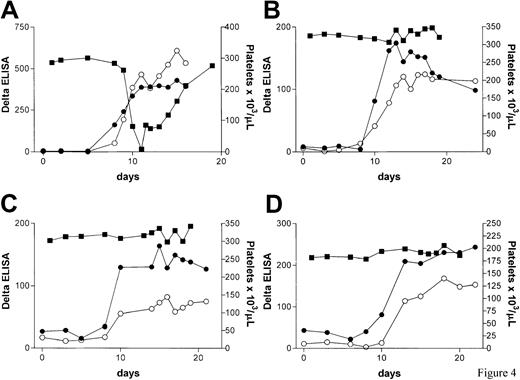
With the aim of capturing free and platelet-bound DDABs to roxifiban in plasma and thereby decreasing the time required to detect a serum conversion in patients not presenting with pre-existing antibodies, a modified DDAB ELISA was implemented in a second phase 2 study. The key change is EDTA treatment of platelets to release platelet-bound DDABs that recognize complex-specific GP IIb/IIIa epitopes (see “Patients, materials, and methods”). Patients with negative prescreen were continuously treated up to 180 days, and additional DDAB testing was performed on days 7, 10, and 14. A total of 5.4% of patients were excluded from the study: 3.0% of patients (15 of 493) were excluded from the study based on a prescreen, and an additional 2.4% (8 of 342) tested positive during either days 7-8, day 10, or day 14-15. Some of the prescreened subjects were not enrolled because other study criteria were not met. We observed one case of thrombocytopenia (Figure 2A). The patient (21013) tested negative for the presence of DDABs prior to drug treatment and received roxifiban for 8 days. DDAB analysis of the day 8 sample was positive (delta = 90). The physician was notified, the patient hospitalized, and drug treatment terminated. A further rapid increase in DDAB concentration was observed, coinciding with a sharp fall in platelet number (Figure 2A). Reanalysis of the predose and day 3 and 5 specimen by both the EDTA (●) and citrate (○) methodologies failed to detect antibodies at earlier time points (Figure 2A). Thus, thrombocytopenia was either elicited by a de novo immune response or the sensitivity of the ELISA was insufficient to detect pre-existing antibodies in this patient.
DDAB development in study DMP 754-017.
Patients with a negative DDAB prescreen using an in vitro platelet antibody elution technique (EDTA plasma) were treated with roxifiban continuously for up to 180 days. Additional antibody tests after platelet elution were performed on days 7, 10, and 14 (panels A-D; ●). Drug treatment in all 4 subjects was discontinued based on a developing antibody concentration. All subjects participated in follow-up antibody testing. In addition, a citrate plasma sample obtained in parallel with the EDTA sample was retrospectively analyzed for the presence of DDABs (○). The platelet count is indicated by ▪. (A) Patient 21013 developed severe thrombocytopenia on day 11. Drug treatment was stopped on day 9 based on a rapidly increasing antibody concentration. The patient was hospitalized for monitoring. (B) Patient 49007 was excluded from the study on day 11. (C) Patient 61003 was excluded from the study on day 11. (D) Patient 7013 was excluded from the study on day 11.
DDAB development in study DMP 754-017.
Patients with a negative DDAB prescreen using an in vitro platelet antibody elution technique (EDTA plasma) were treated with roxifiban continuously for up to 180 days. Additional antibody tests after platelet elution were performed on days 7, 10, and 14 (panels A-D; ●). Drug treatment in all 4 subjects was discontinued based on a developing antibody concentration. All subjects participated in follow-up antibody testing. In addition, a citrate plasma sample obtained in parallel with the EDTA sample was retrospectively analyzed for the presence of DDABs (○). The platelet count is indicated by ▪. (A) Patient 21013 developed severe thrombocytopenia on day 11. Drug treatment was stopped on day 9 based on a rapidly increasing antibody concentration. The patient was hospitalized for monitoring. (B) Patient 49007 was excluded from the study on day 11. (C) Patient 61003 was excluded from the study on day 11. (D) Patient 7013 was excluded from the study on day 11.
Treatment with roxifiban was discontinued in 7 additional patients due to a developing DDAB concentration without a significant reduction in platelet count. The time course of serum conversion of 3 representative patients is depicted in Figure 2B-D. To test whether the EDTA elution facilitated earlier detection of serum conversion, specimens were retrospectively analyzed by both methods (Figure 2). Overall, the time courses of serum conversion were very similar between the EDTA and citrate samples, suggesting that the EDTA procedure had little benefit in detecting a rise in antibody concentration at earlier time points due to elution of platelet-bound IgG.
Studies were performed to delineate whether ELISA-detectable antibodies have the propensity to be pathogenetic (platelet-binding antibodies) or, alternatively, recognize epitopes elicited in GP IIb/IIIa by the immobilization procedure used in the assay procedure. DDAB-containing plasma was incubated with platelets in the presence or absence of roxifiban and reanalyzed in the DDAB ELISA. The results are summarized in Table 2. Platelet absorption reduced the ELISA-detectable antibody concentration in the presence but not absence of roxifiban, indicating the specificity of the platelet absorption. These results indicate that the antibodies have the propensity to bind to intact platelets in a drug-dependent manner. These observations are consistent with the possibility that discontinuation of roxifiban treatment based on the positive DDAB test may have prevented thrombocytopenia.
Platelet absorption of ELISA-detectable DDABs
| . | Δ (no depletion) . | Percentage depletion (minus roxifiban) . | Percentage depletion (plus roxifiban) . |
|---|---|---|---|
| 21013 | 617 | 0 | 77 |
| 49007 | 57 | 0 | 100 |
| 61003 | 64 | 6 | 93 |
| 7013 | 74 | 15 | 100 |
| . | Δ (no depletion) . | Percentage depletion (minus roxifiban) . | Percentage depletion (plus roxifiban) . |
|---|---|---|---|
| 21013 | 617 | 0 | 77 |
| 49007 | 57 | 0 | 100 |
| 61003 | 64 | 6 | 93 |
| 7013 | 74 | 15 | 100 |
Citrated plasma obtained from the indicated patients was tested by the DDAB ELISA (Δ [no depletion]). Parallel samples were incubated with washed platelets in the absence (minus) or presence (plus) of roxifiban. Plasma was recovered by centrifugation and analyzed by the DDAB ELISA. Results are expressed as percentage depletion compared with the samples not treated with platelets.
Effects of prospective testing for DDABs on the frequency of thrombocytopenia
Thrombocytopenia frequencies were compared between clinical trials as a function of the use of the DDAB ELISA to control patient enrollment. All patients included in the comparison presented with advanced atherosclerotic vascular disease. Although different DDAB testing protocols were employed (free DDABs versus free and platelet-bound DDABs), the test results were similar for both test methods (compare free versus free and platelet DDAB time courses in Figure 2). The treatment intervals with respect to the drug holiday were different between the studies. However, the thrombocytopenia observed during the drug holiday (Figure 1), 9 days after discontinuation of treatment, appears to be the result of drug exposure. These considerations suggest that a drug holiday was not fully operative. A 2.1% frequency of thrombocytopenia was observed when 386 patients were enrolled without negative DDAB result as an inclusion criterion. Of the 1044 patients treated after DDAB testing, 2 patients developed thrombocytopenia, with an overall frequency of 0.2%. Statistical analysis revealed a highly significant (P = .0007) reduction of thrombocytopenia when a negative DDAB test was used as an inclusion criterion (Table3), further supporting the immune nature of thrombocytopenia during treatment with roxifiban. In a subgroup analysis, the statistical evaluation was limited to CAD patients. Despite reduced sample size, the reduction in thrombocytopenia frequency by DDAB testing was still highly significant (P = .0078). Taken together, these observations suggest that thrombocytopenia during treatment with roxifiban can be prevented by testing for DDABs.
Contigency table
| . | Thrombocytopenia . | No thrombocytopenia . | Total . |
|---|---|---|---|
| No DDAB test | 8 | 378 | 386 |
| DDAB test | 2 | 1042 | 1044 |
| Total | 10 | 1420 | 1430 |
| . | Thrombocytopenia . | No thrombocytopenia . | Total . |
|---|---|---|---|
| No DDAB test | 8 | 378 | 386 |
| DDAB test | 2 | 1042 | 1044 |
| Total | 10 | 1420 | 1430 |
The number of patients treated with roxifiban without DDAB testing as an exclusion criterion is indicated (no DDAB test). After implementation of a DDAB antibody ELISA, patients were excluded from the studies based on a pre-existing or developing DDAB concentration (DDAB test). The frequency of thrombocytopenia in both groups is indicated. The results were analyzed using a 2-sided Fisher exact test. A statistically significant reduction in the frequency of thrombocytopenia (P = .0007) was observed using the DDAB ELISA as an exclusion criterion.
Discussion
Thrombocytopenia during treatment with roxifiban occurred during 2 time periods, either early in the first 4 days of treatment (early-onset thrombocytopenia) or within 11 to 16 days (delayed-onset thrombocytopenia). No additional cases were observed with up to 6 months of roxifiban treatment. Thus, the critical time frame for the development of thrombocytopenia during treatment with roxifiban is the first 3 weeks. The implication of these findings is that patient safety should be ensured by closely monitoring the platelet counts during this time period or, potentially, using DDAB testing. However, the clinical utility of DDAB testing needs confirmation in large clinical trials. The results presented here further support the immune nature of thrombocytopenia during treatment with the GP IIb/IIIa antagonist roxifiban. This conclusion is based on the observation that controlling patient enrollment using a positive DDAB ELISA test result prior to or during treatment as an exclusion criterion statistically significantly reduced the incidence of thrombocytopenia. In addition, with the exception of one patient, DDABs could be demonstrated by retrospective analysis in all patients with thrombocytopenia when specimens were available. It remains unclear whether this patient experienced a nonimmune-mediated thrombocytopenia or the assay failed to detect this specific antibody. Overall, the frequency of nonimmune-mediated thrombocytopenia during roxifiban treatment must be very low.
The medical decision level employed in these studies was based on a statistically based cutoff level for DDAB concentration derived from nonthrombocytopenic patients of a previous phase 2 trial of roxifiban. The clinical studies were not designed to determine the false-positive rate of the DDAB ELISA, because patients with positive antibody reaction were excluded from the study. However, some estimates as to the false-positive rate can be obtained by comparing clinical trials with and without the prospective use of the DDAB ELISA. For example, between 5% (free and platelet-bound DDABs) and 7% (free plasma DDAB) of patients were excluded from the study based on a positive antibody reaction at prescreen or during treatment. Assuming a thrombocytopenia frequency of 2%, the false-positive rate could be as high as 5%. This false-positive rate would be, in our view, unacceptable for routine clinical use. However, it should be noted that further clinical studies may help to optimize the clinical decision level to reduce the false-positive rate. In addition, patients presenting with a positive DDAB test prior to treatment may benefit from more extensive monitoring. The observed false-negative rate is low (0.1%). The false-negative test result (1 subject) was not due to a nonimmune nature of the thrombocytopenia. Further characterization of this plasma provided evidence for low-affinity antibodies and, after assay modifications to efficiently capture these antibodies, the prescreen was positive. Because of the potential serious sequelae of thrombocytopenia, false positives are preferable over false negatives.
A number of patients developed a positive antibody reaction during treatment with roxifiban, and drug treatment was stopped. Analysis of these time-course plasmas from study by platelet depletion (Table 2) revealed that most of the antibodies bound to platelets. This observation suggests that the patients were at increased risk for thrombocytopenia. Two factors may explain why thrombocytopenia was not observed in these patients. First, DDABs derived during treatment with roxifiban are exquisitely drug dependent,13 and discontinuation of treatment may have reduced the roxifiban blood concentration below levels required for sufficient antibody binding to platelets. Second, none of these plasmas presented with antibody concentrations as high as those observed in thrombocytopenic patients (compare Figure 2 and data by Billheimer et al13).
Whole blood was collected from patients presenting with an increase in antibody concentration in either sodium citrate or EDTA to test whether EDTA dissociation of platelet-bound DDABs that are complex specific alters the time to serum conversion. Generally, we observed an increased DDAB concentration in samples collected in EDTA compared with citrate. However, the increase was modest, and little effect on time to reach the medical decision level was observed. These observations suggest that platelet elution has little advantage and does not justify the additional preassay sample-processing procedures required. This finding is consistent with the idea that antibody-loaded platelets are cleared by the reticuloendothelial system rather than retained in the circulation.
All cases of thrombocytopenia observed with roxifiban fall into 2 categories.
Early-onset thrombocytopenia occurs within the first 4 days of treatment. We observed 4 cases in the absence of DDAB testing, whereas none was observed in the presence of prospective DDAB testing. The available data are consistent with the notion that early-onset thrombocytopenia is immune mediated and that the incidence can be reduced by screening patients for DDAB prior to therapy. Delayed-onset thrombocytopenia occurs within the second or third week of treatment. Of the 6 cases in our roxifiban trials, 3 presented with a pre-existing antibody concentration, whereas 3 developed detectable antibodies during treatment. These observations suggest that a second DDAB test during treatment would be required to maximize the utility of the DDAB testing. We also observed that the time interval between DDAB concentration rise and clinically significant reduction in platelet count varied between patients. Many factors, including “bone marrow reserve,” and DDAB concentration, affinity, and binding sites on GP IIb/IIIa are expected to affect the propensity to develop thrombocytopenia. In one instance, the rise in antibody concentration was closely followed by thrombocytopenia and could not be prevented by discontinuation of drug treatment. In this case, the DDAB testing was positive prior to a significant fall in platelet counts and allowed for improved patient management in a hospital setting. All cases of delayed-onset thrombocytopenia could be detected by a second test on day 8 after initiating drug therapy. Thus, a prescreen and a day 8 test appear sufficient to significantly reduce the incidence of thrombocytopenia. It remains to be established whether the clinical experience and conclusions related to patient management presented here can be extended to other chemical classes of GP IIb/IIIa antagonists.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-02-0471.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dietmar Seiffert, Department of Chemical Enzymology, Bristol-Myers Squibb, Experimental Station, E400/3255, PO Box 80400, Wilmington, DE 19880-0400; e-mail:dietmar.seiffert@bms.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal