Abstract
In the present paper we show that transendothelial migration of a subset of CD14+ circulating leukocytes, coexpressing the CD34 precursor marker, leads to protection from the apoptosis that follows growth factor(s) withdrawal. The resistance of this cell subset to starvation-induced programmed cell death, lasting from 48 to 96 hours, is accompanied by a rise of mitochondrial adenosine triphosphate (ATP), a high nicotinamide adenine dinucleotide (NAD)/reduced nicotinamide adenine dinucleotide (NADH) ratio, and by the up-regulation of expression of the antiapoptotic proteins Bcl-2 and Bcl-X, together with an increase in the cytoplasmic, inactive, form of Bax. This suggests that protection from apoptosis is due to the preservation of mitochondrial function(s). Interestingly, ligation of the platelet endothelial cell adhesion molecule-1 (PECAM-1), which drives CD14+CD34+ transendothelial migration, leads to an increase in Bcl-2 A1 and Bcl-X intracellular content, and to protection from starvation-induced apoptosis. This event is dependent on the engagement of phosphatidylinositol-3 kinase and activation of Akt/PKB that is known to contribute to Bcl-2 and Bcl-X induction. These data point to a critical role of endothelium in preventing the apoptotic program triggered by starvation, possibly inducing a prolonged survival of antigen presenting cell precursors, in order to allow recirculation of these cells and localization to the site of priming of T lymphocytes.
Introduction
It is well known that dendritic cells (DCs), the most potent antigen presenting cells, derive from circulating precursors which extravasate to enter tissues, such as Peyer patches or epidermis, where they complete their differentiation.1 In periphery, DCs undergo maturation upon inflammatory or immunologic stimuli and, after encountering the antigen, recirculate to reach the T-cell areas of lymph nodes.2,3 Once exposed to maturating stimuli, such as tumor necrosis factor-α (TNF-α), interleukin-β, or bacterial lipopolysaccharides,3,4 DCs change the expression of chemokine receptors, altering their response to chemokines and the direction of migration.5,6 Importantly, only cells capable of migrating across the endothelium in the ablumenal-to-lumenal direction (reverse transmigration) become DCs, whereas cells that remain in the subendothelial matrix become macrophages.7
We and others have described a subset of CD14+CD34+ blood precursors of DCs capable of transendothelial migration mainly through a haptotactic gradient mediated via platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), although vascular cell adhesion molecule-1 (VCAM-1)/α4β1 interaction is also involved, as well as matrix invasion and reverse transmigration8,9: upon maturation induced by TNF-α, CD14+CD34+precursors undergo differentiation into immunostimulatory DCs and shift their sensitivity to chemokines from macrophage inflammatory protein 1 alpha (MIP-1α) to MIP-3β, retaining their ability to use PECAM-1 for transmigration.9 10 Thus, DC precursors must survive for a rather long time, in order to allow maturation, recirculation, and priming of T lymphocytes. Little is known, however, about the signal transduction pathways leading to a long-lasting lifespan of DCs and their blood precursors.
Recently, it has been shown that the phosphoinositide-3-OH-kinase (PI-3K) pathway, and its downstream target serine threonine kinase Akt/protein kinase B (PKB), are important in maintaining survival of lipopolysaccharide (LPS)–stimulated monocyte-derived DCs.11 Akt/PKB is an important mediator of metabolic as well as proliferative responses to various growth factors, whose receptors are coupled to PI-3K.12 Akt/PKB is activated by translocation to the plasma membrane when the PI-3K–generated 3-phosphoinositides bind to its pleckstrin homology domain.13 In addition to its metabolic actions, Akt/PKB has been shown to promote cell survival against several apoptotic stimuli14; recently, it has been shown that Akt/PKB activation through PI-3K up-regulates the expression of the antiapoptotic protein Bcl-2, which is a mechanism whereby growth factors induce cell survival.15 Interestingly, we have previously shown that PECAM-1 is coupled to a PI-3K–dependent pathway in granulocytes, where it regulates endothelial cell and subendothelial matrix adhesion.16
In the present paper we show that: (1) upon transendothelial migration CD14+CD34+ precursors are protected against the apoptosis which follows growth factor(s) withdrawal, at variance with the CD14+CD34− counterpart; (2) this is accompanied by high levels of mitochondrial ATP, a high NAD/NADH ratio, and Bcl-2 and Bcl-X up-regulation in migrated precursors; (3) engagement of PECAM-1 leads to Akt/PKB activation, to the increase of Bcl-2 and Bcl-X intracellular content, and to protection from apoptosis in DC precursors.
Materials and methods
Cell sorting and culture
Mononuclear cells were isolated from peripheral blood (PBMCs) of healthy donors' buffy coats, or from cord blood, kindly provided by the Blood Transfusion Department of the IRCCS San Raffaele, by density gradient centrifugation and enriched in CD14+ cells by plastic adherence.9 CD14+CD34+and CD14+CD34− cell subsets were fractionated by transendothelial migration or cell sorting (FACSort; Becton Dickinson, BD Biosciences, Mountain View, CA).9 Before or after transendothelial migration, cells were cultured in RPMI 1640 medium supplemented with 2 mM l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin (Biochrom, Berlin, Germany), 10% heat-inactivated fetal calf serum (FCS; PAA Labour, Linz, Austria), with or without 40 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF; Shering-Plough, Milan, Italy).
Transendothelial migration and cell adhesion assays
Human umbilical vein endothelial cells (HUVECs) were isolated and cultured as described9 and used within 4 passages. Transmigration of CD14+ or sorted CD14+CD34+ cells through HUVECs or adhesion to PECAM-1 transfectants (clone D12P) or NIH3T3 mock-transfected fibroblasts was performed as described,9 using Transwell cell culture chambers (polycarbonate filters, 3-μm pore size; Costar, Cambridge, MA). The CD31−/PECAM-1−ECV304 endothelial cell line or the PECAM-1–transfected P-ECV304 cell clone17 was also used for adhesion experiments. HUVECs were cultured to confluence on the polycarbonate filters; in some experiments, HUVECs were seeded in the lower chamber and naked filters were used for transmigration. After 12 hours of transmigration, migrated or nonmigrated cells were recovered from the lower or upper compartment of the chamber, respectively, cultured for 24, 48, or 72 hours in the presence or absence of GM-CSF, and analyzed. In some samples, cell adhesion was blocked using the F(ab′)2 of the anti–PECAM-1/CD31 monoclonal antibody (mAb) M89D3 or the anti–β2-integrin mAb JT90 (5 μg/106 cells, both IgG2a) or the anti–ICAM-1 mAb 14D12D2 (IgG1).9
Measurement of ATP and NAD/NADH in intact cells and isolated mitochondria
Migrated or nonmigrated cells were collected, after overnight transmigration and an additional 24 hours of culture without growth factors, lysed in a buffer containing 0.01% of Triton-X 100, and spun at 650g for 10 minutes to eliminate nuclei and debris. To obtain purified mitochondria, postnuclear supernatants were subjected to centrifugation at 6500g for 10 minutes. After the first sedimentation of the mitochondria, the pellet was washed twice and resuspended in 0.25 M sucrose; the average protein concentration was determined by the colorimetric method using Coomassie brilliant blue G-250 (BioRad Laboratories, Richmond, CA).18 For ATP determination, 25 μL of cell extracts was added to 0.9 mL of 0.1 M Tris-EDTA (ethylenediaminetetraacetic acid) buffer, pH 7.75, containing 6 mM MgSO4, 100 mM KCl, and 0.1 mM phosphoenolpyruvate. The mixture was kept at 100°C for 3 to 4 minutes and the nucleotide content measured by a luminometer (LKB-Wallac, Turku, Finland) connected to a chart recorder. The concentration of ATP is expressed as nanomoles per 105 cells. NAD+ and NADH contents were assessed in migrated and nonmigrated cell extracts by spectrophotometric analysis (PYE Unicam SP6-550 UV/VIS Spectrophotometer; Cambridge, United Kingdom) at 25°C and results expressed a NAD/NADH ratio per 105 cells, as described.19
Evaluation of apoptosis by flow cytometry and fluorescence microscopy
Cells were collected at different time points after transendothelial migration or cell sorting or after engagement of PECAM-1 or CD54/ICAM-1 or CD34 obtained using the corresponding mAbs at 5 μg/mL (anti–PECAM-1 mAb M89D39 or anti–ICAM-1 mAb 14D12D29 or anti–CD34 HPCA-1, IgG2a; Immunotech, Luminy, Marseille, France), followed by goat anti–mouse (GAM) immunoglobulin (Ig; Sigma Chemical, St Louis, MO), or upon adhesion to PECAM-1+ transfectants (D12P clone or P-ECV304 clone), and cultured for 48 hours in the presence or absence of GM-CSF. Purified mouse Ig (Sigma) was used as a negative control. In some experiments, adhesion was blocked with the F(ab′)2 of the M89D3 mAb or cells were pretreated with the PI-3K blockers LY294002 (20 μM, Sigma) or wortmannin (100 nM, Sigma), the tyrosine kinase inhibitor herbimycin (100 nM; Calbiochem, San Diego, CA), the mitogen-activated protein kinase blocker PD98059 (10 μM; Calbiochem), or the p70/S6 kinase inhibitor rapamycin (10 ng/mL; Sigma). As a control, fully apoptotic cells were obtained by incubation with the anti–Fas mAb CH-11 (IgM, MBL, 0.5 μg/mL; Nagoya, Japan) for 24 hours. To assess apoptosis, cells were stained with 50 μg/mL propidium iodide (PI) solution containing 100 U/mL RNase type A, 10 mM EDTA (to inactivate endogenous nucleases), and 0.0015% nonidet P-40 (NP-40) as previously described.20 In some experiments, cells were stained with fluorescein isothiocyanate (FITC)–conjugated annexin V (Sigma) in order to show the exposure of phospholipids at the external side of the plasma membrane, which occurs in early apoptosis. Samples were analyzed on a FACSort (Becton Dickinson) equipped with laser excitation at 488 nm and 610 nm. A longpass filter was used for the PI fluorescence detection. Calibration was assessed with CaliBRITE particles (Becton Dickinson) using the AutoCOMP computer program (version 2.1.2, Becton Dickinson). At least 104 cells per sample were analyzed and results plotted as a percentage of annexin V+ cells or as a percentage of apoptotic cells (DNA content < 2n-diploid assessed by PI staining). Cell viability and apoptosis was also evaluated in migrated and nonmigrated cells by fluorescence microscopy after in vivo triple staining with the following fluorescent dyes (all from Sigma): acridine orange (AO), which stains living cells, Hoechst 33342 (HO), and PI, specific for early and late apoptotic nuclei, respectively.21 Digital images were taken on an inverted microscope (Nikon Instech, Kawasaki, Japan) equipped with a charged coupled device (CCD) videocamera (Hamamatsu, Osaka, Japan). Microscopy performed at the wavelengths where the contemporary presence of living, early, or late apoptotic and necrotic cells can be assessed, served as a technical control.21
Bcl-2, Bcl-X, and Bax expression by immunofluorescence and Western blot
Bcl-2 and Bcl-X expression was evaluated by cytoplasmic immunofluorescence. Briefly, cells were fixed in 2% paraformaldehyde, permeabilized in 0.01% NP-40, stained with anti–Bcl-2 (BMS162, IgG1; Bender MedSystem, Vienna, Austria) or anti–Bcl-X (2H12, IgG2a; Neomarkers, Fremont, CA) mAbs, followed by fluorescein isothiocyanate–conjugated GAM Ig (FITC-GAM; BD Biosciences), and run on a FACSort. Unrelated FITC-conjugated mAbs, matched for the isotype (BD Biosciences), were used as controls. Results are expressed as arbitrary units (au) of mean fluorescence intensity (MFI). Bcl-2 and Bcl-X, as well as Bax (BMS161, IgG1; Bender MedSystem) or the Bcl-2 homolog A122 (sc-8351, rabbit polyclonal antiserum; Santa Cruz Biotechnology, Santa Cruz, CA), were also evaluated by Western blot. Briefly, cells were allowed to transmigrate across HUVEC monolayers or adhere to NIH3T3 or PECAM-1+ transfectants (D12P), cultured for an additional 48 hours in the absence of growth factor, lysed, and 50 μg of protein/lane was loaded and run on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After electrotransfer onto nitrocellulose and blocking with 5% dry milk solution, samples were blotted with the specific mAbs recognizing Bcl-2, Bcl-X, A1, and the cytosolic form of Bax, followed by horseradish peroxidase (HRP)–conjugated GAM or goat anti–rabbit (GAR) Ig (Sigma), and developed with ECL plus (Amersham, Buckinghamshire, United Kingdom).
PI-3K–dependent Akt activation analysis
PI-3K activity was tested indirectly by analyzing the activation of the serine/threonine kinase Akt, in the absence or presence of the PI-3K inhibitors LY294002 (20 μM) or wortmannin (100 nM), in cell lysates of purified CD14+CD34+ cells with the Akt assay kit, using the specific substate and 32γATP, after immunoprecipitation with the specific anti–Akt antibody (Upstate Biotechnology, Lake Placid, NY).19 Akt activity was tested upon ligation of PECAM-1 obtained by incubation of cells with 5 μg/mL of the specific mAb M89D3 or of the anti–ICAM-1 14D12D2 mAb (both IgG2a)9 (20 minutes at 4°C) followed by 10 μg/mL GAM as described.20 As a further control, cells were exposed to murine Ig followed by GAM or GAM alone. Some experiments were carried out in the presence of the PI-3K inhibitors LY294002 (20 μM) or wortmannin (100 nM), or the mitogen-activated protein kinase blocker PD98059 (10 μM) or the p70/S6 kinase inhibitor rapamycin (10 ng/mL). Results are expressed as counts per minute × 10−3 and are the mean ± SD of 4 independent experiments. Akt activation was also evaluated by Western blot using 2 mAbs recognizing unphosphorylated and phosphorylated Akt (Upstate Biotechnology).
Isolation of RNA and polymerase chain reaction (PCR) amplification
Total RNA was isolated from cell pellets by using the TriPure Isolation reagent (Boehringer Mannheim, Mannheim, Germany) method according to the manufacturer's protocols. RNA (1 μg) was reverse transcribed using MMLV reverse transcriptase (Perkin-Elmer Cetus, Emeryville, CA) and random hexamer primers (Boehringer Mannheim) incubated at 99°C for 5 minutes and 42°C for 1 hour. The PCR mixture, containing 2 μL cDNA, 2 mM MgCl2, 2 mM dNTPs, 50 μM 5′ and 3′ primers, and 2.5 U Ampli Taq Gold Polymerase (Perkin-Elmer Cetus), was amplified by 35 cycles of denaturation at 94°C for 1 minute, annealing at 56°C for 1 minute, and extension at 72°C for 90 seconds in 0.2-mL thin-walled tubes in a total volume of 50 μL. Primers sequences used were β-actin 5′-ACT CCA TCA TgA AgT gTg ACg, β-actin 3′-CCT AgT CgT TCg TCC TCA TAC (228-bp fragment); A1 5′-gAC TAT CTg Cag TgC gTC CTA CAg, and A1 3′-ggg CAA TTT gCT gTC gTA gAA g (286-bp fragment). PCR products were size-fractionated by 8% acrylamide electrophoresis and normalized according to the amount of β-actin detected in the same mRNA samples. All images were acquired by Chemi5500 (Alpha Innotech, San Leonardo, CA) and analyzed by Gel Pro Analyzer 3.1 (Media Cybernetics, Silver Spring, MD).
Results
Protection from starvation-induced apoptosis in migrated CD14+CD34+ precursors
We have reported that circulating CD34+ precursors, coexpressing CD14, are capable of transendothelial migration and differentiation in immunostimulatory DCs.9 In this paper we show that this cell population is long-lived, compared with the CD14+CD34− counterpart. CD14+peripheral blood leukocytes were separated by plastic adherence, allowed to transmigrate overnight across HUVEC monolayers, and cultured with or without GM-CSF; cell survival and apoptosis were evaluated, at different time points, by PI- and annexin V–staining or by triple staining with AO, HO, and PI, and fluorescence analysis. Migrated cells were CD14+CD34+, whereas nonmigrated cells were CD14+CD34− (not shown) in keeping with our previous report.9
Figure 1A shows that more than 50% of nonmigrating (evaluated after 72 hours of culture without growth factors) were annexin V+, indicating the exposure of membrane phospholipids, an early sign of programmed cell death, and more than 40% displayed a PI profile typical of apoptotic cells (compare panel C with panel E, fully apoptotic cells obtained by cross-linking of Fas); conversely, migrated cells were mostly (> 75%) annexin V− (panel A) with a normal PI profile (compare panel B with panel D, freshly isolated cells), suggesting that they are not undergoing programmed cell death.
Survival of transendothelial migrated and nonmigrated cells without growth factors.
PBMCs were allowed to transmigrate overnight across HUVEC monolayers. After 72 hours of culture, nonmigrated and migrated cells were stained with FITC–annexin V (A) or with 50 μg/mL PI solution (B-C). PI staining of freshly isolated PBMCs (D) and of cells treated for 24 hours with 0.5 μg/mL of the anti–Fas mAb CH-11 (E) was also performed. Samples were then analyzed on a FACSort and results plotted as a percentage of annexin V+ cells (A) or as log red fluorescence intensity (au) for PI, versus number of cells (B, migrated cells; C, nonmigrated cells; D, freshly isolated PBMCs; E, CH-11–treated cells). Arrows in panels B-E indicate DNA content less than 2n (apoptotic cells). One representative experiment of 6 is shown.
Survival of transendothelial migrated and nonmigrated cells without growth factors.
PBMCs were allowed to transmigrate overnight across HUVEC monolayers. After 72 hours of culture, nonmigrated and migrated cells were stained with FITC–annexin V (A) or with 50 μg/mL PI solution (B-C). PI staining of freshly isolated PBMCs (D) and of cells treated for 24 hours with 0.5 μg/mL of the anti–Fas mAb CH-11 (E) was also performed. Samples were then analyzed on a FACSort and results plotted as a percentage of annexin V+ cells (A) or as log red fluorescence intensity (au) for PI, versus number of cells (B, migrated cells; C, nonmigrated cells; D, freshly isolated PBMCs; E, CH-11–treated cells). Arrows in panels B-E indicate DNA content less than 2n (apoptotic cells). One representative experiment of 6 is shown.
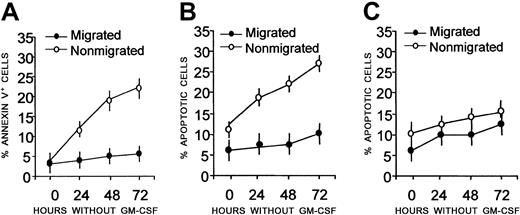
Kinetics experiments showed that more than 90% of migrated cells were annexin V− on day 3 of culture without GM-CSF (Figure2A), indicating that they were still alive (Figure 2B), whereas a large fraction of nonmigrated cells was either annexin V+ (Figure 2A, 20%-25%, early apoptosis) or apoptotic (Figure 2B, 20%-30%, DNA < 2n, by PI staining) by day 3 of culture in the absence of growth factors.
Kinetics of starvation-induced apoptosis after transendothelial migration.
Peripheral blood adherent mononuclear cells were allowed to transmigrate overnight across HUVEC monolayers. Nonmigrated (○) and migrated (●) cells were cultured in the absence of growth factors (A-B), or with GM-CSF (C), collected at the indicated time points, and stained with FITC–annexin V and PI. Samples were then analyzed on a FACSort and results plotted as a percentage of annexin V+(A) or percentage of apoptotic (DNA content < 2n-diploid) cells (B-C). Mean values ± SDs from 6 different experiments are shown.
Kinetics of starvation-induced apoptosis after transendothelial migration.
Peripheral blood adherent mononuclear cells were allowed to transmigrate overnight across HUVEC monolayers. Nonmigrated (○) and migrated (●) cells were cultured in the absence of growth factors (A-B), or with GM-CSF (C), collected at the indicated time points, and stained with FITC–annexin V and PI. Samples were then analyzed on a FACSort and results plotted as a percentage of annexin V+(A) or percentage of apoptotic (DNA content < 2n-diploid) cells (B-C). Mean values ± SDs from 6 different experiments are shown.
Figure 2C shows that, in order to survive, nonmigrated cells needed the addition of GM-CSF. All these results were confirmed by fluorescence microscopy (Figure 3), showing that most of migrated cells, cultured for 48 hours without GM-CSF, were stained with AO, but not with HO or PI (Figure 3A), indicating that they were alive, whereas more than 50% of nonmigrating cells were HO+ or PI+, indicating that they were apoptotic/dead cells (Figure 3B). Interestingly, cells that migrated across naked filters (in the absence of an endothelial monolayer; Figure 3C) are all necrotic, as indicated by AO+and PI+ staining. These data point to a high resistance of CD34+ cells to starvation and suggest that this population receives an antiapoptotic signal during transendothelial migration.
Viability of cells migrated through endothelium.
Peripheral blood CD14+ cells were allowed to migrate across HUVEC monolayers. (A) Migrated cells. (B) Nonmigrated cells. (C) Cells migrated through the naked filter without endothelium. Cells were submitted to triple staining with HO, AO, and PI and analyzed in fluorescence microscopy. Notice in panel A the prevalence of green living cells on red and yellow apoptotic cells. Nonmigrated cells (B) appear mostly as apoptotic-necrotizing cells (yellow nuclei) or late apoptotic–necrotizing cells (red nuclei). Conversely, only orange-stained cellular debris are detectable when cells passed through the naked filter (C). One representative experiment of 4 is shown; original magnification × 200 for all panels.
Viability of cells migrated through endothelium.
Peripheral blood CD14+ cells were allowed to migrate across HUVEC monolayers. (A) Migrated cells. (B) Nonmigrated cells. (C) Cells migrated through the naked filter without endothelium. Cells were submitted to triple staining with HO, AO, and PI and analyzed in fluorescence microscopy. Notice in panel A the prevalence of green living cells on red and yellow apoptotic cells. Nonmigrated cells (B) appear mostly as apoptotic-necrotizing cells (yellow nuclei) or late apoptotic–necrotizing cells (red nuclei). Conversely, only orange-stained cellular debris are detectable when cells passed through the naked filter (C). One representative experiment of 4 is shown; original magnification × 200 for all panels.
CD34+CD14+ cell survival correlates with increased mitochondrial ATP and NAD/NADH ratio
Cell death consequent to starving conditions is usually related to changes in mitochondrial functions, leading to decreased ATP production and reduction of NAD/NADH ratio.23 Thus, ATP content and NAD/NADH ratio were measured in migrated and nonmigrated cells at 24 hours. Figure 4 shows that cells that underwent transendothelial migration (CD34+CD14+) have higher ATP levels than nonmigrated (CD34−CD14+) cells (> 450 nmol/105 cells vs 100 nmol/105 cells; panel A). Along this line, migrated cells displayed a higher NAD/NADH ratio (10 vs 4, panel B), indicating that the resistance to apoptosis of transmigrated cells is linked with a conserved mitochondrial metabolism and a preserved respiratory chain. Indeed, subcellular fractionation showed that mitochondria of migrated cells had increased ATP levels compared with nonmigrated cells (Figure 4C). When cultured with GM-CSF, the 2 cell populations displayed a superimposable content of ATP (500 nmol/105 migrated cells vs 400 nmol/105nonmigrated cells) and a comparable NAD/NADH ratio (not shown). These results support the hypothesis that endothelial cells deliver a positive signal to migrating cells that leads to enhanced mitochondrial function, sustaining both energy production and respiratory chain efficiency.
Increased mitochondrial ATP and NAD/NADH ratio in migrated cells.
ATP content (A,C) and NAD/NADH ratio (B) were measured in migrated (▪) and nonmigrated (■) cells. Results are expressed as nmol/105 cells or as NAD/NADH ratio/105 cells as indicated; in some experiments, ATP was measured in purified mitochondria obtained by subcellular fractionation (C). Results are expressed as the means ± SDs from 4 independent experiments.
Increased mitochondrial ATP and NAD/NADH ratio in migrated cells.
ATP content (A,C) and NAD/NADH ratio (B) were measured in migrated (▪) and nonmigrated (■) cells. Results are expressed as nmol/105 cells or as NAD/NADH ratio/105 cells as indicated; in some experiments, ATP was measured in purified mitochondria obtained by subcellular fractionation (C). Results are expressed as the means ± SDs from 4 independent experiments.
PECAM-1 homophylic interaction is needed for CD34+cell survival
We have previously reported that CD14+CD34+ blood precursors preferentially use PECAM-1/CD31 to transmigrate9; in turn, signaling through CD31 has been shown to protect endothelial cells from apoptosis.24-26 Thus, we investigated whether PECAM-1 contributes to the delivery of the antiapoptotic signal received by our cell population during transendothelial migration. To this purpose, CD31+CD34+ or CD31−CD34+ cell populations were sorted from cord blood (representing 9% ± 3% and 3% ± 1% of cord blood PBMCs, respectively, in 3 different donors examined) and allowed to adhere for 1 hour to the PECAM-1−/CD31−ECV304 endothelial cell line or to the PECAM-1–transfected P-ECV304 clone. Adhesion to P-ECV304 led to protection from starvation-induced apoptosis in CD31+CD34+ cells, but not in CD31−CD34+ cells (Figure5A); this protection was lost by covering PECAM-1 on CD34+ cells with F(ab′)2 of the specific M89D3 mAb; conversely, the anti–ICAM-1 mAb did not exert any effect (Figure 5A). At variance with P-ECV304, the PECAM-1−/CD31−ECV304 cell line did not give any protective signal to CD34+ cells (Figure 5B). This would indicate that PECAM-1–mediated homophilic interaction is involved in the delivery of an antiapoptotic signal to migrating cells. To further confirm this finding, ligation of PECAM-1/CD31, the β2-integrin, or the CD34 molecule was performed as described in “Materials and methods” and survival to growth factor withdrawal evaluated after 48 hours. Figure 5C shows that oligomerization of PECAM-1/CD31, but not of β2-integrin or of the CD34 molecule, can protect CD34+ cells from starvation-induced apoptosis.
Role of PECAM-1 homophilic interaction in inducing survival of CD34+ cells.
CD31+CD34+ (■) or CD31−CD34+ (░) cells were allowed to adhere to PECAM-1+–transfected ECV304 (P-ECV304; A) or to the mock-transfected ECV304 cell line (B), in the absence or presence of the F(ab′)2 of the anti–PECAM-1 (M89D3) or the anti–ICAM-1 (14D12D2) mAbs, then collected after 48 hours of culture, stained with PI as in Figure 2, and run on a FACSort. We analyzed 104 cells per sample; results are plotted as percentages of apoptotic (DNA content < 2n-diploid) cells. (C) Percentage of apoptotic cells, upon ligation of PECAM-1, CD34, β2-integrin (obtained with 5 μg/mL of the M89D3, HPCA-1, or JT90 mAb, respectively, followed by 10 μg/mL GAM). Murine Ig plus GAM were used as controls. Mean values ± SDs from 6 different experiments are shown.
Role of PECAM-1 homophilic interaction in inducing survival of CD34+ cells.
CD31+CD34+ (■) or CD31−CD34+ (░) cells were allowed to adhere to PECAM-1+–transfected ECV304 (P-ECV304; A) or to the mock-transfected ECV304 cell line (B), in the absence or presence of the F(ab′)2 of the anti–PECAM-1 (M89D3) or the anti–ICAM-1 (14D12D2) mAbs, then collected after 48 hours of culture, stained with PI as in Figure 2, and run on a FACSort. We analyzed 104 cells per sample; results are plotted as percentages of apoptotic (DNA content < 2n-diploid) cells. (C) Percentage of apoptotic cells, upon ligation of PECAM-1, CD34, β2-integrin (obtained with 5 μg/mL of the M89D3, HPCA-1, or JT90 mAb, respectively, followed by 10 μg/mL GAM). Murine Ig plus GAM were used as controls. Mean values ± SDs from 6 different experiments are shown.
Regulation of expression of Bcl-2, Bcl-X, and Bax in CD34+ cells upon transendothelial migration or adhesion to PECAM-1+ transfectants
Both Bcl-2 and Bcl-X are known antiapoptotic proteins able to prevent programmed cell death.27-29 Thus, we analyzed the intracellular levels of these antiapoptotic proteins by indirect immunofluorescence in CD14+ cells before transmigration or in migrated (CD14+CD34+) and nonmigrated cells (CD14+CD34−). Table1 shows that migrated cells display higher levels of both Bcl-2 and Bcl-X than nonmigrated or unfractionated cells. This suggests that upon transendothelial migration CD34+ cells receive a signal leading to the activation and up-regulation of these antiapoptotic proteins. To address this point, CD14+CD34+ were sorted and allowed to adhere for 1 hour to murine fibroblasts transfected with PECAM-1 or to mock-transfected NIH3T3 cells; after 36 hours of culture, cells were harvested and tested by indirect immunofluorescence for the intracellular content of Bcl-2 and Bcl-X.
Bcl-2 and Bcl-X expression in migrated and nonmigrated cells
| Cells . | Bcl-2* . | Bcl-X* . | PECAM-1* . |
|---|---|---|---|
| Before migration (CD14+) | 20 ± 10 | 18 ± 9 | ND |
| Migrated (CD14+CD34+) | 180 ± 21 | 160 ± 13 | 210 ± 20 |
| Nonmigrated (CD14+CD34−) | 66 ± 11 | 60 ± 8 | 35 ± 5 |
| Cells . | Bcl-2* . | Bcl-X* . | PECAM-1* . |
|---|---|---|---|
| Before migration (CD14+) | 20 ± 10 | 18 ± 9 | ND |
| Migrated (CD14+CD34+) | 180 ± 21 | 160 ± 13 | 210 ± 20 |
| Nonmigrated (CD14+CD34−) | 66 ± 11 | 60 ± 8 | 35 ± 5 |
Bcl-2 and Bcl-X expression was evaluated by cytoplasmic immunofluorescence and PECAM-1 expression by surface phenotype in unfractionated CD14+ cells or in cells migrated or not through HUVEC monolayers, collected after an additional 36 hours of culture. Cells were fixed in 2% paraformaldehyde, and for cytoplasmic fluorescence permeabilized with 0.01% NP-40, stained with the specific anti–Bcl-2, anti–Bcl-X, or anti–PECAM-1 mAbs followed by FITC-GAM, and run on a FACSort. At least 104 cells/sample were analyzed and results plotted as arbitrary units (au) of mean fluorescence intensity (MFI; mean ± SD from 8 experiments). MFI of cells stained with an FITC-irrelevant mAb was 15 ± 5 au. ND indicates not determined.
As shown in Table 2, adhesion to PECAM-1 tranfectants led to the induction of Bcl-2 and Bcl-X expression, at variance with cells adhered to mock-transfected fibroblasts or NIH3T3 cells transfected with ICAM-1 (not shown). Of note, pretreatment of CD14+CD34+ with the F(ab′)2 of the specific anti–PECAM-1 mAb, before adhesion on D12P transfectants, abolished the up-regulation of both Bcl-2 and Bcl-X, suggesting that the induction of these antiapoptotic proteins is a consequence of PECAM-1–mediated homophilic interactions (Table 2). At variance, an anti–ICAM-1 mAb did not exert any inhibitory effect (not shown). These data were also confirmed by Western blot showing increased intracellular amounts of Bcl-2 and Bcl-X upon interaction with PECAM-1 transfectants (Figure 6), at variance with the Bcl-2 homolog A1, which was not clearly detectable by immunoblotting (not shown). As it has been reported that A1 transcripts increase in endothelial cells after interaction with monocytes,22 A1 mRNA was also examined in CD34+ cells (Figure 6B). We found an increase in A1 transcripts upon migration through HUVECs (lane 3) or through the PECAM-1+ D12P transfectants (lane 4), as well as after ligation of PECAM-1 with the specific mAb followed by GAM (lane 6). Conversely, migration through naked filters (lane 5) or incubation with an isotype-matched mAb and GAM (lane 7) did not exert any effect. Densitometric analysis of the bands in Figure 6B is depicted in Figure6C.
Bcl-2 and Bcl-X up-regulation upon PECAM-1 homophilic interaction
| Cells . | Bcl-2* . | Bcl-X* . |
|---|---|---|
| CD14+CD34+ before adhesion | 16 ± 10 | 6 ± 2 |
| CD14+CD34+ on D12P | 100 ± 11 | 80 ± 13 |
| CD14+CD34+on D12P + anti–PECAM-1 F(ab′)2 | 6 ± 3 | 9 ± 2 |
| CD14+CD34+ on mock NIH3T3 | 20 ± 4 | 10 ± 2 |
| Cells . | Bcl-2* . | Bcl-X* . |
|---|---|---|
| CD14+CD34+ before adhesion | 16 ± 10 | 6 ± 2 |
| CD14+CD34+ on D12P | 100 ± 11 | 80 ± 13 |
| CD14+CD34+on D12P + anti–PECAM-1 F(ab′)2 | 6 ± 3 | 9 ± 2 |
| CD14+CD34+ on mock NIH3T3 | 20 ± 4 | 10 ± 2 |
Bcl-2 and Bcl-X expression was evaluated by cytoplasmic immunofluorescence in CD14+CD34+ cells before or after 1-hour adhesion on D12P (PECAM-1+) or mock-transfected NIH3T3 monolayers, in the absence or presence of the F(ab′)2 of the specific anti–PECAM-1 mAb (5 μg/mL), collected after an additional 36 hours of culture. Cells were fixed in 2% paraformaldehyde, permeabilized with 0.01% NP-40, stained with the specific FITC anti–Bcl-2 or FITC anti–Bcl-X mAbs, and run on a FACSort. At least 104 cells per sample were analyzed and results plotted as arbitrary units (au) of mean fluorescence intensity (MFI, mean ± SD from 8 experiments). MFI of cells stained with an FITC-irrelevant mAb was 10 ± 5 au.
Regulation of Bcl-2, Bcl-X, A1, and Bax in CD34+ cells upon PECAM-1 interactions.
(A) Cells were allowed to adhere to PECAM-1+ transfectants (D12P, lane 3) or to mock-transfected NIH3T3 fibroblasts (mock, lane 2) for 30 minutes, cultured for 36 hours, lysed, and 50 μg of protein per lane was loaded and run on a 12% SDS-PAGE. Lys (lane 1) shows cells before adhesion. After electrotransfer, samples were blotted with the specific anti–Bcl-2, Bcl-X, Bax, and anti–PECAM-1/CD31 mAbs, followed by GAM-HRP, and developed with ECL plus (Amersham). One representative experiment of 4 experiments performed is shown. (B) Normalized aliquots of total RNA were extracted from cells before (CTR, lane 1) or after migration through HUVECs (lane 3), D12P (lane 4), naked filters (lane 5), or upon cross-linking of PECAM-1/CD31 with the specific M89D3 mAb (lane 6), or with control Ig followed by GAM (lane 7). Lane 2: cells treated with 100 ng/mL LPS. Amplification was then performed by PCR with the specific primers for A1 or β-actin and PCR products were size-fractionated by 8% acrylamide electrophoresis. (C) Bands corresponding to A1 or β-actin in panel B were subjected to densitometric analysis and results expressed as pixel number (au).
Regulation of Bcl-2, Bcl-X, A1, and Bax in CD34+ cells upon PECAM-1 interactions.
(A) Cells were allowed to adhere to PECAM-1+ transfectants (D12P, lane 3) or to mock-transfected NIH3T3 fibroblasts (mock, lane 2) for 30 minutes, cultured for 36 hours, lysed, and 50 μg of protein per lane was loaded and run on a 12% SDS-PAGE. Lys (lane 1) shows cells before adhesion. After electrotransfer, samples were blotted with the specific anti–Bcl-2, Bcl-X, Bax, and anti–PECAM-1/CD31 mAbs, followed by GAM-HRP, and developed with ECL plus (Amersham). One representative experiment of 4 experiments performed is shown. (B) Normalized aliquots of total RNA were extracted from cells before (CTR, lane 1) or after migration through HUVECs (lane 3), D12P (lane 4), naked filters (lane 5), or upon cross-linking of PECAM-1/CD31 with the specific M89D3 mAb (lane 6), or with control Ig followed by GAM (lane 7). Lane 2: cells treated with 100 ng/mL LPS. Amplification was then performed by PCR with the specific primers for A1 or β-actin and PCR products were size-fractionated by 8% acrylamide electrophoresis. (C) Bands corresponding to A1 or β-actin in panel B were subjected to densitometric analysis and results expressed as pixel number (au).
In the absence of growth factors, mitochondria are recruited in the apoptotic pathway due to the binding of the protein Bax to the mitochondrial membrane.29 Conversely, Bcl-2 and Bcl-X antagonize this pathway, preventing Bax activation and mitochondrial lysis.30 In keeping with this, adhesion to D12P transfectants led to an increase in the cytosolic (inactive) form of Bax (Figure 6), indicating that PECAM-1–mediated homophilic interactions play a major role in the delivery of antiapoptotic signals during transmigration.
PECAM-1 engagement leads to Akt activation and protection from starvation-induced apoptosis
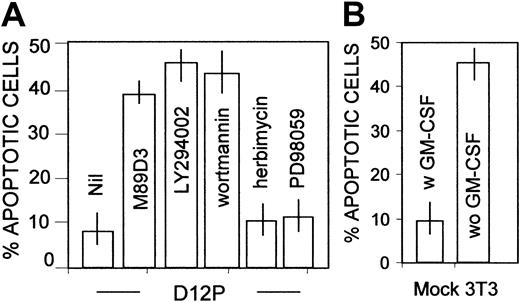
We have shown that PECAM-1 is coupled to PI-3K in granulocytes16; this kinase is involved in Bcl-X induction and regulation of expression of Bcl-2, possibly through the activation of Akt.15 31 Thus, we analyzed the possibility that in our system PECAM-1 ligation led to PI-3K–dependent Akt activation. Figure7 shows that, upon PECAM-1 engagement in sorted CD34+CD14+ cells, Akt rapidly becomes phosphorylated (Figure 7A, lane 2). Interestingly, Akt phosphorylation was impaired in cells exposed to the PI-3K inhibitor LY294002 (Figure7A, lane 4). The activation of Akt in cell lysates of CD14+CD34+ cells was also assessed with the Akt assay kit, using the specific substate and 32γATP, after immunoprecipitation with the specific anti–Akt antibody. Ligation of PECAM-1, at variance with ICAM-1 (not shown), elicited the activation of the serine-threonine kinase, downstream PI-3K, as demonstrated by the finding that Akt induction was not observed in cells exposed to LY294002 or to another PI-3K blocker, wortmannin (Figure 7B). Other kinase inhibitors, such as PD98059 or rapamycin, did not exert any inhibition (Figure 7B). Taken together, these findings point to the existence of a PI-3K–Akt antiapoptotic pathway coupled to PECAM-1. This was further confirmed by the finding that upon adhesion to D12P transfectants (Figure 8A), but not to mock-transfected fibroblasts (Figure 8B), CD34+ cells became resistant to starvation and this protection was abolished by the use of PI-3K blockers (LY294002 or wortmannin), but not of tyrosine-kinase (herbimycin) or MAPK inhibitors (PD98059) (Figure 8A).
Engagement of PECAM-1 leads to Akt activation.
(A) Akt activation evaluated by Western blot with mAbs recognizing unphosphorylated (Akt; bottom) or phosphorylated Akt (P-Akt; top) in purified CD14+CD34+ cells after 2 minutes (lanes 2 and 4) or 4 minutes (lane 3) upon PECAM-1 ligation (incubation with 5 μg/mL of the specific anti–PECAM-1 mAb followed by 10 μg/mL GAM). Lane 1 shows untreated cells (exposed to GAM alone); lane 4, cells exposed to the PI-3K inhibitor LY294002. (B) Akt activation in cell lysates of CD14+CD34+ cells assessed with the Akt assay kit, using the specific substrate and32γATP, after immunoprecipitation with anti–Akt antibody. CTR represents untreated cells. Ig+GAM, cells incubated with murine Ig plus GAM. PECAM-1 ligation was obtained as indicated above in the absence (nil) or in the presence of LY294002, wortmannin, rapamycin, or PD98059. Results are expressed as cpm ×10−3, means ± SDs of 4 independent experiments.
Engagement of PECAM-1 leads to Akt activation.
(A) Akt activation evaluated by Western blot with mAbs recognizing unphosphorylated (Akt; bottom) or phosphorylated Akt (P-Akt; top) in purified CD14+CD34+ cells after 2 minutes (lanes 2 and 4) or 4 minutes (lane 3) upon PECAM-1 ligation (incubation with 5 μg/mL of the specific anti–PECAM-1 mAb followed by 10 μg/mL GAM). Lane 1 shows untreated cells (exposed to GAM alone); lane 4, cells exposed to the PI-3K inhibitor LY294002. (B) Akt activation in cell lysates of CD14+CD34+ cells assessed with the Akt assay kit, using the specific substrate and32γATP, after immunoprecipitation with anti–Akt antibody. CTR represents untreated cells. Ig+GAM, cells incubated with murine Ig plus GAM. PECAM-1 ligation was obtained as indicated above in the absence (nil) or in the presence of LY294002, wortmannin, rapamycin, or PD98059. Results are expressed as cpm ×10−3, means ± SDs of 4 independent experiments.
PECAM-1–induced survival of CD34+ cells is dependent on PI-3K activation.
CD14+CD34+ cells were allowed to adhere to PECAM-1+–transfected fibroblats (D12P; A) or to mock-transfected cells (B), in the absence or presence of the F(ab′)2 of the anti–PECAM-1 antibody (M89D3) or of LY294002 (20 μM), wortmannin (100 nm), herbimycin (100 nM) or PD98059 (10 μM) for 30 minutes, collected after 48 hours of culture, stained with PI as described in the legend to Figure 2, and run on a FACSort. We analyzed 104 cells/sample; results are plotted as percentages of apoptotic (DNA content < 2n-diploid) cells (B-C). Murine Ig was used as control. Mean values ± SDs from 6 different experiments are shown.
PECAM-1–induced survival of CD34+ cells is dependent on PI-3K activation.
CD14+CD34+ cells were allowed to adhere to PECAM-1+–transfected fibroblats (D12P; A) or to mock-transfected cells (B), in the absence or presence of the F(ab′)2 of the anti–PECAM-1 antibody (M89D3) or of LY294002 (20 μM), wortmannin (100 nm), herbimycin (100 nM) or PD98059 (10 μM) for 30 minutes, collected after 48 hours of culture, stained with PI as described in the legend to Figure 2, and run on a FACSort. We analyzed 104 cells/sample; results are plotted as percentages of apoptotic (DNA content < 2n-diploid) cells (B-C). Murine Ig was used as control. Mean values ± SDs from 6 different experiments are shown.
Discussion
Herein we provide evidence that a subset of CD34+circulating precursors receive an antiapoptotic signal during transendothelial migration which renders these cells resistant to starvation due to the up-regulation of Bcl-2, A1, and Bcl-X proteins involved in the control of mitochondrial apoptosis; this signal is delivered via PECAM-1, coupled to PI-3K–dependent Akt activation.
The ability of totipotent progenitor cells to localize to the bone marrow is crucial for the reconstitution of hematopoiesis in therapeutic stem cell transplantation8; on the other hand, tissue localization of antigen-presenting cell precursors is needed for the development of an efficient immune response.4 In both cases, cells must survive for a time sufficient to allow differentiation and maturation; one of the signals involved in the enhancement of the lifespan might be provided to these precursors during transendothelial migration.
Interestingly, the increased resistance of CD34+ migrated cells to the programmed cell death that follows starvation is due to the maintenance of mitochondrial functions; indeed, after transmigration, cells displayed a higher content of mitochondrial ATP and increased cellular NAD/NADH ratio. Both are expression of a conserved integrity of these organelles.32,33 In turn, inhibition of the respiratory chain usually results in mitochondrial membrane permeability transition, cytochrome c release, and apoptosis.23 34 Thus, the vascular endothelium seems to be capable of providing circulating precursors with a survival signal during their localization to peripheral tissues; in other words, interaction with endothelium might substitute for a certain period of time the presence of growth factors.
Among the adhesion molecules possibly involved in this process, integrins may play an important role. In particular, unligated integrins recruit caspase 8 and induce apoptosis, whereas integrin ligation, as might occur during transmigration, disrupts integrin/caspase coupling and increases cell survival.35In our system, however, covering of β2-integrin or its ligand ICAM-1 did not prevent PECAM-1–mediated protection from apoptosis, as observed by masking PECAM-1; thus, although PECAM-1 can activate integrins and enhance cell adhesion,16 β2-integrins do not seem to be primarily involved in preventing starvation-induced apoptosis of CD34+ cells. On the other hand, β2-integrin engagement can both delay and enhance apoptosis in the same cells, due to the activation state of the molecule and the consequent preferential engagement of ERK rather than Akt kinase.36 Along this line, other survival signals involving integrins, such as chemokines, do not affect survival of lymphohematopoietic cells, but rather potentiate cell-matrix adhesion.37
Mitochondrial-related apoptosis is regulated by a number of pro- and antiapoptotic proteins. In particular, Bax interacts with the mitochondrial membrane allowing cytochrome c release, whereas Bcl-2 and Bcl-X act in the opposite way, interfering with the activation of Bax.25,28-30 We found that the signal received by migrated cells leads to the up-regulation of these antiapoptotic proteins, apparently inhibiting Bax activation and eventually leading to an increased resistance to starvation. Ligation of PECAM-1 in adherent endothelial cells has been reported to enhance Bcl-2 transcript levels,25 in keeping with our findings showing increased amounts of Bcl-2 intracellular protein in circulating CD31+CD34+ leukocytes. On the other hand, we also found a rise in Bcl-X protein levels in CD31+CD34+ cells upon PECAM-1 engagement, whereas other studies reported no change in Bcl-XL transcript levels in adherent endothelial cells.24 25 One intriguing explanation for these apparently conflicting results is that PECAM-1 regulates Bcl-X only at the level of protein stability, whereas regulation of Bcl-2 also occurs at the level of mRNA transcription.
Transcription of the Bcl-2 family member A1,22 induced in endothelial cells resistant to serum deprivation after interaction with monocytes,24,25 was also enhanced in CD34+cells upon migration through HUVECs or through the PECAM-1+D12P transfectants, as well as after ligation of PECAM-1 with the specific mAb, further supporting that this molecule is directly involved in protection from starvation-induced apoptosis. Interestingly, that PECAM-1 engagement results in increased A1 mRNA transcription has been reported also in adherent endothelial cells,24 25 pointing to a more general role for A1 in PECAM-1–mediated antiapoptotic function both in vascular endothelium and in circulating lymphocytes. Thus, A1 might represent the main antiapoptotic protein induced via PECAM-1 during transendothelial leukocyte migration, providing both cell types with a strong survival signal.
Of note, the antiapoptotic signal delivered to CD34+precursors during transendothelial migration is confined to PECAM-1+ cells, is inhibited by covering the PECAM-1 molecule, and is mimicked by the interaction with PECAM-1–expressing fibroblasts and P-ECV304 cells. This interaction leads to a series of events: (1) PI-3K is engaged, (2) Akt is phosphorylated and activated, (3) Bcl-2 and Bcl-X are up-regulated, as are the mRNA of A1 and the cytoplasmic inactive form of Bax, and (4) cells are protected from starvation-induced apoptosis. Interestingly, PI-3K is deeply involved in Bcl-X induction and apoptosis inhibition mediated by growth factors31; likewise, Bcl-2 is up-regulated by activation of the PI-3K–dependent serine-threonine kinase Akt.15 Moreover, in our system, the protective effect of the signal delivered via PECAM-1 is prevented by the use of PI-3K inhibitors, but not by other kinase blockers. We are aware that integrins can also activate PI-3K and amplify the antiapoptotic signals occurring during transendothelial migration; in turn, PECAM-1 ligation up-regulates integrin functions.16,36 However, this cross-talk seems to be relevant for adhesion or anchorage-dependent survival,36-38 whereas PECAM-1–mediated direct engagement of PI-3K would contribute to the prevention of starvation-induced apoptosis.
All these data point to a role for PECAM-1, which is now thought to be more than a simple adhesion molecule,39,40 as an antiapoptotic molecule; signaling through PECAM-1/CD31 can protect serum-starved endothelial cells from apoptosis.24,26 Other features make this molecule a good candidate for the role of a protective molecule in CD34+ precursors: the high levels of expression in this cell population; the junctional distribution in vascular endothelium, which allows both haptotactic migration and ligation of PECAM-1 expressed by migrating cells during homophylic interaction; and the association with the PI-3K–dependent Akt activation pathway. It is tempting to speculate that engagement of PECAM-1 during transmigration provides a cross-talk between migrating and endothelial cells, eventually leading to prolonged survival of both cell types.24,26 40
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2002-03-0768.
Supported in part by the Italian Ministero della Sanità2000-2002 (M.R.Z. and A.P.) and by the Italian Association for Cancer Research (AIRC 2002, A.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maria Raffaella Zocchi, Laboratory of Tumor Immunology, Scientific Institute San Raffaele, Via Olgettina 60, I-20132 Milan, Italy; e-mail: zocchi.maria@hsr.it.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal