Abstract
Homeostasis of the extracellular matrix is a delicate balance between degradation and remodeling, the balance being maintained by the interaction of activated matrix metalloproteinases (MMPs) and specific tissue inhibitors of matrix metalloproteinases (TIMPs). Up-regulation of MMP activity, favoring proteolytic degradation of the basement membrane and extracellular matrix, has been linked to tumor growth and metastasis, as well as tumor-associated angiogenesis, whereas inhibition of MMP activity appears to restrict these processes. We have used retroviral-mediated gene delivery to effect sustained autocrine expression of TIMP-3 in murine neuroblastoma and melanoma tumor cells in order to further examine the ability of TIMPs to inhibit angiogenesis in vivo. Growth of both histologic types of gene-modified tumor cells in severe combined immunodeficiency (SCID) mice was significantly restricted when compared with controls. Grossly, these tumors were small and had few feeding vessels. Histologic evaluation revealed that although tumors overexpressing TIMP-3 had an increased number of CD31+endothelial cells, these endothelial cells had not formed functional tubules, as evidenced by decreased vessel continuity and minimal pericyte recruitment. This effect appears to be mediated, in part, by decreased expression of vascular endothelial (VE)–cadherin by endothelial cells in the presence of TIMP-3 as seen both in an in vitro assay and in TIMP-3–overexpressing tumors. Taken together, these results demonstrate that overexpression of TIMP-3 can inhibit angiogenesis and associated tumor growth, and that the antiangiogenic effects of TIMP-3 appear to be mediated through the inhibition of functional capillary morphogenesis.
Introduction
Angiogenesis, the process by which new blood vessels are formed from existing ones, is a fundamental requirement for tumor growth, invasion, and metastasis.1-3 Evidence also exists to suggest that inhibition of tumor-associated angiogenesis can retard tumor growth, prevent spreading, and even cause tumor regression,4-6 making angiogenesis inhibition an attractive anticancer approach. Angiogenesis is an invasive, multistep process that initially involves proteolytic degradation of the basement membrane and extracellular matrix (ECM) surrounding existing blood vessels.7 8 Activated endothelial cells lining these vessels proliferate and migrate into this defect in the ECM where they then, again, tightly adhere to one another and establish functional tubules covered with a new basement membrane.
Angiogenesis and its associated remodeling of the ECM are tightly regulated both temporally and spatially and involve the interaction of various cytokines, adhesion molecules, and proteolytic enzymes. Matrix metalloproteinases (MMPs) are a group of zinc-dependent extracellular proteolytic enzymes that degrade components of the ECM and, therefore, play an essential role in tissue invasion, as required for neovascularization.9 Activated MMPs are selectively inhibited by a group of low-molecular-weight proteins known as tissue inhibitors of matrix metalloproteinases (TIMPs). The TIMP family consists of 4 distinct proteins, TIMP-1, -2, -3, and -4, which are expressed by a variety of cell types and can be found in most tissues throughout the body.10,11 Consistent with the hypothesized role of MMPs in tumor invasion and spread is experimental evidence from various tumor models demonstrating that overexpression of TIMPs or other synthetic MMP inhibitors results in suppression of tumor growth and metastasis.12-14 A precise understanding of the mechanism by which TIMPs affect tumor growth remains uncertain.
TIMP-3 is a unique TIMP family member. It shares only a 25% amino acid homology with TIMP-1 and TIMP-2, demonstrates a close association with the ECM, and has been reported to induce apoptosis in some normal and malignant cell lines.15,16 Overexpression of TIMP-3 has been shown to restrict tumor cell invasiveness in vitro15,16 and to inhibit tumor growth in vivo.17,18 Although TIMP-3 has been shown to inhibit endothelial cell migration and tubule formation in vitro,19 the effect of TIMP-3 overexpression on tumor-induced angiogenesis in vivo has not been investigated. Therefore, in this study we have sought to examine, specifically, the in vivo antiangiogenic effect of enforced expression of TIMP-3 by gene-modified tumor cells.
Materials and methods
Cell lines
The murine neuroblastoma cell line NXS2,20 provided by Dr R. Reisfeld (La Jolla, CA), was maintained in RPMI 1640 medium (Bio-Whittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (Bio-Whittaker), 100 units/mL penicillin-100 μg/mL streptomycin (Life Technologies, Grand Island, NY) and 2 mMl-glutamine (Life Technologies). The murine melanoma cell line B16F10, provided by Dr I. Fidler (Houston, TX) and 293T cells (human embryonic kidney cells expressing SV40 large T antigen)21 were maintained in Dulbecco modified Eagle medium (DMEM, Bio-Whittaker) with the same supplements as in the growth medium for NXS2. Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (Walkersville, MD) and were maintained in endothelial growth medium (EGM).
Retroviral vector construction
A plasmid (pBLAST-mTimp-3) containing cDNA encoding murine TIMP-3 was purchased from InvivoGen (San Diego, CA). A 674-bpSgrAI-NheI fragment containing the Timp-3 transgene was taken from this vector and ligated into pBlueScript (BS) (Stratagene, La Jolla, CA) cut with XmaI and XbaI to make mTimp3-BS. A 710-bp ClaI-SacII fragment from mTimp-3-BS was then ligated into murine stem cell virus-Ires-green fluorescent protein (MSCV-I-GFP) cut withBstBI and SacII. MSCV-I-GFP22 is a retroviral expression plasmid that contains the MSCV long terminal repeat (LTR) driving expression of enhanced green fluorescent protein (Clontech Laboratory, Palo Alto, CA). The resulting retroviral expression plasmid, MSCV-Timp-3-I-GFP, contains the GFP gene linked to an internal ribosome entry site (IRES) from encephalomyocarditis virus23 3′ of the mTimp-3 cDNA.
Tumor cell line transduction
NXS2 and B16F10 tumor cells were transduced with conditioned medium containing high titer, vesicular stomatitis virus G (VSV-G) pseudotyped MSCV-Timp-3-I-GFP or MSCV-I-GFP vector particles, supplemented with 6 μg/mL polybrene. These conditioned media were derived by cotransfection of 293T cells with the respective retroviral vector plasmids and 2 helper plasmids, one containing thegag and pol retroviral genes and the other containing the gene for the VSV-G envelope, as described previously.24 25 Transduced cells were then sorted based on GFP expression by using fluorescence-activated cell sorting (FACS; Becton Dickinson, Bedford, MA) to recover highly transduced cells. An additional subpopulation of less highly transduced NXS2-Timp3-I-GFP was also collected. These subpopulations were then expanded, with GFP expression being confirmed by FACS and TIMP-3 expression being confirmed by Western blot analysis.
TIMP-3 Western blot analysis
Conditioned media from confluent Timp-3-I-GFP-(high and low) and GFP-gene modified tumor cells, and protein lysates from excised tumors were electrophoresed on 12% sodium dodecyl sulfate–polyacrylamide gels after standardizing for total protein. The separated proteins were then transferred onto polyvinyl difluoride (PVDF) membranes (Bio-Rad, Hercules, CA). TIMP-3 was bound with 0.4 μg/mL rabbit anti–human TIMP-3 antibody (Chemicon International, Temecula, CA) and then detected with enhanced chemiluminescence (ECL) Western blotting reagents (Amersham, Buckinghamshire, England) and Hyperfilm ECL (Amersham). Purified Timp-3 (R&D Systems, Minneapolis, MN) was included as a control.
TIMP-3 inhibition of vascular endothelial growth factor–stimulated endothelial cell migration
Endothelial cell migration assays were performed in 24-well transwell plates (Corning COSTAR, Corning, NY) as previously described.26 Briefly, 8-μm polycarbonate membranes were placed over a bottom chamber containing 2% gelatin that had been allowed to settle at room temperature for 30 minutes. Excess gelatin was then aspirated and replaced with an equal volume of DMEM plus 0.1% bovine serum albumin (BSA) and incubated for one hour at 37°C. Excess DMEM was aspirated from the bottom wells and replaced with EGM supplemented with vascular endothelial growth factor (VEGF, 10 ng/mL; R&D Systems) along with conditioned media from confluent Timp-3-I-GFP– or control GFP gene-modified NXS2 tumor cells (total volume 600 μL). HUVECs (50 000) (passage 5, total volume 50 μL) were added to the upper chambers. The plates were incubated for 6 hours at 37°C with 5% CO2 to allow the cells to migrate. Nonmigrated cells on the upper surface of the filter were removed by wiping with a cotton swab. The cells were then fixed with 10% formalin, stained with Harris hematoxylin and after washing away excess dye, the migrated cells were counted. The assays were run in triplicate.
Inhibition of gelatinase-A activity
Inhibition of MMP-2 (gelatinase-A) activity was measured with the Gelatinase Activity Assay Kit (Chemicon International, Temecula, CA) as per the manufacturer's instructions. Conditioned media from NXS2-Timp3-I-GFP or NXS2-I-GFP tumor cells and MMP (active) samples were incubated with a biotinylated gelatinase substrate, added to a biotin-binding plate, and incubated with a 1:3000 dilution of streptavidin-enzyme conjugate. The addition of enzyme substrate resulted in a colored product. The optical density of the product was determined at a wavelength of 450 nm by using a microplate reader (Anthos Labtec, Wals, Austria).
Inhibition of tubule formation
Endothelial tubule formation assays were performed in 24-well cell culture plates (Corning COSTAR). Growth factor–reduced Matrigel (Becton Dickinson) was allowed to polymerize in the presence of 30 ng/mL recombinant basic fibroblastic growth factor (bFGF; R&D Systems) for 1 hour at 37°C (300 μL per well). HUVECs (10 000), serum-starved for 2 hours prior to trypsinization, were resuspended in 100 μL of serum-free conditioned media from confluent NXS2-I-GFP or NXS2-Timp3-I-GFP-(high), together with 30 ng/mL recombinant bFGF. Cells were then seeded onto the polymerized Matrigel (total volume, 400 μL) and incubated for 16 hours at 37°C with 5% C02 to allow tubule formation. The assays were run in triplicate. Representative results were photographed at 4 ×.
Murine tumor models
Tumors were established in C.B-17 SCID mice (Charles River Laboratory, Wilmington, MA) by subcutaneous injection of 1 × 106 cells in 200 μL PBS. Tumor measurements were performed with calipers twice weekly and volumes calculated as width2 × length × 0.5. These experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of St Jude Children's Research Hospital.
Tumor immunohistochemistry
Immunohistochemistry for the endothelial cell marker CD31 (PECAM-1) was performed by indirect immunoperoxidase staining. Tumors were excised from experimental mice 3 weeks following subcutaneous injection, placed in Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC) and snap frozen in liquid nitrogen. Tissue sections (6 μm) were fixed in cold acetone, allowed to air dry, and then place in tris-buffered saline containing 0.05% Tween 20 (pH = 7.6) for at least 10 minutes prior to assay. Endogenous peroxidase activity was blocked with 0.03% hydrogen peroxide for 5 minutes. The slides were then incubated with a monoclonal rat anti–mouse CD31 antibody (25 μg/mL, 60 minutes; PharMingen, San Diego, CA) followed by a biotinylated secondary antibody, rabbit antirat (1:200; Vector, Burlingame, CA). Following a 10-minute incubation with streptavidin conjugated to horseradish peroxidase (DAKO, Carpinteria, CA), a substrate containing the chromagen DAB (3,3′ diaminobenzidine tetrahydrochloride) was added that resulted in deposition of a brown precipitate. Rat IgG2aκ was used as a negative control. Slides were counterstained for 3 minutes with a 1:5 dilution of hematoxylin.
Endothelial cell density was determined using the method described by Weidner et al.27 Stained tumor sections were scanned at low power and areas of greatest CD31+ density were chosen for analysis. Only tumors with adequate staining were evaluated. After identification of the “hot spot,” the number of individual brown staining endothelial cells or clusters were counted at 400 ×. Endothelial cell counts were determined by 3 independent, blinded observers.
Tumor specimens were also analyzed for smooth muscle actin (SMA) content. Sections (5 μm) of formalin-fixed, paraffin-embedded tissues were deparaffinized in xylene. Heat-induced epitope retrieval in citrate buffer (Zymed, South San Francisco, CA), pH 6.0, was performed at more than 95°C for 15 minutes. The slides were cooled and placed in tris-buffered saline containing 0.05% Tween 20 (DAKO) for 10 minutes. Endogenous peroxidase activity was blocked by incubation with 3% H2O2. The specimens were then incubated with biotinylated monoclonal mouse anti–human smooth muscle actin (clone 1A4; DAKO) at a 1:50 dilution for 15 minutes with antibody binding being detected as described above. Slides were counterstained with hematoxylin (DAKO). Individual tumors were analyzed for SMA content and quantitated using the same method used to determine endothelial cell counts.
Tumor specimens were also analyzed by indirect immunoperoxidase staining for the endothelial cell adhesion molecule, vascular endothelial (VE)–cadherin (CD144). Frozen tissue sections (6 μm) were cut, allowed to air dry, and then fixed in 4% paraformaldehyde for 20 minutes prior to analysis. Endogenous peroxidase activity was quenched by the addition of 0.3% H2O2 for 30 minutes. The samples were then washed in PBS buffer followed by incubation in normal rabbit blocking serum (Vector Laboratories, Burlingame, CA) at room temperature for 30 minutes. Specimens were then incubated with purified rat anti–mouse VE-cadherin antibody (1:10, 60 minutes, 37°C; BD PharMingen) followed by addition of the biotinylated secondary antibody, rabbit-antirat (1:200, 45 minutes, 37°C; Vector). Specimens were then incubated with Vectastain Elite ABC reagent for 30 minutes at room temperature. Following detection with DAB, the slides were counterstained with 0.5% methyl green.
Perfusion analysis
Functional tumor perfusion was assessed utilizing the inherent autofluorescent nature of the erythrocytes to determine intratumoral red blood cell density. Paraffin-embedded tumor sections (5 μm) were deparaffinized in xylene and rehydrated in graded ethanol (100%, 95%, and 70%). The samples were then washed with PBS, rinsed in distilled water, and then mounted for analysis. Tumor sections were examined in a Leica TCS Nt SP confocal microscope (Heidelberg, Germany) equipped with 3 lasers including a krypton laser (580 nm-720 nm) to detect the red autofluorescence of erythrocytes. Optical sections (0.5 μm) were made through the center of cells to obtain the images. Representative photographs were taken. Total intratumoral erythrocyte density was determined in a blinded fashion by 3 independent observers after counting the number of individual erythrocytes per field (250 μm × 250 μm) and reporting the results as the number of erythrocytes per mm2.
Intratumoral VEGF expression analysis
Resected tumor specimens were collected and total protein prepared for analysis. The specimens were homogenized using a Dounce (Kontes, Vineland, NJ) homogenizer after the addition of 1 mL phosphate buffered solution (154 mM NaCl, 5 mM Na2HPO4, 1 mM KH2PO4) for every 0.5 g tissue. The homogenates were then centrifuged at 600g at 10°C for 20 minutes, the supernatants collected and then frozen at −70°C for later use. Total protein levels were determined for each specimen using the Bradford Protein Assay (Bio-Rad). The protein extracts from TIMP-3– and GFP-expressing NXS2 and B16F10 tumors were diluted 1:5 and VEGF levels for each tumor specimen were quantitated by enzyme-linked immunoabsorbent assay from R&D Systems as per the manufacturer's instructions. The optical density of the product was determined at a wavelength of 450 nm by using a microplate reader. All samples were run in triplicate and the final data were standardized relative to total protein content and expressed as picograms VEGF/micrograms total protein.
VE-cadherin expression analysis
In vitro expression of VE-cadherin by HUVECs was measured using FACS analysis. Conditioned media from NXS2-TIMP-3-I-GFP or NXS2-I-GFP tumor cells was filtered and then added to confluent HUVECs (passage 6) for 16 hours. Ethylenediaminetetraacetic acid (EDTA) (5 mM) was used to lift the cells from the culture plates. Following a wash with 5 mM calcium chloride (pH = 5.2), the cells were incubated with mouse monoclonal anti–human VE-cadherin antibody (1:20; Chemicon) and then fluorescein isothiocyanate (FITC)–conjugated goat antibody to mouse immunoglobulin (1:200; Becton Dickinson, San Jose, CA). Fluorescence was measured using a Becton Dickinson FACS Caliber flow cytometer. Mouse IgG2a served as isotype control. Individual tumors were analyzed for VE-cadherin expression with the number of cells expressing detectable VE-cadherin being quantitated using the same method as was used to determine endothelial cell counts.
Statistical analysis
Tumor volumes are reported as means plus or minus standard error. The Student t test was used to analyze statistical differences between the tumor volumes at specific time points; intratumoral VEGF levels; and CD31, SMA, and VE-cadherin counts in mice that received control cells (NXS2-I-GFP or B16F10-I-GFP) and those expressing TIMP-3. A P value of less than .05 was considered statistically significant.
Results
Tumor cell transduction
In vitro transduction of NXS2 and B16F10 cells with high-titer VSV-G–pseudotyped MSCV-Timp3-I-GFP or MSCV-I-GFP retroviral vector was highly efficient (> 90% of targeted cells). Following FACS sorting for transduced cells, more than 99% of the tumor cells expressed GFP. Western blot analysis of the conditioned medium from cells transduced with the MSCV-Timp-3-I-GFP vector confirmed secretion of the TIMP-3 protein, seen in both the unglycosylated (24 kDa) and glycosylated forms (30 kDa) (Figure 1). The subpopulation of high-expressing NXS2-Timp-3-I-GFP cells secreted approximately 75 pg TIMP-3/106 cells per 48 hours while the subpopulation of low-expressing NXS2-Timp-3-I-GFP cells secreted approximately 10 pg TIMP-3/106 cells per 48 hours. No expression of TIMP-3 was detectable in conditioned medium from cells transduced with MSCV-I-GFP.
Western blot detection of TIMP-3 protein in conditioned medium from NXS2 or B16F10 tumor cells transduced with MSCV-TIMP-3-I-GFP or MSCV-I-GFP.
Reduced protein bands in both unglycosylated (24 kDa) and glycosylated (30 kDa) forms are seen. TIMP-3 expression from NXS2 cells FACS-sorted for high and low GFP expression is shown. Recombinant TIMP-3 (100 ng) was placed in the first lane.
Western blot detection of TIMP-3 protein in conditioned medium from NXS2 or B16F10 tumor cells transduced with MSCV-TIMP-3-I-GFP or MSCV-I-GFP.
Reduced protein bands in both unglycosylated (24 kDa) and glycosylated (30 kDa) forms are seen. TIMP-3 expression from NXS2 cells FACS-sorted for high and low GFP expression is shown. Recombinant TIMP-3 (100 ng) was placed in the first lane.
Expression of functional TIMP-3
The function of TIMP-3 generated from the retroviral vector MSCV-Timp3-I-GFP was evaluated in vitro. The ability of conditioned media from cells modified with this vector to inhibit endothelial cell migration and tubule formation, as well as MMP-2 (gelatinase-A) activity, was tested, as these are often cited as properties of TIMP-3.17
HUVECs have been shown to be able to migrate through a gelatin matrix that mimics the basement membrane, in response to VEGF.28There was a significant inhibition of endothelial migration in the presence of conditioned medium from NXS2-TIMP-3-I-GFP cells relative to GFP alone (P = .008). This was found to occur in a dose-dependent manner (Figure 2A). No inhibition of VEGF-stimulated endothelial migration was seen in the presence of conditioned medium from NXS2-I-GFP cells when compared with conditioned medium from unmodified parental NXS2 cells.
Effect of conditioned medium from NXS2 tumor cells overexpressing TIMP-3 on VEGF-stimulated HUVEC migration and bFGF-stimulated tubule formation.
(A) Data (means ± SE of triplicates) are expressed as numbers of migrating cells across the filter area. Also shown are the dose-dependent effects of diluting TIMP-3 at ratios of 1:2 and 2:1, the effect of medium with and without VEGF, and the effect of 250 ng/mL recombinant TIMP-3. (B) Tubule formation in the presence of NXS2-I-GFP–conditioned medium (CM). (C) Inhibition of tubule formation in the presence of NXS2-Timp-3-I-GFP CM.
Effect of conditioned medium from NXS2 tumor cells overexpressing TIMP-3 on VEGF-stimulated HUVEC migration and bFGF-stimulated tubule formation.
(A) Data (means ± SE of triplicates) are expressed as numbers of migrating cells across the filter area. Also shown are the dose-dependent effects of diluting TIMP-3 at ratios of 1:2 and 2:1, the effect of medium with and without VEGF, and the effect of 250 ng/mL recombinant TIMP-3. (B) Tubule formation in the presence of NXS2-I-GFP–conditioned medium (CM). (C) Inhibition of tubule formation in the presence of NXS2-Timp-3-I-GFP CM.
Previous in vitro studies have also demonstrated that HUVECs plated on Matrigel will form tubulelike structures in response to bFGF.29 30 The ability of endothelial cells to form tubules in vitro under these conditions after the addition of conditioned medium from NXS2-TIMP-3-I-GFP or NXS2-I-GFP was therefore tested next. TIMP-3–containing conditioned medium markedly suppressed tubule formation relative to conditioned medium from control cells. Although some of the endothelial cells did appear to align in the presence of the TIMP-3 protein, they did not organize themselves into a functional anastamotic network of tubulelike structures (Figure 2B-C). There was no qualitative difference in the tubules formed in the presence of NXS2-I-GFP–conditioned medium plus bFGF compared with bFGF alone.
Finally, the in vitro inhibitory effect of TIMP-3 on MMP-2 cleavage of a biotinylated gelatinase substrate was evaluated. Both unmodified NXS2 and B16F10 cells were found to express MMP-2 and with similar levels of activity. However, the MMP-2 activity of conditioned medium from NXS2 cells expressing TIMP-3 was 5 times less than that of conditioned medium from GFP-expressing control cells (P < .001).
Unaltered growth of TIMP-3–expressing tumor cells in vitro
The growth of NXS2-TIMP-3-I-GFP and B16F10-TIMP-3-I-GFP tumor cells was compared with the growth of unmodified parental cell lines to determine whether TIMP-3 expression had any inherent direct effect on the growth of tumor cells. Although previous studies have suggested that TIMP-3 can induce apoptotic cell death and inhibition of monolayer cell growth in a number of cell lines,15,16,18,31 this has not been found to be true for all tumor cell lines.17 In this study, the in vitro growth of the TIMP-3–expressing NXS2 and B16F10 cells was not significantly different from that of the unmodified parental or control GFP-expressing cell lines (data not shown). This suggests that the target of the TIMP-3 is not the tumor cells themselves but factors in the local milieu of the growing tumor cells in vivo.
Restricted growth of TIMP-3–expressing tumor cells in vivo
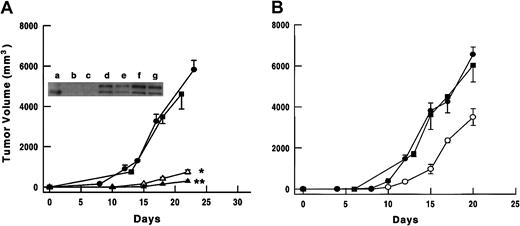
To evaluate the in vivo effects of TIMP-3 overexpression, SCID mice were given subcutaneous injections of either Timp-3-I-GFP– or GFP-modified tumor cells, or unmodified tumor cells. Growth of the NXS2 and B16F10 tumor cells overexpressing TIMP-3 was significantly inhibited when compared with the control cells. The mean volume of NXS2-TIMP-3-I-GFP-(high) tumors (0.3 ± 0.1 cm3) was 5% that of NXS2-I-GFP tumors (5.8 ± 0.5 cm3;P < .00001) 23 days after injection (Figure3A). For NXS2-TIMP-3-I-GFP-(low) tumors, the mean volume (0.7 ± 0.1) was 12% of control (P < .0001), suggesting a dose-response for TIMP-3 expression. That these tumors maintained their TIMP-3 expression in vivo was confirmed by Western blot analysis of protein lysates from tumors harvested after 23 days (Figure 3A). The mean volume of B16F10-TIMP-3-I-GFP tumors (3.5 ± 0.4 cm3) was 53% that of control tumors (6.5 ± 0.4 cm3;P < .001) 20 days after injection (Figure 3B). The growth of tumor cells expressing GFP alone was comparable to that of the unmodified tumor cells for both the neuroblastoma and melanoma cells.
Subcutaneous in vivo tumor growth following injection of parental or gene-modified tumor cells.
(A) Growth of unmodified parental NXS2 (-▪-); NXS2-I-GFP (-●-); NXS2-TIMP-3-I-GFP-(low) (-▵-); or NXS2-TIMP-3-I-GFP-(high) (-▴-) tumor cells in C.B-17 SCID mice (n = 10 mice/group). *P < .0001 comparing NXS2-TIMP-3-I-GFP-(low) with NXS2-I-GFP; **P < .002 comparing NXS2-TIMP-3-I-GFP-(high) with NXS2-TIMP-3-I-GFP-(low). Shown also is a Western blot for TIMP-3 expression in protein lysates of tumors from NXS2-I-GFP (a,c), NXS2-TIMP-3-I-GFP-(low) (d,e), and NXS2-TIMP-3-I-GFP-(high) (f,g). Lane b has recombinant TIMP-3. (B) Growth of unmodified parental B16F10 (-▪-); B16F10-I-GFP (-●-); or B16F10-TIMP-3-I-GFP (-○-) tumor cells in C.B-17 SCID mice (n = 10 mice/group). P < .001 comparing B16F10-TIMP-3-I-GFP with B16F10-I-GFP. Error bars represent SE.
Subcutaneous in vivo tumor growth following injection of parental or gene-modified tumor cells.
(A) Growth of unmodified parental NXS2 (-▪-); NXS2-I-GFP (-●-); NXS2-TIMP-3-I-GFP-(low) (-▵-); or NXS2-TIMP-3-I-GFP-(high) (-▴-) tumor cells in C.B-17 SCID mice (n = 10 mice/group). *P < .0001 comparing NXS2-TIMP-3-I-GFP-(low) with NXS2-I-GFP; **P < .002 comparing NXS2-TIMP-3-I-GFP-(high) with NXS2-TIMP-3-I-GFP-(low). Shown also is a Western blot for TIMP-3 expression in protein lysates of tumors from NXS2-I-GFP (a,c), NXS2-TIMP-3-I-GFP-(low) (d,e), and NXS2-TIMP-3-I-GFP-(high) (f,g). Lane b has recombinant TIMP-3. (B) Growth of unmodified parental B16F10 (-▪-); B16F10-I-GFP (-●-); or B16F10-TIMP-3-I-GFP (-○-) tumor cells in C.B-17 SCID mice (n = 10 mice/group). P < .001 comparing B16F10-TIMP-3-I-GFP with B16F10-I-GFP. Error bars represent SE.
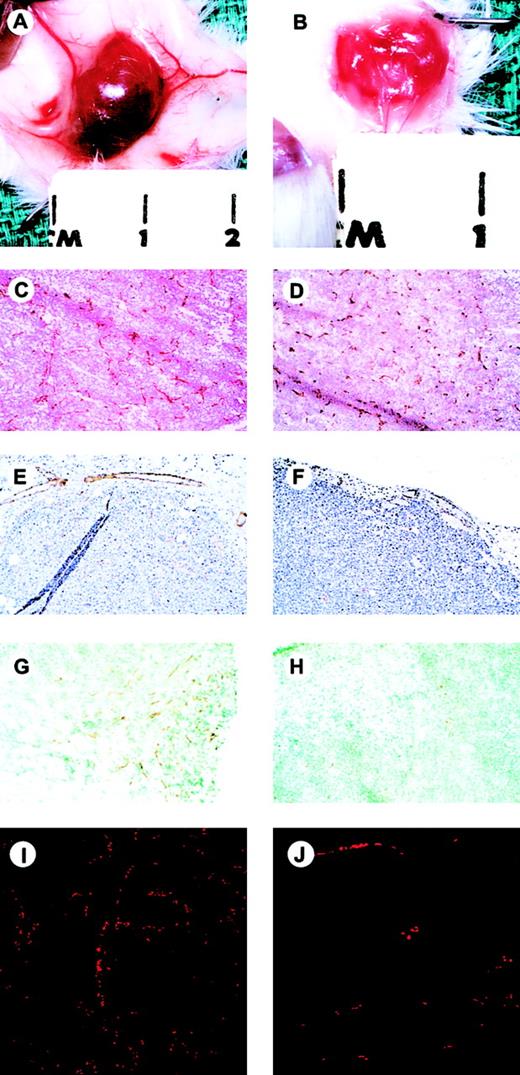
Excision of the TIMP-3–expressing tumors revealed clear evidence that tumor-associated angiogenesis had been suppressed. In addition to being smaller, the TIMP-3–expressing tumors were paler in appearance (neuroblastomas), and markedly less hemorrhagic relative to GFP-expressing controls (Figure 4A-B). Also, there was a significant lack of subcutaneous “feeder” vessels supplying the TIMP-3 tumors compared with GFP controls.
Gross and microscopic appearance of NXS2-I-GFP and NXS2-TIMP-3-I-GFP-(high) tumors.
Gross appearance after reflection of the overlying skin and exposure of the subcutaneous tumors (A,B; marks are 1 cm apart). Immunohistochemical analysis of intratumoral vascularity as assessed by staining with anti–CD31 antibody (C,D; original magnification, × 25); intratumoral vessel SMA content (E,F; original magnification, × 25); intratumoral VE-cadherin expression (G,H; original magnification, × 25); and confocal microscopic analysis of functional vascular status demonstrated by the inherent autofluorescence of intravascular erythrocytes (I,J). NXS2-I-GFP tumors, left panels; NXS2-TIMP-3-GFP-(high) tumors, right panels.
Gross and microscopic appearance of NXS2-I-GFP and NXS2-TIMP-3-I-GFP-(high) tumors.
Gross appearance after reflection of the overlying skin and exposure of the subcutaneous tumors (A,B; marks are 1 cm apart). Immunohistochemical analysis of intratumoral vascularity as assessed by staining with anti–CD31 antibody (C,D; original magnification, × 25); intratumoral vessel SMA content (E,F; original magnification, × 25); intratumoral VE-cadherin expression (G,H; original magnification, × 25); and confocal microscopic analysis of functional vascular status demonstrated by the inherent autofluorescence of intravascular erythrocytes (I,J). NXS2-I-GFP tumors, left panels; NXS2-TIMP-3-GFP-(high) tumors, right panels.
Histopathologic characteristics of TIMP-3–expressing tumors
Immunohistochemical analysis of tumor sections was used to determine the endothelial cell density in both TIMP-3-(high) and GFP-expressing tumors. Following staining with an anti–mouse CD31 antibody, the degree of intratumoral endothelial cell density was assessed by evaluation of endothelial “hot spots.” Although smaller in size than GFP-expressing control tumors, TIMP-3–expressing tumors showed a significant increase in CD31+ cells. The mean endothelial cell count per 400 × field in the NXS2-TIMP-3-I-GFP tumors was 33.1 ± 3.1 but only 24.6 ± 2.1 (P = .034) in the NXS2-I-GFP controls (Figure 4C-D). This represented a 35% increase within the TIMP-3–overexpressing NXS2 tumors relative to GFP controls. Similarly, the mean endothelial cell count in the B16F10-TIMP-3-I-GFP tumors was 43% higher than in the control tumors (22.5 ± 2.0 vs 15.7 ± 1.2; P = .05). Qualitatively, however, despite the increased number of CD31+ endothelial cells within the TIMP-3–overexpressing tumors, these cells appeared to form a dysfunctional network that showed less apparent organization into functional tubules. Conversely, the endothelial cells within the GFP tumors appeared to arborize and align themselves into functional conduits lined by CD31+ endothelial cells.
Immunohistochemical analysis of the control tumors with an anti–SMA antibody suggested that the murine neuroblastoma and melanoma (not shown) microvasculature in these models normally have a high degree of pericyte recruitment (Figure 4E). Yet in those tumors overexpressing TIMP-3, virtually none of the CD31+ endothelial cells had adjacent pericytes (Figure 4F). A quantitative assessment was done which revealed that the mean number of SMA-positive cells per 400 × field in the NXS2-I-GFP control tumors was 11.3 ± 2.3 compared with only 3.7 ± 1.2 (P = .03) in the NXS2-TIMP-3-I-GFP tumors. Thus, SMA staining, a marker of pericyte recruitment within the TIMP-3–expressing tumors, was 67% less than that found in the GFP controls. A “vessel maturity index” was determined by combining this data with the previous endothelial density counts. This was defined as the number of CD31+ endothelial cells per SMA-positive cell, as described previously.32 The maturation index in the TIMP-3–expressing NXS2 tumors (11%) was significantly less than GFP controls (46%), suggesting a failure of maturation of new blood vessels.33 34 There was no significant difference in the vessel maturation index when tumors were harvested and analyzed earlier, 17 days after tumor cell injection (data not shown).
Decreased perfusion of TIMP-3–expressing tumors
Immunohistochemical identification of endothelial cells to determine microvessel density has been used as a marker for quantifying angiogenesis for a variety of different tumor types.35 It may be, however, that a more appropriate determination of the state of ongoing angiogenesis should reflect the functional status of the tumor neovasculature.36 37 The assessment of vascular continuity and tumor perfusion by exploiting the inherent autofluorescence of erythrocytes provides a unique opportunity for assessing the functional status of a tumor's vascular tree. Representative fluorescence photomicrographs are shown in Figure 4. Microscopic evaluation of tumors overexpressing TIMP-3 revealed a marked decrease (85% less) in the erythrocyte content relative to GFP controls (Figure 4I-J) (494 ± 178 vs 3336 ± 434 RBCs/mm2,P < .001). This marked decrease in the erythrocyte density within TIMP-3–expressing tumors reinforced the fact that functional neovascularization with subsequent tumor perfusion was not occurring effectively in those tumors overexpressing TIMP-3.
VEGF expression
Hypoxia is one of the strongest inducers of VEGF expression; high levels of VEGF within a tumor mass suggest that the cells are poorly perfused.38 39 Therefore, the level of VEGF expression in the TIMP-3–overexpressing tumors was determined and compared with control tumors to assess the adequacy of tumor perfusion. VEGF protein levels within TIMP-3-I-GFP– and GFP-expressing tumors were determined by enzyme-linked immunosorbent assay (ELISA) on whole-tumor protein homogenates from 22 different tumor samples. VEGF was found to be present in all tumors tested. The mean VEGF level within the NXS2-TIMP-3-I-GFP tumors (n = 9) was 1326 ± 202 pg/μg total protein (TP), whereas control NXS2-I-GFP tumors (n = 9) had a mean VEGF level of 857 ± 81 pg/μg TP (P < .05). This represented a 55% increase in the intratumoral VEGF levels within the TIMP-3–overexpressing tumors. Determination of VEGF levels for both NXS2-TIMP-3– or GFP-expressing cell lines in vitro revealed no significant difference (6.1 pg/106 cells per 48 hours vs 5.9 pg/106 cells per 48 hours, respectively), suggesting the differences observed in vivo were in response to the hypoxic state of the tumors. In the B16F10-TIMP-3-I-GFP tumors the VEGF levels were 3983 ± 1262 pg/μg TP, whereas control GFP tumors had a mean of 2256 ± 152 pg/μg TP, a 77% increase in intratumoral VEGF (P < .3).
VE-cadherin expression
VE-cadherin, an endothelial-specific adhesion molecule, is vital to cell-to-cell interaction and subsequent vascular morphogenesis.40,41 Since TIMP-3 had been found to inhibit tubule formation in vitro and capillary morphogenesis in vivo we chose to evaluate the effect of TIMP-3 on the expression of VE-cadherin by endothelial cells as this surface protein is known to be involved in endothelial cell-cell interactions.42 43 FACS analysis demonstrated a 30% reduction in mean VE-cadherin expression in HUVECs after in vitro exposure to conditioned media from NXS2-TIMP-3-I-GFP tumors relative to controls. This reduction was similar to that observed when culturing HUVECs with 50 ng/mL recombinant TIMP-3 (data not shown). Immunohistochemical analysis of VE-cadherin by endothelial cells within NXS2-TIMP-3-I-GFP tumors versus I-GFP controls (Figure 4G-H) demonstrated a 55% decrease in expression within the TIMP-3–expressing tumors. The mean number of cells expressing VE-cadherin per 400 × field in the NXS2-I-GFP control tumors was 22.8 ± 1.8 compared with only 10.3 ± 2.0 in the NXS2-TIMP-3-I-GFP tumors (P = .006). This decrease in expression of VE-cadherin further suggests that TIMP-3 does in fact mediate its effects through inhibition of functional vascular development, possibly by down-regulating endothelial cell adhesion molecules and ultimately inhibiting perfusion.
Discussion
Enforced expression of TIMPs by tumor cells has been shown to effect their malignant phenotype, generally resulting in suppression of local and metastatic tumor growth.16,17,19,44,45 The mechanism by which this was effected however, especially for TIMP-3, is less certain. Potential mechanisms have included the ability of TIMP-3 to induce tumor cell apoptosis directly,15,16 to inhibit tumor cell invasion through the surrounding basement membrane,15,16 or to inhibit angiogenesis.19Although the ability of TIMP-3 to alter endothelial cell phenotype has been demonstrated in vitro,17,19 confirmation that TIMP-3–mediated inhibition of tumor-induced angiogenesis occurs in vivo has not been clearly shown. TIMP-3, for example, has been shown in this study and by others to inhibit endothelial cell migration, invasion, and tubule formation in vitro.17 However, it is uncertain whether these in vitro properties can be extrapolated to suggest that TIMP-3 can effect in vivo inhibition of new blood vessel formation.46 There are 2 prior studies that evaluated the effect of TIMP-3 overexpression on in vivo tumor growth that did not examine the effect on tumor-induced angiogenesis specifically.17 19
The results from this study, however, clearly demonstrate the in vivo antiangiogenic effect of TIMP-3 overexpression. Grossly, the TIMP-3–overexpressing tumors were small, consistent with previous reports in which a tumor growth inhibitory effect of TIMP-3 overexpression on other tumor types was observed.17,19 In addition, in our model fewer “feeding” vessels were visible in the subcutaneous tissue surrounding the tumor mass, as compared with the numerous, large vessels investing the control tumors. Despite this grossly apparent antiangiogenic effect, however, immunohistochemical analysis of the tumors demonstrated an increase in the number of CD31+ endothelial cells within the TIMP-3–overexpressing tumors. This is in marked contrast to what has been observed recently with overexpression of TIMP-2. Several studies have shown that enforced expression of TIMP-2 results in in vivo tumor growth inhibition.44,45,47-49 However, 2 recent studies in which angiogenesis was specifically evaluated found that endothelial cell density was diminished, at least in part, due to down-regulation of VEGF expression.48,49 We have shown that the net effect of TIMP-3 overexpression is an inhibition of functional angiogenesis. Despite the greater number of endothelial cells within these tumors, these endothelial cells are not forming physiologic conduits. Decreased intratumoral red cell content confirms what was observed grossly, that there was significantly less blood coursing through these pale tumors despite the presence of abundant but dysfunctional endothelial cells. The observed increase in endothelial cell density despite successful angiogenesis inhibition could be a result of tumor cell “drop-out” or death exceeding endothelial cell “drop-out.”50
Further indirect confirmation of the inhibition of functional angiogenesis is the demonstration that high levels of VEGF were being expressed within the TIMP-3–overexpressing tumors. As hypoxia is one of the strongest signals for up-regulation of VEGF expression, this suggests the presence of significant tissue hypoxia within the tumor mass. This latter finding also provides an alternate explanation for the increased number of endothelial cells in the TIMP-3–overexpressing tumors. The high levels of VEGF likely stimulate increased endothelial cell proliferation, a process that is not inhibited by TIMP-3. This observation that endothelial cell density does not necessarily correlate with functional neovasculature has significant implications for the assessment of tumor angiogenesis, based simply on CD31 staining. Although most studies associate an increase in endothelial cell number with increased angiogenesis, our results suggest that a more functional evaluation may be required to more accurately assess the degree of neovascularization within a tumor. This emphasis on determining functional angiogenesis has also been suggested by others.37 51
Further evidence for the inhibition of functional angiogenesis by TIMP-3 is the finding that the TIMP-3–expressing tumors had a notable paucity of SMA-positive pericytes investing the endothelial cells within the growing tumors, suggesting that there was a failure of vessel maturation. Although, varying degrees of pericyte recruitment to the microvasculature have been observed among tumors of different histologies,36 the SMA staining of control tumors in this study suggests that vessels in both the neuroblastoma and melanoma tumors normally have significant pericyte covering. However, in the tumors overexpressing TIMP-3, there is very little pericyte investment of the endothelial cells. The reason for this lack of pericyte coverage is unclear. It has been shown that TIMP-3 can inhibit vascular smooth muscle cell chemotaxis and invasion, while also promoting vascular smooth muscle cell death by apoptosis through a prodeath domain located within its N-terminal 3 loops.31,52,53 Thus, perhaps, direct inhibition of smooth muscle cell activity by TIMP-3 has inhibited the appropriate support or signaling by these cells required to ensure vessel maturity. Alternatively, high levels of VEGF expression within the TIMP-3–overexpressing tumors may inhibit pericyte recruitment. VEGF is required to initiate the formation of immature vessels during angiogenesis. These are typically enlarged, thin-walled, and pericyte-poor vessels (“mother” vessels).54 These immature mother vessels are transient structures that often mature in response to a variety of cytokines with the formation of a new basement membrane and investment of these vessels with pericytes. As the tumor neovasculature develops and re-establishes oxygen delivery, VEGF levels decrease. This decline in VEGF then promotes smooth muscle–endothelial cell interactions with resultant vessel stabilization.33 Reports on the effect of persistent, high levels of VEGF on vasculogenesis differ. Some studies have shown that persistently elevated VEGF levels result in dysregulated vasculogenesis where recruited endothelial cells fail to integrate into functional, mature vessels,55-57 while others have reported that hypoxia-induced VEGF production leads to the recruitment of endothelial cells and pericytes, resulting in the formation of new microvessels.58 Thus, it is uncertain whether this lack of pericyte recruitment is the cause or a consequence of the elevated levels of VEGF. Nevertheless, the inability of the endothelial cells to form mature tubules, as reflected by a low “vessel maturity index” in the TIMP-3–overexpressing tumors supports the hypothesis that functional capillary morphogenesis in TIMP-3–overexpressing tumors is impaired and is consistent with the previous finding of Hellstrom et al that a lack of pericytes leads to abnormal vascular morphogenesis.59
Although overexpression of TIMP-3 in these tumor models clearly resulted in an inhibition of tumor-associated angiogenesis, the molecular mechanism by which TIMP-3 overexpression has inhibited angiogenesis in these tumor models is unclear. Unlike TIMP-2 overexpression, which also inhibits angiogenesis, TIMP-3 overexpression does not act through VEGF down-regulation. We and others have shown that TIMP-3 can directly inhibit in vitro tubule formation of endothelial cells.17,19 Thus the absence of functional tubules within the TIMP-3–overexpressing tumors provides excellent correlation between the in vitro and in vivo activity of TIMP-3. VE-cadherin has been shown to play a major role in endothelial cell migration, tubule formation, and maintenance of vascular integrity.40-42 The decrease seen in VE-cadherin expression found on endothelial cells in the presence of elevated levels of TIMP-3 supports the concept that these tumors fail to maintain vascular integrity. In fact, these data suggest that TIMP-3 may mediate its effects through the down-regulation of this cell adhesion molecule. Thus, although both TIMP-2 and TIMP-3 inhibit tumor-associated angiogenesis, they appear to do so through distinctly different mechanisms. It is unclear, however, whether this is a direct effect of TIMP-3 or is an indirect effect, perhaps resulting from MMP inhibition.
Designing effective antiangiogenic strategies for cancer therapy will require further understanding of the factors involved in a tumor-induced angiogenic response. Although tumor growth was slowed by overexpression of TIMP-3, eventually tumors did grow to a size where the mice had to be killed. Documented TIMP-3 and GFP expression at the time of tumor harvest confirmed that this was not due to the loss of transgene expression. Instead, it is likely that redundant angiogenic pathways permit continued tumor neovascularization and subsequent tumor growth. This suggests that effective inhibition of tumor growth by restricting neovascularization will require multiple agents acting on different pathways to completely disrupt the process of angiogenesis.
We would like to thank Dorothy Bush, Bonnie Greer, and Adriana Nance for their assistance with immunohistochemistry; Dr Gopal Murti and Ken Barnes for their assistance with fluorescent confocal microscopy; and Dr Richard Ashmun for his assistance with the FACS analysis. We would also like to thank Drs Brian Sorrentino, Arthur Nienhuis, and Stephen Shochat for their critical review of this manuscript.
Supported by grants from the Assisi Foundation of Memphis 94-000, grant no. IRG-87-008-09 from the American Cancer Society, Cancer Center Support CORE grant P30 CA 21765, and American Lebanese Syrian Associated Charities (ALSAC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew M. Davidoff, Department of Surgery, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105; e-mail: andrew.davidoff@stjude.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal