Abstract
We previously observed the presence of anti-human μ-opioid-receptor (anti-hMOR) autoantibodies in IgG pools prepared from several thousand healthy blood donors. These autoantibodies behaved agonistically because of their ability to bind to the first and third extracellular loops of the receptor. In this study, we found that each healthy donor's serum contained anti-hMOR IgG autoantibodies with a specific activity against both the first and the third extracellular loops of the receptor. Because of the inability of IgG to cross the blood-brain barrier, we investigated the effects of the expression of anti-hMOR autoantibodies on immune cells. In analogy to studies of the effects of morphine, we investigated the ability of antibodies to sensitize splenocytes to Fas (CD95)-mediated apoptosis. We took advantage of the high sequence homology between murine MOR and hMOR extracellular loops to estimate the effect on murine splenocytes of anti-hMOR antibodies raised by immunizing mice. Splenocytes from mice injected with Chinese hamster ovary (CHO) cells expressing MOR were sensitized to Fas-mediated apoptosis, whereas those from mice injected with CHO cells or phosphate-buffered saline were not. Similar sensitization to Fas-mediated apoptosis was observed in splenocytes from mice undergoing passive transfer either with IgG from mice previously immunized against CHO cells expressing MOR or with IgG directed against the first and third extracellular loops of the receptor. Together, our data show that anti-MOR autoantibodies are commonly expressed in healthy humans and could participate in the control of lymphocyte homeostasis by promoting Fas-mediated apoptosis.
Introduction
Within an integrated organization, the immune system accommodates a variety of signals originating from the neuroendocrine system1; the immunomodulatory potency of hormones and neuropeptides has been described extensively.2 In turn, cytokines produced mainly by immune cells modify the neuronal activity, as exemplified by the effect of interleukin 1 (IL-1) on the central nervous system.3 These communication pathways have been highlighted by the characterization on immune and nervous cells of common receptors for cytokines, hormones, and neuropeptides.4,5 The interactions between the nervous and immune systems are indicated by the ability of immune cells and neurons to synthesize cytokines, neuropeptides, and hormones.6,7 It was reported that lymphocytes infiltrating inflamed tissues released β-endorphin, which produced analgesia by acting on opioid receptors (7-transmembrane segment, Gi/o-protein–coupled receptors) expressed on peripheral afferent nerves.8 Opioids constitute a good model for a neuroimmunologic relationship because their effects are not restricted to the nervous system but extend to the immune system. Some immunologic functions, such as natural killer activity, lymphocyte proliferation, macrophage motility, and secretion of proinflammatory cytokines, are reduced by opioids, including morphine.9-13 In vivo, the effects of morphine are mainly mediated by the μ opioid receptor (MOR).10,14 Using mice injected with morphine or subjected to stressful conditions, Yin et al demonstrated that opioids promoted Fas-mediated lymphocyte apoptosis in vivo.15 16
Under normal conditions, a large part of circulating immunoglobulins are autoreactive. Antibody activities against soluble, membrane, and intracellular self-antigens have been observed in healthy humans and animals.17 Among the several physiologic roles attributed to these “natural antibodies,” only their contribution to organism protection as the first line of defense against pathogens has been clearly established.18,19 An immunoregulatory role for natural autoantibodies has been proposed on the basis of a description of their ability to prevent ligand-receptor binding involved in immune processes by neutralizing ligands or receptors.20 21
We previously showed that antibodies directed against the human MOR (hMOR) were present in pools of normal human IgG. These anti-hMOR IgG autoantibodies displayed a specific agonistic activity because of their simultaneous binding on the first and third extracellular loops of the receptor.22 23 In this study, anti-hMOR IgG autoantibodies directed toward both the first and third extracellular loops of the receptor were found in serum samples from all healthy blood donors examined. We took advantage of the high sequence homology between murine MOR and hMOR extracellular loops to assess the potency of anti-hMOR IgG to sensitize immune cells to Fas (CD95)-mediated apoptosis in mice. Immunization against the hMOR was performed by injecting mice with recombinant cells in the absence of adjuvant. Hypodiploid DNA fluorescence was used to assess apoptosis levels. Spleen cells from mice immunized against recombinant hMOR-expressing Chinese hamster ovary (hMOR/CHO) cells were sensitized to Fas-mediated apoptosis, whereas those from mice injected with either phosphate-buffered saline (PBS) or untransfected CHO cells were not. Sensitization to Fas-mediated apoptosis in mice injected with recombinant hMOR/CHO cells was associated with a reduced number of splenocytes in those mice compared with control mice injected with CHO cells. Furthermore, splenocytes from mice that underwent passive transfer with either IgG from mice immunized against hMOR/CHO cells or IgG directed against the first and third extracellular loops of the hMOR were sensitized to Fas-mediated apoptosis. Together, our data show that, under normal conditions, anti-hMOR IgG autoantibodies are expressed in human serum and could participate in the control of lymphocyte proliferation by promoting Fas-mediated apoptosis.
Materials and methods
Source of human IgG
Venous blood was collected from 22 healthy donors without recent pathologic events and with no history of drug abuse. Donors had not recently undergone surgery or treatment with antibiotic or anti-inflammatory agents. The study was approved by the institutional review board of the Institut National de la Sante et de la Recherche Medicale (INSERM). Informed consent was provided according to the Declaration of Helsinki. IgG fractions were prepared from serum by affinity chromatography on protein G columns (Pharmacia Fine Chemicals, Uppsala, Sweden). A pool of IgG prepared from plasma samples from 15 000 healthy donors was also used as a source of human IgG (Sandoglobulin; Novartis, Basel, Switzerland). F(ab′)2fragments were prepared from IgG by pepsin digestion (2% wt/wt; Sigma, St Louis, MO) in 0.2 M sodium acetate (pH 4.1) at 37°C for 18 hours. F(ab′)2 fragments were free of intact IgG as assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
Purification of human anti-hMOR antibodies
Antibodies directed against the hMOR were affinity purified on a recombinant hMOR/CHO cell clone as described previously.22Briefly, IgG or F(ab′)2 fragments were incubated with confluent hMOR/CHO cells for 1 hour at 37°C. After washing, bound IgG or F(ab′)2 antibodies were eluted by using 0.1 M sodium citrate (pH 3.5) and recovered by purification on affinity columns with either protein G– or Sepharose-bound sheep anti-human IgG F(ab′)2–specific antibodies (Valbiotech, Paris, France). To remove antibody reactivity toward CHO-K1 cells, a subsequent incubation with untransfected CHO-K1 cells was performed and followed by the appropriate chromatographic analysis. Affinity-purified anti-hMOR IgG or F(ab′)2 antibodies were free of contaminant proteins as assessed by comparing SDS-PAGE immunoblots and goat anti-human IgG F(ab′)2–specific antibody or goat anti-human Fcγ antibody (Jackson Immunoresearch Laboratories, West Grove, PA) immunoblots. Antibodies reactive with nontransfected CHO-K1 cells were prepared from F(ab′)2 fragments as described above. Anti-laminin IgG and anti-laminin F(ab′)2 were purified from a pool of IgG from healthy humans by affinity chromatography on a column of Sepharose-bound laminin, as described previously.24
Cytofluorometric analysis of anti-hMOR antibody activity
Recombinant hMOR/CHO cells and untransfected CHO-K1 cells (106) were incubated with either IgG or F(ab′)2fragments for 45 minutes at 4°C. After washing, the cells were stained with biotin-labeled, goat anti-human IgG F(ab′)2–specific antibodies (Jackson Immunoresearch Laboratories). This was followed by an additional incubation with phycoerythrin (PE)-labeled streptavidin (PharMingen, San Diego, CA).
For inhibition experiments, 2 peptides, corresponding to hMOR extracellular loop 1 (NYLMGTWPFGTILCK [hMOR EL1]) and loop 3 (KALVTIPETTFQT [hMOR EL3]),25 and the encephalitogenic peptide (GSLPQKSQRSQDENPVV), corresponding to amino acid residues 70 to 86 of the guinea pig myelin basic protein,26 were used. Antibodies were incubated with 20 μg/mL peptide for 1 hour at 37°C before being added to the cells for 45 minutes at 4°C. Residual antibody binding was then examined.
Cytofluorometric analysis of spleen cell subpopulations
After lysis of red blood cells with ammonium chloride (150 mM), potassium carbonate (10 mM), and EDTA (ethylenediaminetetraacetic acid; 0.1 mM), spleen cells were incubated with optimal concentrations of fluorescein isothiocyanate–conjugated, PE-conjugated, or biotin-conjugated monoclonal antibody (mAb) for 30 minutes at 4°C in PBS containing 1% fetal-calf serum (FCS), 5 mM EDTA, and 0.1% sodium azide. The following mAbs were used: FITC-GK1.5 anti-CD4 (TIB 207; American Type Culture Collection [ATCC], Rockville, MD), FITC-KT1.5 anti-CD8 (PharMingen), FITC-M1/70 anti-Mac1 (TIB 128; ATCC), FITC- or biotin-34.1.2S anti-Kd (HB79; ATCC), PE-Jo2 anti-CD95 (PharMingen), PE-Ha4/8 anti–keyhole-limpet hemocyanin (KLH; PharMingen), and biotin-RA3-3A1 anti-B220 (TIB146; ATCC). Spleen cells were then washed and stained with streptavidin-CyChrome (PharMingen). Data were collected on 10 000 living cells by observing forward- and side-scatter intensity on an EPICS XL flow cytometer (Beckman Coulter, Hialeah, FL) and were subsequently analyzed by using CellQuest software (Becton Dickinson, Mountain View, CA).
Immunization of mice
All experiments were conducted in conformity with guiding principles for the care and use of laboratory animals. Eight-week-old female BALB/c mice were purchased from Janvier (Le Genest Saint Isle, France). The mice were injected intraperitoneally with either PBS or 15 × 106 CHO cells or the hMOR/CHO cell clone every 2 weeks. Three days after the ninth injection, blood was collected and mice were killed to obtain spleens.
Anti-hMOR EL1 and anti-hMOR EL3 IgG antibodies were raised by immunizing BALB/c mice with either hMOR EL1 peptide or hMOR EL3 peptide in the presence of methylated bovine serum albumin emulsified in complete Freund adjuvant (Difco, Detroit, MI). Subsequent immunizations were performed with incomplete Freund adjuvant.
Determination of anti-hMOR EL IgG antibody activity
Enzyme-linked immunosorbent assay plates were coated with 10 μg/mL of either hMOR EL1 or hMOR EL3 peptide in PBS overnight at 4°C. Uncoated sites were saturated with PBS containing 1% gelatin for 90 minutes at 37°C. Plates were washed and then incubated for 60 minutes at 37°C with antibody samples. Bound antibodies were revealed by using biotin-labeled, goat anti-mouse Fcγ–specific antibodies (Jackson Immunoresearch Laboratories) and peroxidase-labeled streptavidin (Amersham, Slough, United Kingdom). For inhibition experiments, IgG antibodies were incubated with serial dilutions of peptides for 60 minutes at 37°C and residual IgG binding was measured.
IgG transfers
IgG purified from normal and immunized mice was injected intravenously into naive BALB/c mice. The mice were killed 24 hours later for analysis of splenocytes.
Induction of Fas (CD95)-mediated apoptosis
Murine splenocytes were prepared by crushing the spleen. The splenocytes were washed and enumerated by using trypan blue dye exclusion. Red blood cell lysis was performed, and 5 × 105 spleen cells were incubated in RPMI 1640 supplemented with FCS alone or containing 5 μg/mL of either hamster anti-mouse Fas (CD95) IgG mAb (Jo2 clone; PharMingen) or hamster anti-KLH IgG mAb (Ha4/8 clone; PharMingen) for 24 hours at 37°C. To better discern a potential lymphocyte sensitization to Fas-mediated apoptosis, we used a suboptimal concentration (5 μg/mL) of agonistic anti-Fas antibodies that did not induce apoptosis of splenocytes from normal and antigen-primed mice. Splenocytes were harvested, washed, and resuspended in 500 μL hypotonic fluorochrome solution (50 μg/mL propidium iodide, 0.1% sodium citrate, and 0.1% Triton X-100) overnight at 4°C. The DNA content of nuclei was examined by analyzing red fluorescence. The percentage of apoptotic cells was signified by the extent of the subdiploid DNA peak.
Results
Anti-hMOR IgG autoantibodies with a potential agonistic activity are commonly present in serum from healthy blood donors
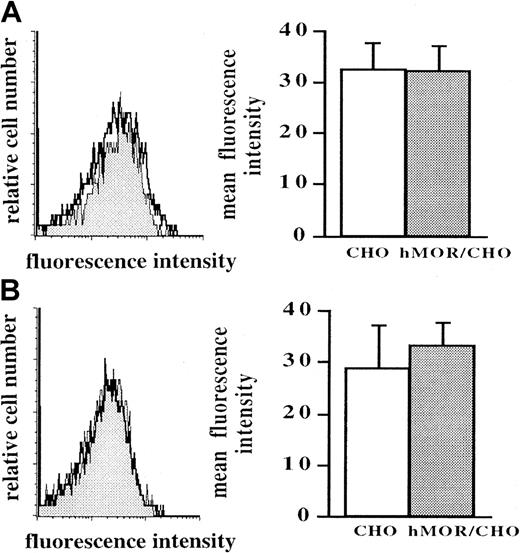
Serum samples from 10 healthy women (22-52-years old; mean age, 31 years) and 12 age-matched healthy men (22-53-years old; mean age, 39 years) were examined for the presence of IgG directed against the hMOR. IgG from each healthy donor was prepared from serum by protein G affinity chromatography. The whole IgG fraction from each donor and control IgG (IgG pool, anti-laminin IgG, and anti–CHO-K1 IgG) showed a similar binding activity toward hMOR/CHO cells and untransfected CHO-K1 cells, as assessed by cytofluorometry (Figure1A). Affinity purification from total IgG was necessary for observation of anti-hMOR antibodies. IgG samples from all donors were subjected to a purification procedure that included isolation of IgG directed against the recombinant hMOR/CHO cell clone, followed by depletion of IgG that reacted with untransfected CHO-K1 cells.22 As shown in Figure 1B, serum IgG from all healthy donors (men and women) contained autoantibodies directed against the hMOR. Mainly because of samples from 2 donors, a greater, though not significantly greater, IgG binding activity to hMOR was observed in men.
Cytofluorometric assessment for the presence of anti-hMOR IgG antibody in 10 healthy women and 12 healthy men.
The presence of anti-hMOR IgG in 22 healthy humans was examined by cytofluorometry after affinity purification of serum IgG on the hMOR/CHO cell clone and depletion of nonspecific antibodies on untransfected CHO cells. Recombinant hMOR/CHO cells and untransfected CHO-K1 cells (106) were incubated with serial dilutions of IgG samples for 45 minutes at 4°C. Bound antibodies were revealed with biotin-labeled, goat anti-human IgG F(ab′)2–specific antibodies and PE-labeled streptavidin. Panel A depicts the binding (filled histogram) to recombinant hMOR/CHO cells (upper panel) and to untransfected CHO-K1 cells (lower panel) of unpurified IgG (i), affinity-purified anti-laminin IgG (ii), affinity-purified anti–CHO-K1 IgG (iii), and affinity-purified anti-hMOR IgG (iv) from a large pool of human IgG at a concentration of 20 μg/mL. A representative binding activity of affinity-purified anti-hMOR IgG from a woman (v) and a man (vi) is shown. The background (open histogram) was cells stained with labeled goat anti-human IgG F(ab′)2–specific antibodies and PE-labeled streptavidin alone. In panel B, data are expressed as mean fluorescence intensity (MFI) obtained from IgG staining with hMOR/CHO cells minus MFI obtained from IgG staining with untransfected CHO-K1 cells. Backgrounds with untransfected and hMOR-transfected CHO-K1 cell clones were similar. The histogram shows, for each donor IgG sample (░), the value obtained with a dilution at which CHO-K1 cell staining was similar to the background. Controls were the specific antibody-binding activity of unpurified IgG (▪), affinity-purified anti-laminin IgG (▤), affinity-purified anti-CHO IgG (■), and affinity-purified anti-hMOR IgG (▨) prepared from a pool of human IgG.
Cytofluorometric assessment for the presence of anti-hMOR IgG antibody in 10 healthy women and 12 healthy men.
The presence of anti-hMOR IgG in 22 healthy humans was examined by cytofluorometry after affinity purification of serum IgG on the hMOR/CHO cell clone and depletion of nonspecific antibodies on untransfected CHO cells. Recombinant hMOR/CHO cells and untransfected CHO-K1 cells (106) were incubated with serial dilutions of IgG samples for 45 minutes at 4°C. Bound antibodies were revealed with biotin-labeled, goat anti-human IgG F(ab′)2–specific antibodies and PE-labeled streptavidin. Panel A depicts the binding (filled histogram) to recombinant hMOR/CHO cells (upper panel) and to untransfected CHO-K1 cells (lower panel) of unpurified IgG (i), affinity-purified anti-laminin IgG (ii), affinity-purified anti–CHO-K1 IgG (iii), and affinity-purified anti-hMOR IgG (iv) from a large pool of human IgG at a concentration of 20 μg/mL. A representative binding activity of affinity-purified anti-hMOR IgG from a woman (v) and a man (vi) is shown. The background (open histogram) was cells stained with labeled goat anti-human IgG F(ab′)2–specific antibodies and PE-labeled streptavidin alone. In panel B, data are expressed as mean fluorescence intensity (MFI) obtained from IgG staining with hMOR/CHO cells minus MFI obtained from IgG staining with untransfected CHO-K1 cells. Backgrounds with untransfected and hMOR-transfected CHO-K1 cell clones were similar. The histogram shows, for each donor IgG sample (░), the value obtained with a dilution at which CHO-K1 cell staining was similar to the background. Controls were the specific antibody-binding activity of unpurified IgG (▪), affinity-purified anti-laminin IgG (▤), affinity-purified anti-CHO IgG (■), and affinity-purified anti-hMOR IgG (▨) prepared from a pool of human IgG.
The hMOR, which is a 7-transmembrane domain receptor, has 4 extracellular segments (1 NH2-terminal segment and 3 extracellular loops) accessible for IgG recognition. Thus, IgG binding to hMOR did not indicate the structures of the receptor recognized and consequently the functional properties of antibodies. Because the morphinelike activity of anti-hMOR antibodies purified from a pool of IgG from healthy humans depends on recognition of the first and third extracellular loops of the hMOR,22 23 we assessed the presence of antibodies directed toward these 2 extracellular segments in anti-hMOR F(ab′)2 fragment preparations. The binding to hMOR/CHO cells of anti-hMOR F(ab′)2 antibodies prepared from a large pool of samples from healthy donors was inhibited by peptides corresponding to the putative sequences of the first and third extracellular loops of the hMOR but not by an irrelevant size-matched peptide (Figure2A). Similar results were obtained with anti-hMOR F(ab′)2 fragments prepared from 2 randomly chosen samples from individual donors (one man and one woman; Figure 2B and2C). In contrast, none of the peptides neutralized the binding of affinity-purified anti-CHO F(ab′)2 fragments to recombinant hMOR/CHO cells (Figure 2D).
Assessment of anti-hMOR F(ab′)2 specificity by cytofluorometry.
Affinity-purified anti-hMOR F(ab′)2 fragments were preincubated with either hMOR EL1 peptide, the hMOR EL3 peptide, or amino acid residues 70 to 86 of the guinea pig myelin basic protein (70-86 MBP) peptide for 1 hour at 37°C. Recombinant hMOR/CHO cells were incubated with 100 μL of the mixture for 45 minutes at 4°C. Bound antibodies were revealed by using biotin-labeled, goat anti-human IgG F(ab′)2–specific antibodies and PE-labeled streptavidin. Shown is the binding to the recombinant hMOR/CHO cell clone of 10 μg/mL affinity-purified anti-hMOR F(ab′)2fragments (thick black line) from either a large pool of samples from healthy donors (A), a sample from one healthy woman (B), or a sample from one healthy man (C) in the absence or presence of 20 μg/mL peptide. The binding activity to recombinant hMOR/CHO cell clones of 5 μg/mL affinity-purified anti–CHO-K1 F(ab′)2 fragments (thick black line) in the absence or presence of 20 μg/mL peptide is shown (D). The background (thin gray line) was the binding activity of anti-hMOR antibodies to untransfected CHO-K1 cells, which was superimposable to the staining of recombinant hMOR/CHO cells obtained by using either anti-laminin F(ab′)2 fragments or labeled goat anti-human IgG F(ab′)2–specific antibodies and PE-labeled streptavidin alone. One representative experiment of 3 is shown.
Assessment of anti-hMOR F(ab′)2 specificity by cytofluorometry.
Affinity-purified anti-hMOR F(ab′)2 fragments were preincubated with either hMOR EL1 peptide, the hMOR EL3 peptide, or amino acid residues 70 to 86 of the guinea pig myelin basic protein (70-86 MBP) peptide for 1 hour at 37°C. Recombinant hMOR/CHO cells were incubated with 100 μL of the mixture for 45 minutes at 4°C. Bound antibodies were revealed by using biotin-labeled, goat anti-human IgG F(ab′)2–specific antibodies and PE-labeled streptavidin. Shown is the binding to the recombinant hMOR/CHO cell clone of 10 μg/mL affinity-purified anti-hMOR F(ab′)2fragments (thick black line) from either a large pool of samples from healthy donors (A), a sample from one healthy woman (B), or a sample from one healthy man (C) in the absence or presence of 20 μg/mL peptide. The binding activity to recombinant hMOR/CHO cell clones of 5 μg/mL affinity-purified anti–CHO-K1 F(ab′)2 fragments (thick black line) in the absence or presence of 20 μg/mL peptide is shown (D). The background (thin gray line) was the binding activity of anti-hMOR antibodies to untransfected CHO-K1 cells, which was superimposable to the staining of recombinant hMOR/CHO cells obtained by using either anti-laminin F(ab′)2 fragments or labeled goat anti-human IgG F(ab′)2–specific antibodies and PE-labeled streptavidin alone. One representative experiment of 3 is shown.
We then assessed the anti-hMOR antibody specificity in IgG samples from 7 women and 7 men. As shown in Figure 3, each donor's anti-hMOR IgG preparation contained antibodies directed against the first and third extracellular loops. These loops were previously identified as targets for activation of the receptor.22 Together, these data show that anti-hMOR antibodies with a potential agonistic activity are commonly present in serum from healthy blood donors.
Inhibition of anti-hMOR antibody binding to hMOR by hMOR EL peptides.
Inhibition of anti-hMOR antibody binding to hMOR by hMOR EL peptides.
Anti-hMOR IgG sensitizes splenocytes to Fas (CD95)-mediated apoptosis
Previous studies demonstrated that both exogenous opioids (morphine) and endogenous opioids promoted Fas (CD95)-mediated lymphocyte apoptosis.15,16 In analogy to these studies, we investigated the ability of anti-hMOR autoantibodies to generate similar effects. For this investigation, we developed a model in which anti-hMOR antibodies were raised by immunizing mice. The proapoptotic effect of induced anti-hMOR (auto) antibodies could be studied in murine splenocytes because IgG-induced MOR activation depends on antibody binding to the first and third extracellular loops,23 which show 93% and 92% amino acid identity, respectively, between humans and mice. In addition, we previously showed that amino acids in the hMOR constituting epitopes recognized by rat agonistic antibodies are conserved in the murine receptor.27 Our immunizing protocol to produce agonistic anti-hMOR antibodies was based on induction of experimental autoimmune Graves disease in BALB/c mice by generating agonistic anti-thyrotropin-receptor antibodies.28 In vitro, Fas-mediated apoptosis was examined on splenocytes from BALB/c mice injected with either PBS, CHO cells, or hMOR/CHO cells. Splenocytes from mice injected with recombinant hMOR/CHO cells were more susceptible to anti-Fas antibody–triggered apoptosis than those from mice injected with PBS or CHO cells (P < .01 andP < .05, respectively, on one-way analysis of variance; Figure 4A and 4B).
Splenocytes from mice immunized against hMOR are sensitized to CD95-mediated apoptosis.
BALB/c mice were injected intraperitoneally with either PBS (n = 8), CHO cells (n = 10), or recombinant hMOR/CHO cells (n = 10). Mice were injected 9 times, with a 2-week interval between each injection. Panel A shows, for one representative mouse from each group, the DNA content of nuclei of splenocytes incubated for 24 hours in the absence (i) or presence of 5 μg/mL of either hamster anti-KLH IgG mAb (clone Ha4/8) (ii) or hamster anti-CD95 IgG mAb (clone Jo2) (iii). DNA content of nuclei was analyzed by cytofluorometry after incubation of the cells in hypotonic medium containing propidium iodide. Each experiment was performed at least in duplicate. The percentage of subdiploid DNA content is indicated. The arrow indicates nuclei with subdiploid DNA content typical of apoptosis. Panel B depicts the anti-Fas antibody–induced apoptosis of splenocytes from each mouse injected with PBS (○), CHO cells (▵), or hMOR/ CHO cells (■). Basal apoptosis of splenocytes in the absence or presence of hamster anti-KLH IgG mAb was 7% ± 3%. One-way analysis of variance was used for statistical analysis.
Splenocytes from mice immunized against hMOR are sensitized to CD95-mediated apoptosis.
BALB/c mice were injected intraperitoneally with either PBS (n = 8), CHO cells (n = 10), or recombinant hMOR/CHO cells (n = 10). Mice were injected 9 times, with a 2-week interval between each injection. Panel A shows, for one representative mouse from each group, the DNA content of nuclei of splenocytes incubated for 24 hours in the absence (i) or presence of 5 μg/mL of either hamster anti-KLH IgG mAb (clone Ha4/8) (ii) or hamster anti-CD95 IgG mAb (clone Jo2) (iii). DNA content of nuclei was analyzed by cytofluorometry after incubation of the cells in hypotonic medium containing propidium iodide. Each experiment was performed at least in duplicate. The percentage of subdiploid DNA content is indicated. The arrow indicates nuclei with subdiploid DNA content typical of apoptosis. Panel B depicts the anti-Fas antibody–induced apoptosis of splenocytes from each mouse injected with PBS (○), CHO cells (▵), or hMOR/ CHO cells (■). Basal apoptosis of splenocytes in the absence or presence of hamster anti-KLH IgG mAb was 7% ± 3%. One-way analysis of variance was used for statistical analysis.
Anti-CHO IgG antibody activity in serum from mice injected with CHO cells and mice injected with recombinant hMOR/CHO cells was similar, as assessed by cytofluorometry (data not shown). The number of spleen cells was increased in mice injected with CHO cells compared with those injected with PBS (P < .01 on Mann-Whitney U test; Figure5A). Spleen cellularity in mice injected with CHO cells was also significantly greater than that in mice injected with recombinant hMOR/CHO cells, whose splenocytes were sensitized to Fas-mediated apoptosis (P < .05 on Mann-Whitney U test; Figure 5A). The percentage of cells negative for H-2 Kd in the spleen was similar in mice injected with CHO cells and those injected with hMOR/CHO cells (5% ± 3% versus 6% ± 3%), indicating that the high number of spleen cells in CHO-injected mice did not result from infiltrating CHO cells. The relative number of CD4 and CD8 T lymphocytes, B lymphocytes, and monocytes was similar in each experimental group of mice (Figure 5B). The number of thymocytes was not modified by the immunization procedures (Figure 5C). The sensitization of splenocytes to Fas-mediated apoptosis observed in hMOR/CHO-injected mice was not associated with an increase in Fas antigens on the surface of CD4 or CD8 T lymphocytes, as assessed by cytofluorometry (Figure6).
Spleen but not thymus cellularity in mice injected with recombinant hMOR/CHO cells is reduced compared with that in mice injected with CHO cells.
Mice injected with recombinant hMOR/CHO cells in which splenocytes were not sensitized to Fas-mediated apoptosis were excluded from the analysis. Panel A shows results when spleen cells from BALB/c mice injected with either PBS (n = 6; ○), CHO cells (n = 7; ▵), or recombinant hMOR/CHO cells (n = 4; ■) were enumerated by using trypan blue dye exclusion. Panel B shows the relative distribution of B lymphocytes (■), CD4 (▨) and CD8 (░) T cells, and monocytes (▪) in the spleen. Panel C depicts enumeration of thymic cells for each group. In panels A and C, results are expressed as means ± SD. Statistical analysis was performed by using the Mann-Whitney U test.
Spleen but not thymus cellularity in mice injected with recombinant hMOR/CHO cells is reduced compared with that in mice injected with CHO cells.
Mice injected with recombinant hMOR/CHO cells in which splenocytes were not sensitized to Fas-mediated apoptosis were excluded from the analysis. Panel A shows results when spleen cells from BALB/c mice injected with either PBS (n = 6; ○), CHO cells (n = 7; ▵), or recombinant hMOR/CHO cells (n = 4; ■) were enumerated by using trypan blue dye exclusion. Panel B shows the relative distribution of B lymphocytes (■), CD4 (▨) and CD8 (░) T cells, and monocytes (▪) in the spleen. Panel C depicts enumeration of thymic cells for each group. In panels A and C, results are expressed as means ± SD. Statistical analysis was performed by using the Mann-Whitney U test.
Cytofluorometric analysis of Fas-receptor expression on spleen CD4 and CD8 lymphocytes from mice injected with CHO or hMOR/CHO cells.
Mice injected with recombinant hMOR/CHO cells in which splenocytes were not sensitized to Fas-mediated apoptosis were excluded from the analysis. Spleen cells from BALB/c mice injected with either untransfected CHO cells or recombinant hMOR/CHO cells were stained by using FITC-labeled anti-CD4 or anti-CD8 antibodies together with either PE-conjugated anti-CD95 or anti-KLH antibodies. Data were analyzed for Fas expression gated on either CD4 (A) or CD8 (B) T lymphocytes. The panel on the left shows a representative Fas staining on CD4 (top) and CD8 (bottom) T lymphocytes from CHO-injected mice (open histogram) and hMOR/CHO-injected mice (filled histogram). Histograms in the right panel show, for CHO-injected mice (n = 5; ■) and hMOR/CHO-injected mice (n = 3; ░), the corresponding means ± SD of the MFI.
Cytofluorometric analysis of Fas-receptor expression on spleen CD4 and CD8 lymphocytes from mice injected with CHO or hMOR/CHO cells.
Mice injected with recombinant hMOR/CHO cells in which splenocytes were not sensitized to Fas-mediated apoptosis were excluded from the analysis. Spleen cells from BALB/c mice injected with either untransfected CHO cells or recombinant hMOR/CHO cells were stained by using FITC-labeled anti-CD4 or anti-CD8 antibodies together with either PE-conjugated anti-CD95 or anti-KLH antibodies. Data were analyzed for Fas expression gated on either CD4 (A) or CD8 (B) T lymphocytes. The panel on the left shows a representative Fas staining on CD4 (top) and CD8 (bottom) T lymphocytes from CHO-injected mice (open histogram) and hMOR/CHO-injected mice (filled histogram). Histograms in the right panel show, for CHO-injected mice (n = 5; ■) and hMOR/CHO-injected mice (n = 3; ░), the corresponding means ± SD of the MFI.
These data suggesting that immunization of mice against the hMOR generated agonistic opioidlike antibodies responsible for an increased sensitivity of lymphocytes to CD95 (Fas)-mediated apoptosis were strengthened by IgG passive-transfer experiments. Splenocytes from mice injected intravenously with IgG purified from pooled serum from mice immunized against hMOR/CHO that showed splenocyte sensitization to apoptosis in vitro were more susceptible to anti-Fas antibody–mediated apoptosis than splenocytes from mice given IgG from PBS- or CHO-injected mice (Figure 7).
Splenocytes from mice that underwent passive transfer with IgG from hMOR/CHO-injected mice are sensitized to CD95-mediated apoptosis.
Purified serum IgG (100 μg) from mice injected with either PBS (○), CHO cells (▵), or recombinant hMOR/CHO cells (■) were transferred by intravenous injection into naive BALB/c mice. Twenty-four hours later, the mice given IgG injections were killed. Shown is the percentage of subdiploid nuclei when splenocytes were incubated in the presence of anti-Fas IgG mAb Jo2 for 24 hours. Nuclei DNA peak was analyzed by cytofluorometry after incubation of the cells in hypotonic medium containing propidium iodide. Each experiment was performed in duplicate. Basal apoptosis of splenocytes in the absence or presence of hamster anti-KLH IgG mAb was 8% ± 2%.
Splenocytes from mice that underwent passive transfer with IgG from hMOR/CHO-injected mice are sensitized to CD95-mediated apoptosis.
Purified serum IgG (100 μg) from mice injected with either PBS (○), CHO cells (▵), or recombinant hMOR/CHO cells (■) were transferred by intravenous injection into naive BALB/c mice. Twenty-four hours later, the mice given IgG injections were killed. Shown is the percentage of subdiploid nuclei when splenocytes were incubated in the presence of anti-Fas IgG mAb Jo2 for 24 hours. Nuclei DNA peak was analyzed by cytofluorometry after incubation of the cells in hypotonic medium containing propidium iodide. Each experiment was performed in duplicate. Basal apoptosis of splenocytes in the absence or presence of hamster anti-KLH IgG mAb was 8% ± 2%.
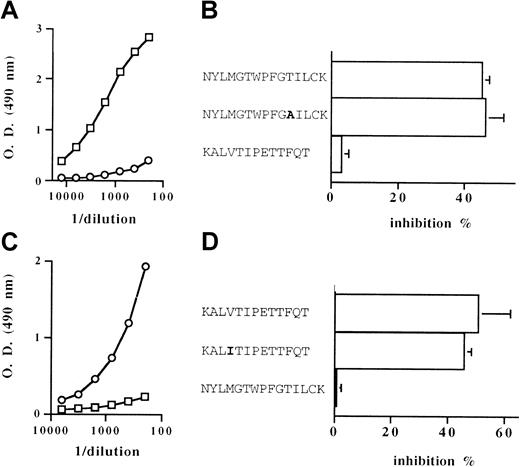
The proapoptotic effect of IgG antibodies directed against the first and third extracellular loops of the hMOR was then investigated. BALB/c mice were immunized against peptides corresponding to the amino acid sequences of either the first (hMOR EL1) or third (hMOR EL3) extracellular loop of the receptor. The IgG binding activity measured in serum from immunized mice was specific to the peptide used as the immunogen. Anti-hMOR EL1 IgG bound to the insoluble hMOR EL1 peptide but not the hMOR EL3 peptide. Reciprocally, anti-hMOR EL3 IgG bound to the hMOR EL3 peptide but not the hMOR EL1 peptide (Figure8A,C). The ability of anti-hMOR EL1 IgG and anti-hMOR EL3 IgG to recognize the human as well as the murine MOR extracellular loop was demonstrated by inhibiting the specific IgG binding with soluble peptides corresponding to human and murine sequences. As shown in Figure 8B, the anti-hMOR EL1 antibody activity was equally neutralized by the hMOR EL1 peptide and a substitute of the murine MOR EL1 amino acid sequence. Peptides corresponding to the human and murine MOR EL3 amino acid sequences inhibited the binding of anti-hMOR EL3 IgG to hMOR EL3 to a similar level (Figure 8D). The indication of an absence of cross-reactivity of anti-hMOR EL1 IgG toward hMOR EL3 and of anti-hMOR EL3 IgG toward hMOR EL1 was supported by the inability of hMOR EL3 peptide and hMOR EL1 peptide to neutralize the binding activity of anti-hMOR EL1 IgG and anti-hMOR EL3 IgG, respectively (Figure 8B,D).
Binding activity of anti-hMOR EL1 IgG and anti-hMOR EL3 IgG raised in mice.
The IgG binding activity toward the hMOR EL1 peptide (A) and the hMOR EL3 peptide (C) in pooled serum from mice immunized against the hMOR EL1 peptide (■) and pooled serum from mice immunized against the hMOR EL3 peptide (○) was estimated by enzyme-linked immunosorbent assay. A representative result from 3 experiments performed in triplicate is shown. The panels on the right show the inhibition of the binding activity of anti-hMOR EL1 IgG (B) and anti-hMOR EL3 IgG (D) by peptides corresponding to the human MOR EL1 sequence (NYLMGWPFGTILCK), to an analog of the murine MOR EL1 sequence (NYLMGWPFGAILCK), to the human MOR EL3 sequence (KALVTIPETTFQT), and to the murine MOR EL3 sequence (KALITIPETTFQT). Results are expressed as means ± SD percentage of inhibition of IgG binding in the presence of peptide at a concentration of 250 μg/mL. At least 3 independent experiments were performed in triplicate. O.D. indicates optical density.
Binding activity of anti-hMOR EL1 IgG and anti-hMOR EL3 IgG raised in mice.
The IgG binding activity toward the hMOR EL1 peptide (A) and the hMOR EL3 peptide (C) in pooled serum from mice immunized against the hMOR EL1 peptide (■) and pooled serum from mice immunized against the hMOR EL3 peptide (○) was estimated by enzyme-linked immunosorbent assay. A representative result from 3 experiments performed in triplicate is shown. The panels on the right show the inhibition of the binding activity of anti-hMOR EL1 IgG (B) and anti-hMOR EL3 IgG (D) by peptides corresponding to the human MOR EL1 sequence (NYLMGWPFGTILCK), to an analog of the murine MOR EL1 sequence (NYLMGWPFGAILCK), to the human MOR EL3 sequence (KALVTIPETTFQT), and to the murine MOR EL3 sequence (KALITIPETTFQT). Results are expressed as means ± SD percentage of inhibition of IgG binding in the presence of peptide at a concentration of 250 μg/mL. At least 3 independent experiments were performed in triplicate. O.D. indicates optical density.
Anti-hMOR EL1 IgG and anti-hMOR EL3 IgG preparations were then assessed alone or together with respect to their potency in favoring lymphocyte apoptosis in vivo. When passively transferred into naive mice, normal IgG and anti-hMOR EL1 IgG had no effect on Fas-mediated splenocyte apoptosis. When compared with mice injected with normal IgG or anti-hMOR EL1 IgG, mice injected with both anti-hMOR EL1 IgG and anti-hMOR EL3 IgG showed a significant splenocyte sensitization to Fas-mediated apoptosis. This suggests the presence of an additive contribution of these 2 antibody preparations with an essential role of anti-hMOR EL3 IgG (P = .02 on Mann-Whitney U test; Figure 9).
Splenocytes from mice that underwent passive transfer with both anti-hMOR EL1 IgG and anti-hMOR EL3 IgG are sensitized to CD95-mediated apoptosis.
Two hundred micrograms of either normal IgG (⋄), anti-hMOR EL1 IgG (○), anti-hMOR EL3 IgG (▵), or anti-hMOR EL1 IgG (100 μg) together with anti-hMOR EL3 IgG (100 μg) (■) were transferred by intravenous injection into naive BALB/c mice, which were killed 24 hours later. Shown is the percentage of subdiploid nuclei when splenocytes were incubated in the presence of anti-Fas IgG mAb Jo2 for 24 hours. Nuclei DNA peak was analyzed by cytofluorometry after incubation of the cells in hypotonic medium containing propidium iodide. Each experiment was performed in triplicate. Basal apoptosis of splenocytes in the absence or presence of hamster anti-KLH IgG mAb was 18% ± 3%. Statistical analysis was performed with the Mann-Whitney U test.
Splenocytes from mice that underwent passive transfer with both anti-hMOR EL1 IgG and anti-hMOR EL3 IgG are sensitized to CD95-mediated apoptosis.
Two hundred micrograms of either normal IgG (⋄), anti-hMOR EL1 IgG (○), anti-hMOR EL3 IgG (▵), or anti-hMOR EL1 IgG (100 μg) together with anti-hMOR EL3 IgG (100 μg) (■) were transferred by intravenous injection into naive BALB/c mice, which were killed 24 hours later. Shown is the percentage of subdiploid nuclei when splenocytes were incubated in the presence of anti-Fas IgG mAb Jo2 for 24 hours. Nuclei DNA peak was analyzed by cytofluorometry after incubation of the cells in hypotonic medium containing propidium iodide. Each experiment was performed in triplicate. Basal apoptosis of splenocytes in the absence or presence of hamster anti-KLH IgG mAb was 18% ± 3%. Statistical analysis was performed with the Mann-Whitney U test.
Together, these data show that anti-hMOR autoantibodies present in serum from healthy blood donors could contribute to sensitivity of immune cells to Fas (CD95)-mediated apoptosis.
Discussion
In a previous study, the presence of anti-hMOR antibodies in all 11 IgG samples examined, including 5 IgG pools each prepared from samples from 4 unrelated healthy blood donors and 6 single IgG fractions prepared from samples from 6 donors, led us to estimate that at least 42% of individuals express anti-hMOR autoantibodies in their serum (for each pool, we had considered that only one individual of 4 showed anti-hMOR IgG).22 In this study, isolation of anti-hMOR IgG from serum from all healthy blood donors studied revealed that these antibodies represent a regular feature of the IgG autoantibody repertoire under normal conditions. In addition, antibody-binding activity toward both the first and third extracellular loops of the hMOR responsible for opioid agonist mimicry23was found in each anti-hMOR autoantibody sample. Thus, the morphinelike antibody activity previously observed in a pool of IgG samples from healthy donors originates from an additive contribution of all donor samples included in the pool. The low levels of circulating serum IgG in antigen-free and germ-free mice compared with mice housed in conventional conditions29 indicate that environment has a role in the occurrence of IgG antibodies under physiologic situations. Thus, it could be speculated that exposure to a wide spectrum of food antigens, drugs, and microbes results in IgG antibody responses that show some (cross-) reactivity with the first and third extracellular loops of the hMOR. Alternatively, the presence of anti-hMOR antibodies could result from a secondary anti-idiotypic response against anti-opiate antibodies30 that is generated by self-opioid or opioid peptides such as casomorphins released from bovine milk casein during digestion.31 Our inability to isolate anti-MOR IgG from pooled serum samples from normal mice, whose nutritional and medicinal intakes differ from those of humans, strengthens the evidence that environmental antigens have a role in the occurrence of detectable anti-hMOR IgG in healthy blood donors. Another possibility is that although external stimuli may enhance the production of some cross-reactive natural autoantibodies, these autoantibodies occur independently of environmental stimuli.32 Accordingly, it has been considered that disease-associated autoantibodies may emerge from the natural autoantibody repertoire.33 In this context, characterization of agonistic anti-G protein–coupled receptor autoantibodies in several pathologic conditions, including Graves disease,34 preeclampsia,35 neonatal lupus congenital heart block,36 and schizophrenia,37 may presume that these kinds of autoantibodies, including anti-hMOR autoantibodies, are a common feature of the natural autoantibody repertoire.
The presence of anti-hMOR IgG antibodies with a potential agonistic activity in the serum of all randomly selected healthy donors studied led us to examine the putative physiologic role of these antibodies by investigating the effects of their overexpression on murine immune status. Taking advantage of the high sequence homology between human and murine extracellular loops of the MOR, we immunized mice against the hMOR to raise antibodies recognizing murine host receptors. MOR expressed on immune cell surfaces constituted the more accessible targets for circulating IgG antibodies that are unable to cross the blood-brain barrier. We focused our investigation on lymphocyte apoptosis. The cell-surface receptor Fas (APO-1/CD95) is a death receptor that plays a key role in immune homeostasis, especially by regulating the immune response to external and internal antigens.38 Increased Fas and Fas ligand expression in activated macrophages and lymphocytes results in self-destruction of those cells and therefore contributes to limitation of clonal expansion.39,40 Similar to findings for morphine, mice immunized with the recombinant hMOR/CHO clone showed an increased spleen cell sensitivity to Fas-mediated apoptosis. The essential role of IgG antibodies in this process was supported by the observation of a similar effect in naive mice injected with purified serum IgG. The increased sensitivity to Fas-mediated apoptosis in mice immunized with the recombinant hMOR/CHO clone was associated with a reduced spleen cellularity compared with that in mice immunized with untransfected CHO cells. This finding could be perceived as an enhancement of the Fas-dependent negative feedback of the xenogeneic anti-CHO immune response by anti-hMOR antibodies. In addition, as was shown in transfer experiments using anti-hMOR EL1 and anti-hMOR EL3 IgG antibodies, the proapoptotic activity of anti-hMOR IgG antibodies could be attributed to the recognition of both the first and third extracellular loops of the receptor. Similar to agonistic anti-β2-adrenergic receptor divalent IgGs, which lose their activity in the monovalent Fab fragment form,41IgG-induced MOR activation could be attributed to the receptor oligomerization. However, in contrast to the β2-adrenergic receptor, the MOR does not seem to require oligomerization for its activation.42 43
The finding of a proapoptotic effect of IgG autoantibodies specifically targeting the MOR was in agreement with results of a previous study in knockout mice showing that the MOR was the main (exclusive) mediator of the immunomodulatory effects of morphine, including spleen hypotrophy.10 As was found in mice given morphine, the reduction in the number of spleen cells observed in mice injected with hMOR/CHO was not associated with a modification in the relative content in CD4 and CD8 T lymphocytes, B lymphocytes, and monocytes.10 Because IgG cannot cross the blood-brain barrier, our results suggest that at least a part of the Fas-mediated cell death induced by opioids is mediated by receptors expressed on splenocytes. No modification of lymphocyte Fas expression was observed. The apparent discrepancy with the study of Yin et al,15which showed an up-regulation of Fas in animals given morphine, could be attributed either to the lower discriminatory ability of the cytofluorometry method we used compared with Northern blot analysis or to the absence of a direct relation between messenger RNA (mRNA) synthesis and protein expression. Alternatively, the increase in mRNA encoding for Fas receptor observed by Yin et al in whole spleen cells from mice given morphine could have been due to an enhancement of Fas expression on cells other than T lymphocytes.44 The nature of spleen cells, which could directly interact with μ-opioid agonistic ligands, is still unclear. Although morphine acts on T-cell hybridomas,15,45 MOR mRNA has been observed clearly only on macrophages.46-48 Thus, the effect of morphine on both spleen cell subpopulations requires that either MOR is expressed on all lymphocyte subsets or Fas-mediated lymphocyte apoptosis is initiated on macrophages and then extends to lymphocytes by means of release of mediators (such as nitric oxide) able to promote up-regulation of Fas,49 50 if expression of MOR is restricted to macrophages.
In contrast to splenocytes, thymocytes were not affected by immunization of mice against hMOR/CHO cells. This observation rules out a direct mechanism involving the MOR. Our results are in agreement with previous findings showing that adrenalectomy abolished the apoptotic effect of morphine on thymocytes but not on splenocytes.16,51,52 Moreover, only δ and κ opioid-receptor subclasses have been observed on thymic cells.53-55
Together, our findings indicate that, under normal conditions or in an IgG pool for therapeutic use, anti-hMOR IgG autoantibodies could participate in immunoregulatory processes by favoring Fas-mediated control of the immune response.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-01-0055.
Supported by INSERM, Université P. Sabatier, Toulouse III (ASUPS UB18CR04), and grants from Association de la Recherche contre le Cancer (ARC; 5646). G.M. is a recipient of a fellowship from ARC (P99/3).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gilles Dietrich, INSERM U563, Hôpital Purpan, Pavillon Lefebvre, Place du Dr Baylac, 31059 Toulouse, France; e-mail: gilles.dietrich@toulouse.inserm.fr.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal