Abstract
Intravenous injection of a lipopolysaccharide (LPS) into mice induces a rapid accumulation of platelets in the lung and liver. When degradation of the accumulated platelets occurs, anaphylactoid shock follows rapidly, the severity of the shock paralleling the quantity of platelets accumulated in the lung. Here we examined the contributions made by LPS structure and the complement system to the platelet response to LPS. BALB/c mice were injected with an LPS fromEscherichia coli O8, O9, O111, or K-12, or from recombinant mutants of K-12. The O-regions of the O8 and O9 LPSs consist of a mannose homopolysaccharide (MHP), while that of O111 consists of a heteropolysaccharide (not including mannose), and K-12 LPS lacks an O-region. O111 LPS was devoid of the ability to induce the platelet response or shock, while the ability of K-12 LPS was weak. The 2 recombinant LPSs—each having an O-region (from O8 or O9) linked to K-12 LPS—exhibited activities similar to or stronger than those of their original LPSs. Mannose-binding lectin (MBL) complexed with MBL-associated serine proteases (MASPs) bound strongly to LPSs containing MHP and caused C4 activation. Moreover, the abilities of these LPSs to activate the complement system corresponded well with their abilities to induce the platelet response and rapid shock. These results suggest that the structure of the O-antigen region is important for the platelet response to LPS, and that activation of the lectin pathway of the complement system is involved in this response.
Introduction
Lipopolysaccharide (LPS) is a structural component of the outer membrane of Gram-negative bacteria. The typical LPS structure—as seen in Salmonella, Klebsiella, andEscherichia coli—consists of an O-antigen region (made up of repeating units of oligosaccharide), an R-core oligosaccharide region, and a lipid region (called lipid A).1 The O-antigen region is devoid of typical endotoxic activities, whereas the lipid A region exhibits most of the activities shown by LPS. On the other hand, the O-antigen region is responsible for the heat-resistant antigenicity of Gram-negative bacteria. Different genes, rfa and rfb, control the synthesis of the R-core and O-antigen regions, respectively.2 Most wild types of bacteria form colonies of a smooth (S) type in culture. On the other hand, most mutant bacteria lacking an O-antigen region form colonies of a rough (R) type.
Intravenous injection of LPS is known to induce a rapid drop in the circulating platelet count, and platelet aggregations have been shown to localize within pulmonary and hepatic capillaries.3 However, because of a lack of adequate methods for the quantification of platelet accumulation in tissues or organs, until a few years ago no quantitative studies had been done of the relationship between the drop in platelet count in the circulation and the accumulation (or aggregation) of platelets in these organs. Then, using serotonin (5-hydroxytryptamine [5HT]) as a marker, we found evidence of a unique response of platelets to LPS.4-6 In brief, within a few minutes of its intravenous injection into mice, LPS induces an accumulation of platelets in the lung and liver (especially in the lung: at maximum, about 80% of the platelets lost from the blood accumulate in the lung).4,6 Following this platelet accumulation in the lung, anaphylactoid shock occurs rapidly, the severity of the shock paralleling the quantity of platelets that have accumulated in the lung.4,6 In mice given a lethal dose of LPS, many degranulated or destroyed platelets can be seen in the lung under the electron microscope.4,6 Thus, the characteristics of this shock differ from those of the well-known endotoxic shock that occurs several hours after an injection of LPS or lipid A and can be manifested by proinflammatory cytokines (interleukin 1 [IL-1] and/or tumor necrosis factor [TNF]).1 The magnitude of the aforementioned platelet response depends on the source of LPS. Of the various LPSs we have tested so far, an LPS from an S type of Klebsiella O3 was the most potent in inducing both the platelet response and the rapid shock.5 On the other hand, the ability of an LPS from an R type of Klebsiella O3 to induce the platelet response was weak, and it did not induce shock.5 The O-antigen region of Klebsiella O3 LPS is made up of repeating units of mannose homopolymers (MHPs),7 although O-antigen regions of many LPSs from Gram-negative bacteria are made up of heteropolysaccharide.8 Thus, the ability of a given LPS to induce the platelet response and rapid shock seemed to us likely to depend on the structure of its O-antigen. In the present study, we examined this idea by comparing the effects of LPSs containing MHP (E coli O8 and O9 and Klebsiella O3) or heteropolysaccharide (E coli O111), of an LPS without an O-region (E coli K-12),8 and of LPSs from 2 recombinant mutants of K-12 each carrying a cloned rfb gene (encoding the O-antigen region of either O8 or O9).7 In this study, we measured not only the 5HT level in the blood, as in our previous studies, but also the platelet count (using a cell counter).
Recently, we reported a line of evidence indicating that the complement system is involved in the LPS-induced accumulation and degradation of platelets in the lung.5 In recent years, it has become clear that some LPSs stimulate the complement system by binding to the complex formed between mannose-binding lectin (MBL) (originally called mannan-binding protein [MBP]) and MBL-associated serine proteases (MASPs); this pathway is called “the lectin pathway.”9-11 In the lectin pathway of human complement, MBL is associated with 3 types of MASP (MASP-1, MASP-2, and MASP-3)12-14 and a truncated protein of MASP-2 (called sMAP or MAp19).15,16 Upon binding of the complex (MBL-MASP) to a certain carbohydrate, such as the mannose andN-acetylglucosamine of pathogens, MASPs cleave complement components with concomitant activation of the complement cascade. The MASP-1 in the complex has a proteolytic activity against C3 and C2, while MASP-2 cleaves C4 and C2.17 In the present study, therefore, we also examined the contribution made by the lectin pathway to the platelet response to LPS.
Materials and methods
Animals and materials
BALB/c mice (male, 6 to 7 weeks old) were provided by the animal facility of our university, while DBA/2 and AKR mice (male, 6 to 7 weeks old) were obtained from SLC Japan (Shizuoka). Two recombinant mutants of E coli K-12 (carrying cloned rfb genes encoding the O-region of either O8 or O9) were produced as previously described.7 The recombinant and parent LPSs are shown in Table 1. They were prepared as previously described.18 Briefly, the LPSs were all prepared by a phenol-water method,19 then dissolved in sterile saline and injected intravenously (0.1 mL/10 g body weight). No protein contamination was detectable in preparations of K-12, recombinant O8 (rO8), and rO9 LPSs by silver staining methods after sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).7 The LPSs from E coliO111:B4 (S type), Klebsiella O3 (KO3) strain LEN-1 (S type), and KO3 strain LEN-113 (R type) were the same as those used in our previous study.5 All experiments were carried out at 27°C to 28°C. An anticomplement agent, K76 monocarboxylic acid,20 was provided by Ohtsuka Pharmaceutical (Tokushima, Japan), and it was dissolved in saline with the addition of enough NaOH solution to bring the pH to about 7. Ovalbumin (OVA) (from chicken, 5 × crystallized) was purchased from Seikagaku (Tokyo, Japan). Human MBL-MASP and human C4 were prepared as previously described.12 21 All experiments complied with the Guidelines for Care and Use of Laboratory Animals in Tohoku University.
Recombinant and parent E coli strains providing the LPSs used in this study
| Strain . | Abbreviation . |
|---|---|
| Wild-typeE coli O8 (S type) | O8 |
| Wild-typeE coli O9 (S type) | O9 |
| E coli K-12 (R type) | K-12 |
| E coli K-12 with rfb gene of E coli O8 (S type) | rO8 |
| E coli K-12 with rfb gene of E coli O9 (S type) | rO9 |
| Strain . | Abbreviation . |
|---|---|
| Wild-typeE coli O8 (S type) | O8 |
| Wild-typeE coli O9 (S type) | O9 |
| E coli K-12 (R type) | K-12 |
| E coli K-12 with rfb gene of E coli O8 (S type) | rO8 |
| E coli K-12 with rfb gene of E coli O9 (S type) | rO9 |
Platelet count
Two or 3 drops of blood from each decapitated mouse were directly collected into a preweighed test tube containing 1.0 mL of 4 mM EDTA (ethylenediaminetetraacetic acid) in 0.01 M phosphate-buffered saline (pH 7.0). The tube plus blood was weighed, and the volume of the blood recovered in the tube was calculated from its weight on the basis of the assumption that the specific gravity of the blood is 1.0. The number of platelets was then ascertained by means of a cell counter, Sysmex SF-3000 (Toa Medical Electronics, Kobe, Japan).
Determination of 5HT and histamine (H)
Briefly, blood (2 or 3 drops) was collected by decapitation into preweighed test tubes containing 3 mL 0.4 M HClO4, 0.1%N-acetyl cysteine-HCl, and 2 mM EDTA-2Na. After the tubes had been weighed, platelets were destroyed by sonication, and each tube was cooled in an ice bath. Lungs and liver were rapidly removed and kept in a jar with dry ice until needed. The determination of the 5HT level in the blood was carried out soon after the blood was collected. The 5HT levels in the liver and lung were determined within 3 days of collection. After 5HT had been separated by column chromatography, it was measured fluorometrically as previously described.22 A portion of the extracts of blood obtained as described above was used for the determination of histamine (H), as described previously.23 The values reported here for 5HT levels are somewhat lower than those shown in our previous reports. This is due to the fact that in the present study we used a newly purchased standard 5HT, while in previous studies we used one purchased about 10 years ago.
Sensitization of mice to antigen
A suspension (0.5 mL) containing OVA (50 μg) and alum (3 mg) was injected intraperitoneally on day 0 and day 10. Experiments involving the induction of anaphylaxis were performed on day 20. The antigen challenge was delivered by intravenous injection (via a tail vein) of OVA dissolved in saline at 2 mg/kg.
Scoring of the rapid shock induced by LPSs
The incidence and the severity of the rapid shock were assessed within 30 minutes of the injection of LPS or OVA. These values and the subsequent mortality (in the first hour after the injection) were recorded as described previously.5 Briefly, the severity of the shock was scored as follows: 0, no signs of shock; 1, staggering; 2, crawling and prostration; 3, prostration and weak convulsions; 4, prostration, strong convulsions, and death.
Assay of the binding of MBL-MASP complex to LPSs
Each LPS was diluted with carbonate buffer (pH 9.6) (40 μg LPS per milliliter). Microtiter plates (Sumitomo Bakelite, Tokyo, Japan) were coated with 0.1 mL of the respective LPS solution. After blocking with Block Ace (0.3 mL) (Dainippon Seiyaku Laboratory, Osaka, Japan), human MBL-MASP solution (0.1 mL)12 diluted with TBST (50 mM Tris [tris(hydroxymethyl)aminomethane], 150 mM NaCl, 10 mM CaCl2, 0.1% Tween 20 [pH 7.5]) was incubated in the wells at 4°C for 2 hours. After the wells had been washed with TBST, biotinylated anti-MBL monoclonal antibody (3E7) (0.1 mL)24 was incubated in the wells at 37°C for 1 hour. After being washed, the plates were incubated with avidin-peroxidase complex (0.1 mL) (VECTOR Laboratories, Burlingame, CA) at 37°C for 1 hour, and developed with azino-di (3-methylbenzthiazoline) sulfonic acid (ABTS) (0.1 mL) (ZYMED, San Francisco, CA). The amount of the MBL-MASP complex deposited was estimated by measuring the absorbance at 405 nm (A405).
Assay of C4 activation by MBL-MASP bound to LPS
Microtiter plates were coated with 0.1 mL of the LPS solution mentioned above, and MBL-MASP was then incubated in the wells as described above. After the wells had been washed with TBST and then with mannitol gelatin veronal buffer (MGVB) (5 mM veronal, 74 mM NaCl, 0.1% gelatin, 2.3% mannitol, 2 mM CaCl2, and 0.5 mM MgCl2 [pH 7.4]), human C4 (0.1 mL)21 was incubated in the wells at 37°C for 1 hour. After the wells were washed with TBST, the C4 deposition in these wells was detected by incubating peroxidase-conjugated polyclonal anti-C4 at 37°C for 1 hour and developing with ABTS (0.1 mL). The amount of C4 deposition was estimated by measuring A405.
Assay of the abilities of LPSs to activate the complement system
Activation of the complement system was assayed by measuring the extent of the hemolysis of antigen-sensitized sheep erythrocytes that was induced by human serum treated with various LPSs. For this, we used Mayer's methods.25 In this assay of hemolytic activity, CH50 (the amount of serum that produces 50% hemolysis) is determined as follows. Briefly, 100 μL LPS solution (100 μg/mL) in 10 mM veronal buffer containing 0.148 M NaCl, 0.1% gelatin, 0.15 mM CaCl2, and 0.5 mM MgCl2(GVB2+, pH 7.4) was incubated with 100 μL human serum at 37°C for 30 minutes. As a control, human serum was incubated with GVB2+ without LPS. The control and LPS-treated sera were then diluted to a total volume of 5.5 mL with GVB2+. Various amounts of the diluted sera (0.5, 0.6, 0.7, 0.8, 1.0, and 1.5 mL) were each made up, to a total volume of 2.6 mL with GVB2+, and 0.4 mL antibody-sensitized sheep-erythrocyte suspension was added to each one. Then, the mixture was incubated at 37°C for 1 hour. After centrifugation, the A541 of the supernatant was measured, and CH50 values were determined for the control and LPS-treated sera (expressed as CH50control and CH50LPS, respectively). The ability of a given LPS to activate the complement system was taken as [(CH50control − CH50LPS) ÷ CH50 control] × 100.
Statistical analysis
Experimental values are given as mean ± SD. The statistical significance of differences was assessed by means of a Student unpaired t test after analysis of variance (ANOVA): P < .05 was considered to indicate significance.
Results
Comparison of the abilities of LPSs to induce rapid shock
At a dose of 2 mg/kg, O8 and O9 LPS both induced rapid shock (Table 2): at this dose, O9 LPS was lethal, but O8 LPS was not. K-12 LPS, at the same dose, was inactive at inducing rapid shock, although at 4 mg/kg it did produce weak shock in about half the mice tested. The rO8 and rO9 LPSs were both more potent at inducing shock than their respective original LPSs.
Ability of LPSs from wild and recombinant strains ofE coli to induce rapid shock
| LPS . | Dose, mg/kg . | Incidence . | Shock score . | Mortality . |
|---|---|---|---|---|
| E coliK-12 | 2 | 0/4 | 0 | 0/4 |
| 4 | 5/9 | 1 | 0/9 | |
| E coliO8* | 0.5 | 0/4 | 0 | 0/4 |
| 1 | 4/4 | 2 | 0/4 | |
| 2 | 4/4 | 2-4 | 0/4 | |
| E coliO9* | 0.25 | 1/4 | 0-1 | 0/4 |
| 0.5 | 4/4 | 2-3 | 0/4 | |
| 1 | 4/4 | 3-4 | 0/4 | |
| 2 | 4/4 | 4 | 3/4 | |
| E coli rO8* | 0.5 | 4/4 | 1 | 0/4 |
| 1 | 4/4 | 2-4 | 0/4 | |
| 2 | 4/4 | 4 | 4/4 | |
| E coli rO9* | 0.25 | 4/4 | 1 | 0/4 |
| 0.5 | 4/4 | 3-4 | 1/4 | |
| 1 | 4/4 | 4 | 3/4 | |
| E coliO111 | 4 | 0/5 | 0 | 0/5 |
| KlebsiellaO3-S* | 0.5 | 4/4 | 4 | 3/4 |
| KlebsiellaO3-R | 4 | 0/4 | 0 | 0/4 |
| LPS . | Dose, mg/kg . | Incidence . | Shock score . | Mortality . |
|---|---|---|---|---|
| E coliK-12 | 2 | 0/4 | 0 | 0/4 |
| 4 | 5/9 | 1 | 0/9 | |
| E coliO8* | 0.5 | 0/4 | 0 | 0/4 |
| 1 | 4/4 | 2 | 0/4 | |
| 2 | 4/4 | 2-4 | 0/4 | |
| E coliO9* | 0.25 | 1/4 | 0-1 | 0/4 |
| 0.5 | 4/4 | 2-3 | 0/4 | |
| 1 | 4/4 | 3-4 | 0/4 | |
| 2 | 4/4 | 4 | 3/4 | |
| E coli rO8* | 0.5 | 4/4 | 1 | 0/4 |
| 1 | 4/4 | 2-4 | 0/4 | |
| 2 | 4/4 | 4 | 4/4 | |
| E coli rO9* | 0.25 | 4/4 | 1 | 0/4 |
| 0.5 | 4/4 | 3-4 | 1/4 | |
| 1 | 4/4 | 4 | 3/4 | |
| E coliO111 | 4 | 0/5 | 0 | 0/5 |
| KlebsiellaO3-S* | 0.5 | 4/4 | 4 | 3/4 |
| KlebsiellaO3-R | 4 | 0/4 | 0 | 0/4 |
Data for LPSs from E coli O111 andKlebsiella O3-S and R are from our previous report.5
The O regions of the LPSs of these bacteria are composed of MHP.
To compare these results with those of other LPSs, our previous results5 are also shown in Table 2. The O-region ofE coli O111 LPS is composed of heteropolysaccharide (not including mannose).8 This LPS was inactive at inducing rapid shock, even at 4 mg/kg. Thus, it should be noted that of the LPSs tested, those that have MHP as their O-antigen all have a powerful ability to induce rapid shock.
Effects of O9 LPS on 5HT, H, and platelet count
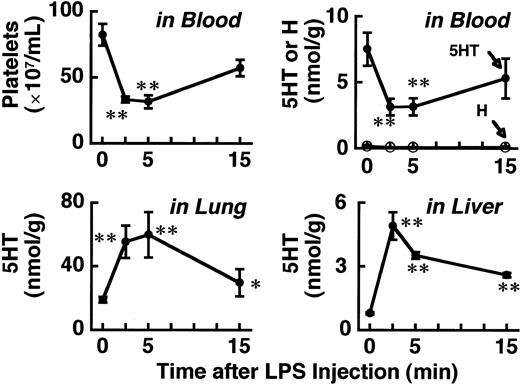
The effects of O9 LPS on 5HT and platelets at a dose of 0.25 mg/kg, which produces only weak signs of shock (score 0-1), are shown in Figure 1. There is a good parallel between the changes in 5HT and platelet count in the blood. Moreover, these changes in the blood are well mirrored by the 5HT increase in the lung (or lung plus liver). The 5HT increases in the lung and liver were maximal at 2.5 to 5 minutes after LPS injection. It should also be noted that there was no detectable increase in H in the blood (Figure1, upper right panel). We previously suggested that platelets that have accumulated in the lung and liver are returned to the circulation provided they are not profoundly damaged.5 This idea is clearly supported by the data shown in Figure 1.
Time course of the effects of O9 LPS on 5HT, H, and platelet count.
Blood and tissues were taken at the indicated times after intravenous injection of the LPS (0.25 mg/kg). Each value is the mean ± SD from 4 mice. *P < .05, **P < .01 versus time 0.
Time course of the effects of O9 LPS on 5HT, H, and platelet count.
Blood and tissues were taken at the indicated times after intravenous injection of the LPS (0.25 mg/kg). Each value is the mean ± SD from 4 mice. *P < .05, **P < .01 versus time 0.
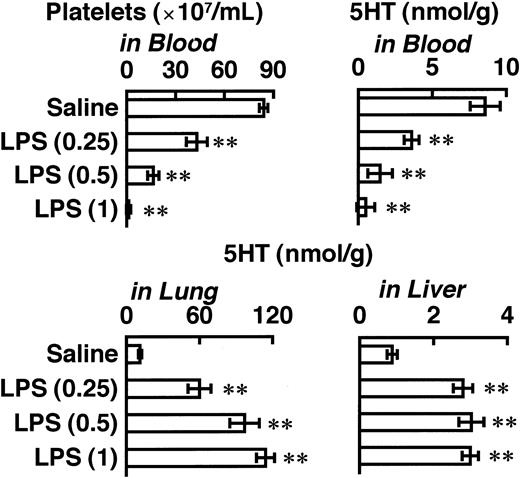
The effects of O9 LPS, at 5 minutes after its injection, on blood 5HT and on the platelet count were dose-dependent and occurred in parallel (Figure 2). Although the 5HT level in the liver had reached a maximum value even at 0.25 mg/kg of this LPS, the 5HT level in the lung increased in a dose-dependent manner at the doses tested. In the following experiments, therefore, we compared the effects of various LPSs (at 0.5 or 1 mg/kg) on 5HT levels in blood and lung at 5 minutes after the injection of LPS.
Dose-dependence of 5HT responses to O9 LPS.
The indicated dose (milligrams per kilogram) was injected intravenously, and blood and tissues were taken 5 minutes later. Each value is the mean ± SD from 4 mice. *P < .05, **P < .01 versus dose 0.
Dose-dependence of 5HT responses to O9 LPS.
The indicated dose (milligrams per kilogram) was injected intravenously, and blood and tissues were taken 5 minutes later. Each value is the mean ± SD from 4 mice. *P < .05, **P < .01 versus dose 0.
Comparison between the rapid shock induced by LPS and anaphylactic (OVA-induced) shock
Intravenous injection of rO9 LPS (0.5 mg/kg) into normal mice and of OVA (2 mg/kg) into sensitized mice induced similar shock signs within 10 minutes of the injection. Under our conditions, this LPS and OVA produced similar shock scores. A marked increase in H in the blood of sensitized mice preceded the shock signs seen after injection of OVA, but there was no detectable increase in blood H after LPS injection (Table 3; Figure 1). Pyrilamine, a histamine antagonist, reduced the shock scores allocated to the anaphylactic (OVA-induced) shock, but not those allocated to the LPS-induced shock.
Shock scores and serum H levels in LPS-induced rapid shock and anaphylactic (OVA-induced) shock
| Injection . | Shock score . | Blood H (nmol/g)3-150 . | Shock scores after pyrilamine3-151 . |
|---|---|---|---|
| OVA | 3-4 (0/4)3-152 | 10.5 ± 1.5 | 2 (0/4) |
| rO9 LPS | 3-4 (1/4)3-152 | < 0.2 | 3-4 (0/4) |
| Injection . | Shock score . | Blood H (nmol/g)3-150 . | Shock scores after pyrilamine3-151 . |
|---|---|---|---|
| OVA | 3-4 (0/4)3-152 | 10.5 ± 1.5 | 2 (0/4) |
| rO9 LPS | 3-4 (1/4)3-152 | < 0.2 | 3-4 (0/4) |
OVA (2 mg/kg) or rO9 LPS (0.5 mg/kg) was injected into sensitized or nonsensitized mice, respectively.
Blood was collected at 2 minutes after injection of OVA or rO9 LPS.
Pyrilamine was injected intraperitoneally (10 mg/kg) 30 minutes before injection of OVA or LPS.
Mortality.
Comparison of the effects of various LPSs on 5HT in blood and lung
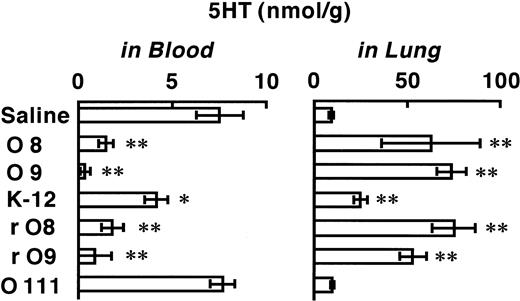
The effects induced by 1 mg/kg of various E coli LPSs on 5HT levels at 5 minutes after their injection are shown in Figure3. The LPSs of O8, O9, rO8, and rO9 each induced a marked reduction in blood 5HT (compared with saline control), with a corresponding large elevation of 5HT in the lung. On the other hand, K-12 LPS produced only a mild decrease in blood 5HT and a corresponding mild increase in the lung, while O111 LPS produced no significant change in 5HT in either blood or lung.
Effects of various LPSs on 5HT levels in blood and lung.
Each column shows the 5HT level at 5 minutes after intravenous injection of a given LPS (or saline). One of the LPSs (1 mg/kg) was injected into a given mouse, and 5 minutes later, blood and lungs were removed and assayed for 5HT. Each value is the mean ± SD from 4 mice. *P < .05, **P < .01 versus saline group.
Effects of various LPSs on 5HT levels in blood and lung.
Each column shows the 5HT level at 5 minutes after intravenous injection of a given LPS (or saline). One of the LPSs (1 mg/kg) was injected into a given mouse, and 5 minutes later, blood and lungs were removed and assayed for 5HT. Each value is the mean ± SD from 4 mice. *P < .05, **P < .01 versus saline group.
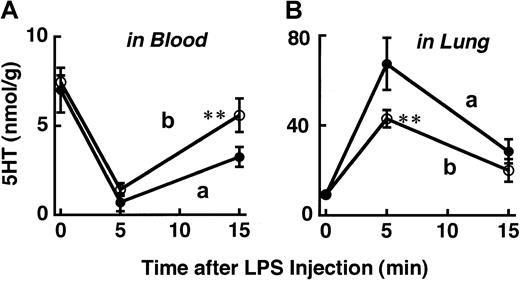
Effects of an inhibitor of complement C5 on the platelet response and rapid shock induced by rO9 LPS
At a dose of 0.5 mg/kg, rO9 LPS produced a shock score of 3 to approximately 4 (Table 2). When this dose of rO9 LPS was injected, the recovery in the blood 5HT level at 15 minutes after LPS was only partial (about 45%) (“a” in Figure4). As shown in Table 2, 1 mg/kg rO9 LPS produced a score-4 shock and was lethal in 3 of 4 mice within 15 minutes of the injection. However, even at this dose of rO9 LPS, prior administration of K76, an inhibitor of complement C5, reduced the platelet accumulation in the lung (“b” in Figure 4B), enhanced the recovery of platelets back into the blood (“b” in Figure 4A), and completely prevented the appearance of shock signs (score 0). K76 itself did not alter the blood 5HT level.
Effects of K76, an inhibitor of complement C5, on 5HT response to rO9 LPS.
The rO9 LPS (0.5 mg/kg) (a) was injected intravenously 1 hour after an intraperitoneal injection of saline. The rO9 LPS (1 m/kg) (b) was injected intravenously 1 hour after an intraperitoneal injection of K76 (100 mg/kg). Each value is the mean ± SD from 4 mice. *P < .05, **P < .01 versus corresponding value in the absence of K76.
Effects of K76, an inhibitor of complement C5, on 5HT response to rO9 LPS.
The rO9 LPS (0.5 mg/kg) (a) was injected intravenously 1 hour after an intraperitoneal injection of saline. The rO9 LPS (1 m/kg) (b) was injected intravenously 1 hour after an intraperitoneal injection of K76 (100 mg/kg). Each value is the mean ± SD from 4 mice. *P < .05, **P < .01 versus corresponding value in the absence of K76.
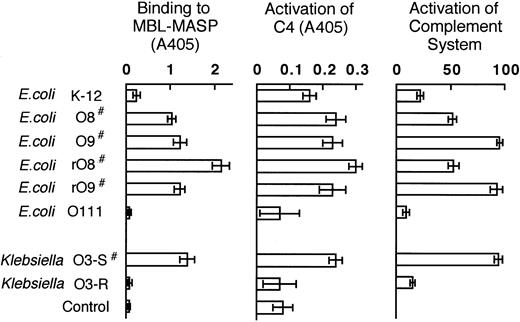
Binding of MBL-MASP to LPSs with concomitant activation of C4
As shown in Figure 5, the MBL-MASP complex became bound to those LPSs having MHP as their O-region (those marked # in Figure 5), but not to LPSs lacking MHP. The MASP-2 in the MBL-MASP complex cleaves C4 into C4a and C4b, the latter of which binds covalently to substances such as LPS (C4 deposition). To determine the binding of MBL-MASP to the LPSs, we examined the activation of complement. First we evaluated C4 deposition. When C4 was incubated in microplate wells that had been coated with LPS and treated with MBL-MASP, the amount of C4b deposited on the plates was more marked for LPSs containing MHP than for the other LPSs (Figure 5). The binding of K-12 LPS to the MBL-MASP complex and the subsequent C4 deposition were both less extensive than those measured for LPSs containing MHP. These results indicate that MBL-MASP binds preferentially to LPSs containing MHP, leading to complement activation.
Ability of various LPSs to bind to a complex of MBL and MASP, together with data on C4 activation by the MBL-MASP complex when bound to various LPSs, and on the ability of LPSs to activate the complement system.
Experiments were carried out as described in “Materials and methods.” #LPS possessing MHP. Control: vehicle instead of LPS. Each value is the mean ± SD from a measurement made in triplicate.
Ability of various LPSs to bind to a complex of MBL and MASP, together with data on C4 activation by the MBL-MASP complex when bound to various LPSs, and on the ability of LPSs to activate the complement system.
Experiments were carried out as described in “Materials and methods.” #LPS possessing MHP. Control: vehicle instead of LPS. Each value is the mean ± SD from a measurement made in triplicate.
Activation of the complement system by LPSs
Activation of the complement system leads to the formation of the assembly C5-C9, also called membrane-attack complex (MAC). The MAC formed in human serum can produce hemolysis of antibody-sensitized sheep erythrocytes in vitro. In this experimental system, the ability of an LPS to induce formation of MAC is reflected by its ability to induce such hemolytic activity. As shown in Figure 5, O9, rO9, and KO3-S LPSs showed the highest levels of activity in this assay system. This is in good agreement with the data showing that these 3 LPSs are more potent at inducing rapid shock than the other LPSs tested (Table 2).
Effects of rO9 LPS on complement C5–deficient mice
DBA/2 and AKR mice are deficient in C5.26 27 As shown in Figure 4, the blood level of 5HT at 15 minutes after an injection of LPS is an index of the recovery of platelets from the lung, where they had previously accumulated. The rO9 LPS was injected into DBA/2 mice at 1 mg/kg, a dose inducing almost complete loss of platelets from the blood (Figure 2) and lethal shock (Table 2) in BALB/c mice. However, in DBA/2 mice, the decrease in 5HT in the blood was only mild, and there was no increase in 5HT in the lung (Figure6). These results indicate that rO9 LPS induces no significant degradation of platelets in DBA/2 mice. Indeed, rO9 LPS produced no signs of rapid shock (score 0). In AKR mice, too, this LPS failed to produce any signs of rapid shock at 1 mg/kg.
Effects of rO9 LPS on 5HT levels in DBA/2 mice.
Samples from blood and lung were taken 15 minutes after an intravenous injection of rO9 LPS (1 mg/kg). Each value is the mean ± SD from 4 mice.
Effects of rO9 LPS on 5HT levels in DBA/2 mice.
Samples from blood and lung were taken 15 minutes after an intravenous injection of rO9 LPS (1 mg/kg). Each value is the mean ± SD from 4 mice.
Discussion
The results obtained in the present study may be summarized as follows. (1) Following intravenous injection of O9 LPS, there was a good parallel between the changes in 5HT and platelet count in the blood in terms of time course and dose response. Moreover, these changes in the blood were well mirrored by the 5HT increase in the lung (or lung plus liver). (2) O9 LPS produced no detectable increase in H in the blood. (3) O111 LPS (composed of heteropolysaccharide) was devoid of the ability to induce the platelet response and rapid shock, while the ability of K-12 LPS (not having an O-region) was weak. The 2 recombinant LPSs, each having an O-region composed of MHP (from O8 or O9) linked to K-12 LPS, exhibited activities similar to or stronger than those of their original LPSs. (4) Administration of a complement C5 inhibitor reduced platelet accumulation in the lung, enhanced the recovery of platelets back into the blood, and prevented the induction of shock signs by rO9 LPS. (5) The MBL-MASP complex became bound to LPSs having an MHP, but not to LPSs lacking MHP. (6) As a result of this binding to the LPSs, MBL-MASP activated C4. (7) The abilities of LPSs having MHP to activate the complement system (assayed by measuring the in vitro hemolysis of antigen-sensitized erythrocytes) corresponded well to their abilities to induce the platelet response and rapid shock. In the following paragraphs, we discuss the implications of these results.
Involvement of platelets, but not mast cells, in LPS-induced rapid shock
Using 5HT as a marker, as described in the “Introduction,” we previously found evidence of a unique response of platelets to LPS. Our present results on the relationship between 5HT levels and platelet count demonstrated this unique platelet response quite clearly. In contrast to the situation with 5HT, the amount of histamine in the blood of mice is much lower than the amounts in the tissues (about 1:100 in molar-ratio terms).22 In anaphylactic shock, the level of histamine in the blood is elevated markedly. In contrast, LPS produced no detectable increase in histamine in the blood. In addition, a typical histamine antagonist, pyrilamine, did not reduce the signs of rapid shock induced by rO9 LPS, although it did reduce the OVA-induced anaphylactic shock. These results strongly suggest that platelets, but not mast cells, are involved in the LPS-induced rapid shock.
Involvement of the lectin pathway in the platelet response to LPS
Those tested LPSs that have an MHP as their O-region were found to possess all 5 of the activities mentioned in Table4, but the LPSs lacking MHP were mostly devoid of these activities. These findings, therefore, strongly suggest that the platelet response and ensuing rapid shock are generally induced by an activation of the complement system through the lectin pathway.
Activities of various LPSs
| LPS . | Platelet response . | Rapid shock . | Binding of LPS to MBL-MASP . | Activation of C4 . | Hemolysis . |
|---|---|---|---|---|---|
| E coliK-12 | + | + | + | + | + |
| E coli O84-150 | ++ ∼ +++ | ++ | ++ | ++ | ++ |
| E coli O94-150 | +++ | +++ | ++ | ++ | +++ |
| E coli rO84-150 | ++ ∼ +++ | ++ | +++ | +++ | ++ |
| E colirO94-150 | +++ | +++ | ++ | ++ | +++ |
| E coliO111 | ± | − | − | − | − |
| KlebsiellaO3-S4-150 | +++ | +++ | ++ | ++ | +++ |
| Klebsiella O3-R | ± | − | − | − | − |
| LPS . | Platelet response . | Rapid shock . | Binding of LPS to MBL-MASP . | Activation of C4 . | Hemolysis . |
|---|---|---|---|---|---|
| E coliK-12 | + | + | + | + | + |
| E coli O84-150 | ++ ∼ +++ | ++ | ++ | ++ | ++ |
| E coli O94-150 | +++ | +++ | ++ | ++ | +++ |
| E coli rO84-150 | ++ ∼ +++ | ++ | +++ | +++ | ++ |
| E colirO94-150 | +++ | +++ | ++ | ++ | +++ |
| E coliO111 | ± | − | − | − | − |
| KlebsiellaO3-S4-150 | +++ | +++ | ++ | ++ | +++ |
| Klebsiella O3-R | ± | − | − | − | − |
The responses were scored as none (−), very weak (±), weak (+), mild (++), and strong (+++).
LPSs with MHP as their O region.
MBL (formerly called MBP; first isolated by Kawasaki et al28) has been shown to bind to the LPSs ofSalmonella and Klebsiella O3, which have an O-region rich in D-mannose.29,30 In addition, Kawakami and his coworkers found a complement-dependent bactericidal factor in nonimmune mouse sera.31-33 Having found that this factor binds to the Ra core region of Salmonella LPS, they decided to call it “Ra-reactive factor” (RaRF). Later, this factor was shown to have 2 components, a complement-activating unit (MASP) and a polysaccharide-binding unit, and the latter unit was soon identified as MBL.11,12,24,34 35 These findings by the pioneers in this field support the view that the abilities of K-12 LPS (an R-type LPS) to induce the platelet response and rapid shock might also be mediated via the lectin pathway, although they are much weaker than those of LPSs having an MHP.
Contribution of the O-region and MHP
The ability of the R-form of the LPS from Klebsiella O3 to induce the platelet response and rapid shock is very weak (Table 2and Shibazaki et al5). In the present study, it was shown that the platelet response and rapid shock induced by O8 and O9 LPSs and by their recombinant LPSs are much stronger than those induced by K-12 LPS.
On the other hand, O-polysaccharide, which was purified on a TSK HW 40(S) column (TOSOH, Tokyo, Japan) after hydrolysis of LPS with 1% acetic acid (100°C, 90 minutes), retains the ability of its parent LPS to inhibit the binding of a monoclonal antibody to its antigen LPS.36 However, the O-region ofKlebsiella O3 LPS (prepared by the same method; ie, MHP itself), even at 2 mg/kg, produced neither platelet response nor signs of rapid shock (Y.O. and Y.E., unpublished data, January 2000), and E coli O9 and Klebsiella O3 strains are claimed to have the same O-polysaccharide.8
To judge from these results, the O-region would appear to play a primary role in stimulating the lectin pathway. However, one must admit the possibilities that (1) the R region may contribute to the construction of the particular conformation of the O-region required for such stimulation, and (2) the R region itself may stimulate the lectin pathway to a certain extent, as seen in K-12 LPS.
It should also be noted that in our previous studies, an LPS preparation from E coli O55:B5 (containing a heteropolysaccharide, but not including mannose as its O-region)8 and Prevotella intermedia LPS (not containing a repeating oligosaccharide unit as its O-region) were also found able to induce the platelet response and rapid shock.4-6 Therefore, it seems unlikely that the possession of a mannose homopolymer is the sole structural prerequisite for inducing the platelet response.
Mechanisms by which platelets accumulate in the lung and liver
At present, we have no available data to help us explain why platelets accumulate predominantly in the lung and liver. However, some collectins or their related proteins have been shown to be present in these organs. For example: (1) surfactant proteins (SPs) are present on the surface of alveoli, and they can bind to LPS,37 and (2) some collectins, including MBL and the complement-activating unit of RaRF, are produced in the liver.31,38-40 The lung and liver are rich in vascular endothelial cells. Interestingly, it has been shown that receptors for collectins, which are identical to those for C1q, are present on many cell types, including vascular endothelial cells, and that MBL and SP can bind to these receptors.9It should also be remembered that the lung is the organ taking in air from the external environment, and that the liver is the organ directly linked through the portal vein to the digestive tract: they are in effect the front line in the body's defense against invasion by bacteria.
Pathologic implications of the platelet response to LPS
As noted by Martin and Silverman,41 approximately 200 000 patients develop Gram-negative sepsis each year in the United States. Of these, about one quarter develop adult (or acute) respiratory distress syndrome (ARDS). Among these patients, mortality is estimated at 60% to 90%. It has been suggested that in addition to neutrophils and macrophages, interaction between platelets and pulmonary endothelial cells may be involved in the pathology of ARDS.42 However, as described above, the mechanisms underlying the accumulation of platelets in the lung (or pulmonary thrombopoiesis) are still not established. Conceivably, our findings might provide an important clue to the mechanism, and the acute shock seen in our mice may, at least in part, model a symptom afflicting ARDS patients.
Conclusions and biological implications of the platelet response to LPS
The present results suggest that the structure of the O-antigen region of LPS is important for inducing the platelet response to LPS, and that the lectin pathway of the complement system is involved in its induction.
During the process of phylogenesis, bacteria acquired a means of self-protection: namely, LPS on their outer membrane.43The immune systems of their targets or hosts then developed 2 different ways to counter the LPS: (1) the recognition of O-antigen regions (the most variable region in LPSs) followed by the production of antibodies against them, and (2) the recognition of lipid A regions (the structurally most conserved region in LPSs) by Toll-like receptor 4,44 followed by the mobilization of bioactive peptides such as cytokines (which induce a variety of metabolic and cellular responses and so protect the host). In addition to these 2 known ways, our findings suggest a third way: namely, the recognition of a certain conformation possessed mainly by the O-antigen region, followed by activation of the complement system. The outstanding property of the third system is that it can work very rapidly (by mobilizing platelets). Interestingly, when platelets are not profoundly damaged, they can return to the circulation. Michelson et al45 also reported that degranulated platelets can continue to circulate and function. Our experimental system may help to elucidate previously unknown strategies against infection employed by platelets as part of the body's innate system of immunity.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-01-0252.
Supported in part by grants for Scientific Research from the Ministry of Education of Japan (Nos. 10877302 and 12877302).
L.Z. and Y.O. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yasuo Endo, Department of Pharmacology, Graduate School of Dentistry, Tohoku University, 4-1 Seiryo-machi, Aoba-ku, Sendai 980-8575, Japan; e-mail: endo@pharmac.dent.tohoku.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal