Multiple myeloma (MM) is a hematologic malignancy characterized by accumulation of plasma cells in the bone marrow (BM). Bone destruction is a complication of the disease and is usually associated with severe morbidity. The balance between receptor activator of nuclear factor-κB (NF-κB) ligand and osteoprotegerin (OPG) is of major importance in bone homeostasis. We have recently shown that serum OPG levels are lower in patients with myeloma than in healthy individuals. Here we show that myeloma cells can bind, internalize, and degrade OPG, thereby providing a possible explanation for the lower levels of OPG in the BM of patients with MM. This process is dependent on interaction of OPG with heparan sulfates on the myeloma cells. The results suggest a novel biologic mechanism for the bone disease associated with MM and that treatment of the bone disease with OPG lacking the heparin-binding domain should be considered.
Introduction
Bone is a dynamic tissue in which the synthesis of bone matrix by osteoblasts and bone resorption by osteoclasts are coupled processes. Osteoclasts differentiate from hematopoietic precursor cells under the control of humoral factors and cell-cell contact with osteoblasts or stromal cells. Key regulators of osteoclastogenesis are members of the tumor necrosis family of receptors and ligands: receptor activator of nuclear factor (NF)–κB (RANK), receptor activator of NF-κB ligand (RANKL), and osteoprotegerin (OPG). RANKL is expressed by activated T cells, stromal cells, and osteoblasts.1-3 When RANKL binds to RANK on osteoclast precursors, maturation and differentiation of the osteoclasts are induced, leading to bone resorption.4,5OPG is a soluble decoy receptor that is secreted by osteoblasts and bone marrow (BM) stromal cells and that competes with RANK for binding to RANKL. Binding of OPG to RANKL inhibits the development of osteoclasts.6,7 The importance of OPG as a negative regulator of osteoclastogenesis is evident from experiments with transgenic mice, where overexpression of OPG leads to severe osteopetrosis and reduced numbers of mature osteoclasts.6In contrast, OPG knockout mice are osteoporotic.8
Multiple myeloma (MM) is a malignancy characterized by accumulation of plasma cells in the BM. Bone destruction is a common complication of the disease and is associated with severe morbidity. A number of osteoclast-activating factors are implicated in myeloma bone disease (for a review, see Callander and Roodman9). However, accumulating data suggest that a disruption of the balance between RANKL and OPG is of major importance. Histologic examination of BM biopsies from patients with MM showed enhanced expression of RANKL in the BM, as well as reduced OPG expression.10,11Furthermore, we have recently shown that serum OPG levels are lower in myeloma patients than in healthy individuals and that myeloma patients with osteolytic lesions have reduced levels of OPG in serum compared to myeloma patients without clinical bone disease.12
Osteoprotegerin has a highly basic heparin-binding domain,13 making interactions with heparin and heparan sulfates possible. A feature of both normal and malignant plasma cells is the abundant expression of syndecan-1,14,15 which is a transmembrane proteoglycan with heparan sulfate side chains. These side chains allow interactions with several macromolecules, including extracellular matrix proteins, growth factors, cytokines, and pathogens (for a review, see Tumova et al16). In addition to modifying the action of its ligands,17,18 syndecan-1 has been shown to mediate the catabolism of several proteins.19
Patients, materials, and methods
MM and control patients
Bone marrow was aspirated from the iliac crest or sternum of 33 patients with MM for diagnostic purposes before treatment (median age, 65 years; 19 men, 14 women). Twenty-seven patients who underwent BM aspiration for diagnostic purposes, but who had normal BM morphology and subsequently were not diagnosed with MM, were included as controls (median age, 60 years; 14 men, 13 women). Patient samples were obtained at the Section of Hematology, University Hospital, Trondheim after informed consent was obtained. The study was approved by the local ethics committee.
OPG measurements
Bone marrow OPG was measured by enzyme-linked-immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Addition of soluble RANKL up to 5 ng/mL, heparin up to 100 μg/mL, or soluble syndecan-1 up to 300 ng/mL did not interfere with the detection of OPG.
Statistical analyses
All statistical analyses were done with the SPSSX/PC computer program (SPSS, Chicago, IL). Results were considered statistically significant with P < .05. Comparisons between groups were performed with the Mann-Whitney U test. Correlation between 2 continuous parameters was estimated by the Spearman method.
Cell surface detection of OPG by flow cytometry
Approximately 200 000 CAG cells (human myeloma cell line received as a kind gift from Dr Joshua Epstein, Little Rock, AK), ARH-77syn-1 cells, or ARH-77TDMcells20 were labeled by incubation with either 10 μg/mL recombinant human OPG-Fc chimeras (R&D Systems) or 10 μg/mL recombinant human OPG (a kind gift from Amgen, Thousand Oaks, CA) for 30 minutes in the absence or presence of heparin as indicated. After washing, cells were stained with 50 μg/mL monoclonal mouse anti-OPG antibody (R&D Systems) for 30 minutes. Bound anti-OPG was detected with 12.5 μg/mL fluorescein isothiocyanate (FITC)–conjugated goat antimouse antibody (Becton Dickinson, Bedford, MA). All steps were performed on ice. Cells were gated for live/dead using propidium iodide (4 μg/mL) and 5000 living cells from each sample were analyzed on a single-cell basis. Primary myeloma cells were stained with OPG-Fc as described above after isolation of BM mononuclear cells by density gradient centrifugation. Plasma cells were identified by staining with phycoerythrin-labeled anti-CD138 (Serotec, Oxford, United Kingdom) and 5000 of these were analyzed for OPG binding on a single-cell basis.
Confocal microscopy
Recombinant human OPG-Fc or recombinant human OPG was conjugated to a fluorochrome (Alexa Fluor 488; Molecular Probes, Eugene, OR) according to the manufacturer's instructions.
To examine the distribution of cell surface-bound OPG, 100 000 CAG cells were labeled with 250 μg/mL Alexa Fluor 488–conjugated OPG for 30 minutes on ice before washing. The cells were either coincubated with 50 nM Lyso Tracker Red DND-99 (Molecular Probes) or 2 mg/mL cholera toxin subunit B (CTB), Alexa Fluor 594 (Molecular Probes). To maximize uptake and focus on the internalized OPG, 100 000 CAG cells were labeled with 20 μg/mL Alexa Fluor 488–conjugated OPG and 50 nM Lyso Tracker Red DND-99 for 1 hour at 37°C. Cell surface–bound OPG was removed by heparin wash before examination of the cells by confocal microscopy (LSM 510; Zeiss, Jena, Germany).
Binding, internalization, and degradation of iodinated OPG
Recombinant human OPG was iodinated by the Iodogen method (Pierce Biosciences Chemical, Rockford, IL). Briefly, 10 μg human OPG was incubated with 10 MBq Na125I (specific activity 644 GBq/mg I; Amersham, Oslo, Norway) in a tube containing 2 μg solid Iodogen reagent in a final volume of 25 μL phosphate-buffered saline (PBS) for 20 minutes on ice. Unbound iodine was separated from human OPG by gel filtration on a Sephadex G25 (Pharmacia, Oslo, Norway) column equilibrated with PBS containing 0.1 mg/mL bovine serum albumin (BSA). The specific activity of the labeled human OPG was 2.3 × 104 cpm/ng and more than 99% of this activity could be precipitated by 20% trichloroacetic acid (TCA).
Cells were incubated with 125I-human OPG (100 ng/mL) on ice for 30 minutes, then washed, reseeded in fresh media in the presence or absence of 25 mM NH4Cl as indicated, and incubated at 37°C for the indicated time periods. The cells were then washed in ice-cold PBS, 1.0 mg/mL BSA, and 100 μg/mL heparin before the cell pellets were dissolved either in 0.1 N KOH and counted in a γ counter or dissolved in sodium dodecyl sulfate (SDS) and subjected to polyacrylamide gel electrophoresis on 15% acrylamide gels under nonreducing conditions. To assess cell binding before incubation at 37°C, an aliquot of cells was washed in PBS, 1 mg/mL BSA, but without heparin, and counted as above. Degraded human OPG was measured by precipitation of proteins from the wash supernatants by adding 0.2 volume ice-cold 20% TCA, centrifugation at 10 000g for 10 minutes, and counting of the supernatant.
Immunohistology of BM biopsies from patients with MM
The BM biopsies were obtained at diagnosis from 1 patient with monoclonal gammopathy of unknown significance (MGUS) and 7 women and 2 men with MM (median age, 76.5 years). Sections (3 μm) of formalin-fixed, paraffin-embedded BM biopsies were deparaffinized, rehydrated, decalcified in formic acid, and treated in a microwave oven in Tris-EGTA (tris(hydroxymethyl)aminomethane-ethyleneglycoltetraacetic acid) buffer. Endogenous peroxidase was quenched by treatment with H2O2. Sections were incubated with either anti-OPG (IMG-103, clone 98A1071, Imgenex San Diego, CA), or as a negative control, CK-7 (Dako, Copenhagen, Denmark). Both antibodies were diluted 1:400 in Tris-buffered saline (TBS), 1% BSA. Immunohistochemical reactions were visualized using biotinylated antimouse immunoglobulin followed by peroxidase-conjugated streptavidin (Histostain TM-Plus Bulk Kits; Zymed Laboratories, South San Francisco, CA) and an AEC-kit (Lab Vision, Fremont, CA). The sections were counterstained with hematoxylin. Myeloma cells were identified by staining with a 1:200 dilution of anti-CD138 (Dako).
Results
Reduced levels of OPG in the BM of patients with MM
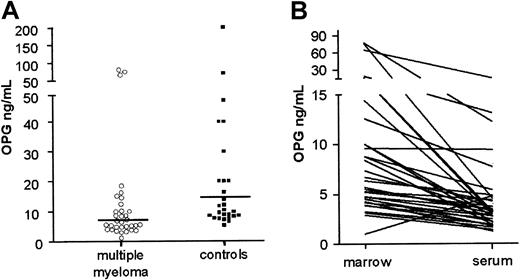
The BM plasma samples of 33 patients with MM and 27 controls were analyzed for OPG content by ELISA. We found that BM OPG levels were lower in MM patients (median 7.6 ng/mL; range 1.0-77.6 ng/mL) than in control patients (median, 10.4 ng/mL; range, 5.3-200 ng/mL; Figure1A). This difference is statistically significant (Mann-Whitney U test, P = .02). Furthermore, when comparing OPG levels in peripheral blood serum with BM plasma in individual patients, we found that the levels of OPG were higher in BM than in blood serum (Figure 1B and data not shown). Median gradient of OPG from BM to blood was 1.9 (range, 0.21-16.9) in MM patients and 2.5 in controls (range, 0.3-15.9). The correlation between OPG serum levels and OPG marrow plasma levels was significant at the 0.01 level (Spearman rho 0.59) in both groups.
Reduced levels of OPG in BM plasma of patients with MM.
(A) BM plasma levels in myeloma (median, 7.6 ng/mL) and control patients (median, 10.4 ng/mL). (B) BM plasma and peripheral blood serum levels of OPG in individual MM patients.
Reduced levels of OPG in BM plasma of patients with MM.
(A) BM plasma levels in myeloma (median, 7.6 ng/mL) and control patients (median, 10.4 ng/mL). (B) BM plasma and peripheral blood serum levels of OPG in individual MM patients.
Heparan sulfate proteoglycans on myeloma cell lines bind OPG
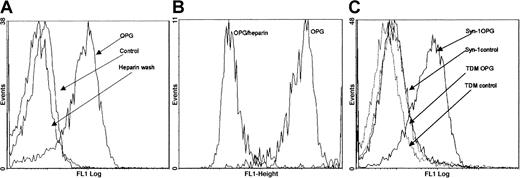
By flow cytometry we found that human OPG bound to the surface of CAG cells (Figure 2A) as well as to other myeloma cell lines such as OH-2, U266, and JJN3 (data not shown). Primary myeloma cells also bound OPG in a similar manner (Figure 2B). Binding was reduced to background levels when it was performed in the presence of heparin (Figure 2B and data not shown). Similarly, washing of the cells in a buffer containing heparin after OPG binding removed cell surface–bound OPG (Figure 2A).
Binding of OPG to myeloma cells.
(A) Binding of OPG to CAG cells, (B) primary myeloma cells, and (C) ARH-77 cells transfected with either intact murine syndecan-1 (Syn-1) or with syndecan-1 lacking heparan sulfate side chains (TDM) examined by flow cytometry. OPG/heparin: preincubation of OPG in the presence of heparin; heparin wash: cells incubated with OPG and subsequently washed with heparin; control: cells incubated with PBS instead of OPG.
Binding of OPG to myeloma cells.
(A) Binding of OPG to CAG cells, (B) primary myeloma cells, and (C) ARH-77 cells transfected with either intact murine syndecan-1 (Syn-1) or with syndecan-1 lacking heparan sulfate side chains (TDM) examined by flow cytometry. OPG/heparin: preincubation of OPG in the presence of heparin; heparin wash: cells incubated with OPG and subsequently washed with heparin; control: cells incubated with PBS instead of OPG.
The predominant proteoglycan on both normal plasma cells and myeloma cells is syndecan-1.14,15 We therefore examined the binding of OPG to the syndecan-1–negative lymphoblastoid ARH-77 cell line transfected with either native syndecan-1 (ARH-77syn-1) or with syndecan-1 lacking heparan-sulfate side chains (ARH-77TDM).20 As shown, cells with native syndecan-1 bound OPG, whereas ARH-77TDM cells did not (Figure 2C). Together, these results indicate that OPG binds heparan sulfates on myeloma cells.
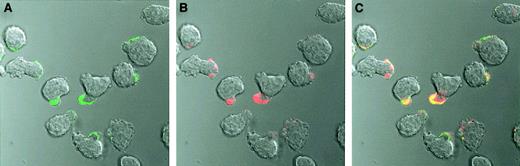
Cell surface binding of OPG was also investigated by confocal microscopy. When we observed CAG cells incubated on ice with Alexa Fluor 488–conjugated OPG, OPG was mainly located on the uropod of polarized myeloma cells (Figure 3A). Furthermore, bound Alexa Fluor 488–conjugated OPG colocalized with Alexa Fluor 594–conjugated CTB (Figure 3C), a known marker for lipid rafts in cell membranes.21 22
Cell surface staining of CAG cells with OPG.
Alexa Fluor 488–conjugated human OPG (green) and Alexa Fluor 594–conjugated CTB (red) colocalize (yellow) on the uropod of polarized CAG cells. The cells were incubated with labeled OPG or CTB or both for 30 minutes on ice, washed, and thereafter examined by confocal microscopy. Original magnification × 630..
Cell surface staining of CAG cells with OPG.
Alexa Fluor 488–conjugated human OPG (green) and Alexa Fluor 594–conjugated CTB (red) colocalize (yellow) on the uropod of polarized CAG cells. The cells were incubated with labeled OPG or CTB or both for 30 minutes on ice, washed, and thereafter examined by confocal microscopy. Original magnification × 630..
OPG binding to heparan sulfate proteoglycans leads to internalization and degradation of OPG
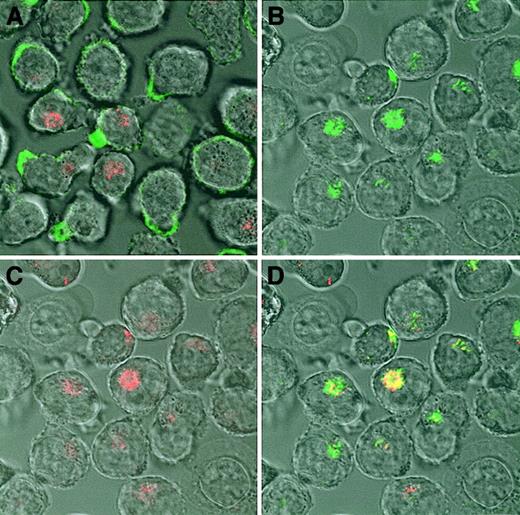
Some ligands are internalized after binding to cell surface heparan sulfate proteoglycans.19 We therefore investigated if internalization and subsequent degradation was the fate of the OPG binding to the surface of myeloma cells. Alexa Fluor 488–conjugated OPG was incubated with CAG cells for one hour at 37°C, then cell surface–bound OPG was removed by heparin wash, and the living cells were examined by confocal microscopy. After 1 hour OPG was clearly internalized, and a substantial part of it colocalized with the lysosomal marker (Figure 4).
Internalization of OPG in CAG cells.
(A) CAG cells incubated on ice for 30 minutes with Alexa Fluor 488–conjugated human OPG (green) shows cell surface binding of OPG. (B-D) Subsequently, the cells were incubated for 1 hour at 37°C, washed, and examined by confocal microscopy. The results show that OPG is internalized, colocalizing (yellow) with the lysosomal marker LysoTracker Red DND-99 (red). Original magnification × 630.
Internalization of OPG in CAG cells.
(A) CAG cells incubated on ice for 30 minutes with Alexa Fluor 488–conjugated human OPG (green) shows cell surface binding of OPG. (B-D) Subsequently, the cells were incubated for 1 hour at 37°C, washed, and examined by confocal microscopy. The results show that OPG is internalized, colocalizing (yellow) with the lysosomal marker LysoTracker Red DND-99 (red). Original magnification × 630.
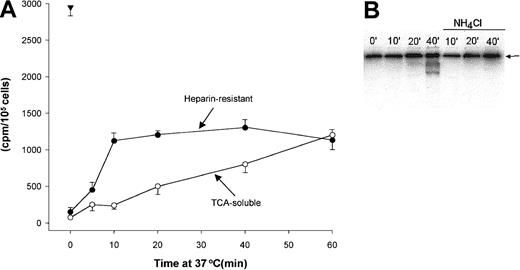
To examine time course of OPG internalization and degradation, CAG cells were incubated with 125I-OPG, and cell surface–bound, internalized, and degraded OPG was measured. Similar to the results obtained by flow cytometry, more than 95% of the bound iodinated OPG could be washed away with heparin when the binding was performed on ice. In contrast, when cells containing surface-bound iodinated OPG were chased by incubation in medium at 37°C, significant amounts of the OPG remained cell bound after heparin wash, indicating that this OPG was internalized (Figure5A). The extent of OPG degradation was estimated by measuring the amount of TCA-soluble iodine released into the medium during the chase period. Little or no OPG was degraded when the cells were incubated on ice, that is, when all cell-associated OPG was on the cell surface. However, with increasing chase periods at 37°C, an increasing amount of iodine was soluble in TCA, indicating that OPG had been degraded to free iodo-tyrosyl residues or to short peptides containing iodo-tyrosyl (Figure 5A). It can be estimated that 106 CAG cells are able to completely degrade in the order of 1 ng OPG/h, suggesting that myeloma cells are able to internalize and degrade a substantial fraction of cell surface–bound OPG.
Internalization and degradation of iodinated OPG in CAG cells.
(A) Cells were labeled with 125I-OPG on ice, washed, and chased by incubation in medium at 37°C as indicated. Heparin-resistant, cell-associated OPG (●) and TCA-soluble radioactivity in cell supernatants (○) were measured as described in “Patients, materials, and methods.” The triangle indicates OPG bound to the cells before the chase period. (B) Polyacrylamide gel electrophoresis of cell pellets containing 125I-OPG. The cells were incubated at 37°C for the indicated time periods in the presence or absence of NH4Cl after labeling on ice. The arrows indicate the position of intact OPG dimers.
Internalization and degradation of iodinated OPG in CAG cells.
(A) Cells were labeled with 125I-OPG on ice, washed, and chased by incubation in medium at 37°C as indicated. Heparin-resistant, cell-associated OPG (●) and TCA-soluble radioactivity in cell supernatants (○) were measured as described in “Patients, materials, and methods.” The triangle indicates OPG bound to the cells before the chase period. (B) Polyacrylamide gel electrophoresis of cell pellets containing 125I-OPG. The cells were incubated at 37°C for the indicated time periods in the presence or absence of NH4Cl after labeling on ice. The arrows indicate the position of intact OPG dimers.
We also analyzed the degradation of OPG by SDS-gel electrophoresis. The dominant band at all time points corresponds to the intact OPG dimer. However with increasing time at 37°C, iodinated degradation products of approximately 60 kDa and 40 kDa could be observed (Figure 5B). When the cells were incubated at 37°C in media containing 25 mM NH4CL, a compound known to increase the pH of intracellular compartments, little or no degradation products of OPG could be observed, indicating that low pH in endosomal/lysosomal compartments is necessary for OPG degradation to occur (Figure 5B).
Myeloma cells in vivo stain for OPG
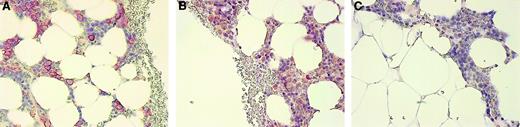
The BM biopsies from 9 patients with MM and 1 with MGUS were evaluated for OPG expression using a monoclonal antibody against OPG. In 6 of the 10 biopsies, plasma cells in the BM stained positive for OPG (Figure 6, and data not shown), indicating that also in vivo, OPG is associated with the plasma cells in the BM of MM patients. Control experiments gave no indication of OPG synthesis by myeloma cells themselves, because both OPG-specific reverse transcription–polymerase chain reaction on myeloma cell line cDNA and Western blots on myeloma cell line extracts stained with anti-OPG were negative (data not shown).
Immunohistologic staining of OPG in a BM biopsy of a myeloma patient.
(A) BM biopsy stained with anti–syndecan-1, (B) anti-OPG, and (C) negative control antibody CK-7 as described in “Patients, materials, and methods.” The staining for OPG was abolished by preincubation of anti-OPG with recombinant human OPG (not shown). Original magnification × 200.
Immunohistologic staining of OPG in a BM biopsy of a myeloma patient.
(A) BM biopsy stained with anti–syndecan-1, (B) anti-OPG, and (C) negative control antibody CK-7 as described in “Patients, materials, and methods.” The staining for OPG was abolished by preincubation of anti-OPG with recombinant human OPG (not shown). Original magnification × 200.
Discussion
In the present study, we demonstrate that OPG levels are reduced in the BM compartment of patients with MM compared to patients without myeloma. Furthermore, we present evidence that the myeloma cells bind, internalize, and degrade OPG, thereby providing a mechanism that may contribute to the reduced BM plasma OPG levels observed in myeloma patients. Moreover, plasma cells in BM biopsies of myeloma patients contain OPG, suggesting that uptake and degradation of OPG by myeloma cells may take place also in vivo.
Normally OPG is present in excess to buffer changes in RANKL expression. A loss of this buffer capacity due to enhanced elimination or reduced production of OPG may render bone homeostasis vulnerable to even minor changes in RANKL expression. Here we propose at least 2 mechanisms for abrogation of the OPG/RANKL balance by the presence of myeloma cells within the BM. First, the degradation of OPG by myeloma cells as shown here may influence the RANKL-OPG balance in favor of increased RANK activation. Second, binding of OPG to syndecan-1 on the surface of myeloma cells may remove OPG from the osteoblast-osteoclast interfaces where it presumably plays its main role. Theoretically, binding of OPG to heparan sulfates per se could inhibit OPG binding to RANKL. However, in a reporter assay using Chinese hamster ovary cells transfected with RANK and stimulated with soluble RANKL, neither soluble syndecan-1 nor heparin affected OPG activity (T.S., unpublished results, December 2000), indicating that occupation of the heparin-binding domain of OPG does not interfere with RANKL binding. Furthermore, the heparin-binding domain is not required for antiresorptive activity in vitro6 or in vivo.23
Recently 2 independent groups10,11 have shown reduced expression of OPG by stromal cells in BM of myeloma patients compared to nonmyeloma patients. Furthermore, data were presented indicating that myeloma cells reduced stromal cell OPG production in vitro,10 11 and this was suggested as a cause of the reduced OPG levels in myeloma marrow. It should be noted that the various proposed mechanisms for OPG reduction are not mutually exclusive. It remains to be determined if they all operate simultaneously within the BM microenvironment, or if any of them play a dominant role, for instance, in certain phases of the bone disease.
The patient material investigated here is too small to allow any conclusions about a possible correlation between clinical stage, signs of osteolysis, and expression of OPG in myeloma cells.
OPG binding to myeloma cells is inhibited by heparin, indicating that binding and internalization is mediated by the major heparan sulfate proteoglycan on myeloma cells, syndecan-1.14 This notion is further supported by the abolished binding of OPG to ARH-77 cells transfected with syndecan-1 lacking the heparan sulfates compared to ARH cells transfected with intact syndecan-1. Heparan sulfate proteoglycans have been shown to mediate the internalization of several extracellular ligands, for example, basic fibroblast growth factor,24 thrombospondin,25follistatin,26Neisseria gonohrroeae,27 and lipoprotein lipase.28,29 Internalization was found to be triggered by clustering of the syndecan transmembrane and cytoplasmic domains,29 and the ligands internalized were directed to degradation in lysosomal compartments.26,29,30 The predominant form of extracellular OPG is a disulfide-linked dimer,6 making cross-linking of syndecan-1 by OPG possible. In our experiments we used both recombinant human OPG-Fc and human OPG, and both forms were efficiently bound, internalized, and degraded (data not shown).
Internalization of lipoprotein lipase by syndecan-1 is mediated by lipid rafts in a mechanism different from the coated pit pathway.28,29 CTB is known to interact with glycosphingolipid,21 a component in lipid rafts, and we therefore used fluorescent-conjugated CTB as a marker of lipid rafts. Borset et al have previously shown that syndecan-1 is localized on the uropod of polarized myeloma cells, and that OPG binds to syndecan-1 on the uropod.31 We observed colocalization of CTB and OPG to the uropod of polarized myeloma cells, indicating that OPG, and thereby probably syndecan-1, is located in lipid rafts. It could thus be that cross-linking of syndecan-1 by OPG induces translocation to lipid rafts, where the internalization process happens. Glycosphingo lipid GM1 was recently shown to be preferentially located to the uropod of polarized T cells,32 and our findings indicate that polarized myeloma cells also show an unequal distribution of GM1.
Serum levels of OPG are shown to be reduced in patients with MM compared to healthy individuals.12 This is in contrast to other diseases associated with increased bone loss, such as postmenopausal osteoporosis33 and seropositive rheumatoid arthritis34 where serum OPG levels are increased. Our finding of a marrow-blood gradient of OPG, both in MM patients and in the control patients, suggests that serum measurements of OPG are informative regarding BM levels of OPG.
Osteoprotegerin has successfully been used to inhibit osteolysis and skeletal tumor burden in various experimental models of bone diseases.35-38 Furthermore, RANK-Fc has proven to be an effective inhibitor of bone resorption in a model of humoral hypercalcemia of malignancy39 and in the severe combined immunodeficiency-human myeloma model.10 The data presented here suggest that it may be of advantage to use OPG lacking the heparin-binding domain in treatment of myeloma bone disease.
We are grateful to Berit Størdal, Birgit Jakobsen, and Hanne Hella for excellent technical assistance.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-04-1190.
Supported by the Norwegian Cancer Society (T.S., C.S., M.B., A.S.); by the Norwegian Research Council (grant 139615/300); by the Cancer Fund, St Olav's Hospital, Trondheim; and by the Danish Medical Research Council (grant 52-00-0374 kg/mp).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Therese Standal, Institute of Cancer Research and Molecular Biology, MTFS, Olav Kyrresgt 3 N-7489 Trondheim, Norway; e-mail: therese.standal@medisin.ntnu.no.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal