Internal tandem duplication (ITD) in the juxtamembrane portion of Fms-like tyrosine kinase 3 (FLT3), a type III receptor tyrosine kinase (RTK), is the most common molecular defect associated with acute myeloid leukemia (AML). The high prevalence of this activating mutation makes it a potential target for molecularly based therapy. Indolinone tyrosine kinase inhibitors have known activity against KIT, another member of the type III RTK family. Given the conserved homology between members of this family, we postulated that the activity of some KIT inhibitors would extend to FLT3. We used various leukemic cell lines (BaF3, MV 4-11, RS 4;11) to test the activity of indolinone compounds against the FLT3 kinase activity of both wild-type (WT) and ITD isoforms. Both SU5416 and SU5614 were capable of inhibiting autophosphorylation of ITD and WT FLT3 (SU5416 concentration that inhibits 50% [IC50], 100 nM; and SU5614 IC50 10 nM). FLT3-dependent activation of the downstream signaling proteins mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription 5 (STAT5) was also inhibited by treatment in the same concentration ranges. FLT3 inhibition by SU5416 and SU5614 resulted in reduced proliferation (IC50, 250 nM and 100 nM, respectively) and induction of apoptosis of FLT3 ITD-positive leukemic cell lines. Treatment of these cells with an alternative growth factor (granulocyte-macrophage colony-stimulating factor [GM-CSF]) restored MAPK signaling and cellular proliferation, demonstrating specificity of the observed inhibitory effects. We conclude that SU5416 and SU5614 are potent inhibitors of FLT3. Our finding that inhibition of FLT3 induces apoptosis of leukemic cells supports the feasibility of targeting FLT3 as a novel treatment strategy for AML.
Introduction
FLT3 (Fms-like tyrosine kinase 3; CD135) is the receptor for the hematopoietic growth factor FLT3 ligand (FL) and belongs to the type III receptor tyrosine kinase (RTK) family that includes KIT (CD117, c-KIT, stem cell factor receptor), and the receptors for macrophage colony-stimulating factor (M-CSF) and platelet-derived growth factor (PDGF). These receptor tyrosine kinases are highly homologous and contain 5 extracellular immunoglobulinlike domains, a single transmembrane domain, and a cytoplasmic tyrosine kinase domain split into 2 subdomains (TK1, TK2). The cytoplasmic juxtamembrane and kinase domains are the most highly homologous regions among members of the type III RTK family and are conserved even between various species.1,2 Binding of ligand to these receptors induces receptor dimerization, stimulation of receptor kinase activity, and initiation of signal transduction. In the absence of ligand binding, these receptors have only minimal kinase activity. However, mutations of these receptors can result in ligand-independent activation of receptor kinase activity.3-7
The most common molecular defect identified in acute myeloid leukemia (AML) patients is an activating mutation of FLT3 tyrosine kinase. In approximately 20% to 30% of patients with AML and 3% of patients with myelodysplasia, FLT3 contains an internal tandem duplication (ITD) of the juxtamembrane domain. More recently, an additional 7% of AML and 3% of myelodysplasia patients were found to have a point mutation in the activation loop of the FLT3 kinase domain.8 These mutations lead to constitutive activation of the FLT3 tyrosine kinase activity and are capable of transforming 32D or BaF3 cells.9-11 BaF3 or 32D cells transfected with an FLT3 ITD are leukemogenic in mice.12In most studies of AML, the presence of this mutation portends a poorer prognosis, regardless of the disease subtype.13-18Although a more aggressive disease phenotype is not observed in every study of FLT3 mutations in AML, loss of the wild-type (WT) allele leaving only the ITD mutation results in both decreased overall survival and higher relapse rates.18
Work to date on mutant FLT3 isoforms has employed either primary AML patient samples or transgenically modified BaF3 or 32D cells. Although both model systems have their merits and are suitable for certain types of experiments, there is only one published report using human AML cell lines with naturally occurring FLT3 ITD mutations. Such cell lines could be useful in the study of FLT3 signaling and the development of novel therapeutics. Indeed, cell models of chronic myelogenous leukemia (CML)19 and acute promyelocytic leukemia (APL)20 have been critical for advancing our understanding of the biology of these diseases and have helped in the development of improved therapeutic approaches. In our experiments, we found that the MV 4-11 biphenotypic leukemia cell line contains a naturally occurring FLT3 ITD mutation, and we report on its uses for the testing of FLT3 inhibitors herein. Our findings with the MV 4-11 cell line confirm the results recently reported by Levis et al.21
SU5416 (Semaxanib; SUGEN, South San Francisco, CA) is an indolinone compound that inhibits the tyrosine kinase activity of flk-1/kinase domain receptor (KDR)22 and KIT.23SU5416 was used in phase 3 clinical trials for the treatment of metastatic colorectal cancer (targeting vascular endothelial growth factor receptor [VEGFR]) and phase 2 trials for leukemia (targeting KIT and/or VEGFR). In preclinical studies, SU5416 treatment of a myeloid cell line that is dependent on ligand-induced KIT signaling resulted in inhibition of KIT receptor autophosphorylation as well as a decrease in cellular proliferation.23Additionally, treatment of KIT-positive AML myeloblasts with SU5416 resulted in apoptosis.23 Given the sequence conservation between the tyrosine kinase domains of KIT and FLT3, we postulated that SU5416 and/or structurally related compounds would have inhibitory effects on FLT3 kinase activity. Unlike tyrosine kinases inhibitors used in previous studies of FLT3,12,24-26 SU5416 is highly specific for flk-1/KDR and KIT and displays minimal effects on epidermal growth factor receptor, insulin receptor, and platelet-derived growth factor receptor.27 As noted above, SU5416 has an established safety record in the clinic.
In this study, we found that SU5416 and the structurally related compound SU5614 inhibit FLT3 tyrosine kinase activity of both wild-type and ITD-mutant isoforms. We demonstrate that both compounds inhibit mutant FLT3 kinase activity and downstream signaling via the mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription 5 (STAT5) pathways. Despite the presence of activating RAS mutations and multiple cytogenetic abnormalities of MV 4-11 cells, both compounds markedly inhibit cellular proliferation and induce apoptosis. These studies indicate that inhibition of FLT3 kinase activity is a novel therapeutic approach to the treatment of AML.
Materials and methods
Cell culture
M-07e cells were graciously provided by Dr Hal Broxmeyer (Indiana University School of Medicine; Indianapolis) and maintained in culture as previously described.28 All other cell lines were obtained through the American Type Culture Collection (ATCC; Manassas, VA). MV 4-11 cells29 and TF-1 cells were maintained in RPMI 1640 (Gibco-BRL, Rockville, MD) supplemented with 10% fetal bovine serum (FBS) and 5 ng/mL recombinant human granulocyte M-CSF (GM-CSF) (Immunex, Seattle, WA). RS 4;11 cells were cultured in RPMI 1640 supplemented with 10% FBS, 4.5 g/L glucose, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 1.0 mM sodium pyruvate. This cell line served as a control as it expresses a WT FLT3 receptor30 and contains a cytogenetic rearrangement similar to MV 4-11. BaF3 cells, a murine interleukin 3 (IL-3)–dependent hematopoietic cell line, were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS and filtered supernatant from WEHI-3 cells (ATCC).
Site-directed mutagenesis and BaF3 mutant transfection
FLT3 cDNA was generously provided by Dr Olivier Rosnet (Molecular Oncology Unit, Institut National de la Santé et de la Recherche Médicale [INSERM], Marseilles, France). Using site-directed mutagenesis, we duplicated bases in the FLT3 cDNA to produce the following amino acid sequence in the FLT3 juxtamembrane domain: Tyr-Glu-Tyr-Asp-Leu-Lys-Tyr-Glu-Tyr-Asp-Leu-Lys-Trp-Glu-Phe-Pro-Arg. This duplicated amino acid sequence is identical to a previous report in a case of human AML.31 The mutant cDNA sequence was confirmed by bidirectional sequencing. The vector encoding the mutant FLT3 ITD was used to create stable transfectants of BaF3 cells.11
Reagents
Indolinone compounds SU5416, SU5614, SU6668, and SU6597 were obtained from SUGEN. These compounds were dissolved in dimethyl sulfoxide (DMSO) to create 10-mM stock solutions that were frozen at −20°C. Human recombinant FL was purchased from R&D systems (Minneapolis, MN).
Polymerase chain reaction
Genomic DNA was obtained from cultured cells with the use of a DNAeasy Tissue Kit (Qiagen, Valencia, CA). The juxtamembrane domain of FLT3 was amplified with the use of the following primer pair: 5′ CTCTTCATTGTCGTTTTAACCC 3′ (sense primer) and 5′ TTCCATTCTTACCAAACTCT 3′ (antisense primer). Polymerase chain reaction (PCR) amplification of genomic DNA was performed with the use of 500 ng whole-cell DNA as previously described.32
WAVE high-performance liquid chromatography (HPLC)
We assessed 5- to 20-μL aliquots of each PCR reaction for FLT3 mutations using a Transgenomic (Omaha, NE) WAVE HPLC system.33 Samples were run at 50°C to distinguish fragments of different lengths. Amplimers were bidirectionally sequenced on an ABI 377 sequencer by means of the BigDye terminator kit (Applied Biosystems, Foster City, CA).32
Antibodies
An anti-FLT3 rabbit polyclonal antibody (C-20; Santa Cruz Biotechnology, CA) was used at 1:1000 dilution. An antiphosphotyrosine antibody (PY20; Transduction Laboratories, Lexington, KY) was used at a 1:1000 dilution. An antiphospho-p44/p42 MAP kinase (Thr202/Tyr204) antibody was used at 1:1000 dilution (New England Biolabs, Beverly, MA). An antiphosphotyrosine-STAT5 antibody (Cell Signaling Technology, Beverly MA) was used at 1:1000 dilution. An antibody to total MAP kinase (New England Biolabs) was used at 1:2000 dilution. A monoclonal antibody to total STAT5 (Santa Cruz Biotechnology) was used at 1:400 dilution. Peroxidase-conjugated goat antimouse antibody and goat antirabbit antibody were used at 1:5000 and 1:10 000 dilutions, respectively (BioRad, Hercules, CA).
Immunoprecipitation and Western blots
Approximately 5 × 107 MV 4-11 or 1 × 107 RS 4;11 cells were serum starved in Opti-Mem (Gibco-BRL) overnight and then exposed to varying concentrations of indolinone compounds (2 hours for SU5416 and 1 hour for SU5614). Cells were then incubated with 100 ng/mL FL for 15 minutes at 37°C before cell pellets were lysed with 100 to 150 μL protein lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane], 150 mM NaCl, 1% Nonidet P-40 [NP-40], 0.25% deoxycholate with added inhibitors aprotinin, AEBSF [4-(2-aminoethyl)-benzenesulfonylfluoride hydrochloride], leupeptin, pepstatin, sodium orthovanadate, and sodium pyruvate). For immunoprecipitation, 1 to 1.5 mg protein from cell lysates was precleared with 5 μL normal rabbit agarose conjugate (Santa Cruz Biotechnology) for 1 hour and then incubated with 5 μL primary antibody overnight at 4°C. Then, 20 μL Protein A/G Plus–Agarose bead (Santa Cruz Biotechnology) was added for 1 hour. This complex was washed 4 times with lysis buffer as listed above, and 15 μL sodium dodecyl sulfate (SDS) sample buffer was added to each. These samples were then analyzed by Western immunoblot assay as previously described.34
Proliferation assays
Cells were added to 96-well plates at densities of 50 000 cells per well for MV 4-11 and RS 4;11, and 20 000 cells per well for BaF3 cells. To decrease growth factor concentration (GM-CSF), MV 4-11 cells were rinsed once in sterile phosphate-buffered saline (PBS) and incubated overnight in DMEM supplemented with 10% FBS. Test compound was added, and proliferation was measured at 72 hours by means of an XTT (2,3-bis[2-methoxyl-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide)–based assay (Roche Molecular Biochemicals, Indianapolis, IN).28
Apoptosis assays
MV 4-11 cells were rinsed with PBS and incubated in DMEM supplemented with 10% FBS. 1 × 106 cells were incubated with SU5416 for 72 hours or SU5614 for 48 hours. Apoptosis was assessed by means of a fluorescein isothiocyanate (FITC)–labeled annexin-V/propidium iodide assay (Immunotech, Marseilles, France).28
Results
SU5416 and SU5614 inhibit proliferation of BaF3 cells expressing a mutant human FLT3 protein
To test the effects of small molecule compounds on the kinase activity of FLT3, we used site-directed mutagenesis to synthetically generate a human FLT3 cDNA with an ITD identical to one previously reported in an AML patient.31 The ITD mutation consisted of an in-frame duplication of the codons encoding for residues 597 through 602 (Tyr-Glu-Tyr-Asp-Leu-Lys).35 Transfection of an expression vector encoding this FLT3 ITD cDNA resulted in factor-independent growth of BaF3 as previously reported.11
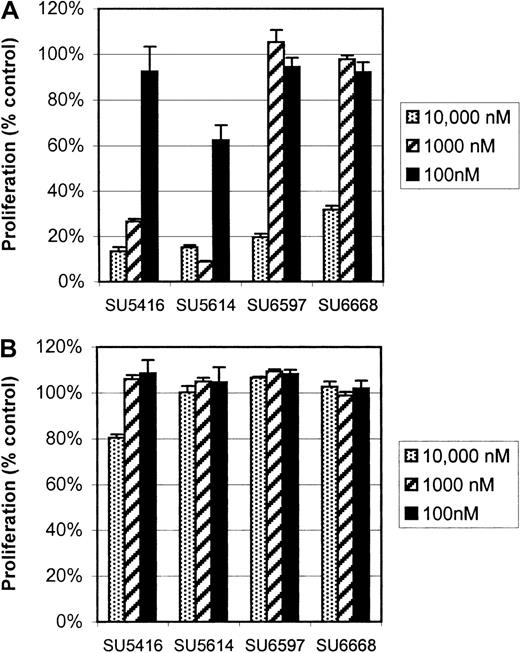
SU5416, SU5614, SU6668, and SU6597 are indolinone compounds selected for their known ability to specifically inhibit KIT23,36and FLK-1/KDR.27 These compounds have varying potency against PDGFR and do not inhibit EGFR or other kinases.37Given that members of the type III and IV receptor tyrosine kinase families share striking homology with highly preserved juxtamembrane and kinase domains,38-40 we postulated that the activity of some of these compounds would extend to FLT3. The 4 compounds were initially screened with our BaF3 ITD-mutant FLT3 cell line with the use of concentrations of 5 μM, 500 nM, and 250 nM. Proliferation of these cells was potently inhibited by SU5416 and SU5614 (Figure1A), with a concentration that inhibits 50% (IC50) for SU5416 at 250 nM. SU5614 was found to be more potent than SU5416; IC50 for this drug was not reached in the screening assay (IC50 < 250 nM). SU6597 and SU6668 affected proliferation only at the highest concentrations tested. In contrast, the proliferation of parental BaF3 cells, grown in the presence of IL-3, was unaffected by any of these 4 compounds even with the use of a dose of 5 μM (Figure 1B).
SU5416 and SU5614 inhibition of proliferation of BaF3 FLT3 ITD-mutant cells but not parental BaF3 cells.
Cells were exposed to inhibitor for 72 hours, and proliferation was assessed with an XTT-based assay. Proliferation is expressed as a percentage of vehicle-treated control cells (error bars indicate standard deviation). (A) In BaF3 FLT3 ITD-mutant cells, SU5416 and SU5614 had the most potent inhibitory effects on proliferation. The IC50 for SU5416 was 250 nM while SU5614 inhibited proliferation at all concentrations tested. By comparison, SU6597 and SU6668 only inhibited proliferation at a 5 μM dose. (B) The proliferation of parental BaF3 cells grown in the presence of IL-3 was not significantly affected by any of the tested concentrations.
SU5416 and SU5614 inhibition of proliferation of BaF3 FLT3 ITD-mutant cells but not parental BaF3 cells.
Cells were exposed to inhibitor for 72 hours, and proliferation was assessed with an XTT-based assay. Proliferation is expressed as a percentage of vehicle-treated control cells (error bars indicate standard deviation). (A) In BaF3 FLT3 ITD-mutant cells, SU5416 and SU5614 had the most potent inhibitory effects on proliferation. The IC50 for SU5416 was 250 nM while SU5614 inhibited proliferation at all concentrations tested. By comparison, SU6597 and SU6668 only inhibited proliferation at a 5 μM dose. (B) The proliferation of parental BaF3 cells grown in the presence of IL-3 was not significantly affected by any of the tested concentrations.
As further proof of the specific nature of the effect of SU5416 and SU5614 on the proliferation of BaF3 ITD-mutant FLT3 cells, we tested the compounds on the proliferation of BaF3 cells transfected with p210 BCR-ABL, NPM-ALK, or v-src (data not shown). None of the 4 compounds had any effect on the proliferation of these factor-independent cells. On the basis of these results, we selected SU5416 and SU5614 for additional study.
MV 4-11 cells have an internal tandem duplication mutation of the FLT3 receptor
In order to further characterize the activity of SU5416 and SU5614, we sought to test these compounds against WT human FLT3 protein kinase activity as expressed in human cell lines. The acute lymphocytic leukemia cell line RS 4;11 has been reported to express wild-type FLT3 and has been previously used to study FLT3 signal transduction.30 We investigated the use of MV 4-11 cells, an AML cell line that contains a similar translocation of chromosomes 4 and 11, as a control in our experiments of FLT3 signal transduction. However, in preliminary experiments to verify FLT3 expression and function, ligand-independent phosphorylation of FLT3 was observed in MV 4-11 but not RS 4;11 cells, suggesting that the MV 4-11 cells might express a mutant FLT3 isoform (data not shown).
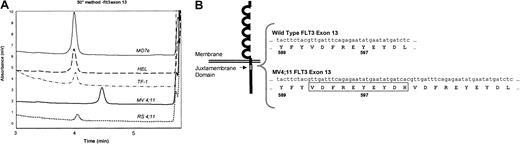
To test this hypothesis, we purified genomic DNA from several human leukemia cell lines, including MV 4-11, RS 4;11, MO7e, and TF-1 cells, and used PCR to amplify the juxtamembrane region of FLT3. The resulting amplicons were then analyzed by high-performance liquid chromatography.33 The FLT3 PCR product obtained from MV 4-11 genomic DNA had a higher molecular weight than that obtained from RS 4;11, MO7e, or TF-1 cells (Figure 2A). Sequencing revealed a homozygous 30–base pair (bp) internal tandem duplication at cDNA position 1803 consisting of GGTGATTTCAGAGAATATGAATATGATCTC.
Genomic DNA ITD mutation of the juxtamembrane domain of FLT3 in MV 4-11 cells.
MV 4-11 cells are homozygous for genomic DNA ITD mutation of the juxtamembrane domain of FLT3. (A) WAVE nondenaturing HPLC of MV 4-11 FLT3 DNA demonstrated a lower mobility peak indicative of a longer amplicon, characteristic for an ITD mutation. The amplimers for RS 4;11, TF-1, MO7e, and HEL were all of similar size and were sequenced and found to be wild type. (B) The MV 4-11 amplicon was sequenced, and the mutation is shown schematically here. MV 4-11 has a 30-bp (10 amino acid) tandem repeat (boxed). Wild-type FLT3 cDNA sequence is provided for comparison.
Genomic DNA ITD mutation of the juxtamembrane domain of FLT3 in MV 4-11 cells.
MV 4-11 cells are homozygous for genomic DNA ITD mutation of the juxtamembrane domain of FLT3. (A) WAVE nondenaturing HPLC of MV 4-11 FLT3 DNA demonstrated a lower mobility peak indicative of a longer amplicon, characteristic for an ITD mutation. The amplimers for RS 4;11, TF-1, MO7e, and HEL were all of similar size and were sequenced and found to be wild type. (B) The MV 4-11 amplicon was sequenced, and the mutation is shown schematically here. MV 4-11 has a 30-bp (10 amino acid) tandem repeat (boxed). Wild-type FLT3 cDNA sequence is provided for comparison.
This in-frame duplication encompasses Tyr597, consistent with previous reports that FLT3 ITD mutations always involve either this codon or Tyr591.41 As seen in Figure 2A, only the mutant FLT3 allele was detected in MV 4-11 cells. The amplicon from RS 4;11 was sequenced and found to be WT. For the reader's convenience, in this manuscript these cell lines will subsequently be referred to as MV 4-11 (FLT3 ITD) and RS 4;11 (FLT3 WT).
SU5416 and SU5614 inhibit the phosphorylation of internal tandem duplication mutant (MV 4-11) and wild-type (RS 4;11) FLT3
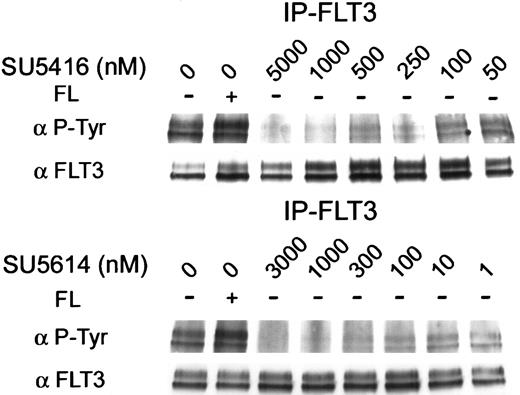
To confirm that SU5416 and SU5614 could inhibit the kinase activity of FLT3 in human leukemia cell lines, we first tested the effect of these 2 compounds on ligand-independent receptor phosphorylation observed in MV 4-11 (FLT3 ITD). This cell line had a low baseline expression of FLT3, requiring immunoprecipitation of FLT3 for our immunoblotting experiments. Antiphosphotyrosine immunoblots of the immunoprecipitated FLT3 protein revealed autophosphorylation of FLT3 protein in the absence of exogenous FL (Figure3). This ligand-independent phosphorylation was inhibited by SU5416 with an IC50 of approximately 100 nM (Figure 3). SU5614 inhibited the ITD-mutant protein phosphorylation with an IC50 of approximately 10 nM (Figure 3). To determine whether SU5416 and SU5614 modulated expression of mutant FLT3 protein, the membrane was stripped and reprobed with anti-FLT3 antibody. There was no change in the expression of FLT3 protein in cells treated with SU5416 or SU5614.
Inhibition of constitutive autophosphorylation of ITD-mutated FLT3 by SU5416 and SU5614.
MV 4-11 (FLT3 ITD) cells were incubated with varying concentrations of inhibitor and stimulated with recombinant human FL. Ligand-independent constitutive autophosphorylation was demonstrated (first 2 lanes), consistent with an activating mutation of FLT3. FLT3 autophosphorylation was ablated by treatment with SU5416 and SU5614 with an IC50 of 100 nM and 10 nM, respectively. The membrane was stripped and reprobed with an antibody to FLT3. FLT3 expression was unaffected by treatment with either inhibitor.
Inhibition of constitutive autophosphorylation of ITD-mutated FLT3 by SU5416 and SU5614.
MV 4-11 (FLT3 ITD) cells were incubated with varying concentrations of inhibitor and stimulated with recombinant human FL. Ligand-independent constitutive autophosphorylation was demonstrated (first 2 lanes), consistent with an activating mutation of FLT3. FLT3 autophosphorylation was ablated by treatment with SU5416 and SU5614 with an IC50 of 100 nM and 10 nM, respectively. The membrane was stripped and reprobed with an antibody to FLT3. FLT3 expression was unaffected by treatment with either inhibitor.
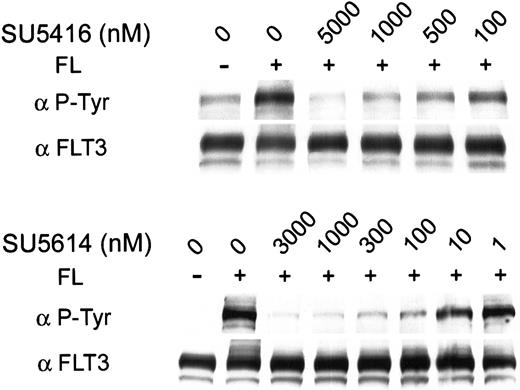
To test whether SU5416 and SU5614 would have similar effects on FL-dependent autophosphorylation of the WT FLT3 receptor, we pretreated RS 4;11 (FLT3 WT) cells with SU5416 or SU5614 before stimulating the cells with FL. FLT3 phosphorylation dramatically increased following treatment with 100 ng/mL recombinant human FL. Pretreatment with SU5416 or SU5614 inhibited ligand-dependent autophosphorylation with an IC50 of 100 nM and 10 nM, respectively (Figure4).
Inhibition of ligand-dependent phosphorylation of wild-type FLT3 by SU5416 and SU5614.
RS 4;11 cells (FLT3 WT) were incubated in varying concentrations of inhibitor and treated with FL. FL-induced receptor autophosphorylation was abrogated by pretreatment with SU5416 or SU5614 with an IC50 of 100 nM and 10 nM, respectively. Total FLT3 protein expression was unaffected by treatment.
Inhibition of ligand-dependent phosphorylation of wild-type FLT3 by SU5416 and SU5614.
RS 4;11 cells (FLT3 WT) were incubated in varying concentrations of inhibitor and treated with FL. FL-induced receptor autophosphorylation was abrogated by pretreatment with SU5416 or SU5614 with an IC50 of 100 nM and 10 nM, respectively. Total FLT3 protein expression was unaffected by treatment.
To confirm that the effect was a result of inhibition of phosphorylation rather than down-regulation of FLT3 expression, we reprobed the blot with an antibody for FLT3. As shown in Figure 4, neither SU5416 nor SU5614 had an effect on expression of total FLT3. Therefore, we conclude that these compounds decrease both mutant and wild-type FLT3 autophosphorylation by decreasing FLT3 kinase activity without down-regulating FLT3 protein expression.
SU5416 and SU5614 inhibit FLT3-dependent phosphorylation of STAT5
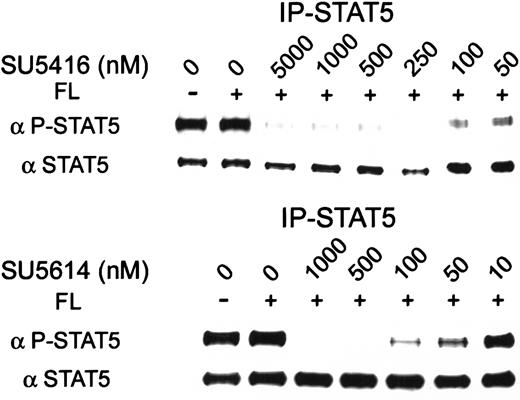
FLT3 signal transduction has been reported to occur via several pathways, including activation of STAT5.9 To test for the effect of these inhibitors on FLT3 signal transduction, we immunoprecipitated STAT5 protein from MV 4-11 (FLT3 ITD) cells treated with various concentrations of inhibitor. Phosphorylation was then assessed with the use of an antiphosphotyrosine-STAT5 antibody. SU5416 and SU5614 inhibited STAT5 phosphorylation with an IC50 of 100 nM and 10 to 50 nM, respectively. Both inhibitors decreased STAT5 phosphorylation without altering total STAT5 expression (Figure 5).
SU5416 and SU5614 inhibition of constitutive STAT5 activation in cells expressing FLT3 ITD.
MV 4-11 (FLT3 ITD) cells were incubated with varying concentrations of inhibitor and stimulated with FL. In this immunoprecipitate using total STAT5 antibody, phosphorylation was ligand-independent but could be inhibited by SU5416 or SU5614 with an IC50 of 100 nM and 10 to 50 nM, respectively. Total STAT5 protein expression was unaffected by treatment.
SU5416 and SU5614 inhibition of constitutive STAT5 activation in cells expressing FLT3 ITD.
MV 4-11 (FLT3 ITD) cells were incubated with varying concentrations of inhibitor and stimulated with FL. In this immunoprecipitate using total STAT5 antibody, phosphorylation was ligand-independent but could be inhibited by SU5416 or SU5614 with an IC50 of 100 nM and 10 to 50 nM, respectively. Total STAT5 protein expression was unaffected by treatment.
Inhibition of STAT5 phosphorylation occurred in an inhibitor dose range similar to that required to inhibit FLT3 activation. These results were consistent with the hypothesis that SU5416 and SU5614 inhibited FLT3 kinase activity required for downstream signaling via the STAT5 pathway.
SU5416 and SU5614 inhibit phosphorylation of MAP kinase
In addition to its effects on the STAT5 pathway, activation of FLT3 kinase also results in activation of MAPK. To assess the effects of SU5416 and SU5614 on the FLT3-dependent activation of MAPK, we probed lysates prepared from inhibitor-treated MV 4-11 (FLT3 ITD) cells with an antibody specific for phosphorylated MAPK. In MV 4-11 (FLT3 ITD) cells, the IC50 for inhibition of MAPK phosphorylation was 100 nM for SU5416 and 10 nM for SU5614 (Figure6A). In contrast to the effect of these compounds on MAPK phosphorylation, the inhibitors had no effect on overall expression of MAPK protein.
Effect of SU5416, SU5614, and GM-CSF on constitutive MAPK activation in cells expressing FLT3 ITD.
SU5416 and SU5614 inhibit constitutive MAPK activation in cells expressing FLT3 ITD; GM-CSF restores signaling. MV 4-11 (FLT3 ITD) cells were incubated with varying concentrations of inhibitor and stimulated with ligand. (A) MAPK was constitutively phosphorylated in these cells, and activation was not augmented by treatment with FL. This constitutive activation was inhibited by both SU5416 and SU5614 with an IC50 of 100 nM and 10 nM, respectively. Despite the presence of activating RAS mutations in MV 4-11 (FLT3 ITD) cells, MAPK activation appeared to be dependent upon FLT3 activation. (B) Ligand-independent MAPK activation was completely inhibited by 1 μM concentrations of either SU5416 or SU5614. Treatment with FL did not induce additional MAPK phosphorylation. Stimulation with GM-CSF restored MAPK activation in cells pretreated with SU5416 or SU5614. Total MAPK protein was unaffected by treatment.
Effect of SU5416, SU5614, and GM-CSF on constitutive MAPK activation in cells expressing FLT3 ITD.
SU5416 and SU5614 inhibit constitutive MAPK activation in cells expressing FLT3 ITD; GM-CSF restores signaling. MV 4-11 (FLT3 ITD) cells were incubated with varying concentrations of inhibitor and stimulated with ligand. (A) MAPK was constitutively phosphorylated in these cells, and activation was not augmented by treatment with FL. This constitutive activation was inhibited by both SU5416 and SU5614 with an IC50 of 100 nM and 10 nM, respectively. Despite the presence of activating RAS mutations in MV 4-11 (FLT3 ITD) cells, MAPK activation appeared to be dependent upon FLT3 activation. (B) Ligand-independent MAPK activation was completely inhibited by 1 μM concentrations of either SU5416 or SU5614. Treatment with FL did not induce additional MAPK phosphorylation. Stimulation with GM-CSF restored MAPK activation in cells pretreated with SU5416 or SU5614. Total MAPK protein was unaffected by treatment.
MV 4-11 (FLT3 ITD) cells, unlike RS 4;11 (FLT3 WT) cells, express a receptor for GM-CSF. To confirm that the observed effects of SU5416 and SU5614 are specific to effects on FLT3 kinase and not the result of nonspecific toxicity, we pretreated MV 4-11 (FLT3 ITD) cells with inhibitor and then stimulated the cells with either 100 ng/mL FL or GM-CSF. As noted above, addition of FL does not induce resultant phosphorylation of MAP kinase after pretreatment with 1 μM SU5416 or SU5614. However, the addition of 100 ng/mL GM-CSF permitted MAPK phosphorylation despite the presence of these inhibitors (Figure6B). We therefore concluded that the observed inhibitory effects of these compounds on MAPK activation was specific to FLT3 signal blockade.
SU5416 and SU5614 inhibit proliferation of MV 4-11 (FLT3 ITD) but not RS 4;11 (FLT3 WT) cells
To test the hypothesis that ligand-independent activation of FLT3 contributes to the growth of MV 4-11 (FLT3 ITD) cells, we tested the effects of SU5416 and SU5614 on these cells in short-term proliferation assays (Figure 7A). Treatment of MV 4-11 (FLT3 ITD) with 1 μM SU5416 or SU5614 decreased proliferation of MV 4-11 (FLT3 ITD) cells by 73% ± 1% and 91% ± 0% respectively as compared with vehicle-treated cells. In the presence of 100 nM SU5614, proliferation was decreased by 38% ± 6% compared with vehicle-treated controls. Similar to the observation in the BaF3-screening cell assays, SU6597 and SU6668 inhibited the proliferation of MV 4-11 (FLT3 ITD) only at the highest inhibitor concentration tested.
Effect of SU5416 and SU5614 on MV 4-11 (FLT3 ITD) and RS 4;11 (FLT3 WT) cell proliferation.
SU5416 and SU5614 inhibit proliferation of MV 4-11 (FLT3 ITD) cells but not RS 4;11 (FLT3 WT) cells. Cells were incubated with inhibitors for 72 hours, and proliferation assessed with an XTT-based assay. Proliferation is expressed as a percentage of vehicle-treated controls (error bars indicate 1 standard deviation). (A) For MV 4-11 (FLT3 ITD), the IC50 was between 100 and 1000 nM for SU5416, while it was approximately 100 nM for SU5614. SU6597 and SU6668 affected proliferation only at the 10-μM dose. (B) RS 4-11 (FLT3 WT) are not dependent on FL for proliferation, and only the highest dose of SU5416 had an effect on proliferation. There was no significant effect of the other compounds on the growth of RS 4;11 (FLT3 WT). These experiments were performed without addition of FL.
Effect of SU5416 and SU5614 on MV 4-11 (FLT3 ITD) and RS 4;11 (FLT3 WT) cell proliferation.
SU5416 and SU5614 inhibit proliferation of MV 4-11 (FLT3 ITD) cells but not RS 4;11 (FLT3 WT) cells. Cells were incubated with inhibitors for 72 hours, and proliferation assessed with an XTT-based assay. Proliferation is expressed as a percentage of vehicle-treated controls (error bars indicate 1 standard deviation). (A) For MV 4-11 (FLT3 ITD), the IC50 was between 100 and 1000 nM for SU5416, while it was approximately 100 nM for SU5614. SU6597 and SU6668 affected proliferation only at the 10-μM dose. (B) RS 4-11 (FLT3 WT) are not dependent on FL for proliferation, and only the highest dose of SU5416 had an effect on proliferation. There was no significant effect of the other compounds on the growth of RS 4;11 (FLT3 WT). These experiments were performed without addition of FL.
In contrast to the results with MV 4-11 (FLT3 ITD) cells, the RS 4;11 (FLT3 WT) cells were only marginally inhibited with the use of a 10-μM dose of SU5416 (Figure 7B). This demonstrated that the indolinone inhibitors exhibit minimal nonspecific toxicity on leukemia cells. The inhibitory effect on the MV 4-11 (FLT3 ITD) cell line in the nanomolar range suggested that a specific proliferative signaling pathway was being inhibited by SU5416 and SU5614. As noted previously, FLT3 phosphorylation, as well as its downstream targets (STAT5 and MAPK), was inhibited in a similar and consistent range. The marked 100-fold difference between specific activity and nonspecific toxicity suggests a favorable therapeutic index.
GM-CSF restores proliferation of FLT3-inhibitor–treated MV 4-11 (FLT3 ITD) cells
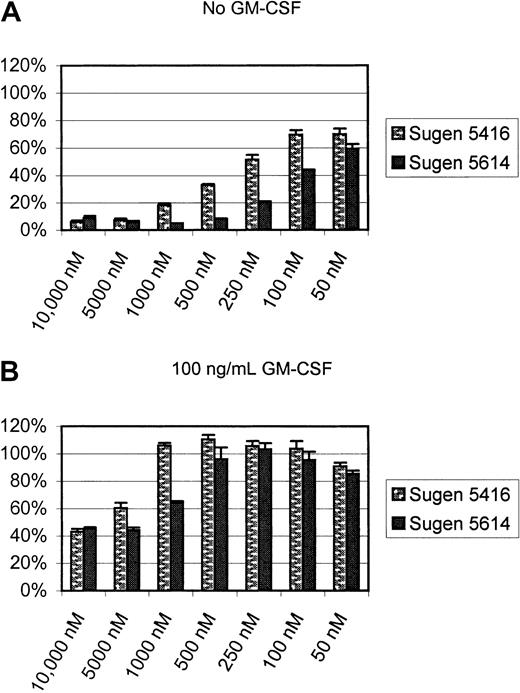
Given the observation that phosphorylation of MAPK could be restored by treatment with GM-CSF, proliferation assays were conducted with and without treatment with this growth factor. As noted above, in the absence of exogenous GM-CSF, the addition of SU5416 or SU5614 dramatically inhibited the proliferation of MV 4-11 (FLT3 ITD) cells with and IC50 of 250 nM and 100 nM, respectively (Figure8A). However, in the presence of supraphysiologic doses (100 ng/mL) of GM-CSF, MV 4-11 (FLT3 ITD) cells were inhibited only by 60%, even in the presence of 10-μM concentrations of each inhibitor (Figure 8B). In the presence of GM-CSF, concentrations of 1 μM for SU5416 and 500 nM for SU5614 had no effect on cellular proliferation. The restoration of proliferation by exogenous GM-CSF further suggests that the antiproliferative effect of these compounds in this experimental system is due to specific inhibition of FLT3 kinase activity.
GM-CSF restores proliferation of MV 4-11 in the presence of FLT3 inhibitors.
Cells were incubated with varying concentrations of inhibitors with or without GM-CSF for 72 hours, and proliferation was assessed with an XTT-based assay. The proliferation values are expressed as a percentage of vehicle-treated controls (error bars indicate standard deviation). (A) In the absence of GM-CSF, the IC50 for SU5416 and SU5614 were similar to our previous experiments (Figure 7). (B) However, in the presence of 100 ng/mL GM-CSF, no more than 60% inhibition of proliferation could be achieved even with a dose of 10 μM.
GM-CSF restores proliferation of MV 4-11 in the presence of FLT3 inhibitors.
Cells were incubated with varying concentrations of inhibitors with or without GM-CSF for 72 hours, and proliferation was assessed with an XTT-based assay. The proliferation values are expressed as a percentage of vehicle-treated controls (error bars indicate standard deviation). (A) In the absence of GM-CSF, the IC50 for SU5416 and SU5614 were similar to our previous experiments (Figure 7). (B) However, in the presence of 100 ng/mL GM-CSF, no more than 60% inhibition of proliferation could be achieved even with a dose of 10 μM.
Treatment with SU5416 and SU5614 results in apoptosis of MV 4-11 (FLT3 ITD) cells
In our proliferation assays, we observed not only a decrease in proliferation but also morphologic features consistent with cellular death (data not shown). On the basis of these observations, we hypothesized that FLT3 inhibition induced apoptosis of MV 4-11 (FLT3 ITD) cells. To test this hypothesis, MV 4-11 (FLT3 ITD) cells were incubated with SU5416 and SU5614 for 72 and 48 hours, respectively. Apoptosis was assayed by means of flow cytometry to measure cellular binding of an annexin V–FITC conjugate as well as uptake of the vital stain propidium iodide. A dose of 2.5 μM SU5416 induced approximately 40% of the MV 4-11 cells to undergo apoptosis (Figure9). SU5614 was more potent; treatment with this compound resulted in apoptosis of 80% of the cells at 2.5 and 1 μM. The IC50 for this effect was approximately 500 nM.
Induction of apoptosis of MV 4-11 (FLT3 ITD) cells by SU5416 and SU5614.
Cells were incubated with SU5416 for 72 hours and SU5614 for 48 hours, and apoptosis was assessed by means of a flow cytometric assay of annexin V binding and propidium iodide exclusion. Values represent the percentage of cells demonstrating early or late stages of apoptosis. SU5416 was less potent, inducing less than 40% apoptosis in the cells treated with 2.5 μM. By comparison, SU5614 displayed more marked effects, with an IC50 of approximately 500 nM. No effects are noted when inhibitors are used at concentrations of less than 250 nM.
Induction of apoptosis of MV 4-11 (FLT3 ITD) cells by SU5416 and SU5614.
Cells were incubated with SU5416 for 72 hours and SU5614 for 48 hours, and apoptosis was assessed by means of a flow cytometric assay of annexin V binding and propidium iodide exclusion. Values represent the percentage of cells demonstrating early or late stages of apoptosis. SU5416 was less potent, inducing less than 40% apoptosis in the cells treated with 2.5 μM. By comparison, SU5614 displayed more marked effects, with an IC50 of approximately 500 nM. No effects are noted when inhibitors are used at concentrations of less than 250 nM.
Discussion
Acute myeloid leukemia remains a difficult disease to treat. Patients typically respond to initial treatment with anthracycline and cytosine arabanoside–based induction chemotherapy, but most patients ultimately experience relapse and die of their disease. The situation is more dire for elderly patients. AML patients in this subset experience substantially greater treatment-related toxicities with fewer durable responses and are rarely cured. In addition, the elderly are usually not candidates for more aggressive therapy using stem cell transplantation. AML in older patients is typically associated with multiple adverse prognostic factors, including high-level multidrug resistance (MDR) expression, preceding myelodysplasia, multiple cytogenetic abnormalities, and FLT3 mutations. FLT3 ITD mutations, found in 20% to 30% of cases of de novo AML, are the most common molecular abnormality in AML, and these mutations may be more common in elderly patients.42
Although it is unclear if an FLT3 ITD mutation alone is sufficient to cause de novo leukemia, such a mutation can cause transformation in cell lines.9-11 Transplantation of murine bone marrow transduced with a retrovirus encoding for FLT3 ITD cDNA into lethally irradiated mice produces a fatal myeloproliferative syndrome.43 Mice receiving FLT3-ITD–transduced stem cells develop splenomegaly and bone marrow hypercellularity owing to massive expansion of myeloid lineage cells. Another model employing a synthetically generated TEL-FLT3 construct also produces splenomegaly and leukocytosis in transgenic mice.44 It should be noted, however, that a TEL-FLT3 fusion gene has never been documented in human disease. These findings suggest that mutations in FLT3 may play a significant, albeit not a singular, role in leukemogenesis, thus making such mutations a target for molecularly based therapy analogous to BCR-ABL in blast-crisis CML. In that disease, impressive response rates were reported in patients treated with a selective tyrosine kinase inhibitor, imatinib mesylate, as a single agent.45
We used 2 complementary cell-based systems to assay small molecules for activity against FLT3 kinase activity. The BaF3 model uses an IL-3–dependent murine cell line rendered factor-independent via transfection of an expression vector encoding an ITD-mutated FLT3 coding sequence. This model system provides a versatile platform for testing of potential FLT3 inhibitors against various classes of FLT3 mutation. Novel insertion or point mutations discovered from genotypic studies of the FLT3 gene in AML specimens can be synthetically generated by means of site-directed mutagenesis of FLT3 cDNA and expressed in BaF3 cells.
Although the use of BaF3 cell–based models is useful in screening compounds for activity against mutant FLT3 isoforms, this model has several shortcomings. First, it is based on the use of murine factor–independent cell lines. Although the human and murine FLT3 proteins are highly conserved, there are a number of potentially confounding biologic differences in downstream signaling pathways.30,46,47 Thus, the expression of human or murine FLT3 in a murine cellular context may not be completely representative of the biology of human FLT3 in a human leukemic cell. In addition, the expression of activated FLT3 renders such cells artificially dependent upon this receptor tyrosine kinase for survival. However, in human AML, the genetic background is more complicated, and mutant FLT3 isoforms are expressed in a cellular context of other molecular abnormalities such as blocked cellular differentiation due to loss of functional activity of individual transcription factors (eg, AML1), RAS mutations, or loss of functional activity of p53 and/or other tumor suppressor genes.42 48
In this respect, the MV 4-11 (FLT3 ITD) cell line serves as a useful experimental model of FLT3 ITD-positive AML. These cells express a homozygous in-frame ITD mutation of FLT3, consistent with those previously reported in 20% to 30% of AML patients.13,15,49,50 In our experiments, in vitro treatment of MV 4-11 (FLT3 ITD) cells with SU5416 and its sister compound SU5614 resulted in nearly complete inhibition of FLT3 autophosphorylation as well as a decrease in downstream FLT3 signal transduction through STAT5 and MAP kinase.9 STAT5 is constitutively phosphorylated in AML myeloblasts, and ITD mutations of FLT3 appear to be the cause of STAT5 activation in many of these cases.51 SU5416 and SU5614 decreased proliferation in similar dose range in terms of their effects on protein phosphorylation. The dose range of antiproliferative effects on MV 4-11 paralleled those of the generated BaF3 ITD cell line and the relative efficacy of the all 4 indolinone compounds in these 2 cell lines is remarkably similar. Treatment with SU5416 and SU5614 induced apoptosis of the MV 4-11 cells, which, although at 10-fold higher concentrations than doses required to inhibit proliferation, is still consistent with the hypothesis that FLT3 inhibition is linked to this event. GM-CSF treatment restored MAPK activation and cellular proliferation of MV 4-11 (FLT3 ITD) cells treated with SU5416 and SU5614, demonstrating that many intracellular signaling pathways remain intact after treatment with these inhibitors. It is conceivable that inhibition of an alternative kinase common to mutant ras and FLT3- but not GM-CSF–dependent signal transduction is responsible for our observations with SU5416 and SU5614. However the similar dose-effect range of these compounds for inhibition of FLT3 kinase activity, MAPK and STAT5 activation, and cellular proliferation suggests that FLT3 inhibition is largely responsible for the antiproliferative effect of these compounds on MV 4-11 (FLT3 ITD) cells. We therefore propose that the MV 4-11 (FLT3 ITD) cell line can serve as a model of an FLT3 ITD-mutant leukemia with uses similar to those of K-562 (chronic myelogenous leukemia)19 and NB4 (acute promyelocytic cell line).20
The finding that FLT3 inhibition using small molecule inhibitors can produce apoptosis of a human leukemia cell line is provocative. Unlike the synthetically generated BaF3 transfects, MV 4-11 (FLT3 ITD) has multiple mutations of the RAS proto-oncogene52 as well as chromosomal translocation involving the MLL gene (t(4;11)(q21:23)) and trisomy of chromosomes 8 and 19. Despite the presence of activating RAS mutations, constitutive MAPK activation in these cells is dependent upon FLT3 activation as inhibition of FLT3 phosphorylation by SU5416 and SU5614 completely blocks MAPK activation. Inhibition of FLT3 signaling in MV 4-11 (FLT3 ITD) cells by these compounds induces apoptosis despite the presence of multiple genetic abnormalities. These findings attest to the feasibility of targeting the FLT3 receptor in leukemic cells with complex genomic and cytogenetic abnormalities, such as are often present in human AML cells.
A phase 2 trial of SU5416 in patients with AML with more than 30% KIT-positive cells in bone marrow or blood has been completed.53 In addition, a patient in second relapse of KIT-positive AML was treated with SU5416 on a compassionate-use protocol.54 The patient had normalization of bone marrow by morphology and flow cytometry and achieved a complete hematological remission (with the exception of a platelet count that rose only to 50 to 80 × 109/L). These data indicate that SU5416 has clinical activity in AML, but the mechanism of action needs to be further elucidated. Because SU5416 also inhibits KDR signaling, the observed responses may result from the antiangiogenic effects of SU5416 rather than its effects on FLT3 and/or KIT.55 SU5416 was the first tyrosine kinase inhibitor with known activity against FLT3 used in clinical trials of AML. Additional compounds that inhibit FLT3, from several companies, are now entering clinical development.21,56 57 These trials have been specifically designed to study the effects of FLT3 inhibition in patients with FLT3 ITD-positive AML and will help clarify the role of inhibiting FLT3 kinase activity in the treatment of this disease.
As with other tyrosine kinase inhibitors tested in clinical trials, SU5416 monotherapy has been well tolerated without significant hematologic toxicity. Responses were seen in a limited number of patients, were of short duration, and were not complete. These results are analogous to blast-crisis CML where treatment with STI571 resulted in initial responses, but the vast majority of patients had a relapse of the disease.45 It is possible that combining targeted therapies with cytotoxic chemotherapy may improve clinical outcome. The most successful application of such a combination in acute leukemia to date has been the use of all-trans retinoic acid (ATRA) in the treatment of acute promyelocytic leukemia. Treatment with ATRA as a single agent results in early remission but unacceptably high relapse rates. However, the use of ATRA in combination with traditional chemotherapeutic agents produces the highest sustained remissions rate of any French-American-British (FAB) subtype of AML.58 59 In this manner, the targeting of FLT3 in combination with other agents may prove useful in the development of more effective therapy for patients with AML.
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2002-02-0531.
Supported in part by a Merit Review Grant from the Department of Veterans Affairs (M.C.H.).
Several of the authors (A.O., B.D.S., J.M.C., G.M.) are employed by a company (SUGEN) whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Heinrich, R&D-19 3710 SW US Veterans Hospital Rd, Portland, OR 97201; e-mail: heinrich@ohsu.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal