Myelodysplastic syndromes (MDSs) are heterogeneous diseases of bone marrow (BM) cell precursors for which immunophenotypic characterization is still considered irrelevant despite the accuracy and sensitivity of flow cytometry techniques. The aim of this study was to determine whether immunophenotypic abnormalities could be defined in MDSs and could correlate with the French-American-British classification and cytogenetics. Analysis was performed on 275 BM samples (207 MDS patients, 68 controls) and 25 control blood samples. Immunophenotyping was based on a primary gating of blast cells, monocytes, and granulocytes according to CD45 antigen expression and side scatter light diffraction. Immunophenotypic hierarchical clustering was performed to analyze the results. The data obtained show that (1) immunophenotypic clustering partly discriminates patients with refractory anemia with excess blasts/refractory anemia with excess blasts in transformation (RAEB/RAEB-T), chronic myelomonocytic leukemia (CMML), and refractory anemia/refractory anemia with ring sideroblasts (RA/RARS) for CD45lo blast cells and patients with RA/CMML, RARS, and RAEB/RAEB-T for CD45hi/side scatterhi (SShi) granulocytes; (2) the most discriminating markers were CD16, CD34, CD36, CD38, CD71, and HLA-DR for blast cells and CD11b, CD13, CD33, CD36, CD38, CD71, and HLA-DR for CD45hi/SShigranulocytes; (3) clusters related to CD34 expression were associated with high levels of blast cells on BM smear; (4) clusters related to high levels of CD36 expression on CD45lo blast cells and CD45hi/SShi granulocytes were associated with a poor International Prognosis Scoring System score; and (5) high levels of CD71 expression on CD45hi/SShi granulocytes were associated with the RARS category. These results show a close relationship between immunophenotypic abnormalities and BM dysplasia and suggest that flow cytometry could be a future tool for the characterization of MDSs.
Introduction
Myelodysplastic syndromes (MDSs) are heterogeneous clonal diseases of myeloid bone marrow (BM) precursor cells. The incidence of MDS is 5/100 000. Its frequency increases with age and the median age at diagnosis is 70 years old.1 Most cases are idiopathic. MDSs are characterized by BM cell dysplasia and persistent cytopenia. The evolution of the disease may be indolent, with a slow decrease of blood cell counts, or it may have a more aggressive course characterized by a worsening severe cytopenia or transformation in acute myeloid leukemia.2 Because of the heterogeneity of the patients with MDS and the poor global outcome of patients with MDS, a major goal in this disease is to define reliable and efficient diagnosis and prognosis markers.
The diagnosis of MDS is based primarily on cytologic analysis of BM smears. The French-American-British (FAB) classification of MDS distinguishes 5 categories: refractory anemia (RA), refractory anemia with ring sideroblasts (RARS), refractory anemia with excess of blasts (RAEB), refractory anemia with excess of blasts in transformation (RAEB-T), and chronic myelomonocytic leukemia (CMML).3,4The threshold is 5% of blasts between RA and RAEB and 20% of blasts between RAEB and RAEB-T. The International Prognosis Scoring System (IPSS) includes cytopenia, the percentage of blasts, and cytogenetic abnormalities.5 The World Health Organization classification of MDS takes into account both cytologic and cytogenetic features. However, by contrast with acute leukemia and lymphoproliferative diseases, immunophenotyping is considered as poorly relevant in the diagnosis of MDS.6
Even though flow cytometry is becoming an increasingly valuable tool in the diagnosis and follow-up of hematopoietic neoplasms, this technique of immunophenotyping is still ill used for MDSs. In a review of the literature, Elghetany7 has pointed out the conflicting results on this matter. More recently, Stetler-Stevenson et al10 have reported an immunophenotypic study of BM cells from patients with MDS, based on an eyeballing analysis of the flow cytometry results.8 9 These authors reached the conclusion that flow cytometry was not useful as a screening procedure for MDS, but stressed its interest for MDS diagnosis in cases in which morphology and cytogenetics are indeterminate. In fact, in addition to the heterogeneity of the cell suspension obtained from BM aspiration of patients with MDS, difficulties arise from the fact that 2 categories of immunophenotypic abnormalities may exist because cell subsets may either express an unexpected marker or express a classical marker with unexpected intensity.
Because of the conflicting results reported in the literature, the Groupe d'Etude Immunologique des Leucémies (GEIL) has conducted a prospective multicenter study on flow cytometry analysis of BM cells from patients with MDSs to evaluate the relationships between myelodysplasia and immunophenotypic abnormalities, assessing the future usefulness of flow cytometry in the staging of patients with MDSs regarding both the diagnosis and the prognosis. Our analysis was based on the quantification of the fluorescence ratio between the measured mean fluorescence intensity of the marker tested and the measured mean autofluorescence of the cells. We show that patients with MDSs can be clustered according to the immunophenotype of both CD45loblast cells and CD45hi/side scatterhi(SShi) granulocytes. Immunophenotypic clustering of patients with MDSs was correlated with the FAB classification, the percentage of blast cells in BM smears, and cytogenetic data. These results evidenced the relationship between BM dysplasia and objective and quantitative immunophenotypic abnormalities. Furthermore, based on the calculated intensity of fluorescence ratios, our results show that expression of CD34 and CD36 on CD45lo blast cells, or expression of CD36 and CD71 on CD45hi/SShigranulocytes, identified specific immunophenotypic subgroups of patients with MDSs.
Patients, materials, and methods
Patients and controls
For this prospective multicenter study, 207 patients were enrolled in 8 different centers between January 1999 and March 2001. The patients were suffering from primary MDS. BM aspiration was performed only for diagnosis or for the cytogenetic staging of the disease. Diagnosis and FAB classification were based on the analysis of BM smears after standard May-Grünwald-Giemsa staining and after Perl staining of erythroblasts. A centralized review of the slides was performed by 2 of us (F.P. and B.C.). Patients' scoring and determination of the cytogenetic risk were performed according to the IPSS5 in all cases where cytogenetic data were available.
The BM samples were also obtained from 68 control subjects without any known hematologic disease and with normal blood cell counts who underwent sternotomy for cardiac surgery and gave their informed consent for BM aspiration during the anesthesia. Control blood samples were obtained from 25 subjects without any known hematologic disease and with normal blood cell counts. This study was approved by the Comité Consultatif de Protection des Personnes en Recherche Biologique (CCPPRB) of Dijon (France).
Flow cytometry analysis of cell specimens
Immunolabeling and flow cytometry analysis of the different BM cell subsets were performed on the whole BM/peripheral blood after red blood cell lysis according to standard procedures recommended by the GEIL11 and after establishing a consensus standardized method. This standardized protocol was based on the experience acquired in the diagnosis of acute leukemia. The panel included 3-color combinations of markers (Table 1). In this panel, the CD45 marker was tested in each combination to allow a primary gating of BM cell subsets based on CD45 antigen expression and SS laser light diffraction. Cell subsets recognized by this method were blast cells (CD45lo/SSlo), lymphocytes (CD45hi/SSlo), monocytes (CD45hi/SSintermediate) and granulocytes (CD45hi/SShi).12Phycoerythrin (PE)–cyanine 5 (Cy5)–conjugated CD45 monoclonal antibody (mAb; clone Immu 19.2) was kindly provided to the group for this specific study by Beckman Coulter France (Villepinte Roissy CDG, France). Clones for the other mAbs were chosen freely by each participant and originated from Becton Dickinson (Le Pont-de-Claix, France), Beckman Coulter, and Dako (Trappes, France). Fluorochromes were assigned for each marker tested as indicated in Table 1. The markers tested in this panel were chosen by reference to markers that have been proven to be efficient in the diagnosis of acute myeloid leukemias (AMLs).13 Internal controls were inserted by testing CD36 twice and CD34 expression 3 times (twice with a fluorescein isothiocyanate [FITC]–conjugated mAb and once with a PE-conjugated mAb). The flow cytometers used were from Becton Dickinson or Beckman Coulter. Data were acquired in linear mode for SS and in log mode for fluorescent signal. The fluorescent light (FL) 4 photomultiplier was adjusted so that lymphocytes were positioned at the beginning of the fourth decade of the FL4 axis on a CD45/SS dotplot. FL1 and FL2 were adjusted so that the peak of autofluorescence appeared within the first decade of the corresponding axis. FL1/FL2 compensations were adjusted on an electronic FL4/SS gate corresponding to lymphocytes after a Cy5-CD45/PE-CD4/FITC-CD8 triple labeling. Each of the 3 gates defining blast cells, monocytes, and granulocytes were to contain at least 2000 events to allow analysis. Contamination of the “blast gate” by unlysed CD45− erythroblasts was estimated by the percentage of CD36+CD11c−events in this gate and had to be below 20% to allow further analysis of blast cells. The expression of each marker was encoded as the ratio between the mean of the fluorescence of the specific signal and the mean of autofluorescence. A qualitative review of the fluorescence graphs was performed by 4 participants (M.M., F.P., B.H., and J.F.) for 170 of 207 patients, demonstrating a globally homogeneous manner of acquisition of flow cytometry data throughout the 8 different centers.
Combinations of markers used for immunophenotypic characterization of MDS, with their respective cellular reactivity in BM
| Cy5 . | Cellular reactivity . | PE . | Cellular reactivity . | FITC . | Cellular reactivity . |
|---|---|---|---|---|---|
| CD45 | Leucocytes | — | — | ||
| CD45 | CD16 | Mature granulocytes, macrophages | CD14 | Monocytes | |
| CD45 | CD11c | Monocytes, macrophages, granulocytes (weak) | CD36 | Monocytes, platelets, erythrocytes | |
| CD45 | CD33 | Myeloid progenitors/precursors, granulocytes (weak), monocytes | CD15 | Mature myeloid cells | |
| CD45 | CD38 | Hematopoietic stem cells, myeloid cell, monocytes, subsets of B and/or T lymphocytes | CD36 | Monocytes, platelets, erythrocytes | |
| CD45 | CD13 | Myeloid cells | CD11b | Myeloid cells, monocytes | |
| CD45 | CD56 | Subset of AML 2 with t(8;21), NK, subset of T cells, plasma cells | DR | Hematopoietic precursors, monocytes, B cells, subsets of activated T lymphocytes | |
| CD45 | CD7 | T-cell lineage, pluripotent hematopoietic stem cells | CD2 | NK and T-cell lineage, some AMLs | |
| CD45 | CD34 | Hematopoietic stem cells from both lymphoid and myeloid lineage | CD71 | Hematopoietic precursors, monocytes, erythrocytes | |
| CD45 | CD19 | B-cell lineage, subset of AML 2 with t(8;21) | CD34 | Hematopoietic precursor from both lymphoid and myeloid lineage | |
| CD45 | CD117 | Myeloid hematopoietic precursors | CD34 | Hematopoietic precursor from both lymphoid and myeloid lineage |
| Cy5 . | Cellular reactivity . | PE . | Cellular reactivity . | FITC . | Cellular reactivity . |
|---|---|---|---|---|---|
| CD45 | Leucocytes | — | — | ||
| CD45 | CD16 | Mature granulocytes, macrophages | CD14 | Monocytes | |
| CD45 | CD11c | Monocytes, macrophages, granulocytes (weak) | CD36 | Monocytes, platelets, erythrocytes | |
| CD45 | CD33 | Myeloid progenitors/precursors, granulocytes (weak), monocytes | CD15 | Mature myeloid cells | |
| CD45 | CD38 | Hematopoietic stem cells, myeloid cell, monocytes, subsets of B and/or T lymphocytes | CD36 | Monocytes, platelets, erythrocytes | |
| CD45 | CD13 | Myeloid cells | CD11b | Myeloid cells, monocytes | |
| CD45 | CD56 | Subset of AML 2 with t(8;21), NK, subset of T cells, plasma cells | DR | Hematopoietic precursors, monocytes, B cells, subsets of activated T lymphocytes | |
| CD45 | CD7 | T-cell lineage, pluripotent hematopoietic stem cells | CD2 | NK and T-cell lineage, some AMLs | |
| CD45 | CD34 | Hematopoietic stem cells from both lymphoid and myeloid lineage | CD71 | Hematopoietic precursors, monocytes, erythrocytes | |
| CD45 | CD19 | B-cell lineage, subset of AML 2 with t(8;21) | CD34 | Hematopoietic precursor from both lymphoid and myeloid lineage | |
| CD45 | CD117 | Myeloid hematopoietic precursors | CD34 | Hematopoietic precursor from both lymphoid and myeloid lineage |
NK indicates natural killer.
Statistical analysis
The mean of the fluorescence signal for each marker tested and the mean of autofluorescence of the corresponding cells were given by the flow cytometer apparatus of each center after setting it according our consensus standardized protocol, as described above. Flow cytometry data were collected by one of us (J.F.) before final statistical analysis. The data fed to a dedicated database system for PC included the measurements of the mean of the fluorescence signal for each marker tested and the mean of autofluorescence for the 3 cell subsets analyzed (blasts, granulocytes, and monocytes) in addition to other clinical and biologic features of the patients, yielding a table of approximately 20 000 numerical values. All fluorescence ratios were calculated before clustering. Clustering of fluorescence ratios was performed with the software created by M. Eisen14 15(http://rana.Stanford.EDU/software/) using the hierarchical ordering of Pearson coefficients method. Clustering analysis allowed recognition of groups of markers that were coexpressed on BM cell subsets, as well as groups of patients with similar blast cell, granulocyte, and monocyte immunophenotypic profiles. This analysis allowed to define positive (+) cells as related to low (lo), or high (hi) levels of fluorescence ratios for the marker tested versus negative (−) cells for that marker. Student t test, χ2 test, variance analysis, and principal components factor analysis were then performed using the Statview software (Berkeley, CA) to compare the mean fluorescence ratios between each group.
Results
Patients
The FAB classification was applied to the 207 patients included in this study (Table 2). The mean percentages of blasts on BM smears were 1.9% (SD = 1.7%), 2.1% (SD = 1.5%), 9.4% (SD = 5.2%), 23.8% (SD = 5.2%), and 7.2% (SD = 10.5%) for RA, RARS, RAEB, RAEB-T, and CMML, respectively (Table 2). Cytogenetics, available for 127 patients (62%), indicated good prognosis in 63% of the cases (including 46% with normal karyotypes), intermediate in 26%, and poor in 11%. Seventeen (13%) patients had a del(5q), as an isolated anomaly in 7 of them. Nine patients had a trisomy 8, 9 had a del(20q), and 4 a del(7). Mean IPPS score of each FAB group is presented in Table 2. No center effect was observed except for patients with an MDS classified as RARS prevalent in the recruitment of one center (F.P.; 13 of 28, 46%).
Classification of patients with MDS according to the FAB system
| FAB subtype . | Frequencies (%) . | Mean and SD of BM blast cell count . | Mean IPSS . |
|---|---|---|---|
| RA | 52 (25) | 1.9% (SD = 1.7%) | 0.32 |
| RARS | 28 (13) | 2.1% (SD = 1.5%) | 0.24 |
| RAEB | 67 (32) | 9.4% (SD = 5.2%) | 1.39 |
| RAEB-T | 20 (10) | 23.8% (SD = 5.2%) | 2.41 |
| CMML | 40 (20) | 7.2% (SD = 10.5%) | 0.85 |
| Total | 207 |
| FAB subtype . | Frequencies (%) . | Mean and SD of BM blast cell count . | Mean IPSS . |
|---|---|---|---|
| RA | 52 (25) | 1.9% (SD = 1.7%) | 0.32 |
| RARS | 28 (13) | 2.1% (SD = 1.5%) | 0.24 |
| RAEB | 67 (32) | 9.4% (SD = 5.2%) | 1.39 |
| RAEB-T | 20 (10) | 23.8% (SD = 5.2%) | 2.41 |
| CMML | 40 (20) | 7.2% (SD = 10.5%) | 0.85 |
| Total | 207 |
General strategy of flow cytometry data analysis
For each marker tested, the corresponding flow cytometry result was defined as the ratio the mean of the fluorescence of the specific signal and the mean of autofluorescence. The aim of the first step of the analysis was to look for similarities between patients to define immunophenotypic groups. We have used a clustering method for this purpose. Clustering algorithms have been proved to be efficient in recognition of similarities in biology and these methods have been applied successfully for the definition of the cluster of differentiations (CDs of mAbs) or for cDNA array analysis, for example. We used a clustering program based on hierarchical ordering of Pearson correlation coefficient developed by Eisen et al14 and Alizadeh et al15 because it allows us to cocluster rows and columns of any data table, giving as an output table a graphic representation of the reordered data. Clustering was applied for CD45lo blast cells, CD45hi/SShi granulocytes, and monocytes of patients with MDS on calculated fluorescence ratios of each marker tested. Clustering analysis was done on both the tested markers (CD clustering) and patients. The significance of the different branches defined using this methodology was assessed by comparison with the immunophenotypic clustering of the same cell subsets from controls, by comparison with the results obtained from the principal component factor analysis and by correlation between the percentage of CD45lo blast cells on BM smears, the FAB classification, and the IPSS score. Such analysis was not conclusive for monocytes of patients with MDS because no significant differences were found with the immunophenotypic profile of normal monocytes and no correlation could be evidenced with the percentage of blasts, the FAB classification, and the IPSS score (not shown). By contrast, immunophenotypic clustering of CD45lo blast cells and CD45hi/SShi granulocytes allowed us to define specific branches for both the markers tested and MDS patients, with correlation between the percentage of blast cells on BM smears, FAB classification, IPSS values, and cytogenetic risk factors.
Immunophenotypic clustering of the antigenic profile of CD45lo BM cells
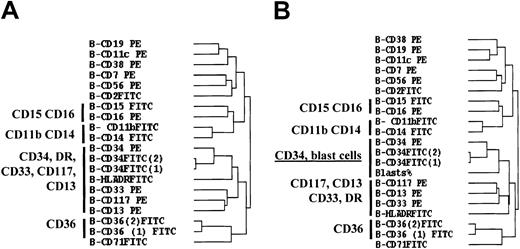
Clustering of the markers expressed by CD45lo BM cells gave rise to a tree that allowed estimation of the proximity between them (CD clustering). As internal controls, CD36 expression was tested twice and CD34 expression 3 times. A first conclusion obtained from this tree of CDs is that, as expected, both FITC-CD34 and both FITC-CD36 fluorescence ratios formed pairs the distance of which indicated a Pearson correlation coefficient close to 1. PE-CD34 fluorescence ratios clustered with those of the 2 FITC-CD34 labelings, but the distance was higher, probably due to the better sensitivity of PE-conjugated mAbs in the detection of surface antigens. Recalculation showed Pearson correlation coefficients of 0.94 and 0.97 for the 2 series of FITC-CD36 and FITC-CD34 fluorescence ratios, respectively, and of 0.73 between the series FITC-CD34 and PE-CD34 fluorescence ratios. Therefore, these controls show that similar immunophenotypic profiles were clustered together.
Within the whole CD tree, one of the major branches clustered CD34, HLA-DR, CD33, CD117, and CD13 together (Figure1A). This branch contained a subbranch with the 3 CD34 markers and a subbranch with the CD13, CD33, and CD117 myeloid markers. The fluorescence ratios of the 3 CD34 labelings coclustered with the percentage of blast cells counted on BM smears in MDS when this criterion was introduced in the CD tree, whereas the CD117, CD33, CD13 myeloid branch became separated from the CD34 branch (Figure 1B). These results are in accordance with the expression of CD34, CD117, CD33, and CD13 on myeloid precursors and suggest that levels of CD34 expression are correlated with blast cell count on BM smears. It is noteworthy that CD15/CD16 and CD36 markers belonged to branches unrelated to the CD34/CD117/CD33/CD13 branch.
Hierarchical clustering analysis of the 20 markers tested on CD45lo blast cells from patients with MDS.
(A) Hierarchical clustering tree of the markers tested on BM CD45lo blast cells of MDS patients. (B) The same clustering analysis was performed after introducing the percentage of blast cells (blasts %) on BM smears. Clustering was performed after median centering and normalizing the fluorescence ratios. Analysis was achieved with the 149 patients for whom at least 16 of the 20 markers of the panel had been tested, the number of events in the CD45lo/SS blast electronic gate was above 2000, and contamination of this gate with CD36+/CD11c−erythroblasts was below 20%.
Hierarchical clustering analysis of the 20 markers tested on CD45lo blast cells from patients with MDS.
(A) Hierarchical clustering tree of the markers tested on BM CD45lo blast cells of MDS patients. (B) The same clustering analysis was performed after introducing the percentage of blast cells (blasts %) on BM smears. Clustering was performed after median centering and normalizing the fluorescence ratios. Analysis was achieved with the 149 patients for whom at least 16 of the 20 markers of the panel had been tested, the number of events in the CD45lo/SS blast electronic gate was above 2000, and contamination of this gate with CD36+/CD11c−erythroblasts was below 20%.
Immunophenotypic clustering of patients according the antigenic profile of their CD45lo BM cells
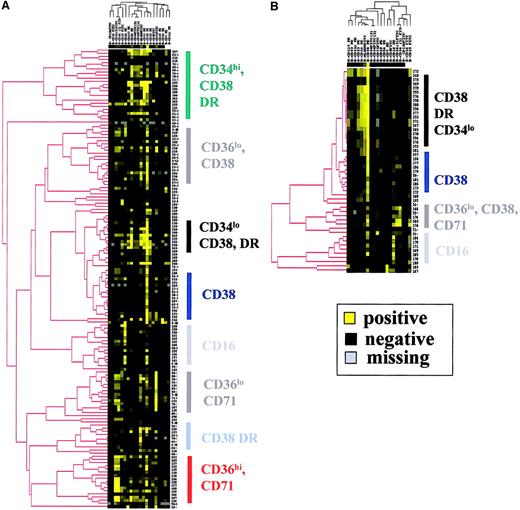
Ascending hierarchical ordering of patients with MDS according to the immunophenotypic profiles of their CD45loblast cells allowed us to identify 8 major clusters according to the most significant markers that were CD16, CD34, CD36, CD38, CD71, and HLA-DR (Figure 2A). The myeloid markers CD13, CD15, CD33, and CD117 were not associated with specific branches even if expressed with various intensities on CD45lo blast cells from a large range of patients. CD2, CD7, CD11b, CD14, and CD19 were barely or not expressed in most cases.
Clustering analysis of patients with MDS and controls according the phenotype of CD45lo blast cells.
(A) Hierarchical clustering analysis of MDS patients. (B) Hierarchical clustering analysis of controls. Analysis was achieved with the 149 patients and 49 controls for whom at least 16 of the 20 markers of the panel had been tested, the number of events in the CD45lo/SS blast electronic gate was above 2000, and contamination of this gate with CD36+/CD11c−erythroblasts was below 20%. Color saturation was obtained for fluorescence ratios above 98 for the 2 clustering analyses.
Clustering analysis of patients with MDS and controls according the phenotype of CD45lo blast cells.
(A) Hierarchical clustering analysis of MDS patients. (B) Hierarchical clustering analysis of controls. Analysis was achieved with the 149 patients and 49 controls for whom at least 16 of the 20 markers of the panel had been tested, the number of events in the CD45lo/SS blast electronic gate was above 2000, and contamination of this gate with CD36+/CD11c−erythroblasts was below 20%. Color saturation was obtained for fluorescence ratios above 98 for the 2 clustering analyses.
Table 3 shows the distribution of patients according to their immunophenotypic profile and the FAB classification. CMML was the major FAB category of the CD16+ cluster. RAEB and RAEB-T were found mainly in the CD34hi/CD38+, CD34lo/CD38+, and CD36hi/CD71+ immunophenotypic clusters (χ2 test, P < .001; Figure3A).
Relationship between immunophenotypic hierarchical clustering of CD45lo blast cells of MDS patients and FAB categories
| Phenotypic cluster . | FAB diagnosis . |
|---|---|
| CD34hi, CD38+DR+ | 14 RAEB, 6 RAEB-T, 2 CMML |
| CD36lo, CD38+ | 14 RA, 4 RAEB, 2 RAEB-T, 3 CMML |
| CD34lo, CD38+, DR+ | 10 RAEB, 3 RAEB-T, 3 CMML |
| CD38+, CD34− | 11 RA, 2 RARS, 3 RAEB, 8 CMML |
| CD16+ | 1 RA, 2 RAEB, 10 CMML |
| CD36lo, CD71+ | 6 RA, 6 RARS, 3 RAEB, 1 CMML |
| CD38+, DR+ | 3 RA, 6 RAEB, 3 RAEB-T, 4 CMML |
| CD36hi, CD71+ | 2 RA, 6 RAEB, 3 RAEB-T, 4 CMML |
| Phenotypic cluster . | FAB diagnosis . |
|---|---|
| CD34hi, CD38+DR+ | 14 RAEB, 6 RAEB-T, 2 CMML |
| CD36lo, CD38+ | 14 RA, 4 RAEB, 2 RAEB-T, 3 CMML |
| CD34lo, CD38+, DR+ | 10 RAEB, 3 RAEB-T, 3 CMML |
| CD38+, CD34− | 11 RA, 2 RARS, 3 RAEB, 8 CMML |
| CD16+ | 1 RA, 2 RAEB, 10 CMML |
| CD36lo, CD71+ | 6 RA, 6 RARS, 3 RAEB, 1 CMML |
| CD38+, DR+ | 3 RA, 6 RAEB, 3 RAEB-T, 4 CMML |
| CD36hi, CD71+ | 2 RA, 6 RAEB, 3 RAEB-T, 4 CMML |
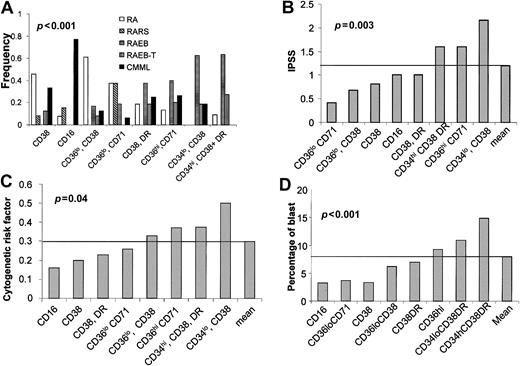
Relationship between the immunophenotypic clustering of patients according the antigenic profile of their CD45hi/SShi BM cells, FAB categories, IPSS score, cytogenetic risk factor, and blast cell counts on BM smears.
(A) Distribution of the FAB categories; (B) mean of the IPSS score; (C) mean of the cytogenetic risk factor; and (D) mean of the blast cell count on BM smears in each of the 8 clusters defined in Figure 2A.P values are indicated for each panel (χ2 test for panel A; variance analysis for panels B-D).
Relationship between the immunophenotypic clustering of patients according the antigenic profile of their CD45hi/SShi BM cells, FAB categories, IPSS score, cytogenetic risk factor, and blast cell counts on BM smears.
(A) Distribution of the FAB categories; (B) mean of the IPSS score; (C) mean of the cytogenetic risk factor; and (D) mean of the blast cell count on BM smears in each of the 8 clusters defined in Figure 2A.P values are indicated for each panel (χ2 test for panel A; variance analysis for panels B-D).
The mean value of IPSS, cytogenetic risk factors, and the blast cell count on BM smears, calculated for the clusters defined in Table 3, were the highest for the CD34hi/CD38+, CD34lo/CD38+, and CD36hi/CD71+ immunophenotypic clusters (Figure 3B-D). Variance analysis evidenced the nonrandom distribution of IPSS, cytogenetic risk factors, and blast cell count among the immunophenotypic clusters (Figure 3).
Expression of CD34 and CD36 on CD45lo BM cells defines specific clusters of patients with MDS
Immunophenotypic hierarchical clustering of CD45loblast cells from MDS patients and controls evidenced frequent corresponding branches in patients and controls (Figure 2B). Yet, 2 branches were found exclusively in the hierarchical clustering tree of MDS patients, respectively, the CD34hi/CD38+ and CD36hi/CD71+ clusters.
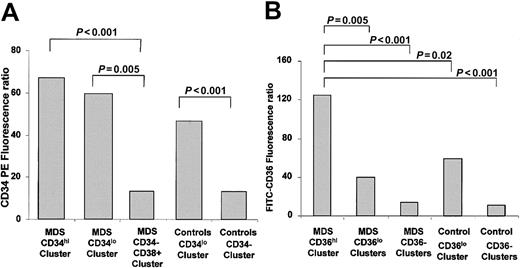
Analysis of the 3 CD34 fluorescence ratios showed that CD34 expression was higher in both the CD34hi/CD38+ and CD34lo/CD38+ branches of MDS patients (Figure4A). CD36 fluorescence ratios were the highest in the CD36hi/CD71+ branch of MDS patients with a significant difference between this immunophenotypic cluster and all other categories (Figure 4B).
CD34 and CD36 fluorescence ratios of CD45loblast cells of MDS patients and controls.
(A) Mean CD34 fluorescence ratios in CD34hi, CD34lo, and CD34− clusters. (B) Mean CD36 fluorescence ratios in clusters defined from CD36 expression. Student t test P values between each category are indicated above the corresponding brackets.
CD34 and CD36 fluorescence ratios of CD45loblast cells of MDS patients and controls.
(A) Mean CD34 fluorescence ratios in CD34hi, CD34lo, and CD34− clusters. (B) Mean CD36 fluorescence ratios in clusters defined from CD36 expression. Student t test P values between each category are indicated above the corresponding brackets.
Figure 5A shows that the levels of CD34 fluorescence ratios were significantly higher for patients with RAEB/RAEB-T than for those with RA/RARS (P < .001), whereas CD117 fluorescence ratios were similar in both groups (P = .67). Figure 5B shows that high levels of CD36 expression were not correlated with CD34 expression in patients with RAEB, suggesting that CD34 and CD36 are independent markers in clustering of CD45lo blast cells of patients with MDS. This was confirmed by principal components factor analysis of the fluorescence ratios for each series of markers (Figure 5C). This analysis is based on the determination of the minimal set of factors (each factor being a particular combination of the variables analyzed) giving the maximum dispersion of the markers tested. This was followed by the mathematical definition of 2 major oblique axes on the projection plan corresponding to the 2 most significant factors. These oblique axes were found to correspond to CD36 and CD34 expression on CD45lo BM cells and were close to the orthogonal axis, suggesting that CD34 and CD36 expressions were unrelated. It should be noted that these results fit fairly well with the CD clustering tree (see above and Figure 1).
Expression of CD34 and CD36 expression on CD45lo blast cells defines 2 different immunophenotypic clusters of patients with MDS.
(A) Comparison of the means of the PE-CD34 and CD117 fluorescence ratios between controls and patients according to their FAB diagnosis. (B) Comparison of the mean of PE-CD34 and FITC-CD36 fluorescence ratios in patients belonging to the CD36hi immunophenotypic cluster, or with a RAEB/RAEB-T versus RA/RARS MDS disease. (C) Two-dimensional scattergram with its oblique axes of the fluorescence ratios for each of the markers expressed by CD45lo blast cells from patients with MDS according to the 2 most significant factors obtained from principal component factor analysis. For panels A and B, Student ttest P values are indicated on the corresponding columns of the histogram. For panel C, the percentage of variance represented by the 2 most significant factors is indicated.
Expression of CD34 and CD36 expression on CD45lo blast cells defines 2 different immunophenotypic clusters of patients with MDS.
(A) Comparison of the means of the PE-CD34 and CD117 fluorescence ratios between controls and patients according to their FAB diagnosis. (B) Comparison of the mean of PE-CD34 and FITC-CD36 fluorescence ratios in patients belonging to the CD36hi immunophenotypic cluster, or with a RAEB/RAEB-T versus RA/RARS MDS disease. (C) Two-dimensional scattergram with its oblique axes of the fluorescence ratios for each of the markers expressed by CD45lo blast cells from patients with MDS according to the 2 most significant factors obtained from principal component factor analysis. For panels A and B, Student ttest P values are indicated on the corresponding columns of the histogram. For panel C, the percentage of variance represented by the 2 most significant factors is indicated.
Immunophenotypic clustering of the antigenic profile of CD45hi/SShi BM cells
Hierarchical CD clustering showed that CD13, CD33, CD38, and HLA-DR fluorescence ratios of CD45hi/SShi BM cells (ie, granulocytes) were correlated with the percentage of blast cells on BM smears (Figure 6A). The levels of fluorescence ratios were indeed increased in RAEB/RAEB-T for these markers (Figure 6B), the difference being statistically significant (P < .01) when compared with RA/RARS using the Student t test.
Hierarchical clustering analysis of the markers tested on CD45+/SShi granulocytes from patients with MDS.
(A) Hierarchical clustering tree of the percentage of blasts on BM smears with the 20 markers tested on BM CD45+/SShi granulocytes of MDS patients. Clustering was performed after median centering and normalizing the fluorescence ratios. Analysis was achieved on the 199 patients for whom at least 16 of the 20 markers of the panel were tested and the number of events in the CD45+/SShi granulocyte electronic gate was above 2000. (B) Mean CD38, CD13, CD33, and HLA-DR fluorescence ratios according the FAB categories.
Hierarchical clustering analysis of the markers tested on CD45+/SShi granulocytes from patients with MDS.
(A) Hierarchical clustering tree of the percentage of blasts on BM smears with the 20 markers tested on BM CD45+/SShi granulocytes of MDS patients. Clustering was performed after median centering and normalizing the fluorescence ratios. Analysis was achieved on the 199 patients for whom at least 16 of the 20 markers of the panel were tested and the number of events in the CD45+/SShi granulocyte electronic gate was above 2000. (B) Mean CD38, CD13, CD33, and HLA-DR fluorescence ratios according the FAB categories.
Immunophenotypic clustering of patients according the antigenic profile of their CD45hi/SShi BM cells
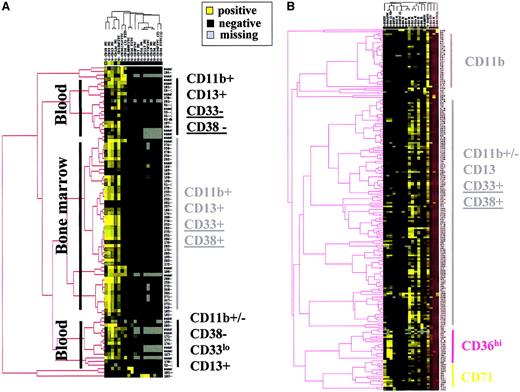
Clustering of controls according to the immunophenotypic profile of their CD45hi/SShi BM cells discriminated roughly 3 major branches according to the origin of the CD45hi/SShi BM cells examined: 1 for the BM and 2 for peripheral blood (Figure 7A). CD45hi/SShi BM cells originating from the BM were characterized by higher levels of CD11b, CD13, CD33, and CD38 expression, suggesting a relationship between the immunophenotypic profile and the maturation stage of the cells (M.M., manuscript in preparation). These results are in accordance with the CD tree of CD45hi/SShi BM cells (Figure 6A), because the percentage of blast cells counted on BM smears was correlated with CD13, CD33, and CD38 expression, and suggests a relationship with the level of immature cells in the BM of patients with MDS.
Clustering analysis of MDS patients and controls according to the immunophenotype of CD45+/SShigranulocytes.
(A) Hierarchical clustering analysis of granulocytes from BM and peripheral blood of control samples. (B) Hierarchical clustering analysis of granulocytes from BM samples of MDS patients.
Clustering analysis of MDS patients and controls according to the immunophenotype of CD45+/SShigranulocytes.
(A) Hierarchical clustering analysis of granulocytes from BM and peripheral blood of control samples. (B) Hierarchical clustering analysis of granulocytes from BM samples of MDS patients.
The immunophenotypic characteristics of normal CD45hi/SShi BM cells were also observed on CD45hi/SShi BM cells from most MDS patients (Figure 7B). It is noteworthy that HLA-DR expression was not observed on CD45hi/SShi BM cells from most controls yet was frequently noted in MDS samples. Even though no specific immunophenotypic branch was associated with this marker, 56% of patients with a fluorescence ratio higher than 5 for HLA-DR on CD45hi/SShi BM cells were classified as RAEB/RAEB-T (P = .03), in agreement with the CD clustering tree of CD45hi/SShi BM cells (Figure 6).
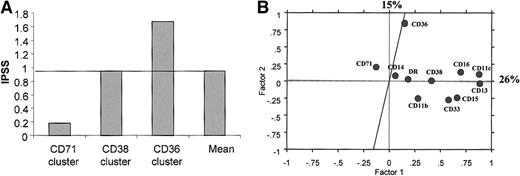
Expression of CD36 and CD71 on CD45hi/SShiBM cells defines specific clusters of patients with MDS
CD36 and CD71 were the only 2 markers expressed at higher levels on CD45hi/SShi BM cells of patients with MDS than on normal CD45hi/SShi BM cells. The CD71hi branch contained 5 patients with RA, 10 with RARS, 1 with RAEB, and 1 with CMML, whereas the CD36hi branch included 4 patients with RA, 6 with RAEB, 3 with RAEB-T, and 2 with CMML. Moreover, this branch included 5 of 17 patients with 5q abnormalities. Correlation with IPSS and cytogenetic risk factors suggests that the expression of CD71 on CD45hi/SShi BM cells is associated with a good prognosis, whereas CD36 expression on CD45hi/SShi BM cells could be a factor of poor prognosis (Figure 8A). Principal components factor analysis of the markers expressed by CD45hi/SShi BM cells showed that CD36 and CD71 expression were poorly related (Figure 8B).
Expression of CD36 and CD71 CD45hi/SShigranulocytes defines 2 different immunophenotypic clusters of patients with MDS.
(A) Mean of IPSS scores in CD36, CD38, and CD71 clusters of CD45hi/SShi granulocytes from patients with MDS. (B) Two-dimensional scattergram with its oblique axes of the fluorescence ratios for each of the markers expressed by CD45hi/SShi granulocytes from patients with MDS according to the 2 most significant factors obtained after principal component factor analysis. The percentage of variance represented by these 2 most significant factors is indicated.
Expression of CD36 and CD71 CD45hi/SShigranulocytes defines 2 different immunophenotypic clusters of patients with MDS.
(A) Mean of IPSS scores in CD36, CD38, and CD71 clusters of CD45hi/SShi granulocytes from patients with MDS. (B) Two-dimensional scattergram with its oblique axes of the fluorescence ratios for each of the markers expressed by CD45hi/SShi granulocytes from patients with MDS according to the 2 most significant factors obtained after principal component factor analysis. The percentage of variance represented by these 2 most significant factors is indicated.
Discussion
Flow cytometry analysis of hematopoietic neoplasms has been recognized for general usefulness and is now widely used. However, this technique is usually considered irrelevant to the characterization of MDS. Here, we present the results of a multicenter study of flow cytometry analysis of MDS showing that immunophenotypic changes of BM cells from patients with MDS were correlated with the FAB classification, IPSS, the blast cell count on BM smears, and the cytogenetic risk factor.
Flow cytometry is a powerful technique allowing analysis of the expression of a wide range of molecules on a high number of cells. Multiple color labeling further allows a refined and complex analysis of cell subsets and provides an accurate image of cell maturation. This complexity may be the weak point of flow cytometry because it may render very difficult the complete numerical description of the results. Usually, flow cytometry results are simplified and expressed for diagnosis in a qualitative or semiquantitative manner. For example, in a recent immunophenotypic study on the usefulness of flow cytometry in the diagnosis of MDS, Stetler-Stevenson and coworkers8expressed flow cytometry data of multiple color labeling as a normal versus abnormal pattern by reference to controls. Flow cytometry analysis of large series of patients raises the question of numerically coding fluorescence signals for further statistical analysis. Here we chose to express fluorescence labeling in a simplified numerical form, corresponding to the fluorescence ratio of the whole cell subset gated for each marker tested. Indeed, fluorescence ratios depend both on the percentage of cells expressing the marker tested and on the intensity of expression. However, this did not allow us to take into account information on possible heterogeneous expressions. This means also that the results were expressed in a monoparametric form, with some loss of the information provided by multiple color labeling. Nevertheless, fluorescence ratios have previously proven sensitive in the analysis of surface molecules expression.16 Therefore, it was hypothesized that constructing and organizing flow cytometry results in such a way that allowed manipulation by statistical software would lead to the emergence of the most significant markers in MDS. Analysis of the data from both patients with MDS and controls was performed according to a clustering technique based on hierarchical ascendant ordering of Pearson correlation coefficients14 15for CD45lo blast cells, CD45hi/SShigranulocytes, and monocytes. Conclusive results were obtained for MDS patients only for CD45lo blast cells and CD45hi/SShi granulocytes. The absence of significant results on monocytes could be due either to an inadequate panel of markers tested regarding monocytic differentiation or a poor gating of monocytes in flow cytometry.
Abnormal immunophenotypic clusters were related to dysregulation in the expression of such markers as CD34, CD36, and CD71, normally expressed on BM cell subsets, rather than related to the expression of an unexpected marker. Regarding blast cells, our data suggest that an increased expression of CD16 and decreased expression of CD117 on CD45lo blast cells is associated with CMMLs. CD34 and CD36 expression may have unique patterns of expression observed only on CD45lo blast cells of some MDS patients.
Increased C34 expression was associated with a poor IPSS, a poor cytogenetic risk factor, and a high blast cell count on BM smears, suggesting a relationship between CD34 expression on CD45loblast cells and the prognosis of MDS patients. Because levels of CD34 normally expressed on CD45lo control blast cells were within the range of those noted in the CD34lo/CD38+ branch of MDS patients, CD34 expression could be significant as a prognosis marker rather than as a diagnosis marker of MDS, being probably related to the immaturity level of CD45lo blast cells.17 18
By contrast, high levels of CD36 on CD45lo blast cells of MDS patients may contribute to better defining such patients because (1) it was not found in controls, (2) it was associated with a subset of MDS patients with a majority of RAEB/RAEB-T, but also CMMLs, and (3) it was associated with a poor IPSS score and an increased cytogenetic risk factor without an increase in CD34 expression.
For CD45hi/SShi granulocytic lineage cells, it was found that high levels of CD71 and CD36 expression allowed us to define 2 MDS branches not observed in controls. The CD71 branch contained 10 (59%) of 17 patients with RARS and 5 (29%) of 17 patients with RA. The expression of CD71, which is the receptor for the iron transporter transferrin, is regulated by iron depletion.19,20 RARS is characterized by DNA mutations of mitochondria resulting in an abnormal metabolism of iron and a defect in its incorporation in the heme structures and in the cytochromec of the respiratory chain of mitochondrion.21This raises the question of whether the up-regulation of CD71 expression on CD45hi/SShi granulocytes of patients with RARS could be an immunophenotypic reflection of the defect in iron metabolism in these cells originated from the BM precursor. Patients with MDS classified as RARS or RA whose CD45hi/SShi granulocytes clustered in the CD71 branch had good IPSS scores and cytogenetic risk factors.
Patients whose CD45hi/SShi granulocytes were in the CD36 branch had poor IPSS scores and cytogenetic risk factors and were classified as RAEB/RAEB-T in 9 (60%) of 15 cases. Among these 15 patients, 5 were found to belong also to the CD36hi branch of the CD45lo blast tree, suggesting that dysregulation of CD36 expression occurs early in the differentiation process of BM cells for these patients.
Regarding the relationships between immunophenotyping and FAB classification of MDS, RAEB and RAEB-T were not discriminated, which suggests that these 2 categories belong to the same continuum at least from an immunophenotypic viewpoint. By contrast, immunophenotypic clustering of CD45lo blast cells partly discriminated patients with RAEB/RAEB-T, CMML, and RA/RARS and clustering of CD45hi/SShi granulocytic lineage cells discriminated patients with RA/CMML, RARS, and RAEB/RAEB-T.
In conclusion, our results show that CD34, CD36 expression on CD45lo blast cells and CD36, CD71 expression on CD45hi/SShi granulocytes are likely to define specific immunophenotypic clusters of patients with MDS, with significant correlation with the well-established IPSS prognosis markers and the FAB classification. Our results fully support the existence of relationships between quantitative immunophenotypic abnormalities and BM dysplasia in a significant proportion of patients with MDS. This will open new perspectives, for both diagnosis and prognosis, based on multiple flow cytometry measurements of molecule expression levels on BM cells, allowing future research strategies and protocols to better define subgroups of MDS patients, who are still a rather heterogeneous group.
We are grateful to the Beckman/Coulter, Becton Dickinson, and Dako companies for providing reagents for this study. We thank Dr Hélène Poirel, EA 3406, Université Paris 13, Bobigny, France and Pr Gilbert Faure, EA 3443, Laboratoire d'Immunologie, Faculté de Médecine de Nancy, France for helpful advice and discussion.
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2002-01-0230.
Supported by the Association pour la Recherche contre le Cancer, contrat no. 5729 and by The Ligue contre le Cancer, ComitéDépartemental de la Seine Saint Denis, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jean Feuillard, Service d'Hématologie Biologique Hôpital Avicenne et EA 3406 ATHSCO, UniversitéParis 13, Bobigny, France; e-mail: jean.feuillard@avc.ap-hop-paris.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal