Posttransplantation lymphoproliferative disorder (PTLD) is a life-threatening Epstein-Barr virus (EBV)–associated B-cell malignancy occurring in 1% to 2% of renal transplantation patients. Host- and PTLD-related factors determining the likelihood of tumor response following reduction of immune suppression (IS) and antiviral therapy remain largely unknown. Standard therapy for PTLD is not well established. Eleven consecutive renal transplantation patients who developed EBV-positive PTLD 8 to 94 months after allografting were uniformly treated with acyclovir and IS reduction. All PTLDs were EBV-positive diffuse large B-cell lymphomas. Ten patients (91%) obtained a durable complete response (CR), and 9 (82%) have remained in continuous CR with a median follow-up of 29 months. Five patients (45%) lost their allograft. Of these, 4 patients had PTLD affecting the transplanted kidney. Peripheral blood CD8+ T cells increased significantly (P = .0078) from baseline in 8 responders available for analysis. One of 2 patients whose absolute CD8+ T-cell count subsequently dropped to baseline after IS reduction relapsed. The expanded CD8+ T cells from 2 responders specifically recognized an immunodominant peptide from the EBV lytic gene BZLF-1. Another lytic EBV gene, thymidine kinase, was expressed in all 8 PTLDs tested. IS reduction and antiviral therapy for PTLD after renal transplantation is a highly successful therapeutic combination, but the risk of graft rejection is significant, particularly in patients with PTLD involving the renal allograft. A sustained expansion of CD8+ T cells and a cellular immune response to EBV lytic antigens may be important for PTLD clearance in renal transplantation patients.
Introduction
Renal transplantation has become the therapy of choice for end-stage renal disease (ESRD), with more than 10 000 renal transplantations performed annually in the United States.1With appropriate histocompatibility matching and long-term immune suppression (IS), most patients now benefit from prolonged graft survival. Prolonged iatrogenic IS, however, carries a substantial risk of both opportunistic infections and secondary malignancies.2 The intimate connection between IS, opportunistic infections, and secondary malignancies in transplant recipients is highlighted by the development of posttransplantation lymphoproliferative disorder (PTLD) following primary infection or reactivation of latent Epstein-Barr virus (EBV) infection.3 The successful eradication of established disease by the adoptive transfer of EBV-specific autologous cytotoxic T lymphocytes (CTLs) suggests a critical role for endogenous CD8+ T cells in controlling PTLD.4
PTLD occurs in 1% to 2% of renal transplantation patients.5 Reported mortality rates range from 50% to 70%, and the optimal treatment approach remains highly controversial.6 Reduction of IS, with or without antiviral drugs, is initially attempted in almost all patients and is reported to result in regression of PTLD lesions in 23% to 50% of patients7-9 (reviewed by Paya et al10). Durable complete responses with this approach, however, are considered rare. The inconsistent response to IS reduction likely reflects the heterogeneity in the type and intensity of IS, the EBV status of the PTLD, the presence of monoclonal versus polyclonal disease, and other tumor- and host-related factors yet to be defined. To our knowledge, studies assessing both the clinical and immunologic response to IS reduction and antiviral drugs as the sole initial intervention for PTLD following renal transplantation in the absence of chemotherapy or biologic therapy are lacking. Cytotoxic chemotherapy, usually reserved for patients who do not respond to IS reduction, has shown activity in selected patients but is associated with significant toxicity.6,11,12 Drugs that target EBV replication, such as acyclovir, ganciclovir, and foscarnet, have occasionally been associated with objective responses when given alone13 and are often used as an adjunct to IS reduction.10 The efficacy of antiviral drugs, however, appears to be limited.14 Moreover, due to the inconsistent detection of lytic EBV gene products in PTLD,15,16 it is often argued that the biologic rationale for the use of antiviral drugs in this disease is weak. Promising preliminary results have been obtained with anti–B-cell monoclonal antibodies in the treatment of PTLD after solid organ transplantation17,18 and with the adoptive transfer of EBV-specific autologous cytotoxic T lymphocytes (CTLs) in bone marrow transplantation–related PTLD.19 More recently, a new and interesting therapeutic approach has been proposed that is based on the systemic administration of arginine butyrate, which induces the expression of the EBV-encoded viral thymidine kinase (vTK), followed by ganciclovir.20 The clinical value of this approach, however, remains to be confirmed. We present here a prospective evaluation of the clinical responses and of the immunomodulatory effects achieved with a limited intervention of IS reduction and acyclovir therapy in 11 consecutive renal transplantation patients with EBV-positive PTLD.
Patients, materials, and methods
Patients and treatment of PTLD
Eleven consecutive cases of EBV-positive PTLD following kidney (10) or kidney-pancreas (1) transplantation diagnosed at The Ohio State University Medical Center (OSUMC) between 1997 and 1999 are included in this study. These patients were not enrolled in a clinical trial. Rather, they were treated prospectively with a standardized and uniform approach based on the recommended initial therapy for PTLD, as recently published in a consensus statement by the American Society of Transplant Surgeons/American Society of Transplant Physicians (ASTS/ASTP) EBV-PTLD Task Force following an International Consensus Development Meeting on EBV-induced PTLD.10 Prior to our current study, the dose and schedule of antiviral therapy and the specific schedule of IS reduction was highly variable. While each patient signed an Ohio State University Medical Center institutional review board (IRB)–approved consent for blood collection and procurement, 3 patients (patient nos. 2, 3, and 5) did not have a baseline absolute lymphocyte count performed and were therefore not fully evaluable for immunologic correlates. All tumors were EBV-positive diffuse large B-cell lymphomas (DLBCLs). Following transplantation, IS consisted of cyclosporin A (CyA; dosed to target serum levels and toxicity), prednisone, and either azathioprine (150-200 mg/d) or mycophenolate mofetil (MMF; 2-3 g/d). Following diagnosis of PTLD, azathioprine or MMF was discontinued, CyA was reduced by 50%, and prednisone was rapidly reduced to 5 to 10 mg daily. Patients were simultaneously started on high-dose intravenous acyclovir (ACV; 10 mg/kg intravenously every 8 hours) for 4 weeks, followed by maintenance oral ACV (800 mg three times daily) for 12 months. Diagnostic tests included computed tomography (CT) scans of the chest, abdomen, and pelvis; gallium-67 single photon emission computed tomography (SPECT) scan, bone marrow aspirate and biopsy, and evaluation of liver and kidney function and serum lactate dehydrogenase (LDH). Evaluation of disease response was performed 4 to 8 weeks after reduction of IS. When possible, repeat biopsies were performed. Complete response (CR) was defined as the disappearance of all lesions present on CT scan, the lack of detectable disease upon rebiopsy, and/or the resolution of all gallium-avid lesions on SPECT scan. The progression-free survival (PFS) was the time from the day IS was first reduced to the time of relapse, death, or last follow-up. The overall survival (OS) was the time from the day IS was first reduced to the time of death.
In situ reverse transcription–polymerase chain reaction (IS-RT-PCR) was performed as previously described.21,22Forward and reverse primers for EBV thymidine kinase were 5′-GAACCCGCATGCTCTCCTT-3′ and 5′-TCTGGATGATGCCCAAGACA-3′, respectively. The primers employed for heavy and light chain mRNA analysis and the criteria used to define clonality have been described elsewhere.23 For each specific mRNA, primers were used as a nested set. After an initial incubation at 65°C for 30 minutes, followed by denaturation at 94°C for 3 minutes, 20 cycles of PCR were performed at 60°C for 1 minute and then 94°C for 30 seconds. Control conditions were analyzed on the same slides. The presence of a new B-cell clone in subsequent samples from the same patient was identified by the detection of different light and/or heavy chain gene expression. The detection of mRNA of 2 different heavy or light chains in different areas of the same slide was defined as biclonal.
T-cell subset analysis and tetramer assay
T-cell subsets were assessed on fresh blood samples by standard 3-color flow cytometry. EBV-reactive CD8+ T cells were detected by flow cytometry using HLA-B8 tetramers complexed with immunodominant EBV peptides derived from the latent gene, EBNA-3A (FLRGRAYGL), or the immediate early lytic gene, BZLF-1 (RAKFKQLL).24 Frozen patient peripheral blood mononuclear cells (PBMCs) were viably thawed, incubated overnight at 37°C, and then purified by Ficoll-Hypaque density gradient centrifugation to remove debris. Cells were stained with phycoerythrin (PE)–conjugated murine anti-CD8 and fluorescein isothiocyanate (FITC)–conjugated murine anti-CD3 antibodies (both purchased from BD Pharmingen, San Diego, CA) and allophycocyanin (APC)–conjugated HLA-matched tetramer reagent or a nonreactive control. Approximately 105 lymphocyte-gated (based on forward and side scatter) events were collected for each flow analysis.
Statistical analysis
Nonparametric procedures were performed. The Wilcoxon signed rank test was used to assess the change in CD8+ T-cell counts before and after IS reduction.25
Results
Patients
Clinical data are summarized in Table1. Median age was 45 years (range 24-77 years). The median time from transplantation to development of PTLD was 17 months (range, 8-94 months). In 8 patients (73%), PTLD was diagnosed more than 1 year after transplantation. In 3 patients both nodal and extranodal sites were involved, and in 5 patients the kidney allograft was the predominant site of disease. One patient had histologically proven PTLD of the bladder without an obvious mass. Gallium-67 SPECT scans demonstrated gallium-avid disease in 10 of 11 patients. One patient had histologically proven PTLD of the liver that was gallium-negative.
Disease characteristics of 11 renal transplantation patients with PTLD
| Patient no. . | Age/sex . | Disease site . | Time to PTLD, mo . | Clonality . | Immune suppression . | Graft rejection . | Duration of response, mo . |
|---|---|---|---|---|---|---|---|
| 1 | 59/F | Liver/spleen | 13 | NA | Reduced | No | 29+ |
| 2 | 57/M | Kidney/colon | 24 | Monoclonal | Reduced D/C | No† | NR† |
| 3 | 24/M | Kidney/LN | 16 | Monoclonal | Reduced | Yes | 39+ |
| 4* | 39/M | Skin | 18 | NA | Reduced | No | 36+ |
| 5 | 77/M | Kidney/colon | 9 | NA | Reduced | Yes | 35+ |
| 6 | 44/F | Small bowel | 94 | Monoclonal | Reduced | No | 25‡ |
| Small bowel‡ | Monoclonal | D/C1-153 | Yes | 5+ | |||
| 7 | 46/M | Liver/spleen | 13 | Monoclonal | Reduced | No | 28+ |
| 8 | 34/M | Kidney/LN | 8 | Biclonal | Reduced D/C | Yes | 29+ |
| 9 | 31/M | Skin | 44 | Biclonal | Reduced | No | 17+ |
| 10 | 51/M | Kidney/spleen | 10 | Monoclonal | Reduced D/C | Yes | 15+ |
| 11 | 57/F | Bladder | 89 | Monoclonal | Reduced | No | 14+ |
| Patient no. . | Age/sex . | Disease site . | Time to PTLD, mo . | Clonality . | Immune suppression . | Graft rejection . | Duration of response, mo . |
|---|---|---|---|---|---|---|---|
| 1 | 59/F | Liver/spleen | 13 | NA | Reduced | No | 29+ |
| 2 | 57/M | Kidney/colon | 24 | Monoclonal | Reduced D/C | No† | NR† |
| 3 | 24/M | Kidney/LN | 16 | Monoclonal | Reduced | Yes | 39+ |
| 4* | 39/M | Skin | 18 | NA | Reduced | No | 36+ |
| 5 | 77/M | Kidney/colon | 9 | NA | Reduced | Yes | 35+ |
| 6 | 44/F | Small bowel | 94 | Monoclonal | Reduced | No | 25‡ |
| Small bowel‡ | Monoclonal | D/C1-153 | Yes | 5+ | |||
| 7 | 46/M | Liver/spleen | 13 | Monoclonal | Reduced | No | 28+ |
| 8 | 34/M | Kidney/LN | 8 | Biclonal | Reduced D/C | Yes | 29+ |
| 9 | 31/M | Skin | 44 | Biclonal | Reduced | No | 17+ |
| 10 | 51/M | Kidney/spleen | 10 | Monoclonal | Reduced D/C | Yes | 15+ |
| 11 | 57/F | Bladder | 89 | Monoclonal | Reduced | No | 14+ |
Each PTLD lesion had diffuse large B-cell lymphoma histology.
NA indicates tissue not available for analysis; D/C, discontinued; LN, lymph nodes; NR, no response.
Kidney/pancreas transplantation.
Patient died 4 weeks after diagnosis of PTLD.
Relapsed PTLD.
Patient received rituximab at relapse.
Molecular characterization of tumors
All patients had EBV-positive PTLD (Table2). Nine patients were EBV-positive by in situ hybridization (ISH) for Epstein-Barr–encoded RNA (EBER) or by immunohistochemistry (IHC) for latent membrane protein-1 (LMP-1). Samples from patient nos. 10 and 11 were negative for EBER and for LMP-1 but were positive for vTK by IS-RT-PCR. The type of EBV latency present in the PTLD was not extensively investigated, and Table 2reports all the EBV studies performed on diagnostic and subsequent PTLD tissue samples. For the 8 patients whose initial diagnostic tissue was available for additional analysis, tumor clonality was established by IS-RT-PCR for immunoglobulin heavy and light chain mRNA. Each of the 8 diagnostic PTLD specimens available for molecular analysis were monoclonal or biclonal (ie, 2 distinct but dominant clones in 1 sample; Table 2), and each expressed EBV vTK mRNA by IS-RT-PCR (Table 2 and Figure 1). In 5 of these 8 patients, additional tumor sampling during response allowed a serial analysis of clonality and EBV gene expression. In 4 of the 5 cases, different B-cell clones were demonstrated after IS reduction (Table 2). In addition, there was variation in the number of tumor cells expressing vTK mRNA among different clones from the same patient (Table2).
Tumor clonality and EBV gene expression in 11 renal transplantation patients with PTLD
| Patient no. . | EBER (ISH) . | LMP-1 (IHC) . | Clone . | κ/λ . | Immunoglobulin heavy chain . | EBV vTK (IS-RT-PCR) . |
|---|---|---|---|---|---|---|
| 1 | + | − | NA | NA | NA | |
| 2 | ND | + | κ | HC2 | 2+ | |
| 3 | + | − | 1 | κ | HC1 | 3+ |
| 2 | λ | HC3 | 1+ | |||
| 4 | ND | + | NA | NA | NA | |
| 5 | + | − | NA | NA | NA | |
| 6 | ND | + | 1 | κ | HC6 | 3+ |
| 2 | λ | HC6 | 3+ | |||
| 3 | κ | HC1 | 1+ | |||
| 7 | + | − | λ | HC2 | 3+ | |
| 8 | ND | + | 1 | Both | HC4/HC2 | 1+/2+ |
| 2 | Both | HC2;6/HC4 | 1+/1+ | |||
| 9 | + | − | Both | HC3/HC5 | 3+ | |
| 10 | − | − | 1 | λ | NA | 2+ |
| 2 | λ | HC5 | 1+ | |||
| 3 | κ | HC5 | 1+ | |||
| 11 | − | − | 1 | λ | HC3 | 3+ |
| 2 | κ | HC3 | 2+ | |||
| 3 | λ | HC4/HC2 | 2+ |
| Patient no. . | EBER (ISH) . | LMP-1 (IHC) . | Clone . | κ/λ . | Immunoglobulin heavy chain . | EBV vTK (IS-RT-PCR) . |
|---|---|---|---|---|---|---|
| 1 | + | − | NA | NA | NA | |
| 2 | ND | + | κ | HC2 | 2+ | |
| 3 | + | − | 1 | κ | HC1 | 3+ |
| 2 | λ | HC3 | 1+ | |||
| 4 | ND | + | NA | NA | NA | |
| 5 | + | − | NA | NA | NA | |
| 6 | ND | + | 1 | κ | HC6 | 3+ |
| 2 | λ | HC6 | 3+ | |||
| 3 | κ | HC1 | 1+ | |||
| 7 | + | − | λ | HC2 | 3+ | |
| 8 | ND | + | 1 | Both | HC4/HC2 | 1+/2+ |
| 2 | Both | HC2;6/HC4 | 1+/1+ | |||
| 9 | + | − | Both | HC3/HC5 | 3+ | |
| 10 | − | − | 1 | λ | NA | 2+ |
| 2 | λ | HC5 | 1+ | |||
| 3 | κ | HC5 | 1+ | |||
| 11 | − | − | 1 | λ | HC3 | 3+ |
| 2 | κ | HC3 | 2+ | |||
| 3 | λ | HC4/HC2 | 2+ |
ISH indicates in situ hybridization; IHC, immunohistochemistry; IS-RT-PCR, in situ RT-PCR; NA, tissue not available for analysis; ND, not done.
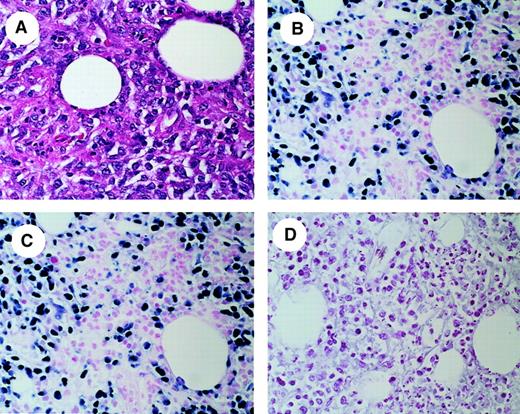
In situ RT-PCR analysis of vTK expression in a representative PTLD tumor sample.
(A) Hematoxylin and eosin (H & E) stain of tumor biopsy showing diffuse infiltration by atypical large lymphocytes. (B) IS-RT-PCR detection of EBER-1 and EBER-2 mRNA. Most of the lymphoma cells in the field are positive for the expression of these abundant EBV transcripts, confirming the presence of EBV. (C) IS-RT-PCR analysis of EBV vTK mRNA. vTK expression is present in a number of lymphoma cells equivalent to those expressing EBER-1 and EBER -2. (D) RNase digestion after IS-RT-PCR analysis of vTK mRNA demonstrates that the signal present in panel C is RNA-based. Original magnification, × 400.
In situ RT-PCR analysis of vTK expression in a representative PTLD tumor sample.
(A) Hematoxylin and eosin (H & E) stain of tumor biopsy showing diffuse infiltration by atypical large lymphocytes. (B) IS-RT-PCR detection of EBER-1 and EBER-2 mRNA. Most of the lymphoma cells in the field are positive for the expression of these abundant EBV transcripts, confirming the presence of EBV. (C) IS-RT-PCR analysis of EBV vTK mRNA. vTK expression is present in a number of lymphoma cells equivalent to those expressing EBER-1 and EBER -2. (D) RNase digestion after IS-RT-PCR analysis of vTK mRNA demonstrates that the signal present in panel C is RNA-based. Original magnification, × 400.
Response to IS reduction and acyclovir
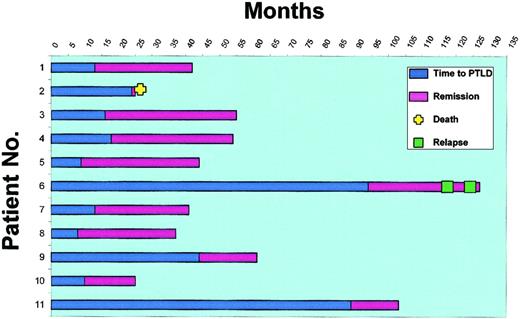
All 11 PTLD tumors were of diffuse large B-cell histology, and each of the 8 samples available for molecular analysis showed either clonal or biclonal disease. Nonetheless, 10 (91%) of 11 patients achieved a durable CR without the need for additional therapy. Patient no. 2 died of intra-abdominal sepsis 4 weeks after diagnosis of PTLD without radiologic evidence of response. An autopsy was not performed. The time to development of PTLD and the duration of CR for each patient following IS reduction and acyclovir are shown in Figure2. The progression free survival (PFS) and overall survival (OS) are presented in Figure3. Overall, the median time to achieve CR following the initiation of IS reduction was 16 weeks (range 7-26 weeks). Eight patients achieved CR without complete discontinuation of IS. With follow-up ranging from 16 to 41 months (median 29 months), 9 of the 10 responders (82% overall) are in continuous CR. Five have maintained a functional renal allograft on a regimen of moderate IS, while 5 (45% overall) have lost their kidney allograft. Four of the 5 patients who lost their allograft following IS reduction presented with PTLD that was primarily located at the site of the allograft. The fifth patient to reject the renal allograft initially presented with PTLD of the small bowel and achieved CR with IS reduction and acyclovir (patient no. 6). She remained in CR with good renal function for 25 months but, as noted below, failed to sustain an increase in her CD3+CD8+ T-cell count. She relapsed with PTLD at the site of her previous disease (small bowel) and was treated with rituximab and complete discontinuation of IS. This was accompanied by renal allograft rejection and by a rise in the CD8+ T-cell count, which peaked at 810/μL (Figure4B). This patient remained progression-free for 5 months and then relapsed again in the same site (small bowel). At the time of her second relapse, the CD8+T-cell counts had dropped to 49/μL despite no IS. She was then treated with combined high-dose antiviral therapy with zidovudine (AZT) and ganciclovir. Again she achieved a complete response, and her CD8+ T cells increased to 350/μL. Of note, response to AZT and ganciclovir was associated with a decrease in whole blood EBV viral load from 971 copies per milliliter to an undetectable level (data not shown). The patient remains radiologically and virologically disease-free after 5 months of follow-up. Thus, 10 (91%) of 11 PTLD patients in this study are currently in CR.
Clinical outcome in 11 renal transplantation patients with PTLD treated with decreasing immunosuppression and acyclovir.
The blue bar represents the time from kidney transplantation to the diagnosis of PTLD. The purple bar represents the time from initiation of therapy to last follow-up, death, or relapse (progression-free survival). Patient no. 2 died of sepsis 4 weeks after diagnosis of PTLD without a documented response. Patient no. 6 relapsed after a 25-month CR with IS taper and acyclovir. A second CR was achieved after withdrawal of IS and institution of rituximab therapy. A second relapse was then treated with high-dose antiviral therapy (zidovudine and ganciclovir), which led to a third CR (clinical, radiologic, and virologic).
Clinical outcome in 11 renal transplantation patients with PTLD treated with decreasing immunosuppression and acyclovir.
The blue bar represents the time from kidney transplantation to the diagnosis of PTLD. The purple bar represents the time from initiation of therapy to last follow-up, death, or relapse (progression-free survival). Patient no. 2 died of sepsis 4 weeks after diagnosis of PTLD without a documented response. Patient no. 6 relapsed after a 25-month CR with IS taper and acyclovir. A second CR was achieved after withdrawal of IS and institution of rituximab therapy. A second relapse was then treated with high-dose antiviral therapy (zidovudine and ganciclovir), which led to a third CR (clinical, radiologic, and virologic).
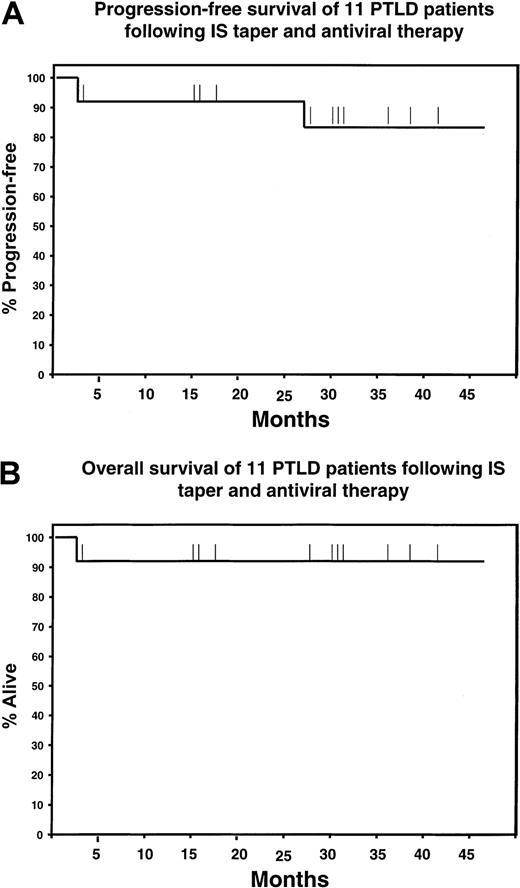
Survival following IS taper and acyclovir in 11 renal transplantation patients with PTLD.
(A) Progression-free survival (PFS). (B) Overall survival (OS). Ten patients are alive and in CR, and 9 patients are progression-free with a median follow-up of 29 months. Patient no. 2 died shortly after diagnosis from overwhelming sepsis, and patient no. 6 relapsed twice after a complete response (CR) of 25 months but is again in CR.
Survival following IS taper and acyclovir in 11 renal transplantation patients with PTLD.
(A) Progression-free survival (PFS). (B) Overall survival (OS). Ten patients are alive and in CR, and 9 patients are progression-free with a median follow-up of 29 months. Patient no. 2 died shortly after diagnosis from overwhelming sepsis, and patient no. 6 relapsed twice after a complete response (CR) of 25 months but is again in CR.
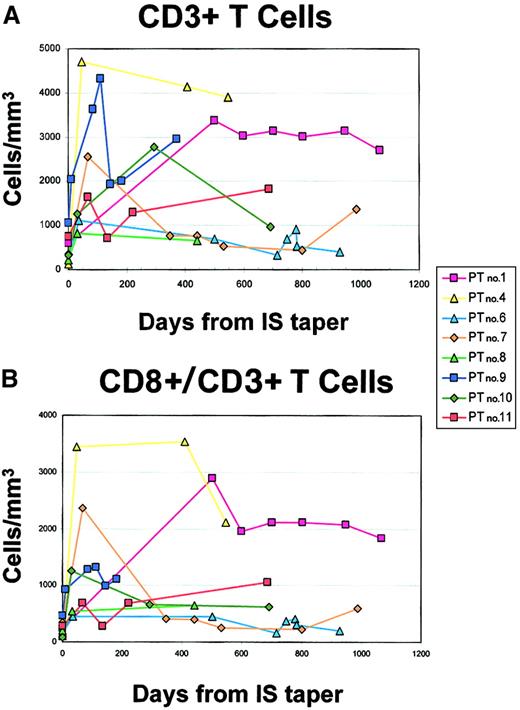
Serial analysis of peripheral blood T cells in 8 evaluable patients with PTLD treated with decreasing immunosuppression and acyclovir.
Freshly collected PBMCs were analyzed for the presence of T cells by flow cytometry. Analyses were performed at multiple time points for each patient. (A) Absolute CD3+ T cells per microliter. (B) Absolute CD8+/CD3+ T cells per microliter.
Serial analysis of peripheral blood T cells in 8 evaluable patients with PTLD treated with decreasing immunosuppression and acyclovir.
Freshly collected PBMCs were analyzed for the presence of T cells by flow cytometry. Analyses were performed at multiple time points for each patient. (A) Absolute CD3+ T cells per microliter. (B) Absolute CD8+/CD3+ T cells per microliter.
Expansion of CD8+ CTLs in vivo
To characterize the dynamics of immune restoration following IS withdrawal, we analyzed peripheral blood T-cell subsets by flow cytometry at the diagnosis of PTLD and during follow-up (Figure 4). In 3 patients IS reduction was initiated without a baseline CD8+ T-cell count (patient nos. 2, 3, and 5); these patients are therefore excluded from this analysis. All 8 patients for whom data are available initially had low numbers of CD3+ T cells (Figure 4A) and CD3+CD8+ T cells (Figure4B) at baseline while on IS. All 8 patients experienced a significant increase in the number of CD3+CD8+ T cells following IS reduction (Figure 4B, P = .0078). The CD3+CD8+ T-cell expansion ranged from 2.5-fold (patient no. 11) to 32.8-fold (patient no. 4) and was sustained at the time of last follow-up in all but 2 patients (patient nos. 6 and 7), 1 of whom relapsed (patient no. 6), as discussed above. The CD3+CD8+ T-cell count of patient no. 7 peaked early after initiation of IS reduction but has since fluctuated at or slightly above the baseline, now more than 28 months from diagnosis. This patient has no clinical evidence of recurrence.
Tetramer analysis of CD8+ CTLs
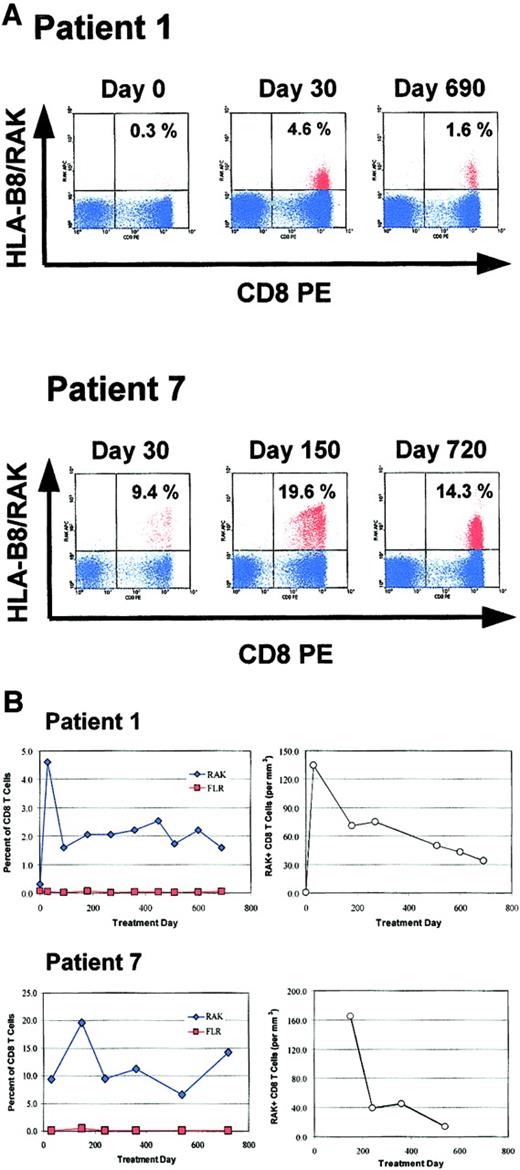
To assess the EBV specificity of the expanded CD3+CD8+ T cells and to identify EBV epitopes recognized during the immune clearance of PTLD, we performed HLA peptide tetramer staining of PBMC samples from 2 HLA-B8+patients (patient nos. 1 and 7). HLA-B8 tetramers complexed with RAK or FLR peptide were used to evaluate an immune response to the EBV lytic gene BZLF-1 or latent gene EBNA-3A, respectively (Figure5). Both patients had an easily detectable CTL response against the RAK peptide product of the EBV BZLF-1 immediate early gene, with peak RAK-specific CTL numbers occurring early in their clinical response, constituting up to 19.6% of all CD3+CD8+ cells (Figure 5A). Patient no. 1 had a detectable number of RAK-specific T cells at the time of diagnosis of PTLD (0.3% of all CD3+CD8+ cells) and experienced a 15-fold increase in the percentage or a 191-fold increase in the absolute number of these cells in the first 30 days of treatment (Figure 5B). The percentage of RAK-specific cells has remained well above the baseline of 0.3% of CD8+ T cells for nearly 2 years during continued CR, with the absolute number remaining at least 48-fold greater than that on day 0. A population of FLR-specific CTLs could not be reliably detected in this patient's blood at any time (Figure 5B). A sample from diagnosis was not available for this analysis for patient no. 7, who had 9.4% of an expanding population of CD8+ T cells specific for RAK at day 30 (Figure 5A). By day 150 this had increased to 19.6% of CD8+ T cells, corresponding to 165 RAK-specific CTLs per microliter. Thereafter, this expanded population of cells has been sustained between 5% and 15% of all CD8+ T cells during ongoing CR (Figure 5B). While detectable at all time points at a low level, the percentage and absolute number of FLR-specific CTL did not increase appreciably throughout the patient's course (Figure5B).
Quantification of EBV-specific T cells in 2 HLA-B8+ patients with HLA tetramers.
Serial PBMC samples from 2 HLA-B8+ patients were analyzed by flow cytometry with APC-conjugated MHC/peptide tetramers. Representative results obtained at 3 time points with the HLA-B8 tetramer containing the RAKFKQLL peptide (HLA-B8/RAK) from the EBV immediate early gene BZLF-1 are shown in panel A. CD3+ events occurring in a lymphocyte gate are shown in blue. The percentage of CD8+ HLA-B8/RAK+ events is indicated in the upper right quadrant of each plot. (B) The serial analysis of RAK+ CD8+ cells in each patient, with RAK-specific CD8+ T cells represented as the percentage of CD8+ T cells (left) and as the absolute number (per microliter) in peripheral blood, calculated from the absolute CD8+ T-cell number determined by flow cytometry of fresh peripheral blood when available (right). Treatment day 0 is defined as the day when reduction of immunosuppression was initiated. Analysis with the additional tetramer reagent, HLA-B8/FLR (FLRGRAYGL; from the EBV latent gene EBNA-3A), is also shown.
Quantification of EBV-specific T cells in 2 HLA-B8+ patients with HLA tetramers.
Serial PBMC samples from 2 HLA-B8+ patients were analyzed by flow cytometry with APC-conjugated MHC/peptide tetramers. Representative results obtained at 3 time points with the HLA-B8 tetramer containing the RAKFKQLL peptide (HLA-B8/RAK) from the EBV immediate early gene BZLF-1 are shown in panel A. CD3+ events occurring in a lymphocyte gate are shown in blue. The percentage of CD8+ HLA-B8/RAK+ events is indicated in the upper right quadrant of each plot. (B) The serial analysis of RAK+ CD8+ cells in each patient, with RAK-specific CD8+ T cells represented as the percentage of CD8+ T cells (left) and as the absolute number (per microliter) in peripheral blood, calculated from the absolute CD8+ T-cell number determined by flow cytometry of fresh peripheral blood when available (right). Treatment day 0 is defined as the day when reduction of immunosuppression was initiated. Analysis with the additional tetramer reagent, HLA-B8/FLR (FLRGRAYGL; from the EBV latent gene EBNA-3A), is also shown.
Discussion
To our knowledge, this is the first prospective clinical and immunologic analysis of a therapeutic intervention limited to reduction of IS plus antiviral drugs in renal transplantation patients with PTLD. We believe that the observations presented in this study provide several novel leads that may improve our understanding of the mechanisms underlying the development and the immune clearance of PTLD. With a median follow-up of 29 months, the observed 82% progression-free survival (Figure 3A) and 91% overall survival (Figure3B) compare very favorably with those described in previous studies of patients with clonal and late-occurring PTLD treated with cytotoxic chemotherapy12 or with anti–B-cell monoclonal antibodies (mAbs).17,18 Previous reports of the treatment of PTLD in renal transplantation patients have included reduction of IS and antiviral therapy, but in all of these studies a substantial fraction of patients were treated with other modalities.26 27 Therefore, the contribution of immune restoration and/or antiviral drugs to the clinical outcome could not be clearly elucidated. In contrast, the present study provides strong evidence that immune restoration and antiviral therapy are highly effective and produce a very high clinical complete response rate in monoclonal and late-occurring PTLD without additional therapy.
The PTLD patients who experienced a sustained increase in the absolute number of CD3+CD8+ T cells underwent a complete and durable clinical response. This may not be dissimilar to the ability of CD3+CD4+ T-cell counts to predict clinical outcome in HIV-infected patients.28 The emergence and expansion of oligoclonal CD3+CD8+ T cells during regression of PTLD has been previously described in an allogeneic bone marrow transplantation patient who had a dramatic complete response following IS withdrawal.29 In that report, the expanded CD3+CD8+ T cells displayed major histocompatibility complex (MHC) class I–restricted cytotoxic activity against autologous EBV-positive lymphoblasts in vitro, suggesting a direct role for endogenous EBV-specific CD8+ T cells in the regression of PTLD in vivo. The prospective HLA tetramer analysis of CD3+CD8+ T cells performed in 2 responding patients in this study complements and extends those observations. We show that, after IS reduction, the number of CD3+CD8+ T cells displaying HLA-restricted recognition of EBV immunodominant peptides can increase more than 190-fold. Despite a long follow-up (> 2 years), the small sample size and the limited number of events in this study preclude any conclusive statement about the association between CD3+CD8+ T-cell counts and response. It remains to be determined whether a critical threshold for the number of peripheral blood CD3+CD8+ T cells exists and whether such a threshold might be a good predictor of response for most patients. Indeed, while both patient nos. 6 and 7 experienced a significant drop in their CD3+CD8+ T-cell counts, only patient no. 6 relapsed. Patient no. 7 had a much greater initial expansion of CD3+CD8+ T cells and showed an upward trend in CD3+CD8+ T cells at last follow-up. More importantly, tetramer analysis of the lymphocytes from patient no. 7 was possible and showed a persistent expansion of EBV-specific CD3+CD8+ T cells despite decreased absolute numbers of CD3+CD8+ T cells. Thus, the number of cells specific for EBV immunodominant peptides may be a more important factor in maintaining a response than the overall number of CD3+CD8+ T cells. Following the lead provided by these observations, further studies may determine whether a decline of CD3+CD8+ T cells to baseline levels is predictive of pending relapse, alerting the treating physician to the need for further reduction of IS or additional therapy. Furthermore, a broader application of tetramer analysis will likely clarify the clinical relevance of the quantification of EBV-specific CD8+ CTLs following therapeutic intervention in PTLD.
We have shown in this study that a lytic rather than a latent EBV peptide was a predominant target for the expanded CD3+CD8+ T cells in 2 responding PTLD patients. It has been generally accepted that PTLD represents a type III latent EBV infection, characterized by the expression of EBNAs 1-6 and LMPs 1 and 2.30 It was therefore logical to think that the immunodominant target antigens for EBV-specific CTLs in this disease would be derived from this group. Recently, a heterogeneity in EBV latent gene expression has been documented in PTLD lesions, with more restricted patterns of gene expression seen in some patients.31-34 In addition, it has become clear that there is often heterogeneity of viral gene expression within a single PTLD lesion.32-34 The expression of EBV lytic gene products has been detected in PTLD,33,34 but the proportion of cells found to be entering into the lytic cycle has typically been small and the relevance of EBV replication to the pathogenesis of PTLD has remained obscure.35 Here we provide direct evidence of the lytic EBV gene vTK (BXLF-1) mRNA expression in all 8 PTLD tumors examined. Importantly, we also provide evidence that, in parallel with the clearance of the PTLD, a population of CD8+ T cells specific for the lytic antigen BZLF-1 expands several-fold following reduction of IS. These data suggest that control of an ongoing EBV lytic cycle may be important not only for resolution of primary EBV infection36 and prevention of reactivation37but also for immune clearance of PTLD. To our knowledge, this report provides the first evidence of a CD8+ T-cell response to the immunodominant lytic gene peptide RAK in PTLD, suggesting an important role for the BZLF-1 antigen both in the pathogenesis of PTLD and in virus-host interaction.
In addition to vTK,38 another EBV-encoded protein kinase, BGLF-4,39 is often expressed during early lytic infection. While we have not performed a detailed analysis of BGLF-4 mRNA expression in PTLD, both the vTK and the BGLF-4 gene products have been detected in EBV-positive lymphoblasts during early lytic infection.40 While EBV vTK has been shown to have a narrow substrate specificity, selectively phosphorylating only thymidine nucleoside analogs (5-bromodeoxyuridine [5-BrdU] and AZT),41 BGLF-4 is assumed to have the capacity to phosphorylate guanosine analogs (acyclovir, ganciclovir), although this has not been formally demonstrated.40 It is tempting to speculate that the presence of both gene products in PTLD might facilitate the selective targeting of actively replicating or reactivated EBV-positive B cells with combination antiviral therapy.42 43 A more complete characterization of the coexpression of vTK and BGLF-4 gene products in PTLD is likely required before the use of multiple antiviral agents for prophylaxis and/or treatment of PTLD could be broadly advocated.
Finally, our in situ molecular analysis demonstrates that distinct B-cell clones are often detectable in different areas of the same PTLD specimen as well as at different times in serial PTLD specimens from the same patient. These observations confirm and extend a previous report44 and may have direct relevance to the issue of lytic versus latent EBV cycle in PTLD, particularly when combined with our observations on the expression of vTK. Continuous B-cell transformation by EBV might occur with ongoing viral replication (evidenced by vTK expression) and might be facilitated by cell-to-cell viral transmission, with minimal systemic release of infectious virions. Intratumoral selection might then lead to the emergence of different B-cell clones at different times or in different locations. Gradual recovery of lytic antigen-specific CD8+ T cells (possibly with the help of antiviral drugs) could result in the control of the lytic EBV cycle, in the prevention of new B-cell transforming events, and ultimately in the clearance of PTLD. Thus, the study of the molecular mechanisms underlying the observed “plasticity” of EBV-positive B-cell populations in PTLD and the identification of the selective forces operating in vivo during immune restoration and antiviral therapy are likely to improve our general understanding of the immune response to virus-induced cancers.
Despite the very encouraging clinical responses achieved in this study and the fact that immune restoration and antiviral therapy is currently recommended as front-line treatment for all PTLD following solid organ transplantation by the ASTS/ASTP,10 a number of questions regarding this approach remain unanswered, particularly in regard to its safety and its integration with other treatment modalities. Comparative data on allograft outcomes in other patients with PTLD treated with immune restoration and antiviral drugs are scattered and incomplete, but the incidence of allograft failure observed in our study was unexpectedly high and concerning. The modality of IS reduction we adopted was consistent with the ASTS/ASTP recommendations,10 and the reason for the high rejection rate in our patients is currently not known. A strong correlation between the presence of PTLD in the allograft and the occurrence of rejection was noted in our patients, and we plan additional analyses to investigate the association. Transplantation physicians at this time should be aware that patients with PTLD affecting the allograft might have a higher risk of rejection following immune restoration and antiviral drugs. A crucial issue in this regard is the relative importance of each individual component (immune restoration vs antiviral therapy) in determining clinical response. If a substantial role for antiviral therapy in PTLD could be demonstrated, and greater knowledge about the role of EBV gene expression and replication in PTLD could be gathered, alternative approaches could be devised that would rely less on reduction of IS and more on antiviral strategies, therefore reducing the risk of rejection. The data presented in our study are a step forward in this direction because they suggest a greater importance of lytic viral activity in PTLD than was previously recognized.
In the last few years several additional treatment modalities have emerged for PTLD, adding further complexity to an already difficult management decision. The anti-CD20 mAb rituximab has shown significant activity in PTLD, with response rates averaging 50% to 60%.18 The administration of rituximab based on quantitative blood EBV viral loads is gaining acceptance in the early management of PTLD after allogeneic stem cell transplantation,45 46 and similar approaches are being adopted in solid organ transplantation patients as well. Follow-up data are not very mature, but the safety profile of rituximab appears very good, and detrimental effects on the allograft have not yet been reported. While it is conceivable that early administration of rituximab might eradicate PTLD in solid organ transplantation patients without the need for reduction of IS, a substantial number (40%-50%) of patients fail to respond to rituximab, suggesting that more than one modality of therapy will be required to control this disease in most patients. In light of the emerging link between immune effector cells and the in vivo clinical efficacy of mAbs, a better understanding of the immune response to EBV in PTLD patients will be necessary for both the development of safe immune restorative strategies and the optimization of mAb-based therapy in EBV-induced malignancies.
Prepublished online as Blood First Edition Paper, June 7, 2002; DOI: 10.1182/blood-2002-01-0210.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael A. Caligiuri, The Ohio State University, A-458 Starling Loving Hall, 320 W 10th Ave, Columbus, OH 43210; e-mail: caligiuri-1@medctr.osu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal