Abstract
Arsenic trioxide (As2O3; ATO) has recently been found to be very effective for relapsed acute promyelocytic leukemia. Several articles reported prolongation of QT interval or ventricular arrhythmias in patients receiving ATO. However, the QT-prolonging effect has not been confirmed and the direct membrane effect of ATO has never been studied. In the present investigation, using conventional action potential recording technique, we found that ATO dose dependently prolonged action potential duration (APD) in guinea pig papillary muscle with a slow pacing frequency. Parenteral administration of ATO prolonged QT interval and APD in guinea pig hearts. Intravenous infusion of clinically relevant doses of ATO prolonged QT interval and APD dose dependently. These studies suggest that ATO has a direct effect on cardiac repolarization. Patients who are receiving ATO should avoid concomitant administration of other QT-prolonging agents or conditions in favor of delaying cardiac repolarization.
Introduction
Arsenic trioxide (As2O3; ATO) has recently been found to be very effective in relapsed or refractory acute promyelocytic leukemia (APL).1-3Several articles have reported prolongation of the QT interval in patients receiving ATO for relapsed APL.4,5 However, the conclusive evidence for ATP to prolong cardiac repolarization is lacking. Some of the reported cases with torsade de pointes have hypokalemia or hypomagnesemia or both.4-6 Most of the patients with APL receiving ATO have been heavily treated with chemotherapeutic agents, including anthracycline and all-trans-retinoic acid. Thus, cardiac damage is likely to be universal before ATO therapy begins.2,4,6 Some reported cases have monomorphic ventricular tachycardia instead of polymorphic tachycardia, which is a prototypical arrhythmia of QT prolongation.4 Shen and colleagues did not report QT prolongation in 15 patients receiving ATO.1 Thus, the causal relationship of the use of ATO and prolongation of QT interval is questionable.7,8 There is a need to unravel the effect of ATO on cardiac repolarization.
In the present study, we demonstrated that ATO prolonged action potential duration (APD) and QT interval in guinea pig heart. This is the first comprehensive study of effect of ATO on cardiac repolarization.
Study design
Action potential recording
The care and handling of the animals in this study was in full compliance with the American Association for the Accreditation of Laboratory Animal Care. Adult Hartley guinea pigs (weighing 400-500 g) of either sex were used. Action potentials were recorded with conventional intracellular recording technique.9 The action potential duration at 90% repolarization (APD90) was measured. This is the most commonly measured parameter of action potential for the purpose of studying the effect of QT-prolonging agents10-12 because this time period encompasses the interval from the beginning of phase 0 depolarization to the late repolarization, and the prolongation of the ADP90 was usually accompanied by a parallel lengthening of the effective refractory period.13 Effects of different concentrations of ATO (1, 10, and 25 μM) under different stimulation frequencies (0.1, 1, and 2 Hz) were examined. ATO was purchased from Sigma (St Louis, MO).
Electrocardiographic recording
Adult guinea pigs were anesthetized with intraperitoneal urethane (1.2 g/kg). Needles (27-gauge) were fixed subcutaneously in each limb and in correct positions on the chest. Three-channel electrocardiograms (ECGs; lead II, V1, and V5) were recorded for off-line measurement done by another person who was blinded to the experimental procedures. The QT interval was measured from the beginning of the QRS complex to the end of the T wave, which was defined as the return to the TP baseline (between the end of the T wave and the following P wave).14-16 When the heart rate was rapid, the QT interval was estimated by extrapolating the downslope of the T wave.14 The QT interval was recorded at baseline condition just before the administration of various doses of ATO (10, 30, and 50 mg/kg) or water (control group) via nasogastric tube, and every 30 minutes until 3 hours (acute feeding group). Our pilot study showed that ATO with doses more than 100 mg/kg killed guinea pigs before the end of the 3-hour recording period. For the chronic feeding group, arsenic ATO, 5 mg/kg, or water (control group) was administered (via nasogastric tube) every other day for 4 times until the eighth day. We did not administer higher doses of ATO nor administer the drug every day because guinea pigs could not survive to the end of the eighth day with either method. The QT interval was measured every other day immediately before the administration of ATO. The corrected QT interval (QTc) was calculated with the Bazett formula17: QTc = QT/(RR)1/2, an accepted method for correcting QT interval for rate in guinea pigs.18 Lead II is used to measure QT interval because its T wave ending is usually discrete.16 19 For the chronic feeding group, guinea pigs were killed at the eighth day immediately after the measurement the QT interval. The action potentials of right ventricular papillary muscles were measured.
The effect of intravenous ATO was also tested. ATO, 0.15 mg/kg, a dose commonly used in humans,2,3 6 was infused for 2 hours. To test the dose-dependent effect, higher doses (0.45 and 1.5 mg/kg) were also used. The control group received equivalent volume of saline. The QT interval was measured immediately before infusion, at 10 minutes after, and every 30 minutes after infusion for a total of 2 hours. Guinea pigs were killed thereafter and action potential was recorded. Intravenous ATO was a gift from TTY Biopharm (Taiwan).
The data are expressed as mean ± SEM. Paired t test was used for the comparison of the effect in the experimental group versus the control group. The intraobserver and interobserver agreement was verified with the Bland-Altman method.20 The difference was considered statistically significant withP < .05.
Results and discussion
ATO prolonged cardiac action potential
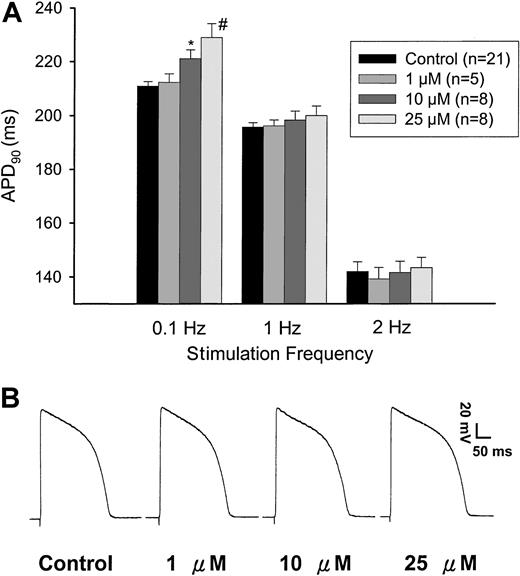
Arsenic trioxide prolonged action potential duration when the stimulation frequency was slow (0.1 Hz). As shown in Figure1A, this effect was not significantly different when the stimulation frequencies were faster. The APD-prolonging effect was-dose dependent. Furthermore, the percent prolongation was greater when the stimulation frequency was 0.1 Hz compared with that of 1 Hz (4.8% ± 0.3% versus 1.3% ± 0.2% for 10 μM, P < .01; 8.6% ± 1.2% versus 2.2% ± 0.3% for 25 μM, P < .01), suggesting a reverse frequency–dependent effect. Figure 1B shows a typical example of the original action potential tracings.
ATO prolonged cardiac action potentials.
(A) Dose-dependent and frequency-dependent prolongation of APD. After action potential was stably stimulated with a frequency of 1 Hz in control condition, ATO (1 μM) was perfused and the effects were recorded. The stimulation frequency was then changed to 0.1 Hz, and then to 2 Hz. The perfusate was then shifted to that containing 10 μM ATO. The changes induced by 3 different stimulation frequencies were recorded. Finally the effect of 25 μM ATO was tested in the same way. Using conventional action potential recording technique, we demonstrated dose-dependent prolongation of APD90 in guinea pig papillary muscle by ATO when the muscle was stimulated with a frequency of 0.1 Hz. The changes in APD90 by faster frequencies (1 and 2 Hz) did not reach statistical significance. *P < .05 versus control; #P < .01 versus control. (B) A typical illustration of action potentials. ATO dose dependently prolonged action potential duration. The stimulation frequency was 0.1 Hz.
ATO prolonged cardiac action potentials.
(A) Dose-dependent and frequency-dependent prolongation of APD. After action potential was stably stimulated with a frequency of 1 Hz in control condition, ATO (1 μM) was perfused and the effects were recorded. The stimulation frequency was then changed to 0.1 Hz, and then to 2 Hz. The perfusate was then shifted to that containing 10 μM ATO. The changes induced by 3 different stimulation frequencies were recorded. Finally the effect of 25 μM ATO was tested in the same way. Using conventional action potential recording technique, we demonstrated dose-dependent prolongation of APD90 in guinea pig papillary muscle by ATO when the muscle was stimulated with a frequency of 0.1 Hz. The changes in APD90 by faster frequencies (1 and 2 Hz) did not reach statistical significance. *P < .05 versus control; #P < .01 versus control. (B) A typical illustration of action potentials. ATO dose dependently prolonged action potential duration. The stimulation frequency was 0.1 Hz.
Enteral (or oral) ATO prolonged QTc and APD
Acute feeding of ATO did not significantly change the heart rate (Table 1). But the QT interval showed time-dependent and dose-dependent prolongation (Table 1). As shown in Figure 2A, during the 3-hour observation, QTc progressively prolonged and was significantly longer than that in the control ones after 60 minutes; the magnitude of prolongation was dose-dependent as well.
The changes in heart rate and QT interval in the acute feeding group
| Time, min . | Heart rate, bpm . | QT, ms . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control, n = 6 . | 10 mg/kg, n = 7 . | 30 mg/kg, n = 8 . | 50 mg/kg, n = 5 . | Control, n = 6 . | 10 mg/kg, n = 7 . | 30 mg/kg, n = 8 . | 50 mg/kg, n = 5 . | |
| 0 | 310.9 ± 15.2 | 317.0 ± 11.2 | 309.3 ± 13.2 | 300.2 ± 9.1 | 107.8 ± 2.1 | 107.4 ± 3.8 | 106.8 ± 2.9 | 108.9 ± 0.9 |
| 30 | 300.1 ± 18.3 | 307.4 ± 13.2 | 320.5 ± 20.1 | 303.2 ± 18.2 | 109.3 ± 1.9 | 109.1 ± 3.2 | 105.7 ± 2.1 | 108.9 ± 3.0 |
| 60 | 297.0 ± 16.2 | 301.4 ± 20.2 | 308.3 ± 13.8 | 310.4 ± 20.0 | 110.7 ± 2.0 | 112.4 ± 4.1 | 110.9 ± 2.3 | 113.8 ± 1.3* |
| 90 | 309.3 ± 11.2 | 306.8 ± 18.3 | 304.6 ± 23.0 | 312.5 ± 16.5 | 107.2 ± 3.1 | 112.9 ± 2.9 | 113.2 ± 3.1* | 114.8 ± 3.1* |
| 120 | 292.7 ± 20.2 | 298.2 ± 22.4 | 295.8 ± 19.1 | 303.2 ± 14.3 | 111.2 ± 2.4 | 116.4 ± 4.2* | 116.1 ± 3.2* | 118.0 ± 1.8* |
| 150 | 295.6 ± 13.2 | 296.3 ± 18.2 | 294.3 ± 17.3 | 298.8 ± 16.7 | 110.9 ± 3.2 | 116.2 ± 3.1* | 118.8 ± 3.9* | 121.9 ± 2.1* |
| 180 | 291.3 ± 17.1 | 292.1 ± 20.9 | 296.8 ± 22.1 | 301.9 ± 23.2 | 110.9 ± 3.3 | 117.5 ± 4.0* | 123.3 ± 2.1* | 130.7 ± 5.3† |
| Time, min . | Heart rate, bpm . | QT, ms . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control, n = 6 . | 10 mg/kg, n = 7 . | 30 mg/kg, n = 8 . | 50 mg/kg, n = 5 . | Control, n = 6 . | 10 mg/kg, n = 7 . | 30 mg/kg, n = 8 . | 50 mg/kg, n = 5 . | |
| 0 | 310.9 ± 15.2 | 317.0 ± 11.2 | 309.3 ± 13.2 | 300.2 ± 9.1 | 107.8 ± 2.1 | 107.4 ± 3.8 | 106.8 ± 2.9 | 108.9 ± 0.9 |
| 30 | 300.1 ± 18.3 | 307.4 ± 13.2 | 320.5 ± 20.1 | 303.2 ± 18.2 | 109.3 ± 1.9 | 109.1 ± 3.2 | 105.7 ± 2.1 | 108.9 ± 3.0 |
| 60 | 297.0 ± 16.2 | 301.4 ± 20.2 | 308.3 ± 13.8 | 310.4 ± 20.0 | 110.7 ± 2.0 | 112.4 ± 4.1 | 110.9 ± 2.3 | 113.8 ± 1.3* |
| 90 | 309.3 ± 11.2 | 306.8 ± 18.3 | 304.6 ± 23.0 | 312.5 ± 16.5 | 107.2 ± 3.1 | 112.9 ± 2.9 | 113.2 ± 3.1* | 114.8 ± 3.1* |
| 120 | 292.7 ± 20.2 | 298.2 ± 22.4 | 295.8 ± 19.1 | 303.2 ± 14.3 | 111.2 ± 2.4 | 116.4 ± 4.2* | 116.1 ± 3.2* | 118.0 ± 1.8* |
| 150 | 295.6 ± 13.2 | 296.3 ± 18.2 | 294.3 ± 17.3 | 298.8 ± 16.7 | 110.9 ± 3.2 | 116.2 ± 3.1* | 118.8 ± 3.9* | 121.9 ± 2.1* |
| 180 | 291.3 ± 17.1 | 292.1 ± 20.9 | 296.8 ± 22.1 | 301.9 ± 23.2 | 110.9 ± 3.3 | 117.5 ± 4.0* | 123.3 ± 2.1* | 130.7 ± 5.3† |
P < .05 versus control.
P < .01 versus control.
Parenteral ATO prolonged QTc and APD.
(A) Acute feeding of ATO resulted in progressive dose-dependent prolongation of QTc. *P < .05 versus control; #P < .01 versus control; +P < .001 versus control. (B) Chronic feeding of ATO produced time-dependent prolongation of QTc. *P < .05 versus control; #P < .01 versus control. (C) APD of papillary muscle after eighth day chronic feeding of ATO, showing longer APD90 in ATO-treated ones. #P < .01 versus control; +P < .001 versus control.
Parenteral ATO prolonged QTc and APD.
(A) Acute feeding of ATO resulted in progressive dose-dependent prolongation of QTc. *P < .05 versus control; #P < .01 versus control; +P < .001 versus control. (B) Chronic feeding of ATO produced time-dependent prolongation of QTc. *P < .05 versus control; #P < .01 versus control. (C) APD of papillary muscle after eighth day chronic feeding of ATO, showing longer APD90 in ATO-treated ones. #P < .01 versus control; +P < .001 versus control.
Table 2 shows the changes in the heart rate and the QT interval in the chronic feeding group. The heart rate did not change significantly, but the QT interval progressively prolonged. QTc also progressively prolonged (Figure 2B). Figure2C demonstrates the APD90 of papillary muscle at the eighth day. APD90 was significantly longer than that in the control group for all the stimulation frequencies being used. Again, the degree of prolongation suggested reverse frequency-dependent relationship (35.4% for 0.1 Hz, 32.8% for 1 Hz, and 30.3% for 2 Hz).
Changes in heart rate and QT interval in the chronic feeding group
| Time, d . | Heart rate, bpm . | QT, ms . | ||
|---|---|---|---|---|
| Control, n = 6 . | 5 mg/kg QOD, n = 8 . | Control, n = 6 . | 5 mg/kg QOD, n = 8 . | |
| 0 | 303.2 ± 12.1 | 301.8 ± 10.1 | 111.9 ± 2.1 | 112.9 ± 1.9 |
| 2 | 301.4 ± 10.2 | 301.0 ± 11.8 | 113.0 ± 1.8 | 118.5 ± 3.3* |
| 4 | 298.5 ± 5.8 | 299.2 ± 8.6 | 112.2 ± 3.1 | 117.3 ± 1.7* |
| 6 | 308.1 ± 13.1 | 306.2 ± 10.4 | 111.9 ± 2.9 | 119.2 ± 1.8* |
| 8 | 300.3 ± 11.0 | 301.8 ± 7.3 | 111.7 ± 2.5 | 121.1 ± 2.8* |
| Time, d . | Heart rate, bpm . | QT, ms . | ||
|---|---|---|---|---|
| Control, n = 6 . | 5 mg/kg QOD, n = 8 . | Control, n = 6 . | 5 mg/kg QOD, n = 8 . | |
| 0 | 303.2 ± 12.1 | 301.8 ± 10.1 | 111.9 ± 2.1 | 112.9 ± 1.9 |
| 2 | 301.4 ± 10.2 | 301.0 ± 11.8 | 113.0 ± 1.8 | 118.5 ± 3.3* |
| 4 | 298.5 ± 5.8 | 299.2 ± 8.6 | 112.2 ± 3.1 | 117.3 ± 1.7* |
| 6 | 308.1 ± 13.1 | 306.2 ± 10.4 | 111.9 ± 2.9 | 119.2 ± 1.8* |
| 8 | 300.3 ± 11.0 | 301.8 ± 7.3 | 111.7 ± 2.5 | 121.1 ± 2.8* |
P < .05 versus control.
Intravenous ATO prolonged QTc and APD
Intravenous ATO did not change the heart rate, but prolonged the QT interval dose dependently and time dependently (Table3). Figure3A shows that QTc also progressively prolonged in the 2-hour period of intravenous infusion of ATO. The prolongation was dose-dependent. When the APD90 was measured in the papillary muscle after the guinea pigs were killed after 2 hours, it was shown that APD90 was longer in the drug-treatment group than that in the control group (Figure 3B). The prolongation was dose-dependent and reverse frequency–dependent.
Changes in heart rate and QT interval by intravenous ATO
| Time, min . | Heart rate, bpm . | QT, ms . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control, n = 6 . | 0.15 mg/kg, n = 8 . | 0.45 mg/kg, n = 7 . | 1.5 mg/kg, n = 8 . | Control, n = 6 . | 0.15 mg/kg, n = 8 . | 0.45 mg/kg, n = 7 . | 1.5 mg/kg, n = 8 . | |
| 0 | 298.3 ± 13.2 | 302.4 ± 18.2 | 308.6 ± 11.7 | 299.3 ± 10.6 | 113.5 ± 1.8 | 112.3 ± 2.1 | 110.1 ± 1.2 | 113.6 ± 2.3 |
| 10 | 308.2 ± 10.9 | 305.8 ± 11.4 | 310.2 ± 9.3 | 310.2 ± 12.4 | 110.2 ± 1.6 | 112.9 ± 3.0 | 113.6 ± 2.2 | 122.6 ± 1.93-150 |
| 30 | 297.0 ± 9.4 | 301.9 ± 13.6 | 307.5 ± 13.1 | 298.3 ± 8.5 | 113.9 ± 2.1 | 118.1 ± 2.73-150 | 118.1 ± 1.23-150 | 129.8 ± 3.0# |
| 60 | 302.4 ± 15.1 | 300.3 ± 11.7 | 301.4 ± 15.7 | 307.6 ± 12.3 | 112.2 ± 2.5 | 119.0 ± 2.13-150 | 120.7 ± 3.23-150 | 130.6 ± 2.8# |
| 90 | 308.2 ± 6.9 | 310.6 ± 9.8 | 303.6 ± 11.9 | 296.8 ± 18.3 | 112.1 ± 1.8 | 118.2 ± 2.83-150 | 123.7 ± 2.93-150 | 138.2 ± 2.7# |
| 120 | 301.1 ± 11.3 | 303.4 ± 12.9 | 299.8 ± 10.1 | 300.7 ± 21.8 | 113.5 ± 3.1 | 119.5 ± 2.93-150 | 126.7 ± 4.13-151 | 139.0 ± 3.8# |
| Time, min . | Heart rate, bpm . | QT, ms . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control, n = 6 . | 0.15 mg/kg, n = 8 . | 0.45 mg/kg, n = 7 . | 1.5 mg/kg, n = 8 . | Control, n = 6 . | 0.15 mg/kg, n = 8 . | 0.45 mg/kg, n = 7 . | 1.5 mg/kg, n = 8 . | |
| 0 | 298.3 ± 13.2 | 302.4 ± 18.2 | 308.6 ± 11.7 | 299.3 ± 10.6 | 113.5 ± 1.8 | 112.3 ± 2.1 | 110.1 ± 1.2 | 113.6 ± 2.3 |
| 10 | 308.2 ± 10.9 | 305.8 ± 11.4 | 310.2 ± 9.3 | 310.2 ± 12.4 | 110.2 ± 1.6 | 112.9 ± 3.0 | 113.6 ± 2.2 | 122.6 ± 1.93-150 |
| 30 | 297.0 ± 9.4 | 301.9 ± 13.6 | 307.5 ± 13.1 | 298.3 ± 8.5 | 113.9 ± 2.1 | 118.1 ± 2.73-150 | 118.1 ± 1.23-150 | 129.8 ± 3.0# |
| 60 | 302.4 ± 15.1 | 300.3 ± 11.7 | 301.4 ± 15.7 | 307.6 ± 12.3 | 112.2 ± 2.5 | 119.0 ± 2.13-150 | 120.7 ± 3.23-150 | 130.6 ± 2.8# |
| 90 | 308.2 ± 6.9 | 310.6 ± 9.8 | 303.6 ± 11.9 | 296.8 ± 18.3 | 112.1 ± 1.8 | 118.2 ± 2.83-150 | 123.7 ± 2.93-150 | 138.2 ± 2.7# |
| 120 | 301.1 ± 11.3 | 303.4 ± 12.9 | 299.8 ± 10.1 | 300.7 ± 21.8 | 113.5 ± 3.1 | 119.5 ± 2.93-150 | 126.7 ± 4.13-151 | 139.0 ± 3.8# |
P < .05 versus control.
P < .01 versus control.
Intravenous ATO prolonged QTc and APD.
(A) Continuous ECG monitoring revealed progressive prolongation of QTc by intravenous ATO in a dose-dependent manner. *P < .05 versus control; #P < .01 versus control; +P < .001 versus control. (B) APD after 2-hour drug infusion. The prolongation was dose-dependent. *P < .05 versus control; #P < .01 versus control; +P < .001 versus control.
Intravenous ATO prolonged QTc and APD.
(A) Continuous ECG monitoring revealed progressive prolongation of QTc by intravenous ATO in a dose-dependent manner. *P < .05 versus control; #P < .01 versus control; +P < .001 versus control. (B) APD after 2-hour drug infusion. The prolongation was dose-dependent. *P < .05 versus control; #P < .01 versus control; +P < .001 versus control.
Both the intraobserver and the interobserver agreement in the measurement of QTc were excellent. The mean intraobserver difference in the measurement of QTc (n = 426) was 0 ± 3 ms and 95% CI was between −1 and 1 ms. The mean interobserver difference was 0 ± 4 ms and 95% CI was between −1 and 1 ms.
In the present study, we have demonstrated that ATO has direct membrane effect, and dose dependently prolonged QTc and APD90 in guinea pig hearts. This report provides direct evidence that ATO delays cardiac repolarization and the rationale for the occurrence of torsade de pointes in susceptible patients.4 5 Patients who are receiving ATO for relapsed APL should avoid concomitant administration of other QT-prolonging agents, or conditions in favor of delaying cardiac repolarization, such as bradycardia, hypokalemia, or hypomagnesemia.
It is unclear why ATO prolongs cardiac repolarization. Although examination of the effects on APD90 of guinea pig papillary muscle is a common strategy to look for the QT-prolonging effects of drugs,12,21 there are still differences between human and guinea pig repolarizing currents. From the particular finding of reverse frequency–dependent effect by ATO, it is suggested that ATO might be able to block the rapid component of the delayed rectifier K+ channel (IKr),22 like other QT-prolonging agents.23 The structural uniqueness enablesIKr the most common target for drugs capable of delaying cardiac repolarization.24 Future study on the ionic mechanisms will be needed to unravel the mechanism of its QT prolongation.
The authors gratefully acknowledge TTY Biopharm Company for kindly providing intravenous arsenic trioxide to us.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-02-0598.
Supported, in part, by Taiwan Society of Cardiology, institutional research grants from Taipei Veterans General Hospital (VGH 90-067 and VGH 90-300), and National Science Council (NSC 90-2314-B-075-041).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chern-En Chiang, Division of Cardiology, Taipei Veterans General Hospital 201, Section 2, Shih-Pai Rd, Taipei 112, Taiwan; e-mail: cechiang@vghtpe.gov.tw.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal