Abstract
Sézary syndrome is a leukemic form of epidermotropic cutaneous T-cell lymphoma related to the malignant proliferation of clonal CD4+ T cells. Extracorporeal photochemotherapy may induce a transient improvement of the clinical signs, but its efficiency is discussed. To investigate the frequency of the T-cell clone in the peripheral blood of patients with Sézary syndrome and to monitor its evolution in patients treated using extracorporeal photopheresis or chemotherapy, we used the immunoscope technique. In one patient, we observed a decrease of the relative frequency of the clone from 15.6% to 0%, paralleling a complete remission of the clinical disease and a disappearance of the circulating Sézary cells. In the other cases, the evolution of the relative frequency paralleled the initial improvement of the clinical status and the absence of long-term efficiency in patients treated with extracorporeal photopheresis or chemotherapy. We observed a quick-acting direct cytotoxicity of the association 8MOP + UVA on the T-cell clone. The immunoscope technique appears to be an efficient tool to appreciate the amount of tumoral cells and to monitor the evolution of the clonal component in the Sézary syndrome.
Introduction
Sézary syndrome (SS) is characterized by exfoliative erythroderma, palmoplantar keratoderma, partial alopecia, abnormal lymph nodes, and pruritus. SS is a leukemic form of epidermotropic cutaneous T-cell lymphoma (CTCL) related to the malignant proliferation of clonal CD4+ T cells. Sézary cells present a typical cerebriform nucleus. The count of cells in peripheral blood usually exceeds 1000 per microliter (10% of total circulating leukocytes). Topical treatments of SS include the use of nitrogen mustard or PUVA therapy, but they have most often proved to be unable to control the clonal component of the peripheral blood. Systemic treatments of SS such as extracorporeal photochemotherapy (ECP), interferon alfa (IFN-α), or antineoplastic polychemotherapy (methotrexate, CHOP) often induce a transient improvement of the clinical signs.
However, the efficiency of either group of treatments is low and controversial. Indeed, the rate of complete remission (CR) with IFN-α treatment was 18% in a recent study of 11 Sézary patients.1 The efficiency of antineoplastic chemotherapy was found variable, with a mean CR rate of 38% and a relapse-free survival time varying from 5 to 41 months,2depending on the protocol used. ECP may induce complete remission in certain patients with Sézary syndrome.3 However, a recent study showed that the mean survival time with ECP was not longer than that induced by other treatments.4 Recently, it has been suggested that the association of IFN-α with ECP was more effective than ECP alone, on the clinical course of the disease.5 6
The search for the best treatment of SS is impeded by the lack of unequivocal biologic parameters to be monitored. Quantitative molecular follow-up of the malignant clone is probably the best method to evaluate the effect of the treatments. Southern blot analysis has been used in a few studies to monitor the evolution of the clonal component in the blood of SS patients treated with IFN7 or ECP.8 The latter study showed the disappearance of the peripheral blood clonal component in 2 of 10 patients with partial remission of the disease. Because of the limitations of this radioactive and insensitive technique, polymerase chain reaction (PCR) techniques—10 times more sensitive but not quantitative—using α, β, and γ T-cell–receptor (TCR) genes have been developed for the diagnosis of CTCL. Indeed PCR-γ,9PCR-DGGE-γ,10,11 PCR-PAGE-γ,12PCR-SSCP-γ,13 and PCR-β14,15 have been developed, whereas these techniques could not been used for the semiquantitative follow-up of the clonal component in SS. In other experiments the clonal population could be observed with a clonotype-specific probe.16
To investigate the frequency of the T-cell clone in the peripheral blood of patients with SS and to monitor its evolution in patients treated with ECP or chemotherapy, we used a semiquantitative and highly sensitive (0.01%) RT-PCR–based method. This technique, called immunoscopy, permits determination of the CDR3 length and of the BV and BJ segments of the expanded clones, and it permits monitoring of the expanded population of interest. CDR3-length spectratyping has been used by other authors to describe this technique.17 Immunoscopy has been widely used in the monitoring of specific T-cell clones in physiological and pathologic situations.18 19
Patients and methods
Patients
Fifteen patients—10 men and 5 women—were included in the present study. Ages ranged from 46 to 79 years (mean, 64 years). Characteristics of the 15 patients and the different treatments received are listed in Table 1. Approval was obtained from the institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. The diagnosis of Sézary syndrome was established on the basis of clinical criteria (exfoliative erythroderma, pruritus, palmoplantar keratoderma, lymph nodes) and biologic parameters including the presence of typical circulating Sézary cells (more than 1000/μL), histologic data (cutaneous epidermotropic T-cell lymphoma), and detection of a T-cell clone in the blood and in the skin by qualitative PCR DGGE γ. Eight patients were treated with ECP (4 patients according to the protocol described by Edelson et al3 and 4 patients according our own intensive protocol as described hereafter), and 2 patients were treated with CHOP chemotherapy (cyclophosphamide, Adriamycin, vincristine, prednisone). The T-cell clonal component was monitored in the peripheral blood during clinical evolution of the disease. For 3 patients treated with ECP, we were able study the T-cell clone before and just after UVA irradiation, with an interval of 30 to 45 minutes between each sample collection.
Clinical characteristics of the 15 patients
| Patient . | Sex/age . | Symptoms . | No. Sézary cells mm3 (%) . | Delay of diagnosis . | Previous treatment . | Current treatment . |
|---|---|---|---|---|---|---|
| N | F/76 | Erythroderma, PPK, nail dystrophy | 400 (6) | Few wk | 0 | Chlormetine |
| Bro | M/61 | Pruritus, erythroderma | 1 750 (25) | 10 mo | Chlormetine, IFN, BCNU, MTX, MTX + pUVA | ECP: 2 per wk then 1 per 15 d then 2 per wk every 3 wk |
| T | F/79 | Pruritus, purpuric erythema | (24) | 3 mo | pUVA, UVA + UVB, chlormetine, ECP + MTX | IFN + CS |
| Ve | F/69 | Pruritus, discreet erythema, then erythroderma, lymph nodes | 1 500 (12) | 4 y | Chlormetine | PUVA |
| S | M/53 | Exfoliative erythroderma, PPK | 100 (1) | 18 mo | PUVA | IFN |
| G | F/46 | Pruritus, exfoliative erythroderma, PPK | 1 200 (9) | 8 mo | PUVA, chlormetine, IFN, MTX, ECP | ECP (intensive) + CS |
| V | M/88 | Exfoliative erythroderma, PPK, inguinal lymphadenopathy | 194 (2) | 4 mo | Chlormetine, BCNU | ECP (intensive) then CHOP 6 cures because of histologic transformation |
| Sa | M/62 | Pruritus, erythroderma | 1 000 (9) | 18 mo | PUVA | ECP (classical) alone then ECP + IFN |
| Vd | M/65 | Pruritus, exfoliative erythroderma, PPK, nail dystrophy, lymphadenopathy | 8 000 (43) | 3 y | 0 | ECP (intensive) then MTX |
| Bra | M/70 | Pruritus, reticular erythema | (4) | — | Chlormetine, CS, pUVA then pUVA+, chloraminophen, IFN alone then + cytapheresis and acitretin | ECP (classical) + acitretin |
| Ciz | M/62 | Exfoliative erythroderma, PPK | 2 200 (22) | 1 y | CS, chlormetine, chloraminophen, IFN, MTX | ECP (classical) |
| Sy | F/75 | Pruritus, xerosis, annular erythematous lesions, then exfoliative erythroderma | 26 000 (88) | 1 y | pUVA | ECP (intensive) |
| Sv | M/60 | Exfoliative erythroderma, pruritus, PPK, nail dystrophy | (56) | 6 y | Cytapheresis, chlormetine, pUVA, IFN | ECP (classical) then 2 per d for 15 d then 1 per d for 15 d, ECP + IFN |
| R | M/59 | Pruritus, erythroderma, axillary lymphadenopathy | 2 000 (25) | 3-4 y | PUVA, chlormetine | ECP (intensive then classical) |
| D | M/53 | Exfoliative erythroderma, pruritus, PPK, fever, systemic signs (pulmonary, hepatic, intestinal, neurologic) | 1 100 (14) | Few mo | 0 | CHOP > 6 cures |
| Patient . | Sex/age . | Symptoms . | No. Sézary cells mm3 (%) . | Delay of diagnosis . | Previous treatment . | Current treatment . |
|---|---|---|---|---|---|---|
| N | F/76 | Erythroderma, PPK, nail dystrophy | 400 (6) | Few wk | 0 | Chlormetine |
| Bro | M/61 | Pruritus, erythroderma | 1 750 (25) | 10 mo | Chlormetine, IFN, BCNU, MTX, MTX + pUVA | ECP: 2 per wk then 1 per 15 d then 2 per wk every 3 wk |
| T | F/79 | Pruritus, purpuric erythema | (24) | 3 mo | pUVA, UVA + UVB, chlormetine, ECP + MTX | IFN + CS |
| Ve | F/69 | Pruritus, discreet erythema, then erythroderma, lymph nodes | 1 500 (12) | 4 y | Chlormetine | PUVA |
| S | M/53 | Exfoliative erythroderma, PPK | 100 (1) | 18 mo | PUVA | IFN |
| G | F/46 | Pruritus, exfoliative erythroderma, PPK | 1 200 (9) | 8 mo | PUVA, chlormetine, IFN, MTX, ECP | ECP (intensive) + CS |
| V | M/88 | Exfoliative erythroderma, PPK, inguinal lymphadenopathy | 194 (2) | 4 mo | Chlormetine, BCNU | ECP (intensive) then CHOP 6 cures because of histologic transformation |
| Sa | M/62 | Pruritus, erythroderma | 1 000 (9) | 18 mo | PUVA | ECP (classical) alone then ECP + IFN |
| Vd | M/65 | Pruritus, exfoliative erythroderma, PPK, nail dystrophy, lymphadenopathy | 8 000 (43) | 3 y | 0 | ECP (intensive) then MTX |
| Bra | M/70 | Pruritus, reticular erythema | (4) | — | Chlormetine, CS, pUVA then pUVA+, chloraminophen, IFN alone then + cytapheresis and acitretin | ECP (classical) + acitretin |
| Ciz | M/62 | Exfoliative erythroderma, PPK | 2 200 (22) | 1 y | CS, chlormetine, chloraminophen, IFN, MTX | ECP (classical) |
| Sy | F/75 | Pruritus, xerosis, annular erythematous lesions, then exfoliative erythroderma | 26 000 (88) | 1 y | pUVA | ECP (intensive) |
| Sv | M/60 | Exfoliative erythroderma, pruritus, PPK, nail dystrophy | (56) | 6 y | Cytapheresis, chlormetine, pUVA, IFN | ECP (classical) then 2 per d for 15 d then 1 per d for 15 d, ECP + IFN |
| R | M/59 | Pruritus, erythroderma, axillary lymphadenopathy | 2 000 (25) | 3-4 y | PUVA, chlormetine | ECP (intensive then classical) |
| D | M/53 | Exfoliative erythroderma, pruritus, PPK, fever, systemic signs (pulmonary, hepatic, intestinal, neurologic) | 1 100 (14) | Few mo | 0 | CHOP > 6 cures |
The clinical course was followed using clinical cutaneous criteria (pruritus, erythroderma, nodules, palmoplantar keratoderma, alopecia) and systemic clinical criteria (sweat, fever, weight loss, abnormal nodes, and other visceral involvement). Sézary cells were counted.
Mean follow-up was 28 months (range, 6-64 months). Average number of blood samples studied during the follow-up period was 4.5 per patient (range, 3-6 samples). The date of sampling corresponded to changes in the clinical course of the disease (ie, relapse or improvement).
Extracorporeal photopheresis protocols
Nine patients were treated with ECP. Four patients were treated according to the Edelson et al3 protocol of 2 treatments per week at 4 week-intervals.3 Five other patients were treated intensively according to a new protocol (developed by Dr Bussel, Dr Baccard, and Dr Moulonguet, Hopital Saint-Louis, Paris). Patients received a first cycle of 3 ECP treatments per week for 1 month (total, 12 treatments) followed by a 2-month period of rest. A second cycle of 3 treatments per week for 2 weeks was performed and repeated according to the clinical evolution. In this intensive protocol, methoxsalen was directly added to the cytapheresis product (200 μg/mL) instead of being taken by mouth by the patient 2 hours before the treatment.3
cDNA and PCR reaction
Peripheral blood lymphocytes (PBLs) were isolated from heparinized venous blood samples by centrifugation over Ficoll-Hypaque (Eurobio, Paris, France). Four-millimeter punch skin biopsy samples were taken and snap-frozen in liquid nitrogen for subsequent analysis.
Total RNA was extracted using the phenol-chloroform technique. Three micrograms total RNA was reverse transcribed according to Clontech (Palo Alto, CA) recommendations.
For each patient, the resultant cDNA was PCR-amplified for 40 cycles (94°C at 30 seconds; 60°C at 30 seconds; 72°C at 30 seconds) using 24 BV-specific primers and one BC-specific primer,24Taq polymerase from Promega (Madison, WI), 10 mM dNTP (Boehringer Mannheim), and 25 mM Mg2+ (Promega). The 24 BV-BC amplifications were individually performed. After checking the quality of amplification on a 2% agarose gel, we submitted each of the 24 BV-BC PCR-resultant products for 3 cycles of a run-off elongation reaction (same PCR conditions as above) using a nested BC primer labeled at its 5′ end with a fluorescent dye Fam (Eurogentec Oligold).
Immunoscope analysis
Immunoscope analysis has been described in detail elsewhere.18 Briefly, after the addition of 10 mL solution of 20 mM EDTA (ethylenediaminetetraacetic acid)–formamide, each of the 24 resultant runoff products was loaded onto a 6% acrylamide sequence gel and analyzed using an automatic sequencer and the Immunoscope software package (Applied Biosystems, Palo Alto, CA). The intensity of fluorescence of each band was determined. Each BV-BC runoff product appeared as a family of peaks characterized by the usage of a definite CDR3 length. A polyclonal distribution that reflects the absence of expanded T-cell clone(s) generated a Gaussian-like pattern, whereas clonal expansion of a T-cell clone was revealed by a distortion of the Gaussian pattern. The 24 BV-BC profiles were automatically inserted into a TCR-β repertoire sheet.18 The relative frequency of the T-cell clone was calculated by dividing the fluorescence intensity of the studied peak by the sum of fluorescence intensities of all peaks of the 24 BV families as previously described.25 Direct sequencing of the CDR3 region of the clonal expansion was performed using the Sequenase kit (Amersham).
Immunohistochemical analysis
Immunohistochemical analysis of frozen 4-mm punch skin biopsy specimens was performed for 3 patients. Immunohistochemical staining was performed using antibodies directed against CD3 (total T cells), BV regions (Ultratech; Immunotech, Marseilles, France), and the Ki67 protein for the detection of tumor cells. Biotinylated antibodies and peroxidase-labeled streptavidin (Ultratech HRP streptavidin-biotin universal detection system; Immunotech). Horseradish peroxidase enzyme activity was revealed using amino-ethyl-carbazole substrate. Results were expressed as the percentage of the positively stained cells in the cutaneous infiltrate. In one patient, the double-staining procedure was performed as follows: in the first step, sections were incubated successively with anti-BV17 mAbs (1 of 5, 45′; Immunotech) and by goat fluorescein-conjugated anti–mouse IgG (1 of 40, 30′; Immunotech), then with anti-Ki67 mAbs (30′; Immunotech) revealed by goat cyanine 3-conjugated anti–mouse IgG (1 of 200, 30′; Sigma, St Quentin Fallavier, France). Sections were examined on a light fluorescence microscope.
Results
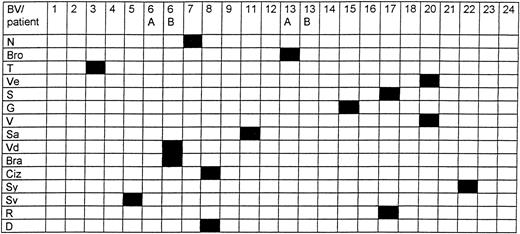
The complete BV repertoire was determined in PBLs from each patient. TCR-β repertoire analysis showed the presence of an expanded peak belonging to one BV family in the blood samples of the 15 studied patients, associated with Gaussian-like usage of the other BV families. This peak was indicative for an oligoclonal or a monoclonal expansion of T cells sharing the usage of the same BV and CDR3 length. There was no preferential or common BV usage accounting for the T-cell expansions. We observed a large diversity of rearranged BV regions, as summarized in Figure 1. For 8 patients (patients D, S, Sa, Vd, Bra, Ciz, R, and Sv), direct sequencing of the CDR3 of the dominant clone was carried out. All CDR3 sequences were found to be different, and no common CDR3 motif was shared by the different sequences (data not shown). We also did not find any preferential usage of any BJ segment. Deduced amino acid sequences of the CDR3 regions of the clones were extensively analyzed using advanced BLAST software, and no homology was detected of any T-cell clone already reported in the literature. In addition to the dominant clones present during the evolution of the disease, we observed transitory peaks corresponding to expanded T-cell populations varying with time.
Complete TCR-β repertoire analysis.
Presence of an expanded peak in one BV family in the blood samples of the 15 studied patients (in black), associated with Gaussian-like usage of the other BV families (in white).
Complete TCR-β repertoire analysis.
Presence of an expanded peak in one BV family in the blood samples of the 15 studied patients (in black), associated with Gaussian-like usage of the other BV families (in white).
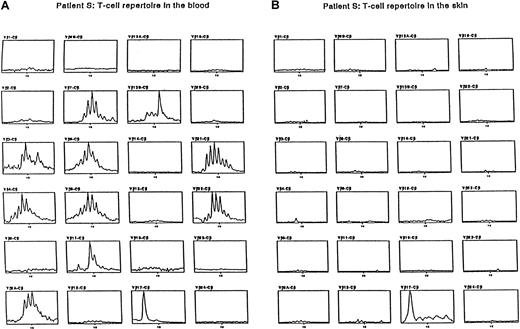
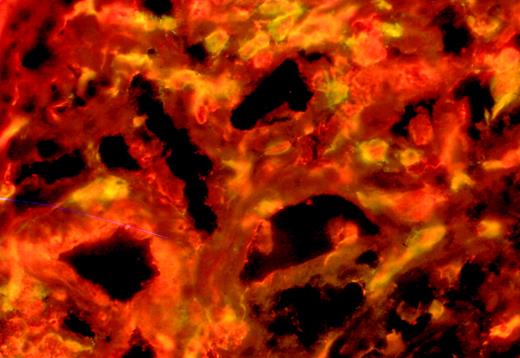
Expression of the BV chains found expanded in the blood was analyzed in the skin. Expression of the expanded BV chains was analyzed using immunoscopy and immunohistochemistry in the lesional skin samples of 3 patients. To normalize the amount of lymphoid RNA obtained from skin biopsy samples, the amount of infiltrating T cells was assessed by immunohistochemistry using anti-CD3 antibodies before immunoscope analysis. Monoclonal antibodies directed against expanded BV regions of the TCR-β chain detected in blood were used. Most skin lymphocytes that expressed the BV region were expanded in the blood of the 3 patients. In contrast, in previous works concerning inflammatory skin diseases, this approach permitted in situ amplification of the entire BV repertoire using the immunoscope technique.20 21Patient S showed BV17 T-cell expansion in blood and skin (Figure2). Moreover, the double staining of skin sections of patient S with an anti-BV17 antibody and an anti-Ki67 mAb evidenced the association of the 2 markers on the same tumoral cells (Figure 3).
Immunoscope analysis.
Patient S showed BV17 T-cell expansion in blood and skin.
Double staining of skin sections.
Patient S with an anti-BV17 antibody (fluorescein) and an anti-Ki67 antibody (cyanine).
Double staining of skin sections.
Patient S with an anti-BV17 antibody (fluorescein) and an anti-Ki67 antibody (cyanine).
Relative frequency of the dominant T-cell clone was determined for each sample taken from each patient
In patient Sy, we observed a decrease in the relative frequency of the clone from 15.6% to close to 0%, paralleling a complete clinical remission and a disappearance of the circulating Sézary cells (Figure 4). In the 7 other patients, ECP was found inefficient and the clinical condition of the patients worsened, sometimes after a period of stability or of short partial improvement. In these 7 patients, the relative frequency of the clone paralleled the clinical condition, except in one patient at the onset of ECP treatment (patient R). Results for the 8 patients treated with ECP are summarized in Table 2, and the evolution for patient Vd is given in Figure5. Surprisingly, the relative frequency of the clone did not follow the Sézary cell count evolution (Table 2).
Relative frequency.
Clone in patient Sy decreased from 15.6% to close to 0%.
Clinical and molecular evolution in patients treated with ECP and CHOP
| Patient . | Treatment . | Clinical course . | Evolution of Sézary cells . | Evolution of relative frequency of clone . |
|---|---|---|---|---|
| V | ECP | Improvement (before histologic transformation) | 7/2% | 9/0/0.5% |
| V | CHOP | Progressive worsening | 2/0% | 0.5/6% |
| Sa | ECP | Stable, then worsening | 10/31% | 9/30% |
| Vd | ECP | Progressive worsening | 42/26% | 9/18% |
| Bra | ECP | Improvement, then worsening | 16/19% | then 84/8/37/20% |
| Ciz | ECP | Partial efficiency, then worsening | 7/5% | Fluctuations 18/20% |
| Sy | ECP | Complete remission | 27/0% | 16% disappearance of the peak |
| Sv | ECP | Worsening | — | Fluctuations 18/10/41/6% |
| R | ECP | Worsening | 2/4% | then 20/2/8.5% |
| D | CHOP | Partial remission | 14/0% | then 43/7/16% |
| Patient . | Treatment . | Clinical course . | Evolution of Sézary cells . | Evolution of relative frequency of clone . |
|---|---|---|---|---|
| V | ECP | Improvement (before histologic transformation) | 7/2% | 9/0/0.5% |
| V | CHOP | Progressive worsening | 2/0% | 0.5/6% |
| Sa | ECP | Stable, then worsening | 10/31% | 9/30% |
| Vd | ECP | Progressive worsening | 42/26% | 9/18% |
| Bra | ECP | Improvement, then worsening | 16/19% | then 84/8/37/20% |
| Ciz | ECP | Partial efficiency, then worsening | 7/5% | Fluctuations 18/20% |
| Sy | ECP | Complete remission | 27/0% | 16% disappearance of the peak |
| Sv | ECP | Worsening | — | Fluctuations 18/10/41/6% |
| R | ECP | Worsening | 2/4% | then 20/2/8.5% |
| D | CHOP | Partial remission | 14/0% | then 43/7/16% |
Evolution of T-cell repertoire.
Patient Vd during PCE. The clonal population is defined by the usage of BV6B.
Evolution of T-cell repertoire.
Patient Vd during PCE. The clonal population is defined by the usage of BV6B.
To determine the effect of chemotherapy on the clonal T-cell population, we included 2 Sézary patients (D, V) treated with CHOP. In patient D, CHOP was the first-line treatment because of a serious systemic extension of the disease (intestinal, hepatic, pulmonary). He entered partial remission after the first cycle of treatment. The relative frequency of the clone decreased from 43% to 6.7%. The description of the complete TCR-β chain repertoire evidenced the reappearance of a polyclonal population. After the fifth cycle, because of T-cell lymphopenia, the relative frequency of the clone increased from 6.7% to 16%, paralleling partial remission of the cutaneous and systemic disease. In patient V, 1 year of ECP treatment proved effective in the evolution of the erythroderma, and the relative frequency of the clone decreased from 8.7% to 0.5%. Chemotherapy (CHOP) had been chosen because of histologic transformation to nodular large T-cell lymphoma. The relative frequency of the clone increased to 5.8% and remained stable for the following months and cure cycles (6.3% after the fifth cure). The evolution of the relative frequency of the clonal population paralleled the absence of efficiency of CHOP (Tables 1, 2).
Relative frequency of the clone was determined, before and immediately after UVA irradiation
PBLs were harvested just before UVA irradiation and in cytapheresis products 30 minutes after UVA irradiation. The T-cell repertoire was analyzed using immunoscopy. In 3 of 3 patients, we observed a decrease in the relative frequency of the expanded clone. Analysis of the T-cell repertoire 4 months after treatment showed that the relative frequency of the clone had increased to the previously determined level before irradiation (Table3).
Relative frequency of the T-cell clone before, 30 minutes after, and 4 months after UVA irradiation
| Patient . | Before irradiation, % . | 30 minutes after irradiation, % . | 4 months later, % . |
|---|---|---|---|
| Sa | 17 | 4 | 17 |
| Bra | 19 | 8 | 37 |
| Ciz | 20 | 15 | 18 |
| Patient . | Before irradiation, % . | 30 minutes after irradiation, % . | 4 months later, % . |
|---|---|---|---|
| Sa | 17 | 4 | 17 |
| Bra | 19 | 8 | 37 |
| Ciz | 20 | 15 | 18 |
Discussion
To determine the BV usage of clonal Sézary cells, the complete BV repertoire was determined in PBLs of 15 patients. Using the highly sensitive immunoscope technique, we were able to detect the presence of a well-defined, dominant T-cell clone in 15 of 15 patients. Our results confirm that SS is related to the proliferation of clonal T cells. These results contrast with a previous published study using less sensitive techniques that detect oligoclonal or polyclonal expansion in CTCL patients.22 The Vβ-Cβ RT-PCR analysis used in this study was not sensitive enough to detect clonal expansion.23 In addition, we did not find a preferential or common BV usage shared by all patients, ruling out the hypothesis of a superantigenic-dependent T-cell stimulation. These results confirm earlier reports on the high diversity of the BV usage by Sézary cells.14,26 27 We also did not observe preferential usage of the BJ segment. Moreover, no homology was found between the CDR3 β chain sequence and a T-cell clone reported in the literature.
In 10 patients, disease evolution was monitored using the immunoscope technique. The expanded clone persisted throughout the survey period, including the treatment. In addition to the dominant clones, we observed variable peaks corresponding to expanded clones varying with time. The significance of such clonal populations remains to be elucidated, but they might reflect nontumoral-reactive lymphocytes. In previous works,24,28 we have found that the BV T-cell repertoire is polyclonal and presents a Gaussian distribution in normal blood. The sensitivity of the technique was previously determined by dilution experiments with a clonal T-cell line. A T-cell clone with a frequency among peripheral blood mononuclear cells of 5 × 10−4 could be detected.29
We sought to determine whether the dominant T-cell clones observed in peripheral blood corresponded to tumor cells. The persistence of the same clone in collected samples after an interval of several months strongly indicated that this clonal population belongs to the malignant Sézary cell compartment. Moreover immunoscopy of skin biopsy specimens and of PBLs showed that the same β chain was used by the expanded circulating T cells and by T cells recovered from the biopsy specimens. In Sézary patients, we used antibodies directed against the BV segments found associated with clonal expansion to study skin biopsy specimens. Expression of the BV chains was found predominantly associated with lymphocytes showing the phenotype of tumor cells. Monoclonal antibodies directed against BV regions of the TCR-β chain have been used by other authors and resulted in the staining of most of the skin lymphocytes in 11 of 15 patients with CTCL (mycosis fungoides and Sézary syndrome), whereas no reactivity was observed in patients with benign dermatoses.30 Moreover, double staining of the biopsy sections of patient S with the anti-BV17 antibody and anti-Ki67 mAb showed the association of the 2 markers with the same cells. It can thus be concluded that the persistent and expanded clones correspond to the malignant T-cell population. However in our study, the Sézary cell count was not correlated with the relative frequency of the monoclonal population. The relative frequency is likely to be more accurate than the optical counting of Sézary cells, which may be subjective,31 and to be an unfaithful reflection of the tumoral population because all tumoral lymphocytes do not have the typical aspect of Sézary cells and because Sézary cells may also be reactive cells that can be found in benign dermatoses.32
We have used the above-defined indicator to monitor the evolution of the T-cell clones in 8 patients treated with ECP. The relative frequency of the dominant T-cell clone was determined. In patient Sy, we observed a decrease in the relative frequency of the clone from 15.6% to 0%, paralleling a 4-year complete remission of the clinical disease and disappearance of the circulating Sézary cells. In the 7 other patients, the relative frequency of the clone paralleled the clinical status. The evolution of the relative frequency paralleled the initial improvement of the clinical status and the absence of long-term efficiency in patients treated with CHOP.
To ascertain the effect of PCE on the tumoral population, the relative frequency of the clone was determined immediately before and after UVA irradiation. We observed a decrease in relative frequency of the expanded clone 30 minutes after UVA irradiation. A few months after treatment, in vivo analysis revealed that the relative frequency of the clone had increased to the level achieved before UVA irradiation. Direct cytotoxicity of the association 8MOP + UVA might induce an immediate decrease in clone frequency.33 However, cytotoxicity must have been transient because the relative frequency of the clone had increased again in samples taken 4 months later.
In conclusion, we have shown that immunoscopy could be used to monitor accurately the evolution of the Sézary cells throughout ECP and during polychemotherapy treatments. In all ECP- and CHOP-treated patients, parallelism between the relative frequency of the T-cell clone and clinical evolution was observed. This finding argues for the role of the T-cell clone in the phenotypic expression of the disease. In addition, the relative frequency of the clone was found to decrease between the beginning and the end of UVA irradiation, a finding suggestive of direct cytotoxicity of the treatment onto Sézary cells. Finally, immunoscopy appears to be an efficient tool to determine the amount of tumoral cells and to monitor the evolution of the clonal component in Sézary syndrome. Because treatments used in patients with Sézary syndrome are likely to induce T-cell death in the tumoral and nontumoral population, the immunoscopy technique allows semiquantitative evaluation of tumoral and nontumoral compartments during treatment. Moreover, this technological strategy could be used to evaluate the efficiency of a treatment and to monitor the antitumoral T-cell response. In the present study we have shown that molecular monitoring is correlated with clinical evolution but that follow-up of the clonal population could not replace clinical monitoring of the disease. Additional studies are required to analyze the clonal component in other T-cell cutaneous lymphomas and pseudolymphomas.
We thank Drs I. Moulonguet and F. Mouly for clinical assistance and Profs D. Charron, and F. Sigaux and Dr C. Rabian for PBL storage.
Supported by grants from INSERM and the Association de la Recherche en Transfusion.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Philippe Musette, Institut de Recherche sur la Peau, INSERM U532, Hôpital saint Louis, 75475 Paris Cedex 10 France.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal