Abstract
Arp2/3 complex is believed to induce de novo nucleation of actin filaments at the edge of motile cells downstream of WASp family proteins. In this study, the signaling pathways leading to Arp2/3 complex activation, actin assembly, and shape change were investigated in platelets isolated from patients with Wiskott-Aldrich Syndrome (WAS), that is, who lack WASp, and in WASp-deficient mouse platelets. WASp-deficient human and mouse platelets elaborate filopodia, spread lamellae, and assemble actin, identical to control WASp-expressing platelets. Human platelets contain 2 μM Arp2/3 complex, or 8600 molecules/cell. Arp2/3 complex redistributes to the edge of the lamellae and to the Triton X-100–insoluble actin cytoskeleton of activated WASp-deficient platelets. Furthermore, the C-terminal CA domain of N-WASp, which sequesters Arp2/3 complex, inhibits by half the actin nucleation capacity of octylglucoside-permeabilized and activated WAS platelets, similar to its effect in WASp-expressing cells. Along with WASp, platelets express WAVE-2 as a physiologic activator of Arp2/3 complex and a small amount of N-WASp. Taken together, our findings show that platelets activate Arp2/3 complex, assemble actin, and change shape in the absence of WASp, indicating a more specialized role for WASp in these cells.
Introduction
Arp2/3 complex is believed to induce de novo nucleation of actin filaments at the edge of motile cells. This complex, originally found in Acanthamoeba castellanii, consists of 2 actin-related proteins, Arp2 and Arp3, and 5 novel proteins, p41-, p34-, p21-, p20-, and p16-Arc.1,2 Arp2/3 complex initiates the actin-based motility of intracellular parasites such as Listeria monocytogenes and Shigella flexneri,3-5 branches actin filaments in vitro,6,7 and localizes at the leading edge of crawling cells such as carcinoma cells and Xenopus laeviskeratocytes.8 9
The actin nucleation activity of Arp2/3 complex can be initiated by WASp family proteins, 5 of which are expressed in mammals: WASp, N-WASp, and WAVE-1, -2, and -3. WASp is expressed only in hematopoietic cells,10 notably in monocytes, lymphocytes, and platelets, whereas N-WASp is widely expressed.11 WAVE-1 (also called Scar) was first identified in Dictyostelium discoideum, then in vertebrates,12,13 and 2 homologues, WAVE-2 and -3, were subsequently cloned.14 WASp and N-WASp are thought to activate Arp2/3 complex–mediated actin nucleation activity to induce filopodia formation downstream of the small guanosine triphosphatase (GTPase) Cdc42,11,15-19 whereas WAVE-1, -2, and -3 are believed to orchestrate lamellae spreading downstream of Rac.20 Recently, cortactin and Abp1 (or drebrin) have also been added to the list of proteins that can activate Arp2/3 complex in mammalian cells.21-25
In vitro observations have elucidated the biochemical and structural mechanisms by which WASp family proteins activate Arp2/3 complex. The conserved C-terminal VCA domain of WASp and N-WASp binds and brings together actin monomers and Arp2/3 complex to stimulate its actin nucleation activity, whereas the CA domain alone sequesters and inhibits Arp2/3 complex.26-28 However, WASp and N-WASp are unable to activate Arp2/3 complex until signaling molecules such as Cdc42 and polyphosphoinostides (ppIs) release intramolecular inhibitory interactions to expose the VCA domain.29,30Listeria ActA resembles the VCA domain of WASp proteins and activates Arp2/3 complex with some similarity, whereasShigella IcsA recruits endogenous N-WASp from the host cell.31
Mutations in the WASP gene encoding WASp in humans results in Wiskott-Aldrich Syndrome (WAS), a severe inherited X-linked recessive hematopoietic disorder that is characterized by thrombocytopenia, immunodeficiency, and eczema.10 WAS platelets have short circulation times and are generally smaller, a phenotype that is partially reversed by splenectomy.32,33Importantly, all described mutations in the WASP gene have been shown to lead to the complete absence of WASp expression in platelets.34,35 In this study, aspects of the signaling pathways leading to Arp2/3 complex activation and actin assembly were investigated in human WAS platelets and WASp-deficient mouse platelets. Consistent with previous observations,36 37 WASp-deficient human and mouse platelets activate Arp2/3 complex and redistribute it to the cell cortex, and elaborate filopodia, spread lamellae, and assemble actin normally when activated. Thus, WASp is unnecessary for a robust actin assembly reaction, suggesting a more specialized role for WASp in platelets.
Patients, materials, and methods
Patients
Patients were diagnosed with WAS based on male sex, thrombocytopenia with small platelets, immunodeficiency, and eczema, and identification of a mutation on their WASP gene. All patients were examined after splenectomy and have platelet counts and sizes close to normal. Their platelets lack WASp (Figure 9) and have normal discoid shape while circulating (data not shown). The phenotype of patient P3434 35 is moderate, with a history of infections, but no eczema. His WASP gene has a G1305 deletion in exon 10, and his B and T cells express a truncated form of WASp at 5% to 20% of normal level. The phenotype of patient P39 is moderate, with a history of infections and mild eczema. HisWASP gene has a G→A mutation in intron 6 at position +5. Full-length WASp is expressed at 5% to 20% of normal level in his T cells but is absent in his B cells. Patient P32 presents a mild phenotype diagnosed as X-linked thrombocytopenia. His WASPgene has a G291→A mutation in exon 2, and his B and T cells express 5% to 20% of full-length WASp.
Approval was obtained from the institutional review boards of both Brigham and Women's Hospital and the Center for Blood Research (Harvard Medical School), and informed consent was approved according to the Declaration of Helsinki.
Animals and reagents
The WASp-deficient mice were provided by Drs Catherine T. Yan, Scott B. Snapper, and Frederick W. Alt (Center for Blood Research, Harvard Medical School, Boston, MA).38 The collagen-related peptide (CRP; amino acids GCP*(GPP)10GCP*G, where P* means hydroxyproline), prepared and cross-linked as previously described,39 was a gift from Drs Michael J. Barnes, Richard W. Farndale, and C. Graham Knight (Department of Biochemistry, University of Cambridge, Cambridge, England). The thrombin receptor PAR-1–activating peptide (TRAP; amino acids SFLLRNPNDKYEPF) was purchased from Bachem Bioscience (King of Prussia, PA). Arp2/3 complex, purified from human platelets as described,40 was a gift from Dr Fumihiko Nakamura (Division of Hematology, Brigham and Women's Hospital, Boston, MA). Affinity-purified rabbit polyclonal antibodies directed against the last 19 amino acids of bovine Arp3 and the amino acids 179-204 of human p34-Arc were a gift from Dr Matthew D. Welch (Department of Molecular and Cell Biology, University of California, Berkeley, CA).3,41 Blocking experiments were performed using the peptides to compete for Arp2/3 complex binding. Rabbit polyclonal antibody B4, directed against the amino acids 224-238 of human WASp (amino acids 226-240 of mouse WASp), was a gift from Drs Marco Lopez and Tomas Kirchhausen (Center for Blood Research, Harvard Medical School).42 Rabbit polyclonal antibody directed against full-length N-WASp and the vector encoding GST-CA derived from N-WASp (amino acids 450-505) were a gift from Drs Le Ma, Rajat Rohatgi, and Marc W. Kirschner (Department of Cell Biology, Harvard Medical School).26 GST-CA was expressed and purified as described.43 Goat polyclonal antibody D-16, directed against an internal region of human WAVE-2, was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Positive control for WAVE-2 included Jurkat T-cell lysate provided by the manufacturer. Rabbit skeletal muscle actin was isolated and labeled with pyrene as described.44 All other reagents were also of the highest purity available (Sigma, St Louis, MO, and Bio-Rad, Hercules, CA).
Platelet preparation
Blood from healthy volunteers and from WAS patients was collected in 0.1 volume of Aster-Jandl anticoagulant.44Human platelet-rich plasma (PRP) was prepared by centrifugation of the blood at 100g for 20 minutes. Blood was collected from wild-type and WASp-deficient mice by retro-orbital plexus bleeding and anticoagulated in Aster-Jandl anticoagulant. Mouse PRP was obtained by centrifugation of the blood at 100g for 6 minutes, followed by centrifugation of the supernatant and the buffy coat at 100g for 6 minutes. Human and mouse platelets were isolated from PRP using a metrizamide gradient.45 Platelet concentration was adjusted to 3 × 108/mL, and platelets were allowed to rest for 30 minutes at 37°C before use.
DIC light microscopy
Attachment and activation of platelets on CRP-coated surface were followed by differential interference contrast (DIC) light microscopy.45 Coverslips were coated with 6 μg/mL CRP in phosphate-buffered saline (PBS) for 2 hours followed by extensive blocking with 0.5% bovine serum albumin (BSA) in PBS. Petri dishes were maintained at 37°C with a Harvard Apparatus (Holliston, MA) temperature controller TC-202. Platelets were imaged on a Zeiss IM-35 inverted microscope with DIC optics and × 100 oil immersion objective. Images were captured with a Hamamatsu C2400 CCD camera (Hamamatsu, Japan), processed for background substraction and frame averaging with a Hamamatsu ARGUS image processor and digitally recorded on Macintosh computer equipped with a SCION frame grabber LG-3 (Frederick, MD).
Immunofluorescence analysis
Platelets were centrifuged at 280g for 5 minutes onto poly-l-lysine–coated coverslips, fixed in 4% formaldehyde, permeabilized with 0.5% Triton X-100, and blocked with 0.5% BSA in PBS. Arp2/3 complex sites were exposed by treating the fixed and blocked cytoskeletons with 0.1% sodium dodecyl sulfate (SDS) for 1 minute. Specimens were washed in PBS and labeled with a mixture of rabbit anti-Arp3 and anti–p34-Arc antibodies, each at 4 μg/mL. Images were obtained using a Zeiss Axiovert S100 microscope with a × 100 oil immersion objective. The peptides used to raise the antibodies3 41 were mixed with the anti-Arp2/3 antibodies as controls (each at 50 μg/mL) and blocked all detectable antibody binding (Figure 5B).
Electron microscopy and immunogold labeling
Platelets isolated from WAS patients were attached to 5-mm round poly-l-lysine–coated coverslips by centrifugation at 280g for 5 minutes at room temperature. Resting platelets isolated from wild-type and WASp-deficient mice were attached to antiglycoprotein Ibα (GPIbα) antibody-coated coverslips, and activated with 1 U/mL thrombin for 10 minutes at 37°C. Human and mouse platelet cytoskeletons were prepared by extraction with 0.75% Triton X-100 in 60 mM PIPES (piperazine-N,N′-bis-2-ethanesulfonic acid), 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 10 mM EGTA (ethyleneglycoltetraacetic acid) and 2 mM MgCl2, pH 6.9, containing protease inhibitors and 2 μM phalloidin (PHEM buffer), as described.46 Extracted platelets were briefly washed in PHEM buffer and fixed with 1% glutaraldehyde in PHEM buffer for 10 minutes. Unreacted aldehydes were blocked with 1 mg/mL sodium borohydride in PHEM buffer for 1 minute. Arp2/3 complex sites were exposed by treating the fixed and blocked cytoskeletons with 0.1% SDS in PHEM buffer for 1 minute. There was no specific labeling in the absence of SDS (data not shown), showing the epitopes to be sequestered when Arp2/3 complex was not denatured. Cytoskeletons were washed with PHEM buffer and incubated in 1% BSA in 150 mM NaCl, 20 mM Tris (TBS), pH 8.2. Cytoskeletons were labeled with a mixture of rabbit anti-Arp3 and anti–p34-Arc antibodies, each at 4 μg/mL. Unbound antibodies were washed away with TBS/1% BSA, pH 8.2, and cytoskeletons were incubated with 10 nm colloidal gold coated with goat antirabbit IgG for 60 minutes. Coverslips were washed thrice with TBS, then fixed with 1% glutaraldehyde in PBS for 10 minutes. Controls included omitting the primary antibody and using nonspecific rabbit antibodies instead of the affinity-purified anti-Arp3 and anti–p34-Arc antibodies. In the absence of specific primary antibodies there was no detectable signal (data not shown). Cytoskeletons were washed thrice with double-distilled water just before freezing. Samples were rapid-frozen, freeze-dried, and rotary-shadowed with 1.2 nm tantalum-tungsten followed by 2.5 nm carbon. Specimens were examined and photographed in a JEOL 1200-EX electron microscope (Tokyo, Japan) using an accelerating voltage of 100 kV.
SDS-PAGE and immunoblot analysis
Platelet proteins were lysed in SDS–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 5% β-mercaptoethanol. After boiling for 5 minutes, proteins were separated on 8% or 10% polyacrylamide gels and transferred onto an Immobilon-P membrane (Millipore, Bedford, MA). Membranes were incubated overnight in 100 mM NaCl and 20 mM Tris, pH 7.4, containing 1% BSA, then probed with antibodies directed against proteins of interest. Detection was performed with an enhanced chemiluminescence system (Pierce, Rockford, IL).
For the Arp2/3 complex depletion, human platelets were lysed in 1% Igepal CA-630 (also called Nonidet P-40), 50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL leupeptin, and 10 μg/mL aprotinin, as described.47 After centrifugation at 14 000g for 10 minutes at 4°C, the Igepal CA-630–soluble fraction was passed twice over a GST-CA column.40 This procedure resulted in more than 99% depletion of Arp2/3 complex (Figure 3B), as described for neutrophils.48
For isolation of the actin cytoskeleton, platelets were lysed with 0.1% Triton X-100 in PHEM buffer. Filamentous actin (F-actin) was isolated by centrifugation at 100 000g for 30 minutes at 4°C in a Beckman Optima TL ultracentrifuge (Palo Alto, CA) using polycarbonate tubes and a TLA 100 rotor. Triton X-100–insoluble and –soluble fractions were resolved by SDS-PAGE, as described above.
Measurement of platelet F-actin content and barbed-end nuclei numbers
Resting or activated platelets were fixed in 3.4% formaldehyde and permeabilized with 0.1% Triton X-100 in PHEM buffer in the presence of 2 μM fluorescein isothiocyanate (FITC)–phalloidin.45 Bound FITC-phalloidin was quantitated by FACS analysis using a Becton Dickinson FACSCalibur flow cytometer (Franklin Lakes, NJ). A total of 10 000 events was analyzed for each sample.
Platelets were activated and extracted with 0.1% Triton X-100 in PHEM buffer44 or permeabilized with 0.25% octylglucoside (OG) in PHEM buffer and activated.49 The OG-platelet suspension was mixed and the platelets were allowed to extract for 30 seconds at 37°C. When required, GST-CA was added prior to activation, as described.43 The polymerization rate assay started by the addition of 185 μL 100 mM KCl, 2 mM MgCl2, 0.5 mM adenosine triphosphate (ATP), 0.1 mM EGTA, 0.5 mM dithiothreitol, and 10 mM Tris, pH 7.0, to 100 μL platelet extract, and the subsequent addition of 15 μL 20 μM monomeric pyrene-labeled rabbit skeletal muscle actin. Pyrene-actin fluorescence was recorded using a spectrofluorimeter LS50 (Perkin-Elmer Analytical Instruments, Norwalk, CT). Excitation and emission wavelengths were 366 and 386 nm, respectively. The number of barbed-end nuclei was determined as described.49
Results
Platelets change shape normally in the absence of WASp
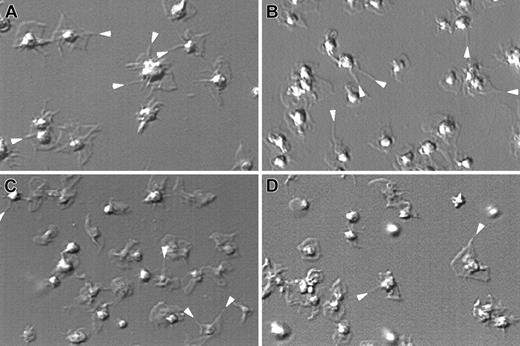
The morphology and organization of the actin cytoskeleton of human WAS platelets and WASp-deficient mouse platelets, activated on a CRP-coated surface, specific for the collagen receptor GPVI,45 were investigated by DIC light microscopy (Figure1). The attachment and spreading process of WASp-deficient human and mouse platelets were identical to control platelets. Both WASp-expressing and WASp-deficient platelets generated filopodia and lamellae when exposed to CRP-coated surface.
Platelets change shape normally in the absence of WASp.
Platelets isolated from a healthy volunteer (A), WAS patient P39 (B), and wild-type (C) and WASp-deficient mice (D) were allowed to adhere on CRP-coated glass coverslips. Images shown are captured 10 minutes after the initial adhesion and activation reaction. Results shown are representative of the 3 patients studied. Filopodia are indicated by a white arrow.
Platelets change shape normally in the absence of WASp.
Platelets isolated from a healthy volunteer (A), WAS patient P39 (B), and wild-type (C) and WASp-deficient mice (D) were allowed to adhere on CRP-coated glass coverslips. Images shown are captured 10 minutes after the initial adhesion and activation reaction. Results shown are representative of the 3 patients studied. Filopodia are indicated by a white arrow.
Platelets assemble actin normally in the absence of WASp
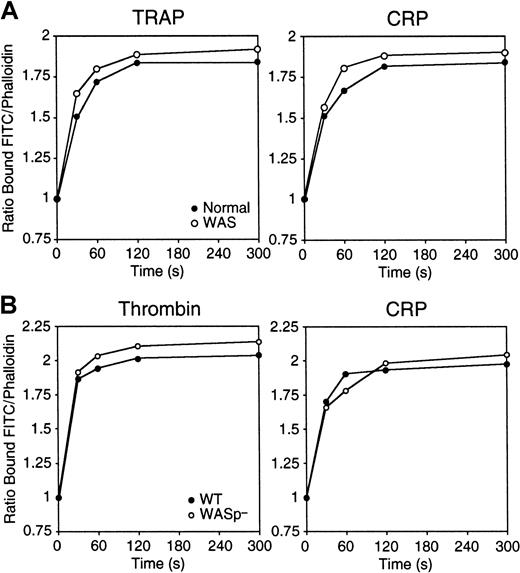
We addressed whether WASp expression is required for platelet actin assembly. Platelets isolated from WAS patients increased their F-actin content normally after ligation of the thrombin receptor PAR-1 with 25 μM TRAP or of the collagen receptor GPVI with 3 μg/mL CRP (Figure 2A), consistent with previous observations.36 37 Similarly, WASp-deficient mouse platelets showed no defects in actin assembly in response to 1 U/mL thrombin or to 3 μg/mL CRP (Figure 2B).
Platelets assemble actin normally in the absence of WASp.
(A) Platelets isolated from a healthy human volunteer (●) and WAS patient P34 (○) were activated with 25 μM TRAP or 3 μg/mL CRP. (B) Platelets from wild-type (●) and WASp-deficient (○) mice were activated with 1 U/mL thrombin or 3 μg/mL CRP. Platelets were fixed with 3.4% formaldehyde, extracted with 0.1% Triton X-100 in PHEM buffer containing 2 μM FITC-phalloidin, and analyzed by FACS. Results shown are expressed as the ratio between the fluorescence of activated cells versus resting cells and are representative of 3 to 4 experiments.
Platelets assemble actin normally in the absence of WASp.
(A) Platelets isolated from a healthy human volunteer (●) and WAS patient P34 (○) were activated with 25 μM TRAP or 3 μg/mL CRP. (B) Platelets from wild-type (●) and WASp-deficient (○) mice were activated with 1 U/mL thrombin or 3 μg/mL CRP. Platelets were fixed with 3.4% formaldehyde, extracted with 0.1% Triton X-100 in PHEM buffer containing 2 μM FITC-phalloidin, and analyzed by FACS. Results shown are expressed as the ratio between the fluorescence of activated cells versus resting cells and are representative of 3 to 4 experiments.
Human platelets contain 2 μM Arp2/3 complex
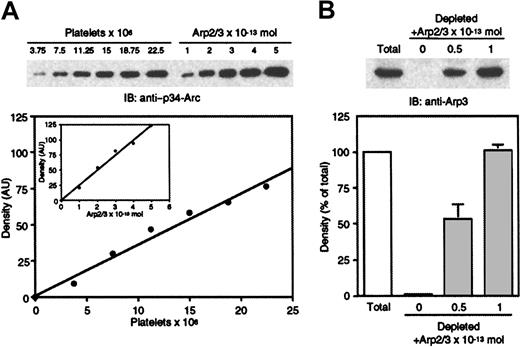
We determined the amount of Arp2/3 complex expressed in human platelets. By densitometric analysis of immunoblots against the Arp3 and p34-Arc subunits of Arp2/3 complex, using purified platelet Arp2/3 complex as a standard,40 we estimated that human platelets contain 14.3 × 10−21 mol Arp2/3 complex, or 8600 molecules/cell (Figure 3A). To verify that the presence of other platelet proteins in whole lysates does not affect the Arp2/3 complex signal on immunoblots, purified Arp2/3 complex was added back to platelet lysates depleted of Arp2/3 complex by sequential passages over a GST-CA column. GST-CA removed more than 99% of Arp2/3 complex from the extract. Similar intensity signals were obtained with lysates corresponding to 7 × 106 platelets and their depleted counterparts complemented with 1 × 10−13 mol Arp2/3 complex, as estimated (14.3 × 10−21mol/platelet × 7 × 106 platelets; Figure 3B). Because the average platelet volume is 7 × 10−15 L, human platelets contain 2 μM Arp2/3 complex.
Platelet Arp2/3 complex level.
(A) Increasing numbers of human platelets were compared to known amounts of purified Arp2/3 complex by SDS-PAGE and probed with a rabbit polyclonal antibody directed against the p34-Arc subunit of Arp2/3 complex. The graph plots the density of the detected bands against the platelet counts and the amount of purified Arp2/3 complex (inset) obtained from a single immunoblot. The amount of Arp2/3 complex divided by the platelet count was determined as the ratio between first-order equation slopes best fitting the data. Human platelets contain 14.3 × 10−21 mol Arp2/3 complex. (B) Lysates corresponding to 7 × 106 platelets (■) were compared by anti-Arp3 immunoblot to their Arp2/3 complex-depleted counterparts complemented or not with 0.5 and 1 × 10−13 mol purified Arp2/3 complex (░). The amount of Arp2/3 complex was quantified by densitometric analysis of the immunoblots. The histogram plots the percentage ± SD of the density relative to total from 3 independent assays.
Platelet Arp2/3 complex level.
(A) Increasing numbers of human platelets were compared to known amounts of purified Arp2/3 complex by SDS-PAGE and probed with a rabbit polyclonal antibody directed against the p34-Arc subunit of Arp2/3 complex. The graph plots the density of the detected bands against the platelet counts and the amount of purified Arp2/3 complex (inset) obtained from a single immunoblot. The amount of Arp2/3 complex divided by the platelet count was determined as the ratio between first-order equation slopes best fitting the data. Human platelets contain 14.3 × 10−21 mol Arp2/3 complex. (B) Lysates corresponding to 7 × 106 platelets (■) were compared by anti-Arp3 immunoblot to their Arp2/3 complex-depleted counterparts complemented or not with 0.5 and 1 × 10−13 mol purified Arp2/3 complex (░). The amount of Arp2/3 complex was quantified by densitometric analysis of the immunoblots. The histogram plots the percentage ± SD of the density relative to total from 3 independent assays.
Normal Arp2/3 complex redistribution in the actin cytoskeleton of WASp-deficient platelets
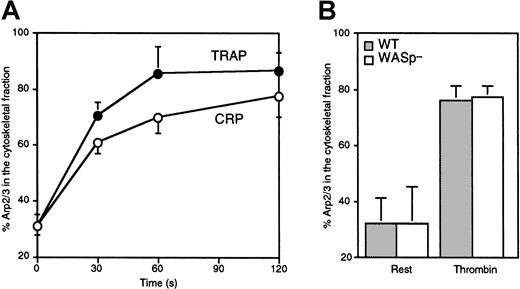
The redistribution of Arp2/3 complex to the actin cytoskeleton of WASp-deficient platelets was evaluated. As is always observed for WASp-expressing platelets (H.F., K.M.H., R.N., et al, submitted manuscript), Arp2/3 complex redistributed to the actin cytoskeleton of WASp-deficient activated platelets. Thirty percent of the total platelet Arp2/3 complex was associated with the Triton X-100–insoluble actin cytoskeleton of resting platelets (Figure4). This amount increased to 80% after stimulation of human WAS platelets with 25 μM TRAP and 3 μg/mL CRP or after activation of WASp-deficient mouse platelets with 1 U/mL thrombin, identical to normal WASp-expressing platelets. WASp was always Triton X-100 soluble in platelets maintained under nonaggregating conditions (data not shown). WASp redistribution to the actin cytoskeleton requires platelet aggregation50 to cross-link the fibrinogen receptor αIIbβ3, a condition that is not required for Arp2/3 complex redistribution, actin assembly, and shape change.
Arp2/3 complex incorporation into the platelet cytoskeleton.
(A) WAS platelets were activated with 25 μM TRAP (●) or 3 μg/mL CRP (○) for the indicated times. (B) Wild-type (░) and WASp-deficient (■) mouse platelets were activated with 1 U/mL thrombin for 1 minute. F-actin–associated Arp2/3 complex was collected by centrifugation of Triton X-100 platelet lysates at 100 000g for 30 minutes at 4°C. F-actin–associated and soluble fractions were displayed by SDS-PAGE, transferred onto an Immobilon-P membrane, and probed with a rabbit polyclonal antibody directed against Arp3. The amount of Arp2/3 complex in the cytoskeletal fraction relative to total was quantified by densitometric analysis of the immunoblots. Values are mean of 3 to 4 experiments ± SD.
Arp2/3 complex incorporation into the platelet cytoskeleton.
(A) WAS platelets were activated with 25 μM TRAP (●) or 3 μg/mL CRP (○) for the indicated times. (B) Wild-type (░) and WASp-deficient (■) mouse platelets were activated with 1 U/mL thrombin for 1 minute. F-actin–associated Arp2/3 complex was collected by centrifugation of Triton X-100 platelet lysates at 100 000g for 30 minutes at 4°C. F-actin–associated and soluble fractions were displayed by SDS-PAGE, transferred onto an Immobilon-P membrane, and probed with a rabbit polyclonal antibody directed against Arp3. The amount of Arp2/3 complex in the cytoskeletal fraction relative to total was quantified by densitometric analysis of the immunoblots. Values are mean of 3 to 4 experiments ± SD.
The Arp2/3 complex redistributes to the cell cortex of spread platelets
Platelets spread over glass surfaces using large circumferential lamellae, although some filopodia are extended.44 Fluorescence microscopy and immunogold electron microscopy were performed using a mixture of 2 rabbit polyclonal antibodies directed against the Arp3 and p34-Arc subunits of Arp2/3 complex followed by either tetrarhodamine isothiocyanate (TRITC)–labeled antirabbit IgG or 10-nm colloidal gold particles coated with goat antirabbit IgG. In the light microscope, Arp2/3 complex becomes concentrated at the periphery of the active platelet (Figure 5A). Internal labeling is also found, concentrated in a number of foci near the center of the spread platelet.
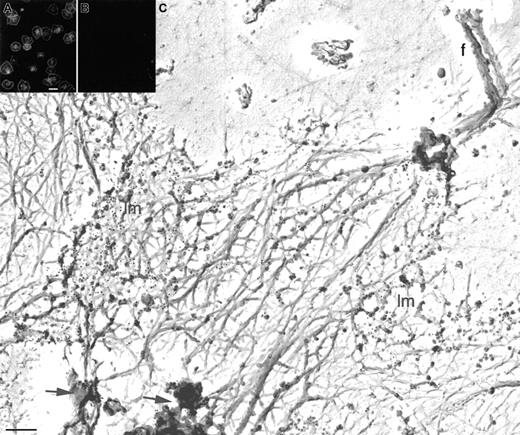
Arp2/3 complex distribution in the cytoskeleton of active human platelets.
Human platelets were activated by centrifugation onto poly-l-lysine–coated glass coverslips, permeabilized with 0.5% Triton X-100 in PBS, and fixed and treated with 0.1% SDS. The actin cytoskeleton is labeled with a mixture of affinity-purified rabbit polyclonal anti-Arp3 and anti–p34-Arc antibodies and TRITC-labeled goat antirabbit IgG (A). Anti-Arp2/3 complex staining concentrated at the cell periphery and at foci in the center of the cytoskeletons. The peptides (50 μg/mL) used to raise the antibodies were used mixed with the anti-Arp2/3 complex antibodies as controls and blocked all detectable antibody binding (B). Human platelets were activated by centrifugation onto poly-l-lysine–coated glass coverslips, permeabilized with 0.75% Triton X-100 in PHEM buffer, fixed, and treated with 0.1% SDS (C). Cytoskeletons were labeled with a mixture of rabbit polyclonal anti-Arp3 and anti–p34-Arc antibodies followed by 10 nm colloidal gold coated with goat antirabbit IgG (black spheres). Cytoskeletons were fixed, washed into water, rapidly frozen, freeze-dried, and metal-coated. Immunogold recognizing Arp2/3 complex densely labels spread lamellar regions (lm) of the active cytoskeletons. Some internal structures are also labeled (arrows). The tips of the filopodia are poorly labeled (f). Note that the electron and light microscopy patterns are identical and that there are no filopodia labeled in panel A. Bars represent 5 μm (A) and 200 nm (C).
Arp2/3 complex distribution in the cytoskeleton of active human platelets.
Human platelets were activated by centrifugation onto poly-l-lysine–coated glass coverslips, permeabilized with 0.5% Triton X-100 in PBS, and fixed and treated with 0.1% SDS. The actin cytoskeleton is labeled with a mixture of affinity-purified rabbit polyclonal anti-Arp3 and anti–p34-Arc antibodies and TRITC-labeled goat antirabbit IgG (A). Anti-Arp2/3 complex staining concentrated at the cell periphery and at foci in the center of the cytoskeletons. The peptides (50 μg/mL) used to raise the antibodies were used mixed with the anti-Arp2/3 complex antibodies as controls and blocked all detectable antibody binding (B). Human platelets were activated by centrifugation onto poly-l-lysine–coated glass coverslips, permeabilized with 0.75% Triton X-100 in PHEM buffer, fixed, and treated with 0.1% SDS (C). Cytoskeletons were labeled with a mixture of rabbit polyclonal anti-Arp3 and anti–p34-Arc antibodies followed by 10 nm colloidal gold coated with goat antirabbit IgG (black spheres). Cytoskeletons were fixed, washed into water, rapidly frozen, freeze-dried, and metal-coated. Immunogold recognizing Arp2/3 complex densely labels spread lamellar regions (lm) of the active cytoskeletons. Some internal structures are also labeled (arrows). The tips of the filopodia are poorly labeled (f). Note that the electron and light microscopy patterns are identical and that there are no filopodia labeled in panel A. Bars represent 5 μm (A) and 200 nm (C).
Because 80% of total Arp2/3 complex is retained in the active cytoskeleton, the localization of Arp2/3 complex was further investigated in the electron microscope in labeled and freeze-dried specimens (Figure 5C). Once again, in the electron microscope, Arp2/3 complex redistributes into lamellae at the cell periphery and is bound predominantly in the short actin filament networks found in these regions (lm). As observed in the light microscope, Arp2/3 complex is also found to rigorously decorate certain dense structures within the cytoskeleton (arrows). One noticeable exception is the tips of filopodia that are poorly labeled with anti-Arp2/3 complex immunogold (f). Actin filaments that enter, and become bundled, in filopodia derive from filaments originating in the cytoskeletal center. Because some Arp2/3 complex labeling is observed throughout the cytoskeleton, as well as the foci of dense labeling that are found internally, some of Arp2/3 complex may be associated with the origins of filaments that eventually become collected in the filopodia. Labeling of Arp2/3 complex was completely dependent on adding denaturating compounds (SDS or methanol) and was not observed in the absence of primary antibodies or with nonspecific primary antibodies substituted (data not shown).
Similar to normal platelets, WASp-deficient platelets spread and elaborate filopodia, fingerlike extensions composed of long actin fibers, and robustly fill the spaces between the filopodia with lamellae composed of short actin filaments organized into dense orthogonal networks (Figures 6 and7). Arp2/3 complex redistributes normally to the cortex as WASp-deficient human and mouse platelets spread on glass, in identical fashion to that observed in the control platelets. Once again, some labeling of densities in the cytoskeletal center was observed and the bundles of actin filaments composing filopodia were poorly decorated with anti-Arp2/3 complex immunogold.
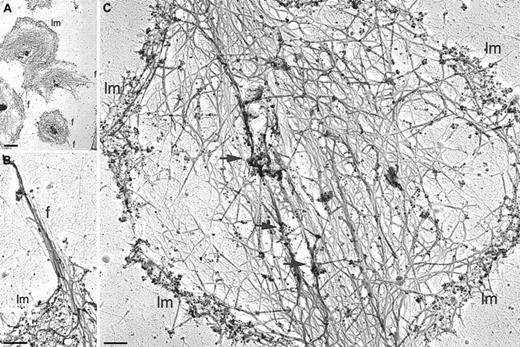
Arp2/3 complex incorporation into the cytoskeleton of the active WAS platelet.
Platelets isolated from WAS patient P50 were activated by centrifugation onto poly-l-lysine–coated glass coverslips and permeabilized with 0.75% Triton X-100 in PHEM buffer. Cytoskeletons were labeled with a mixture of rabbit polyclonal anti-Arp3 and anti–p34-Arc antibodies and 10 nm colloidal gold coated with goat antirabbit IgG. Cytoskeletons were fixed, washed into water, rapidly frozen, freeze-dried, and metal- coated. Bars represent 2 μm (A) and 200 nm (B,C). Lm indicates lamellae; f, filopodia; arrows, foci of gold labeling.
Arp2/3 complex incorporation into the cytoskeleton of the active WAS platelet.
Platelets isolated from WAS patient P50 were activated by centrifugation onto poly-l-lysine–coated glass coverslips and permeabilized with 0.75% Triton X-100 in PHEM buffer. Cytoskeletons were labeled with a mixture of rabbit polyclonal anti-Arp3 and anti–p34-Arc antibodies and 10 nm colloidal gold coated with goat antirabbit IgG. Cytoskeletons were fixed, washed into water, rapidly frozen, freeze-dried, and metal- coated. Bars represent 2 μm (A) and 200 nm (B,C). Lm indicates lamellae; f, filopodia; arrows, foci of gold labeling.
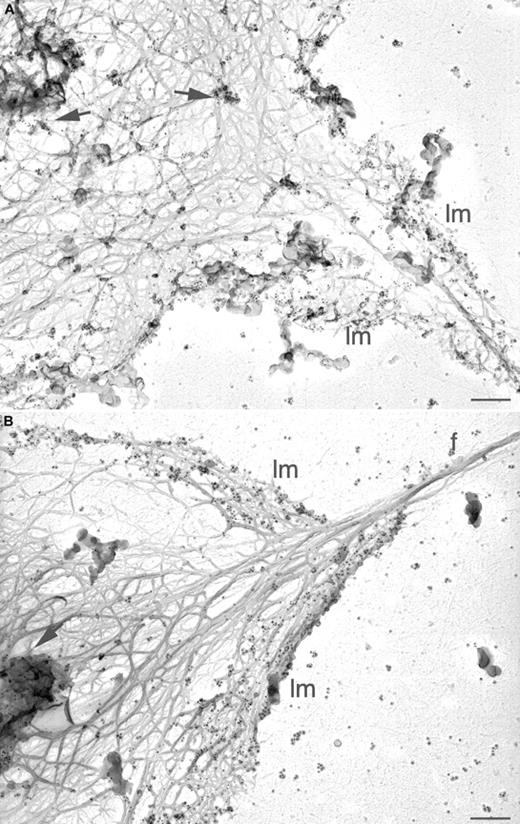
Arp2/3 complex distribution in the active mouse platelet cytoskeleton.
Wild-type (A) and WASp-deficient (B) mouse platelets were activated by 1 U/mL thrombin on anti-GPIbα antibody-coated glass coverslips and permeabilized with 0.75% Triton X-100 in PHEM buffer. Cytoskeletons were labeled with a mixture of rabbit polyclonal anti-Arp3 and anti–p34-Arc antibodies and 10 nm colloidal gold coated with goat antirabbit IgG. Cytoskeletons were fixed, washed into water, rapidly frozen, freeze-dried, and metal-coated. Bars represent 200 nm; lm indicates lamellae; f, filopodia; arrows, foci of gold labeling.
Arp2/3 complex distribution in the active mouse platelet cytoskeleton.
Wild-type (A) and WASp-deficient (B) mouse platelets were activated by 1 U/mL thrombin on anti-GPIbα antibody-coated glass coverslips and permeabilized with 0.75% Triton X-100 in PHEM buffer. Cytoskeletons were labeled with a mixture of rabbit polyclonal anti-Arp3 and anti–p34-Arc antibodies and 10 nm colloidal gold coated with goat antirabbit IgG. Cytoskeletons were fixed, washed into water, rapidly frozen, freeze-dried, and metal-coated. Bars represent 200 nm; lm indicates lamellae; f, filopodia; arrows, foci of gold labeling.
Arp2/3 complex contributes to the actin nucleation activity of WAS platelets
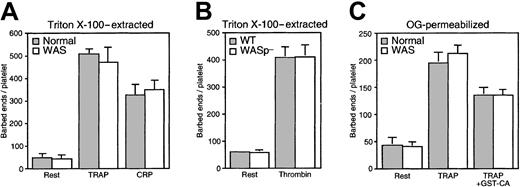
Figure 8A shows that platelets isolated from healthy human volunteers and activated for 1 minute with 25 μM TRAP or 3 μg/mL CRP increase their barbed-end nuclei content from 49 ± 18 (mean ± SD, n = 4) per cell at rest to 509 ± 22 and 326 ± 48, respectively. In resting human WAS platelets, 43 ± 18 barbed-end nuclei were detected per cell (n = 3). This number increased to 471 ± 67 and 348 ± 44 after 1 minute of activation with TRAP and CRP, respectively. Similarly, WASp-deficient mouse platelets showed no defects in barbed-end nuclei production in response to stimulation (Figure 8B), increasing their barbed-end nuclei number from 56 ± 12 (mean ± SD, n = 4) per cell at rest to 408 ± 45 after 1 minute of activation with 1 U/mL thrombin.
Contribution of Arp2/3 complex activity to the production of actin filament barbed ends in WAS platelets.
(A) Barbed-end counts were determined in Triton X-100 lysates of normal (░) and WAS (■) platelets activated with 25 μM TRAP or 3 μg/mL CRP for 1 minute using the pyrene-labeled actin assembly assay, as indicated. (B) Barbed-end counts were determined in Triton X-100 lysates of wild-type (░) and WASp-deficient (■) mouse platelets activated with 1 U/mL thrombin for 1 minute as above. (C) Platelet isolated from healthy volunteers (░) and from WAS patients (■) were permeabilized with 0.25% OG in PHEM buffer. The number of barbed ends in OG-permeabilized platelets was determined without any addition (rest), after incubation with 25 μM TRAP for 1 minute (TRAP), or after incubation with GST-CA (3μM) followed by TRAP (TRAP+GST-CA).
Contribution of Arp2/3 complex activity to the production of actin filament barbed ends in WAS platelets.
(A) Barbed-end counts were determined in Triton X-100 lysates of normal (░) and WAS (■) platelets activated with 25 μM TRAP or 3 μg/mL CRP for 1 minute using the pyrene-labeled actin assembly assay, as indicated. (B) Barbed-end counts were determined in Triton X-100 lysates of wild-type (░) and WASp-deficient (■) mouse platelets activated with 1 U/mL thrombin for 1 minute as above. (C) Platelet isolated from healthy volunteers (░) and from WAS patients (■) were permeabilized with 0.25% OG in PHEM buffer. The number of barbed ends in OG-permeabilized platelets was determined without any addition (rest), after incubation with 25 μM TRAP for 1 minute (TRAP), or after incubation with GST-CA (3μM) followed by TRAP (TRAP+GST-CA).
To further determine whether Arp2/3 complex participates in the actin nucleation activity of WASp-deficient platelets, the C-terminal CA domain of N-WASp, which sequesters and inhibits Arp2/3 complex,26 was added to WAS platelets permeabilzed with OG (Figure 8C). TRAP (25 μM) induced the exposure of 195 ± 20 and 213 ± 15 barbed-end nuclei/platelet (mean ± SD, n = 3) after 1 minute of activation in normal and WAS platelets, respectively, compared to 42 ± 15 and 41 ± 8 at rest. GST-CA (3 μM) inhibited by approximately 50% barbed-end nuclei production in WAS platelets (134 ± 12) similar to its effect in WASp-expressing cells (134 ± 16).43 Thus, WASp expression is not required for activation of Arp2/3 complex in platelets and Arp2/3 complex contributes in 50% of the barbed-end nuclei in activated OG-permeabilized platelets.
Platelet WASp family proteins
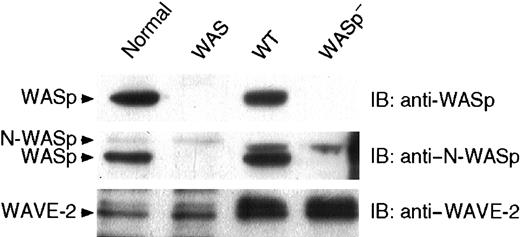
Because platelets change shape, assemble actin, and activate Arp2/3 complex in the absence of WASp, the expression of other WASp family proteins was investigated by immunoblot analysis in control and WASp-deficient platelets. Figure 9confirms that human and mouse platelets express WASp as a protein of 64 kDa. WASp was not detected in platelets isolated from WAS patients, as described,34-37 nor in platelets isolated from WASp-deficient mice. Anti–N-WASp antibody recognized a faint polypeptide of slightly higher molecular weight (66 kDa) in human platelets and cross-reacted with WASp, as evidenced by the presence of the 64-kDa band absent in WASp-deficient human and mouse platelets. We and others51 failed to detect N-WASp in human platelets using other available antibodies (data not shown). Human and mouse platelets express WAVE-2 as a protein of apparent 84 kDa. Expression of WAVE-1 and -3 remains to be determined.
Platelet WASp family proteins.
Lysates corresponding to 5 × 106 platelets isolated from a healthy volunteer (normal), WAS patient P34 (WAS), and wild-type (WT) and WASp-deficient (WASp−) mice were displayed by SDS-PAGE, transferred onto an Immobilon-P membrane, and probed with specific antibodies directed against WASp, N-WASp, and WAVE-2, as indicated. Results shown for the WAS platelets are representative of the 3 patients studied.
Platelet WASp family proteins.
Lysates corresponding to 5 × 106 platelets isolated from a healthy volunteer (normal), WAS patient P34 (WAS), and wild-type (WT) and WASp-deficient (WASp−) mice were displayed by SDS-PAGE, transferred onto an Immobilon-P membrane, and probed with specific antibodies directed against WASp, N-WASp, and WAVE-2, as indicated. Results shown for the WAS platelets are representative of the 3 patients studied.
Discussion
De novo nucleation of actin by Arp2/3 complex is a widely recognized mechanism for promoting barbed-end–directed actin filament assembly associated with intracellular propulsion of microorganisms. This system is also thought to be responsible for actin remodeling at the cell edge during spreading, locomotion, and phagocytosis downstream of WASp family proteins. Human platelets contain 2 μM Arp2/3 complex, or 8600 molecules/cell, approximately half the estimated amount of WASp (4.7 μM).52 However, our findings, consistent with those of others,36,37 show that WASp-deficient human and mouse platelets elaborate filopodia, spread lamellae, and assemble actin, all indistinguishable from WASp-expressing platelets. They also incorporate Arp2/3 complex into the actin cytoskeleton and redistribute it to the edge of the actin-rich lamellae following activation, demonstrating that WASp expression is not required for Arp2/3 complex translocation into the cortex. Human platelets may also express a small amount of N-WASp, as recently described.52 However, because recombinant N-WASp must be added to lysates of human platelets before the motility of Escherichia coli expressingShigella IcsA initiates,51 there is insufficient N-WASp to have an important physiologic role in platelets.
WASp and N-WASp are thought to activate Arp2/3 complex actin nucleation activity to induce filopodia formation downstream of Cdc42 and ppIs, based on in vitro experiments and transfection into cultured cells.11,16,17,26-28 WASp overexpression leads to formation of actin clusters in nonhematopoietic cultured cells,15 and inducible recruitment of WASp to a membrane receptor triggers actin polymerization that results in filopodia formation.18,19 However, endogenous WASp and N-WASp are not required for filopodia formation in platelets, consistent with recent reports that show filopodia formation and actin assembly to be independent of WASp or N-WASp expression in stimulated fibroblasts.53,54 Besides platelets, WASp is expressed in macrophages, dendritic cells, and T and B cells where its deficiency results in impaired podosome formation, cell polarization, motility, and phagocytosis.55-60 WASp may be involved in the formation of supramolecular activation clusters or immunologic synapses, and Arp2/3 complex activation by WASp may be singularly important in such systems for these highly specialized actin polymerization responses. WASp may also have more specialized functions in immune cells, such as in transcriptional activation, proliferative responses, and cytokine production,61 62 functions that are not shared by platelets.
Lamellipodial actin assembly is classically thought to be regulated by the small GTPase Rac, rather than Cdc42.63,64 Arp2/3 complex localizes at the edge of the lamellae of platelets spread on glass, as does Rac.65 Pathways leading to Arp2/3 complex from Rac include the WAVE proteins and the activation of phosphoinositide (PI) kinases, particularly PI 5-kinases.49,66 WAVE proteins have been shown to activate Arp2/3 complex downstream of Rac and IRSp53.20 Human and mouse platelets express WAVE-2, consistent with a recent report showing WAVE-2 to be widely expressed, notably in hematopoietic cells.14 Another contributor may also be cortactin. Cortactin is involved in the formation of lamellae downstream of Rac and PAK in cultured cells, where it colocalizes with Arp2/3 complex.67,68 Cortactin activates Arp2/3 complex21-23 and also redistributes to the cortex of spread platelets.69 Rac also mediates the production of ppIs at the cytoplasmic surface of the plasma membrane that recruits actin regulatory proteins and causes the uncapping of the barbed ends of the actin filaments previously severed by Ca++-activated gelsolin in platelets.44 49 These ends, once uncapped, are subsequently amplified by Arp2/3 complex to drive actin assembly (H.F., K.M.H., R.N., et al, submitted manuscript). Hence, Arp2/3 complex activity is potentiated by actin filament barbed-end uncapping at the edge of the platelet lamellae.
Recently, the small GTPase Rho has also been shown to activate and participate in the production of ppIs that regulate actin assembly required for platelet shape change.70 Rho mediated events are highlighted in Gαq-deficient platelets where Rac is not activated.71 Under normal conditions, Rac activation may predominate, explaining the C3-toxin insensitivity of the platelet actin assembly response.72 Rac is by far a more potent activator of barbed end exposure than Rho in vitro permeabilized platelets.49 Rho may also inactivate ADF/cofilin in platelets by mediating its dephosphorylation.73,74ADF/cofilin has been shown to regulate Arp2/3 complex activity in vitro by producing actin filament barbed ends,75 as does gelsolin in platelets. The roles of Rho and ADF/cofilin in platelet lamellae spreading and Arp2/3 complex activation are unknown.
In conclusion, our findings demonstrate that the actin assembly system remains fully functional in platelets in the absence of WASp, suggesting that proteins other than WASp, probably WAVE-2 or cortactin or both, activate Arp2/3 complex in these cells. The origin of the thrombocytopenia and the short platelet circulation times associated with WAS remains unclear. WASp appears to be required for normal platelet integrity. Thus, WASp deficiency may affect the surface organization of platelets such that clearance is accelerated.
We thank the WAS patients for participating in this study. We thank Drs Thomas P. Stossel, Fred S. Rosen, and Raif S. Geha for valuable discussions and ideas. We thank Dr Joseph E. Italiano Jr for immunofluorescence expertise, our colleagues and collaborators for sharing reagents and animals, and Karen Vengerow for editorial assistance.
Supported by National Institutes of Health grants HL-56949 and HL-56252, Edwin W. Hiam, and the Edwin S. Webster Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hervé Falet, Division of Hematology, Brigham and Women's Hospital, 221 Longwood Ave, LMRC 301, Boston, MA 02115; e-mail: hfalet@rics.bwh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal