Abstract
Ischemia-reperfusion (I/R) leads to organ injury and organ dysfunction in a variety of clinical disorders. Preclinical investigations examining leukocyte adhesion molecules in I/R provided overwhelming evidence that blocking the function of leukocyte adhesion molecules would be highly effective in improving outcome in clinically relevant diseases. Unfortunately, all 9 of the recently completed phase 2 and 3 clinical trials examining antiadhesion therapy have failed. In this report, we show that a modest increase in ischemic time results in conversion from a CD18-dependent to a CD18-independent injury. This fundamental change in the mechanism of injury can be reduced by inhibition of caspases leading to blockade of apoptosis. Muscle injury resulting from aortic clamping was measured by release of creatine kinase. I/R injury following ischemia of 60 minutes or less and 3 hours of reperfusion was significantly reduced by pretreatment with anti-CD18 monoclonal antibody. However, 90 minutes of ischemia resulted in a marked increase in injury that was not reduced by CD18 blockade. Importantly, the injury resulting from 90 or 120 minutes of ischemia was reduced by the pancaspase inhibitor z-VAD. We propose that the length of ischemia can result in a fundamental change in the mechanism of injury and that all preclinical investigations of I/R must be evaluated with increasing ischemia if they are to model the clinical disease. The result showing CD18-independent I/R injury is not unique; likewise, protection by caspase inhibitors is not unique. However, we show for the first time that caspase inhibitors are effective when CD18 blockade is not.
Introduction
Ischemia, with or without reperfusion, leads to organ injury and organ dysfunction in a variety of clinical disorders, including myocardial infarction, stroke, hemorrhagic shock, and organ transplantation. Experimental investigations of ischemia-reperfusion (I/R) revealed that neutrophil depletion results in decreased injury in a variety of organs (for a review, see Thiagarajan et al1). Also, a number of preclinical studies have shown that adhesion molecule blockade (eg, anti-CD18, anti-selectin, anti–ICAM-1) results in a remarkable reduction of injury associated with I/R (for a review, see Cornejo et al2). These promising preclinical data led to 9 phase 2 or 3 clinical trials in putative I/R diseases (hemorrhagic shock, myocardial infarction, ischemic stroke, pulmonary thrombolectomy, and neonatal cardiopulmonary bypass). However, in spite of the promising preclinical data, the results of these trials have been disappointing.3
We reviewed the preclinical studies in order to understand why the clinical trials may have failed. We noted that the ischemia time for most experiments was less than 1.5 hours, with the majority examining ischemia times between 30 and 60 minutes. On the other hand, the ischemia time in the clinical setting is generally much longer. There are reports where adhesion blockade did not protect from I/R injury, and these studies suggest that duration of ischemia may account for this failure.4-7 More recently, caspase inhibitors were shown to be protective in I/R injury; however, the ischemia times were again relatively short.8-13 Therefore, in the experiments described here we investigated the protective effects of leukocyte CD18 blockade and caspase inhibition on I/R injury of the hind limb of the mouse as ischemia time was progressively increased with a fixed reperfusion time of 3 hours. We found that injury following 60 minutes or fewer of ischemia was partially leukocyte-dependent in that it was attenuated by CD18 blockade by monoclonal antibody (mAb). Remarkably, brief prolongation of ischemia from 60 to 90 minutes resulted in a marked increase in tissue injury, which was not affected by pretreatment with anti-CD18 mAb but was reduced by the caspase inhibitor z-VAD-fmk.
Notably, prolonging tissue ischemia from 60 to 90 minutes was associated with significant apoptosis following 3 hours of reperfusion. The broad-spectrum caspase inhibitor z-VAD-fmk prevented apoptosis of all ischemic tissue and reduced tissue injury observed after 90 or 120 minutes of ischemia, indicating that the injury with longer ischemia might be attributed to apoptosis. Therefore, we propose 2 types of I/R injury: an injury due to relatively short ischemia mediated by CD18-dependent leukocyte adherence and a leukocyte-independent injury attributed to apoptosis when the ischemia time is prolonged.
Materials and methods
Animals
All experimental protocols were approved by the University of Washington Animal Care and Use Committee and conform to the National Institutes of Health guidelines for use of experimental animals. Balb/c mice were used in all experiments.
Experimental protocol
Mice were anesthetized with 0.2 to 0.3 mL of ketamine/xylacine mixture (10 mg/mL ketamine, 1mg/mL xylacine in saline) by intraperitoneal injection. A 3-cm midline abdominal incision was made, the abdominal contents gently retracted, and a small incision made into the retroperitoneum. The vena cava and aorta were carefully separated and a small microvascular clamp (size 1-2 mm) was applied to the aorta distal to the renal arteries. Skin and peritoneum were closed in one layer with 4-0 silk sutures. The mice were placed in an incubator at 30°C and anesthesia maintained with 1% to 2% halothane during the period of ischemia (15 to 120 minutes). Following ischemia the abdomen was opened and the clamp was removed to allow reperfusion. The skin and peritoneum were closed and the mice were allowed to recover in the 30°C incubator. Reperfusion was continued for 3 hours, then leg function was evaluated, and the mice were killed by exsanguination under anesthesia. Blood samples were taken for serum creatine kinase (CK) determination via automated analysis. Muscle samples were taken from the left leg, fixed in 4% formalin, and processed for histological evaluation, including terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling (TUNEL) assay.
Pretreatment
CD18 mAb.
Mice were given the CD18 mAb (hamster anti–mouse CD18, clone 2E6, Research Diagnostics, Flanders, NJ) by intravenous injection (2 μg/g body weight in 300 μL saline) 16 hours before the aorta was clamped. Control mice were given hamster IgG (Pharmingen, San Diego, CA; 2 μg/g body weight in 300 μL saline).
z-VAD.
The cysteine protease inhibitor z-VAD (Z-Val-Ala-DL-Asp-fluoromethylketone; Bachem, Torrance, CA) was given at 5 μg/g body weight in 300μL saline by intravenous injection 30 minutes prior to clamping the aorta. Control mice were given an equivalent amount of dimethyl sulfoxide (DMSO; 0.1 μL/g body weight) in 300 μL saline.
Leg function grading
A scoring system was devised to evaluate function of the mouse hind limb following I/R. Leg function was scored by the angle at which the mice carried their legs. Normally, the toes point straight up toward the head; when the legs are totally nonfunctional, the toes point to the tail. The normal position was graded as 7 and the nonfunctional position was graded as 1. Both hind legs were scored and the scores were averaged to determine the leg score for each animal.
TUNEL assay
Paraffin-embedded sections of leg muscle were examined for DNA strand breaks by TUNEL assay (In Situ Cell Death Kit, Roche Molecular Biochemicals, Indianapolis, IN) as described by the manufacturer. Labeled endothelial, skeletal muscle, and smooth muscle cells were examined.
Statistical analysis
The data are presented as the mean ± SEM. Statistical analysis was performed with the 2-tailed t test. Differences associated with P values of less than .05 were considered statistically significant.
Results
CD18 blockade prevents tissue injury with brief but not prolonged ischemia
Ischemia for 15, 30, 45, 60, or 90 minutes, followed by 3 hours of reperfusion, resulted in elevated plasma CK concentration (Figure1). CK concentration was increased over time in both groups and the increase was most dramatic following 90 minutes of ischemia, suggesting a severe injury. CK concentration was significantly less in the CD18 mAb–pretreated mice than in control-treated mice after 30, 45, and 60, but not 90, minutes of ischemia (48.8% reduction at 30 minutes, 67.9% reduction at 45 minutes, 57.3% reduction at 60 minutes). CK concentration following 90 minutes of ischemia was similar in the CD18 mAb–pretreated group (64 214 ± 11 244 IU/L) and the control IgG–pretreated group (65 300 ± 21 819 IU/L).
The effect of CD18 blockade on I/R injury diminished with increasing ischemic times.
Mouse hind limb was subjected to varying periods of ischemia by aortic cross-clamping followed by 3 hours of reperfusion. CK concentration in plasma was determined following 3 hours of reperfusion following varying ischemic times. Solid bars show results for mice pretreated with CD18 mAb (2 μg/g body weight, administered intravenously, at 16 hours prior to cross-clamping) and open bars show results for controls treated with hamster IgG (2 μg/g body weight). Values represent means ± SEM. *P < .05 vs IgG-pretreated.
The effect of CD18 blockade on I/R injury diminished with increasing ischemic times.
Mouse hind limb was subjected to varying periods of ischemia by aortic cross-clamping followed by 3 hours of reperfusion. CK concentration in plasma was determined following 3 hours of reperfusion following varying ischemic times. Solid bars show results for mice pretreated with CD18 mAb (2 μg/g body weight, administered intravenously, at 16 hours prior to cross-clamping) and open bars show results for controls treated with hamster IgG (2 μg/g body weight). Values represent means ± SEM. *P < .05 vs IgG-pretreated.
Prolonged ischemia induces apoptosis
Apoptosis has been implicated as a cause of cell death in I/R injury, and we investigated whether apoptosis was associated with the increased CK in these experiments. Limb tissue following I/R was stained by the TUNEL technique to detect DNA strand breaks in the nuclei of cells (Figure 2).
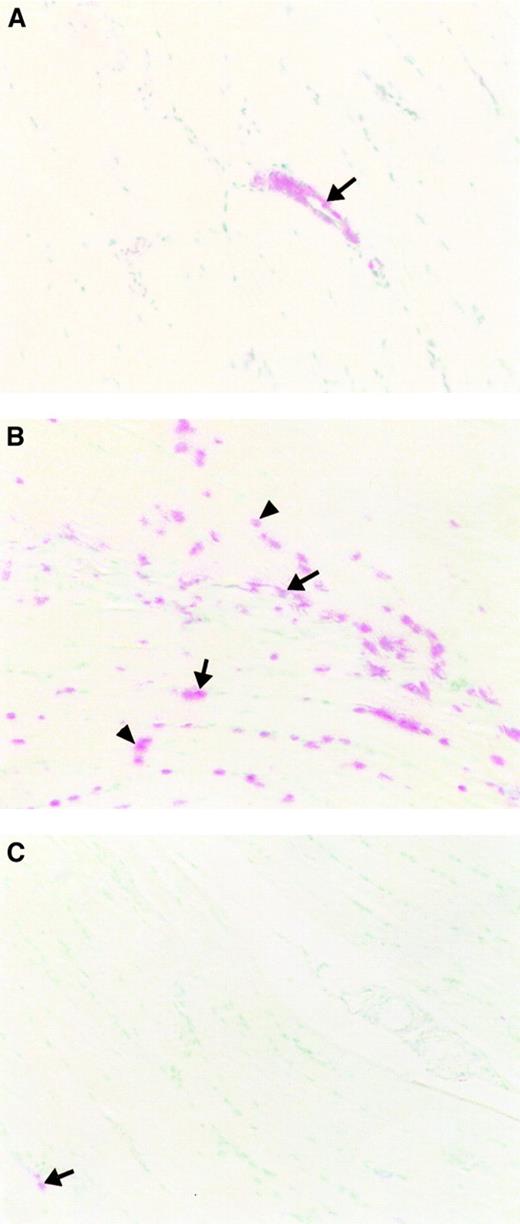
Ischemia-reperfusion induces apoptosis.
Following reperfusion, muscle tissue subjected to varying periods of ischemia as a result of aortic cross-clamping followed by 3 hours of reperfusion was sectioned and stained with the TUNEL technique. Methyl green was used as a nuclear counterstain. Original magnification, × 200. (A) Minimally injured tissue with 45 minutes of ischemia (DMSO treatment) shows TUNEL positivity only in vascular endothelial cells (arrow). (B) More extensive tissue injury with 90 minutes of ischemia (DMSO treatment) with TUNEL-positive nuclei in endothelial cells (arrow) and skeletal muscle fiber cells (arrow head). (C) Pretreatment with z-VAD resulted in only a few TUNEL-positive cells in capillary endothelium of the muscle tissue subjected to 90 minutes of ischemia (arrow).
Ischemia-reperfusion induces apoptosis.
Following reperfusion, muscle tissue subjected to varying periods of ischemia as a result of aortic cross-clamping followed by 3 hours of reperfusion was sectioned and stained with the TUNEL technique. Methyl green was used as a nuclear counterstain. Original magnification, × 200. (A) Minimally injured tissue with 45 minutes of ischemia (DMSO treatment) shows TUNEL positivity only in vascular endothelial cells (arrow). (B) More extensive tissue injury with 90 minutes of ischemia (DMSO treatment) with TUNEL-positive nuclei in endothelial cells (arrow) and skeletal muscle fiber cells (arrow head). (C) Pretreatment with z-VAD resulted in only a few TUNEL-positive cells in capillary endothelium of the muscle tissue subjected to 90 minutes of ischemia (arrow).
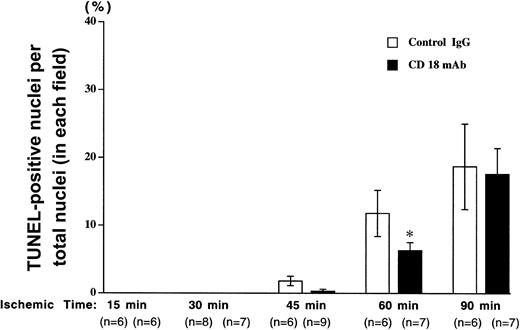
TUNEL-positive nuclei were counted and expressed as a percentage of the total number of nuclei (Figure 3). Very few positive cells were detected following 15 or 30 minutes of ischemia; however, the number of DNA strand breaks increased with longer ischemia time. DNA strand breaks following 45 or 60 minutes of ischemia were reduced in CD18 mAb-pretreated animals to approximately 50% of those seen in the animals pretreated with irrelevant IgG antibody. The number of TUNEL-positive cells was further increased in both groups following 90 minutes of ischemia and differences between the 2 groups were not significant, indicating a CD18-independent injury at this longer ischemic time.
Pretreatment with CD18 mAb reduces apoptosis following brief ischemia but not prolonged ischemia and reperfusion.
The mouse hind limb was subjected to varying periods of ischemia by aortic cross-clamping followed by 3 hours of reperfusion. The percentage of TUNEL-positive nuclei relative to total nuclei in each field was determined following pretreatment with CD18 mAb or hamster IgG. Values represent means ± SEM. Solid bars show results for mice pretreated with CD18 mAb (2 μg/g body weight, administered intravenously, at 16 hours prior to cross-clamping) and open bars show results for controls treated with hamster IgG (2 μg/g body weight). Values represent means ± SEM. *P < .05 vs IgG-pretreated.
Pretreatment with CD18 mAb reduces apoptosis following brief ischemia but not prolonged ischemia and reperfusion.
The mouse hind limb was subjected to varying periods of ischemia by aortic cross-clamping followed by 3 hours of reperfusion. The percentage of TUNEL-positive nuclei relative to total nuclei in each field was determined following pretreatment with CD18 mAb or hamster IgG. Values represent means ± SEM. Solid bars show results for mice pretreated with CD18 mAb (2 μg/g body weight, administered intravenously, at 16 hours prior to cross-clamping) and open bars show results for controls treated with hamster IgG (2 μg/g body weight). Values represent means ± SEM. *P < .05 vs IgG-pretreated.
Z-VAD prevents apoptosis and tissue injury with prolonged ischemic times
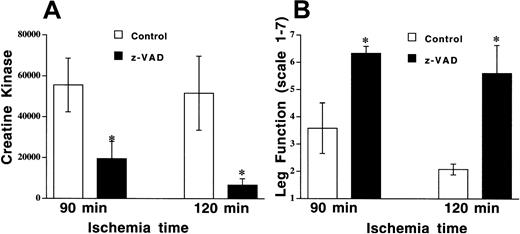
DNA strand breaks in apoptotic cell death result from activation of a caspase cascade, and this process can be inhibited by the broad-spectrum cysteine protease inhibitor z-VAD. Nuclei with DNA strand breaks were examined in endothelial cells, muscle fiber, and vascular smooth muscle cells. TUNEL-positive nuclei were observed in all cell types, with approximately 30% of all cells having DNA strand breaks following 90 minutes of ischemia in the control (DMSO, 0.1 μL/g) group (Figure 2B, Figure 4). Z-VAD (5 μg/g) resulted in fewer than 10% TUNEL-positive cells when all cells were counted, and there were similar reductions in all cell types (Figure 2C, Figure 4). However, there were some fields where endothelial cells were the only cell type that was TUNEL-positive (Figure 2A), but there were no fields where either smooth muscle or skeletal muscle cells were positive with normal endothelial cells. In addition to preventing apoptosis, pretreatment with z-VAD reduced tissue injury, as assessed by CK release (19 466 ± 6343 IU/L with z-VAD vs 55 843 ± 13 137 IU/L with DMSO, P= .046; Figure 5A). Leg function was also significantly preserved in the mice pretreated with z-VAD compared with control mice (mean score of 6.3 ± 0.3 vs 3.6 ± 0.9,P = .029; Figure 5B).
Z-VAD reduces apoptosis following prolonged ischemia followed by reperfusion.
Mouse hind limb was subjected to 90 minutes of ischemia by aortic cross-clamping followed by 3 hours of reperfusion. TUNEL-positive nuclei per total nuclei in a field were counted in endothelial cells (solid), skeletal muscle fiber cells (hatched), and smooth muscle cells (open) in animals pretreated with z-VAD (5 μg/g body weight) or DMSO (0.1 μL/g body weight). Values represent means ± SEM. *P < .05 vs DMSO.
Z-VAD reduces apoptosis following prolonged ischemia followed by reperfusion.
Mouse hind limb was subjected to 90 minutes of ischemia by aortic cross-clamping followed by 3 hours of reperfusion. TUNEL-positive nuclei per total nuclei in a field were counted in endothelial cells (solid), skeletal muscle fiber cells (hatched), and smooth muscle cells (open) in animals pretreated with z-VAD (5 μg/g body weight) or DMSO (0.1 μL/g body weight). Values represent means ± SEM. *P < .05 vs DMSO.
Z-VAD reduces tissue injury and preserves leg function following prolonged ischemia and reperfusion.
Mouse hind limb was subjected to 90 or 120 minutes of ischemia by aortic cross-clamping followed by 3 hours of reperfusion. (A) CK concentrations were determined in animals pretreated with z-VAD (5 μg/g body weight) or DMSO (0.1 μL/g body weight). Values represent means ± SEM. *P < .05 vs DMSO. (B) Leg function was scored as 1 (nonfunctional) to 7 (functional), as described in “Materials and methods.”
Z-VAD reduces tissue injury and preserves leg function following prolonged ischemia and reperfusion.
Mouse hind limb was subjected to 90 or 120 minutes of ischemia by aortic cross-clamping followed by 3 hours of reperfusion. (A) CK concentrations were determined in animals pretreated with z-VAD (5 μg/g body weight) or DMSO (0.1 μL/g body weight). Values represent means ± SEM. *P < .05 vs DMSO. (B) Leg function was scored as 1 (nonfunctional) to 7 (functional), as described in “Materials and methods.”
Discussion
We have shown for the first time that the caspase inhibitor z-VAD reduces I/R injury at ischemic durations at which blocking CD18-mediated leukocyte adhesion is no longer effective. CD18 blockade was effective in reducing I/R injury following 60 minutes or fewer of ischemia but was not effective for ischemia times of 90 minutes. Importantly, the extended ischemia did not result in irreversible injury, since caspase blockade with z-VAD still reduced injury. Protection afforded by CD18 antibodies at relatively short but not longer ischemic times may in part account for the failure of clinical trials that investigated myocardial infarction, stroke, and hemorrhagic shock resulting from severe traumatic injury.
The failure of adhesion molecule blockade (including CD18 blockade) in clinical trials of putative I/R injury in spite of overwhelming preclinical data suggests a need for more predictive preclinical models. At present there are no models in which a therapeutic intervention that is protective in the experimental setting has subsequently been proven to be effective in a clinical trial. Thus, determination of an appropriate model is difficult; however, if a therapy fails clinically but is protective in a preclinical model, then the model must be questioned. On the other hand, if the therapy fails both clinically and experimentally, the model itself has a chance of being predictive. In the studies reported here, we have shown protection with z-VAD in a model in which CD18 blockade, which failed in the clinic, is ineffective. Thus, caspase inhibitors may represent reasonable candidate drugs for future clinical trials.
Several previous studies have reported that leukocyte adherence blockade was not effective in reducing I/R injury. CD18 blockade failed to attenuate myocardial I/R injury following 90 minutes of ischemia in dogs.6,7 However, in similar experiments, others have reported protection by adhesion blockade in dogs following the same length—90 minutes—of myocardial ischemia.14 Palazzo et al reported that mice deficient in CD18, ICAM-1 or P-selectin showed marked reduction in myocardial infarction after 30 minutes of ischemia followed by reperfusion, but there was no beneficial effect after 60 minutes of ischemia and reperfusion.4 5 Although the time frame in Palazzo et al's study was a bit shorter than ours, it is interesting that those researchers also observed that the protective effects of CD18 blockade were dependent on length of ischemia with myocardial injury.
Interestingly, we found that endothelial cells were the only type of cell that was TUNEL-positive in some fields, and smooth muscle and skeletal muscle cells were never found to be TUNEL-positive unless endothelial cells were TUNEL-positive. This finding is consistent with endothelial cells' being the first cells to become apoptotic in I/R injury and with a report by Scarabelli et al that endothelial cells were the first to become apoptotic in I/R heart.15
Caspase inhibitors were reported to reduce apoptotic cardiomyocyte death and infarct size when the inhibitors were administrated 5 to 30 minutes prior to 30 minutes of ischemia9,11,12 or after the onset of ischemia.10 However, to our knowledge, there have been no investigations of prolonged ischemic times using caspase inhibitors. There is another report that caspase inhibitor reduced early onset of renal apoptosis and inflammation after 45 minutes of ischemia.8 In the present study, z-VAD significantly reduced injury to the hind limb and improved leg function when the leg was subjected to prolonged ischemia that was CD18-independent. Thus, the extended ischemia of this study did not produce irreversible injury, suggesting the possibility for development of therapeutic interventions directed at cysteine proteases.
In summary, we have shown that tissue injury following a short period of ischemia followed by reperfusion is leukocyte dependent in that an anti-CD18 mAb can reduce the injury. However, I/R injury following extended ischemia is CD18 independent, perhaps accounting for the failure of antiadhesion therapy in clinical trials. Importantly, the injury following extended ischemia and subsequent reperfusion can be inhibited by cysteine protease inhibitors. Thus, we have shown for the first time that caspase inhibitors are more effective than adhesion blockade in reducing ischemia-reperfusion injury.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2002-03-0752.
Supported by National Institutes of Health grants GM42686 and GM53226.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Akiko Iwata, Harborview Medical Center, Department of Surgery, Box 359796, 325 9th Ave, Seattle, WA 98104; e-mail:aiwata@u.washington.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal