Abstract
The Mpl receptor plays an important role at the level of adult hematopoietic stem cells, but little is known of its function in embryonic and fetal hematopoiesis. We investigated the signals sent by the MPL cytoplasmic domain in fetal liver hematopoietic progenitors and during embryonic stem (ES) cell hematopoietic commitment. Mpl was found to be expressed only from day 6 of ES cell differentiation into embryoid bodies. Therefore, we expressed Mpl in undifferentiated ES cells or in fetal progenitors and studied the effects on hematopoietic differentiation. To avoid the inadvertent effect of thrombopoietin, we used a chimeric receptor, PM-R, composed of the extracellular domain of the prolactin receptor (PRL-R) and the transmembrane and cytoplasmic domains of Mpl. This allowed activation of the receptor with a hormone that is not involved in hematopoietic differentiation and assessment of the specificity of responses to Mpl by comparing PM-R with another PRL-R chimeric receptor that includes the cytoplasmic domain of the erythropoietin receptor (EPO-R) ([PE-R]). We have shown that the cytoplasmic domain of the Mpl receptor transduces exclusive signals in fetal liver hematopoietic progenitors as compared with that of EPO-R and that it promotes hematopoietic commitment of ES cells. Our findings demonstrate for the first time the specific role of Mpl in early embryonic or fetal hematopoietic progenitors and stem cells.
Introduction
Mpl is the receptor for thrombopoietin (TPO), the primary regulator of megakaryocytopoiesis and platelet production.1,2 TPO controls the number and ploidy of megakaryocytes and supports their full maturation into platelet-producing cells.1-3 TPO also stimulates the development of megakaryocyte colonies from marrow progenitors in vitro,4 but several reports indicate that TPO, like other hematopoietic growth factors, has a broader range of activities. TPO has been shown to enhance erythroid cell proliferation5-7and to accelerate red blood cell recovery if administered after myelosuppressive therapy.6,8,9 Furthermore, TPO administration led to an expansion of colony-forming units granulocyte-macrophage (CFU-GMs) and burst-forming units-erythroid (BFU-Es) in normal mice8 and to an increase of CFU-mix in rhesus monkeys.10 More recently, several studies have shown that TPO alone or in combination with early-acting growth factors can stimulate ex vivo expansion of early hematopoietic progenitors, which maintain their capacity for multilineage differentiation.11-15 The phenotype of c-mpl– and TPO-deficient mice was also consistent with a role of TPO and its receptor on early hematopoietic progenitors. Indeed, in addition to the expected thrombocytopenia and defect in megakaryocytes and megakaryocytic progenitors, these mice had a reduced number of myeloid progenitors16,17 and a dramatic shortage in hematopoietic stem cells.18 Clues as to the role of Mpl and TPO in primitive hematopoietic progenitors may have come from the initial biologic properties of myeloproliferative leukemia virus (MPLV): in this virus, the truncated form of the Mpl receptor, v-mpl, which contains the transmembrane and cytoplasmic domains and only 43 amino acids of the extracellular domain, was able to promote the proliferation and terminal differentiation of both committed and multipotential stem cells.19 20
If the role of Mpl within adult hematopoietic stem cells is now well established, nothing is known about their embryonic or fetal counterparts. Therefore, we decided to investigate signals transduced by the Mpl cytoplasmic domain first in fetal liver hematopoietic progenitors and then during embryonic stem (ES) cells hematopoietic commitment. In the in vitro model of hematopoietic differentiation of ES cells, Mpl was found to be expressed only from day 6 of differentiation in embryoid bodies (EBs).21Accordingly, we expressed Mpl in undifferentiated ES cells and studied the consequences of this early expression on hematopoietic differentiation. To prevent the unwanted effect of TPO during cell culture, in both models we used a chimeric receptor, PRL-R/MPL (thereafter named PM-R), composed of the extracellular domain of PRL receptor and the transmembrane and cytoplasmic domain of Mpl. By using the cytoplasmic and transmembrane domains of c-mpl in the chimeric PM-R receptor, it allowed us to study a kind of activatable v-mpl receptor, which could be triggered by a hormone that does not play a role in hematopoietic differentiation (PRL). We assessed the specificity of fetal liver hematopoietic progenitor and ES cell responses to Mpl by comparing PM-R with another PRL-R chimeric receptor that includes the cytoplasmic domain of erythropoietin-R (EPO-R) (PE-R, for PRL-R/EPO-R) and that has been already used to investigate the signaling specificity of cytokine receptors in fetal liver hematopoietic progenitors. From these former studies, it was concluded that the cytoplasmic domains of cytokine receptors are interchangeable for their ability to support the terminal differentiation of hematopoietic progenitor cells.22
Data presented herein show that in fetal liver, the cytoplasmic domain of the MPL receptor is indeed active only in immature hematopoietic progenitors and transduces unique signals compared to the cytoplasmic domain of EPO-R. In addition, this region of MPL promotes hematopoietic commitment of ES cells.
Materials and methods
Hormones, antibodies, and cytokines
Ovine prolactin (O-PRL) was obtained from Dr A. F. Parlow (National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Pituitary Program, Torrance, CA). M110 mouse monoclonal antibody against the extracellular domain of rabbit PRL-R was a kind gift from Dr Jean Djiane (Institut National de la Recherche Agronomique, Jouy-en Josas, France). Murine stem cell factor (SCF) was from Genzyme (Cambridge, MA), human interleukin-6 (IL-6) and human granulocyte colony-stimulating factor (G-CSF) from Roche-Boehringer (Meylan, France), murine IL-3 and human IL-11 from PreproTech (Rocky Hill, NJ), and basic fibroblast growth factor (bFGF) was from Gibco, Life Technologies (Cergy-Pontoise, France). EPO was a gift from Roche-Boehringer. TPO was a gift from Kirin (Japan).
Plasmids and DNA constructs
The cDNA coding for chimeric receptors (PM-R and PE-R) and for rabbit PRL-R (P-R) was subcloned into murine stem cell virus 2.1 (MSCV 2.1) retroviral vector23 at theEcoRI/XhoI polylinker cloning sites for P-R and PE-R and at the SalI site for PM-R. Construction of the PE-R receptor, composed of the extracellular domain of rabbit PRL-R and transmembrane and cytoplasmic domains of EPO-R, has been described previously.24 The mutagenesis procedure described by Kunkel et al25 has been used to generate the PM-R receptor, constituted by the fragment delineated at the 3′ end by theSacI restriction site coding for the extracellular domain of rabbit PRL-R and the cDNA fragment coding for the transmembrane and cytoplasmic domains of MPL.
Full-length SalI murine MPL cDNA26 was introduced in an antisense orientation at the XhoI site of the MSCV vector. Orientation of the insert was checked byBamHI digestion.
ES cell culture
R1 ES cells (kindly provided by Andras Nagy)27 and CJ7 ES cells28 were maintained in an undifferentiated state on gelatinized tissue culture dishes in Dulbecco modified essential medium (DMEM, Gibco, Life Technologies, Cergy-Pontoise, France), supplemented with 15% selected fetal calf serum (FCS) (Biological Industries, Kibbutz Beit Haemek, Israel), 2 mM glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco, Life Technologies), 150 μM monothioglycerol (Sigma, France), and 1000 U/mL of recombinant leukemia inhibitory factor (LIF) (Gibco, Life Technologies).
ES cell electroporation
ES cell culture medium was replaced with fresh medium 3 hours before electroporation. ES cells were then trypsinized, and 7.106 cells were resuspended in 800 μL of electroporation buffer29 in the presence of 40 μg of linearized plasmid MSCV, MSCV P-R, MSCV PE-R, or MSCV PM-R. Electroporation was performed at 250 V and 500 μF (Gene Pulser, Biorad, Brussels, Belgium). After a 10-minute incubation at 37°C, cells were resuspended in ES medium and plated in 6 gelatinized Petri dishes (10 cm in diameter) in the presence of 200 μg/mL geneticin (Gibco, Life Technologies). ES medium plus geneticin was then replaced at day (D)1, D2, D3, D5, and D7. Individual colonies were picked around D8 to D10 and plated in 96-well plates. When cells became confluent, they were trypsinized and split in half in two 96-well plates. One plate was used for freezing the clones, and the other for expression analysis by whole cell blot of the transgene.30
Production of retroviruses and retroviral infections
Retrovirus production.
Retroviral infection of ES cells.
Freshly passaged ES cells were resuspended at 3 × 105cells in 4 mL viral supernatant + 1000 U/mL LIF with 8 μg/mL polybrene, plated in 60-mm gelatinized dishes, and incubated as usual with one change of the viral supernatant over 24 hours. After infection, adherent cells were rinsed with phosphate-buffered saline (PBS) (Gibco, Life Technologies), and fresh medium was added for a further 24-hour culture period. At 48 hours after infection, ES cells were transferred to fresh dishes with 200 μg/mL geneticin (Gibco, Life Technologies). Geneticin-resistant polyclonal populations were then expanded prior to further studies.
Infection of fetal liver hematopoietic progenitor cells
Single-cell suspensions of fetal livers were prepared from day-12.5 C57/Bl6 or PRL-R KO fetuses, respectively.335.106 nucleated cells were resuspended in 10 mL of viral supernatant in the presence of 8 μg/mL polybrene (Sigma, France) and incubated for 3 to 4 hours at 37°C with retroviral supernatant. Following infection, cells were washed in Iscove modified Dulbecco medium (IMDM) (Gibco, Life Technologies) and plated in methylcellulose medium for clonogenic cell assays. A fraction of cells from each infection (4.106 cells) was cultured for 48 hours in IMDM with 20% FCS, in the presence of 500 ng/mL O-PRL and 50 ng/mL mSCF for fluorescence-activated cell-sorter (FACS) analysis of receptor expression.
Receptor expression analysis
Reverse transcription–polymerase chain reaction (RT-PCR).
Undifferentiated ES cells, EBs, or individual hematopoietic colonies were lysed with Trizol (Gibco, Life Technologies) (1 mL per 106 cells or 100 μL per hematopoietic colony), and total RNA was extracted as recommended by the manufacturer. Then 1 μg or less of RNA was incubated for 30 minutes at 37°C with 0.2 U RQ1 DNAse (Promega, Madison, WI) in reverse transcriptase (RT) buffer (Gibco, Life Technologies) in a final volume of 20 μL. MoMulV RT (Gibco, Life Technologies) was added, and the mixture was further incubated for 1 hour at 37°C. The volume of cDNA was then adjusted up to 200 μL, depending of the initial amount of RNA used in the reaction. Samples were submitted to 35 cycles of amplification with Taq Polymerase (Gibco, Life Technologies) under conditions recommended by the manufacturer. Then 5 μL of sample was used with primers specific for β-actin and 10 μL with primers specific for chimeric receptors and rabbit PRL receptor. Sequences for β-actin primers have been previously described.26 The 5′ primer (5′-TTCTCAGCTTATATCCAGGA-3′) is located in the PRL-R extracellular domain and the 3′ primer in the cytoplasmic domain of PRL-R (5′-CAGCAGTGGGAGGGAAGTC-3′), EPO-R 5′-TCACGCTGCAGCAGCCACAG-3′) and MPL 5′-TGTGTGGTGCAGCAGGACCCCGTC-3′).
RNase mapping.
15 μg total RNA was precipitated in ethanol with 105 cpm of a 320-bp riboprobe fragment of the extracellular domain of PRL-R and 5.104 cpm of actin riboprobe (250 bp), in the presence of 0.5 M ammonium acetate. After centrifugation, pellets were resuspended in the hybridization buffer (RPAII kit, Ambion, Austin, TX), heated 3 to 4 minutes at 90°C, and incubated overnight at 45°C (hybridization). Then 200 μL of digestion buffer containing 3 U RNase A and 125 U RNase T1 was added. After a 30-minute incubation at 37°C, the stop/precipitation solution provided with the kit was added; double-stranded RNAs were precipitated 15 minutes at −20°C and loaded on a 6% acrylamide gel, 8 M urea. After a 3-hour migration at 250 V in 1 × TBE buffer, the gel was dried and exposed on Hyperfilm MP (Amersham, France).
FACS analysis.
105 fetal liver or ES infected cells were incubated with 50 μg/mL monoclonal antibody M110 for 30 minutes at 4°C. Cells were then washed 2 times in PBS-0.5% BSA and incubated 30 minutes with a phycoerythrin (PE)–conjugated goat anti–mouse F(ab′)2. After 2 more washes in PBS-0.5% BSA, cells were analyzed by 2-color flow cytometry on a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA).
ES cell differentiation
ES cells were induced to differentiate toward hematopoiesis following the classical 2-step model.21 ES cells were washed twice and incubated in ES medium without LIF 1 to 2 hours prior to differentiation. After trypsinization, 3000 cells were suspended in 1.5 mL complete medium for hematopoietic differentiation and plated in duplicate 35-mm bacterial Petri dishes (Greiner, Frickenhausen, Germany). This differentiation medium consisted of 1.1% methylcellulose (Methocel high viscosity, Fluka AG, Buchs, Switzerland) in IMDM-Glutamax (Gibco, Life Technologies) supplemented with 15% FCS (Biological Industries), 10 μg/mL insulin (Sigma, France), 300 μg/mL transferrin (Roche-Boerhinger), 10 μM hemin, 450 μM monothioglycerol, 50 U penicillin, and 500 μg/mL streptomycin (Gibco, Life Technologies). ES cells were allowed to form EBs for 10 to 12 days at 37°C, in modular incubator chambers (Billups-Rothenberg, Del Mar, CA), in a gas mixture composed of 5% CO2 and 6% O2 (Air Liquide, Mitry-Mory, France) in 100% humidity. To optimize hematopoietic differentiation, a mixture of 7 cytokines (7 GF), composed of 50 ng/mL SCF, 25 ng/mL IL-3, 10 ng/mL IL-6, 10 ng/mL IL-11, 10 ng/mL G-CSF, 2 U/mL EPO, and 50 ng/mL bFGF was added to the medium. In some experiments, a more restricted combination composed of 2 U/mL EPO, 50 ng/mL SCF, 25 ng/mL IL-3, and 500 ng/mL O-PRL (EIPS) has been used. EBs obtained after 10 to 12 days of differentiation were collected and resuspended in 500 μL of a dissociation medium containing IMDM with 10% FCS, 0.2% collagenase B (Roche-Boehringer), and 200 U/mL deoxyribonuclease 1 (Roche-Boehringer) and kept at 37°C in a water bath for 30 minutes. Dissociation of EBs was achieved by vigorous pipetting, and cells were then washed twice in IMDM. After counting, cells were submitted to hematopoietic progenitor cell assays in methylcellulose.
Hematopoietic progenitor cell assays
Clonogenic cultures were performed as previously described.26 Briefly, cells were plated in 1 mL 1% methylcellulose in IMDM supplemented with 30% FCS, 1% crystallized bovine serum albumin (Sigma), and 100 μM 2-mercaptoethanol (Sigma, France) in the presence of appropriate cytokines. For fetal liver cells, assays were performed with either 2 U/mL EPO or 500 ng/mL O-PRL or with 2 U/mL EPO, 10 ng/mL IL-3, and 20 ng/mL TPO (EIT). For ES cells, assays were performed with either the 7 GF or EIPS cocktail. Samples were plated in duplicate 35-mm bacterial Petri dishes for each experimental point and incubated at 37°C in a humidified incubator containing 5% CO2.
Results
PM-R infection of fetal liver hematopoietic cells does not efficiently support mature erythroid colony development, but allows generation of all types of myeloid hematopoietic colonies in the presence of PRL
To study the signals sent by the MPL cytoplasmic domain into hematopoietic progenitor cells from fetal origin, we expressed a chimeric PM-R receptor in fetal liver progenitors after retroviral infection. This receptor is composed of the extracellular domain of rabbit prolactin receptor (P-R) and the transmembrane and cytoplasmic domains of MPL (Figure 1A). The use of such a chimeric receptor exhibits 2 advantages: first, it allows activation of MPL-dependent pathways in the absence of TPO with a hormone that is not involved in hematopoiesis; and second, a chimeric receptor composed of the extracellular domain of PRL-R and of the cytoplasmic domain of EPO-R (PE-R) has already been used successfully in fetal liver cells.22,34 35 cDNAs encoding PM-R, PE-R, and P-R (used as control) (Figure 1A) were subcloned into the MSCV retroviral vector, and high-titer retroviral supernatants of MSCV P-R, MSCV PE-R, and MSCV PM-R were produced. We first checked the expression and function of these receptors in the IL-3–dependent Ba/F3 cell line. Infected cells proliferated when culture medium was supplemented with 10 ng/mL O-PRL instead of IL-3, while parental Ba/F3 or Ba/F3 infected with MSCV did not (data not shown).
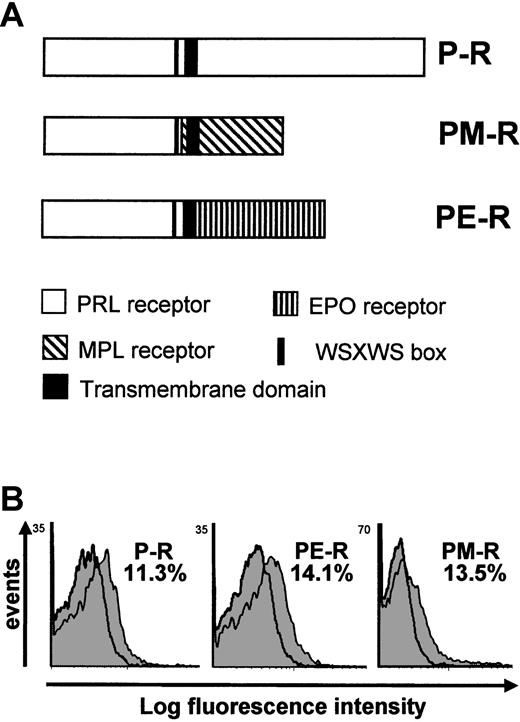
Schematic representation and FACS analysis of chimeric receptors expression.
(A) Schematic representation of P-R and chimeric receptors PM-R and PE-R. P-R is the normal rabbit prolactin receptor; PM-R contains the extracellular part of the rabbit prolactin receptor fused to the transmembrane and the intracellular domain of murine MPL; PE-R is composed of the extracellular part of the rabbit prolactin receptor fused to the transmembrane and the intracellular domain of murine EPO-R.22 All constructs were subcloned into the MSCV retroviral vector. (B) FACS analysis of the expression of the chimeric receptors in fetal liver cells. Cells were stained with the anti–PRL-R monoclonal antibody M110 (gray peak) or isotype control antibody (open peak) 48 hours after infection. Second-step antibody was a PE-conjugated goat anti–mouse F(ab′)2. The percentage of fetal liver cells expressing P-R, PE-R, and PM-R is indicated.
Schematic representation and FACS analysis of chimeric receptors expression.
(A) Schematic representation of P-R and chimeric receptors PM-R and PE-R. P-R is the normal rabbit prolactin receptor; PM-R contains the extracellular part of the rabbit prolactin receptor fused to the transmembrane and the intracellular domain of murine MPL; PE-R is composed of the extracellular part of the rabbit prolactin receptor fused to the transmembrane and the intracellular domain of murine EPO-R.22 All constructs were subcloned into the MSCV retroviral vector. (B) FACS analysis of the expression of the chimeric receptors in fetal liver cells. Cells were stained with the anti–PRL-R monoclonal antibody M110 (gray peak) or isotype control antibody (open peak) 48 hours after infection. Second-step antibody was a PE-conjugated goat anti–mouse F(ab′)2. The percentage of fetal liver cells expressing P-R, PE-R, and PM-R is indicated.
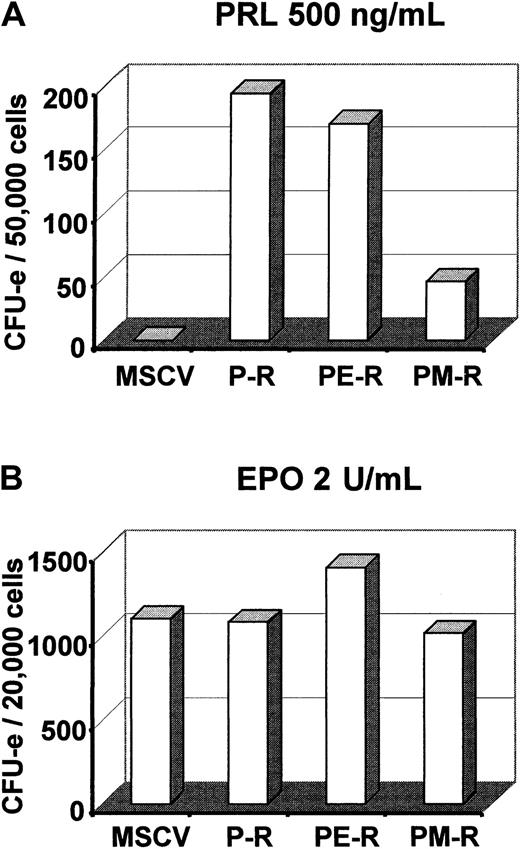
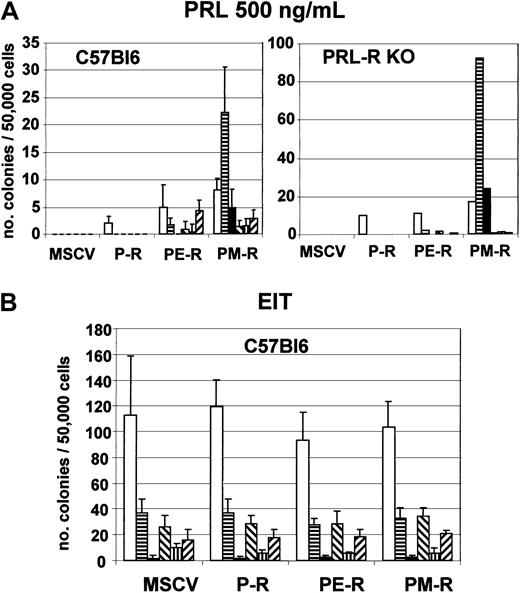
Next we introduced PM-R, P-R, or PE-R receptors into day-12.5 fetal liver hematopoietic precursor cells by retroviral infection and examined the ability of infected cells to generate colonies when cultured in semisolid methylcellulose medium in the presence of EPO or PRL. An aliquot was cultured in parallel in liquid medium with PRL and SCF for 36 to 48 hours and used to measure the percentage of infected cells by quantifying the fraction of M110-positive cells by FACS analysis. As shown in Figure 1B, the same proportion of fetal liver cells (11% to 14%) were found to express P-R, PE-R, or PM-R, indicating that the transduction efficiency was roughly the same for the 3 viruses. Colonies were observed 2 and 7 to 8 days later. As described previously,22 both P-R and PE-R gave rise to similar numbers of CFU-e–derived colonies, equally hemoglobinized, in the presence of PRL. Interestingly enough, PM-R supported only a restricted number of less hemoglobinized CFU-e–derived colonies (Figure 2A). No significant difference was observed in CFU-e numbers between the different receptors in the presence of EPO (Figure 2B). Cultures grown in the presence of PRL were incubated further and observed 5 to 6 days later for the formation of colonies derived from more primitive progenitors. As shown in Figure3A, only PM-R–infected cells were able to generate all types of myeloid colonies (GM, E, E/MK, G/MK, MK, and GEMM), in significant numbers. The same results were obtained with 12.5-day fetal liver hematopoietic precursor cells from PRL-R KO mice (Figure 3A), which do not exhibit any hematopoietic defect (M.S. and N.B., unpublished data, May 1997). In the presence of a mixed myeloid stimulus (EPO + IL-3 + TPO), however, no significant difference was observed between the different receptors (Figure 3B). In addition, when the colonies grown under these conditions were analyzed by RT-PCR for the presence of the chimeric receptors, 8% were found positive for P-R, 26% for PE-R, and 13% for PM-R. Therefore, the ability of PM-R–infected cells to generate all types of myeloid colonies in the presence of O-PRL cannot be explained by a better infection of fetal liver hematopoietic progenitors by the retroviral vector carrying PM-R. Taken together, these results indicate that in 12.5-day fetal liver, the MPL cytoplasmic domain signals in rather primitive hematopoietic progenitors.
PM-R receptor does not efficiently support CFU-e colony formation in the presence of PRL.
Day-12.5 fetal liver hematopoietic progenitors were infected with MSCV retroviruses encoding the various receptors (P-R, PE-R, and PM-R). (A) 5 × 104 cells were cultured in duplicate in methylcellulose medium with 500 ng/mL ovine prolactin and no EPO. (B) 2.104 cells were cultured with 2 U/mL EPO. Colonies were scored 2 to 3 days later. The result of 1 experiment, representative of 3 independent experiments, is shown.
PM-R receptor does not efficiently support CFU-e colony formation in the presence of PRL.
Day-12.5 fetal liver hematopoietic progenitors were infected with MSCV retroviruses encoding the various receptors (P-R, PE-R, and PM-R). (A) 5 × 104 cells were cultured in duplicate in methylcellulose medium with 500 ng/mL ovine prolactin and no EPO. (B) 2.104 cells were cultured with 2 U/mL EPO. Colonies were scored 2 to 3 days later. The result of 1 experiment, representative of 3 independent experiments, is shown.
PM-R receptor supports formation of myeloid hematopoietic colonies in the presence of PRL.
Day-12.5 fetal liver hematopoietic progenitors from C57Bl6 and PRL-R KO mice were infected with MSCV retroviruses encoding the various receptors (P-R, PE-R, and PM-R), and 5 × 104 cells were cultured in duplicate in methylcellulose medium with 500 ng/mL ovine prolactine (PRL) (panel A) or with EIT cocktail (panel B). Colonies were scored after 7 to 10 days. The results shown are the mean of 2 independent experiments. Types of colonies (CFU): ■, granulocyte-macrophage (-GM); ▤, burst-forming unit-erythroid (BFU-E); ▪, mixed (-GEMM); ▧, erythro-megakaryocyte (-EMK); ▥, granulo-megakaryocyte (-GMK); and ▨, megakaryocyte (-MK).
PM-R receptor supports formation of myeloid hematopoietic colonies in the presence of PRL.
Day-12.5 fetal liver hematopoietic progenitors from C57Bl6 and PRL-R KO mice were infected with MSCV retroviruses encoding the various receptors (P-R, PE-R, and PM-R), and 5 × 104 cells were cultured in duplicate in methylcellulose medium with 500 ng/mL ovine prolactine (PRL) (panel A) or with EIT cocktail (panel B). Colonies were scored after 7 to 10 days. The results shown are the mean of 2 independent experiments. Types of colonies (CFU): ■, granulocyte-macrophage (-GM); ▤, burst-forming unit-erythroid (BFU-E); ▪, mixed (-GEMM); ▧, erythro-megakaryocyte (-EMK); ▥, granulo-megakaryocyte (-GMK); and ▨, megakaryocyte (-MK).
Introduction of chimeric receptors into ES cells
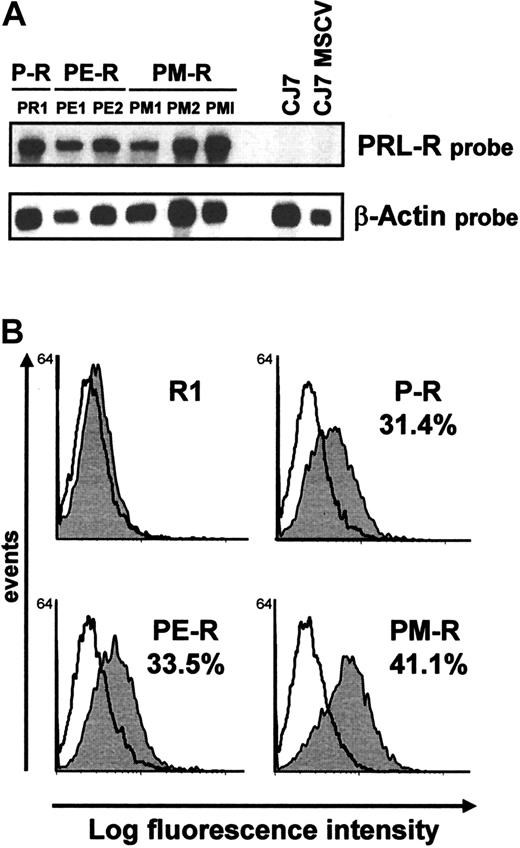
We next compared the signals sent by the cytoplasmic domains of MPL and EPO-R at the earliest stages of development and differentiation of the hematopoietic system by introducing the same chimeric receptors into ES cell lines. Undifferentiated CJ7 ES cells were transduced with MSCV (control), and MSCV P-R, MSCV PE-R, or MSCV PM-R by electroporation or by retroviral infection. After neomycin selection, 3 PM-R– and 2 PE-R–electroporated clones were selected by whole cell blot for high expression of chimeric receptors and amplified. Comparison by RNase mapping of PM-R expression in these clones and in the bulk PM-R–infected population (PMI) demonstrated that retroviral infection was the most rapid and efficient way to induce prominent expression of the chimeric receptor in undifferentiated ES cells, as shown for PM-R in Figure 4A. All receptors were also introduced by retroviral infection into undifferentiated R1 ES cells, and all of the experiments described thereafter were performed in parallel in CJ7 and R1 ES cells. Expression of the receptors at the surface of undifferentiated ES cells was checked by flow cytometry using M110 monoclonal antibody 10 days after infection (at the end of geneticin selection). As illustrated in Figure 4B, 30% to 40% of the R1 ES cell population was found to express the chimeric receptors at their surface.
Analysis of chimeric receptor expression in undifferentiated ES cells by RNase mapping and by FACS analysis.
(A) Expression of chimeric receptors (clone PR1 for P-R, clones PE1 and PE2 for PE-R, clones PM1 and PM2 for PM-R) after CJ7 cell electroporation, by RNase mapping. CJ7 and CJ7 MSCV RNAs were used as negative controls. Actin RNA expression was used as an internal control. A comparison of PM-R expression after ES cell electroporation or after viral infection (PMI) is also shown. (B) ES cell surface expression of chimeric receptors was determined by FACS analysis using M110 monoclonal antibody. Undifferentiated ES cells were dissociated with Accutase (PAA Laboratories, Les Mureaux, France) prior to incubation with the antibody. The percentage of ES cells expressing P-R, PE-R, and PM-R is indicated.
Analysis of chimeric receptor expression in undifferentiated ES cells by RNase mapping and by FACS analysis.
(A) Expression of chimeric receptors (clone PR1 for P-R, clones PE1 and PE2 for PE-R, clones PM1 and PM2 for PM-R) after CJ7 cell electroporation, by RNase mapping. CJ7 and CJ7 MSCV RNAs were used as negative controls. Actin RNA expression was used as an internal control. A comparison of PM-R expression after ES cell electroporation or after viral infection (PMI) is also shown. (B) ES cell surface expression of chimeric receptors was determined by FACS analysis using M110 monoclonal antibody. Undifferentiated ES cells were dissociated with Accutase (PAA Laboratories, Les Mureaux, France) prior to incubation with the antibody. The percentage of ES cells expressing P-R, PE-R, and PM-R is indicated.
Expression of PM-R chimeric receptor enhances hematopoietic differentiation of ES cells after PRL stimulation
Transduced ES cells were then induced to differentiate toward hematopoiesis following the classical 2-step model in the presence of the 7-growth factor cocktail (7GF cocktail) (no O-PRL added). By RT-PCR analysis, chimeric receptor expression was at least identical or higher 12 days after the initiation of differentiation (D12) than in the starting population of ES cells (D0) (Figure5A), indicating that the expression of chimeric receptors was maintained during the first steps of hematopoietic differentiation. Hematopoietic progenitors included within D12 embryoid bodies (EBs) were analyzed in parallel in semisolid medium with the 7GF cocktail. EBs derived from P-R and PE-R ES cells contained an identical number of CFU-GM, BFU-E, and CFU-Mix progenitors as MSCV EBs. In contrast, an influence of the PRL-R/MPL receptor already could be detected both on the number of E and early Mix colonies (90 ± 16.2 BFU-E in PM-R EBs vs 54 ± 23.2 in MSCV EBs, and 28.6 ± 10.9 CFU-Mix in PM-R EBs vs 15.8 ± 13.4 in MSCV EBs) and on the size and appearance of all colonies (Figure 5B). This has been observed in all subsequent experiments. When transduced CJ7 ES cells were induced to differentiate under the same conditions, an increased number of CFU-Mix colonies was also observed (data not shown). The weak amount of prolactin contained within fetal bovine serum (FBS), although much lower than 500 ng/mL (between 6 ng/mL and 50 ng/mL in the lots of FBS used in these experiments), seemed sufficient to trigger the chimeric receptors expressed at the surface of ES cells under those conditions.
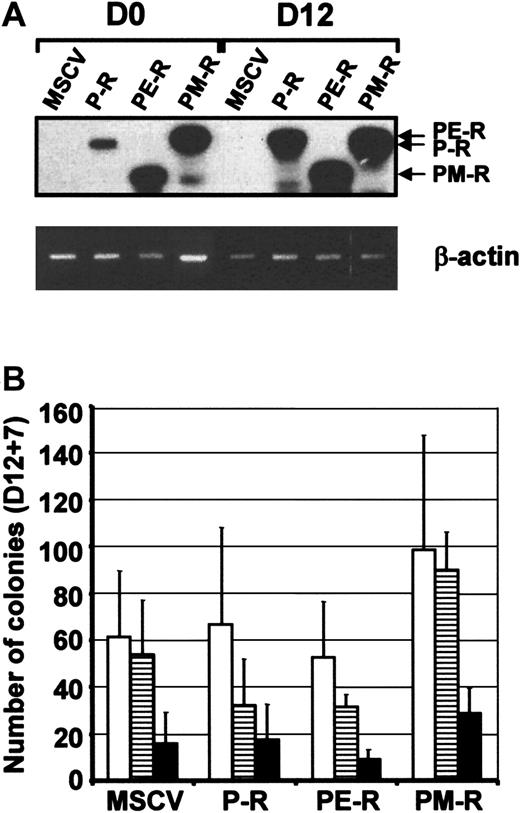
Expression of PRL-R chimeric receptors and hematopoietic progenitor levels after in vitro hematopoietic differentiation of ES cells.
(A) Chimeric receptor expression was determined in undifferentiated ES cells (D0) and in embryoid bodies (EBs) 12 days after induction of differentiation (D12) by RT-PCR. Thirty-five cycles of PCR were performed, and the specificity of the PCR products was confirmed by Southern blot analysis with a specific internal oligonucleotide. (B) Hematopoietic progenitor content analysis of D12 EBs. EBs were collected, dissociated in collagenase solution and their hematopoietic progenitor content established by clonogenic assays in the presence of 7GF cocktail 12 days after induction of hematopoiesis. Colonies were scored 7 days later (D12 + 7). Types of colonies (CFU): ■, granulocyte-macrophage (-GM); ▤, burst-forming unit-erythroid (BFU-E); ▪, mixed (-Mixt).
Expression of PRL-R chimeric receptors and hematopoietic progenitor levels after in vitro hematopoietic differentiation of ES cells.
(A) Chimeric receptor expression was determined in undifferentiated ES cells (D0) and in embryoid bodies (EBs) 12 days after induction of differentiation (D12) by RT-PCR. Thirty-five cycles of PCR were performed, and the specificity of the PCR products was confirmed by Southern blot analysis with a specific internal oligonucleotide. (B) Hematopoietic progenitor content analysis of D12 EBs. EBs were collected, dissociated in collagenase solution and their hematopoietic progenitor content established by clonogenic assays in the presence of 7GF cocktail 12 days after induction of hematopoiesis. Colonies were scored 7 days later (D12 + 7). Types of colonies (CFU): ■, granulocyte-macrophage (-GM); ▤, burst-forming unit-erythroid (BFU-E); ▪, mixed (-Mixt).
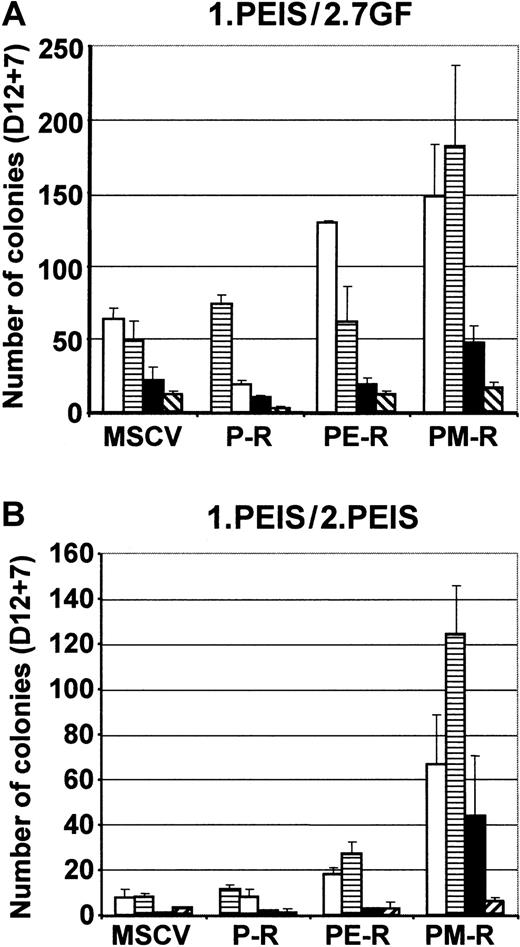
To demonstrate the impact of Mpl signaling on ES cell hematopoietic commitment, control MSCV, P-R, PE-R, and PM-R ES cells were also induced to differentiate in the presence of 500 ng/mL of PRL in a minimal cocktail (PEIS). This allowed activating the chimeric receptors from the beginning of differentiation. No difference in the number and morphology of D12 EBs was observed under those conditions. EBs were then dissociated and analyzed in semisolid medium in the presence of either the 7GF or PEIS cocktail. In the presence of the 7GF cocktail, EBs derived from PM-R ES cells gave rise to higher numbers of CFU-GM, BFU-E, and CFU-Mix than MSCV- and P-R–derived EBs. They also generated higher numbers of BFU-E and CFU-Mix than PE-R (Figure 6, 1.PEIS/2.7GF). Interestingly, no such increase was observed for CFU-megakaryocytes (CFU-MKs). This difference was even greater when the content of EBs was analyzed in PEIS, since under these conditions, only PM-R ES cells were found to give rise to all kinds of hematopoietic progenitors, with a substantial number of CFU-Mix (44 ± 27 CFU-Mix per 105 EB cells plated) (Figure 6, 1.PEIS/2.PEIS). The results presented in Figure 6 are representative of 2 independent experiments conducted with ES R1 cells. However, it is important to note that this increase in the content of hematopoietic progenitors in PM-R EBs has been consistently observed, regardless of which ES cell line (R1 or CJ7) or conditions for hematopoietic differentiation were used. These data indicate that early Mpl signaling favored the hematopoietic differentiation of ES cells by stimulating the production of progenitors.
Enhancement of hematopoietic differentiation of PM-R ES cells in the presence of PRL.
MSCV, P-R, PE-R, and PM-R R1 ES cells were induced to differentiate in the presence of PRL (500 ng/mL) (cocktail PEIS) for 12 days. The hematopoietic progenitor content of EBs was determined by clonogenic assays in methylcellulose, in the presence of 7GF cocktail (1.PEIS/2.7GF) or in the presence of PEIS (1.PEIS/2.PEIS). The results of 2 independent experiments are reported. Types of colonies (CFU): ■, granulocyte-macrophage (-GM); ▤, burst-forming unit-erythroid (BFU-E); ▪, mixed (-Mixt); and ▧, -EMK, or -GMK.
Enhancement of hematopoietic differentiation of PM-R ES cells in the presence of PRL.
MSCV, P-R, PE-R, and PM-R R1 ES cells were induced to differentiate in the presence of PRL (500 ng/mL) (cocktail PEIS) for 12 days. The hematopoietic progenitor content of EBs was determined by clonogenic assays in methylcellulose, in the presence of 7GF cocktail (1.PEIS/2.7GF) or in the presence of PEIS (1.PEIS/2.PEIS). The results of 2 independent experiments are reported. Types of colonies (CFU): ■, granulocyte-macrophage (-GM); ▤, burst-forming unit-erythroid (BFU-E); ▪, mixed (-Mixt); and ▧, -EMK, or -GMK.
Introduction of antisense MPL into CJ7 ES cells inhibits their hematopoietic differentiation in vitro
To check for the functional relevance of Mpl signaling in ES cell differentiation, we used an antisense strategy. Full-length murine Mpl cDNA was subcloned in MSCV, in an antisense orientation. MSCV MPL antisense (MPLAS) DNA was introduced into ES CJ7 cells by electroporation, and 3 clones expressing high levels of MPLAS RNA were isolated after neomycin selection, as performed for the PRL-R/MPL receptor.
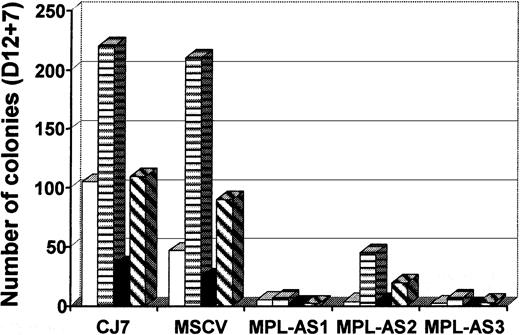
We first checked whether MPLAS expression influenced the formation of EBs. As shown in Table 1, except in one clone (MPLAS-3), MPLAS did not affect the total number of D12 EBs. However, a decrease in red (hematopoietic) EBs was observed in all clones. This decrease was dramatic in MPLAS-1 and MPLAS-3 (87% and 79%, respectively, compared to MSCV) and reached 32% for MPLAS-2. This was consistently observed, even when we optimized the conditions for hematopoietic differentiation by introducing MS5 stromal cells during the first step of differentiation.21 Upon analysis of hematopoietic progenitors present in these EBs, the number of hematopoietic colonies derived from MPLAS ES clones was sharply decreased (Table 2). MPLAS-2 was the clone that produced the highest number of colonies, although this was still reduced by 84%. As shown in Figure7, all types of colonies decreased in all clones (89% to 96% decrease for CFU-GM, 79% to 97% for BFU-E, 84% to 100% for CFU-Mix, and 78% to 98% for mixed colonies with MKs). Production of megakaryocytes was also severely impaired in these cultures (97% to 99.7%) (Table 2).
Effect of antisense Mpl expression on the formation of CJ7 EBs
| . | CJ7 . | MSCV . | MPL-AS1 . | MPL-AS2 . | MPL-AS3 . |
|---|---|---|---|---|---|
| Total EBs | 334 | 362 | 384 | 533 | 85 |
| Hematopoietic EBs | 59 | 47 | 6 | 32 | 10 |
| . | CJ7 . | MSCV . | MPL-AS1 . | MPL-AS2 . | MPL-AS3 . |
|---|---|---|---|---|---|
| Total EBs | 334 | 362 | 384 | 533 | 85 |
| Hematopoietic EBs | 59 | 47 | 6 | 32 | 10 |
Total number of embryoid bodies obtained after 12 days in culture. Two thousand CJ7 cells per dish were mixed with 15 000 MS-5 cells and cultured in methylcellulose using the differentiation medium described in “Materials and methods.” EBs containing red cells are scored as hematopoietic EBs.
Effect of antisense Mpl expression on the hematopoietic differentiation of CJ7 cells
| Day 12 + 7 . | CJ7-T . | Vector . | MPL-AS1 . | MPL-AS2 . | MPL-AS3 . |
|---|---|---|---|---|---|
| Total number of hematopoietic colonies | 474 | 371 | 14 | 59 | 11 |
| Number of MKs | 3667 | 1107 | < 10 | 107 | < 10 |
| Day 12 + 7 . | CJ7-T . | Vector . | MPL-AS1 . | MPL-AS2 . | MPL-AS3 . |
|---|---|---|---|---|---|
| Total number of hematopoietic colonies | 474 | 371 | 14 | 59 | 11 |
| Number of MKs | 3667 | 1107 | < 10 | 107 | < 10 |
Total number of hematopoietic colonies generated from 2000 initial CJ7 cells. EBs were dissociated and cells were cultured in secondary semisolid cultures with 7GFs 12 days after initiation of hematopoietic differentiation in the presence of the 7GFs cocktail. Hematopoietic colonies were scored 7 days later (day 12 + 7). Megakaryocytes were counted after immunofluorescence staining of cytospin preparations from CFU-MK colonies with the rat anti-murine platelet monoclonal antibody 4A5.
MPLAS expression inhibits hematopoietic differentiation of ES cells.
Three independent MPLAS CJ7 ES clones (MPL-AS1, MPL-AS2, and MPL-AS3), obtained after electroporation, were tested for in vitro hematopoietic differentiation in the presence of the 7GF cocktail and MS-5 cells. Analysis of D12 EB content of hematopoietic progenitors, for MPLAS clones as well as for parental and MSCV CJ7 ES cells, is shown. Types of colonies (CFU): ■, granulocyte-macrophage (-GM); ▤, burst-forming unit-erythroid (BFU-E); ▪, mixed (-Mixt); and ▧, -EMK, or -GMK.
MPLAS expression inhibits hematopoietic differentiation of ES cells.
Three independent MPLAS CJ7 ES clones (MPL-AS1, MPL-AS2, and MPL-AS3), obtained after electroporation, were tested for in vitro hematopoietic differentiation in the presence of the 7GF cocktail and MS-5 cells. Analysis of D12 EB content of hematopoietic progenitors, for MPLAS clones as well as for parental and MSCV CJ7 ES cells, is shown. Types of colonies (CFU): ■, granulocyte-macrophage (-GM); ▤, burst-forming unit-erythroid (BFU-E); ▪, mixed (-Mixt); and ▧, -EMK, or -GMK.
Discussion
During the past few years, an accumulation of data has demonstrated that the Mpl receptor and its ligand, TPO, which were first described to be involved in the primary regulation of megakaryopoiesis and thrombopoiesis, also played an important role at the level of adult hematopoietic stem cells.11,12,36-41This concept has been supported by the effects of inactivation of the Mpl/TPO pathway.16,17 c-mpl−/− adult mice are not only deficient in hematopoietic progenitors, but also demonstrate a dramatic decrease in hematopoietic stem cells.18-20 This deficiency seems to develop at approximately the time of birth, since 12.5-day fetal livers from c-mpl−/− mice contain normal numbers of hematopoietic progenitors.16 However, Mpl expression is essential for fetal liver progenitors to exhibit hematopoietic repopulating ability.20 In the present work, we investigated the signals sent by the Mpl cytoplasmic domain in fetal hematopoietic progenitors and in embryonic stem cells induced to commit toward hematopoiesis.
We introduced chimeric receptors composed of the extracellular domain of PRL-R and the cytoplasmic domain of Mpl or EPO-R (PM-R and PE-R), as well as the parental rabbit PRL-R, into hematopoietic progenitors prepared from C57Bl6 or PRL-R−/− 12.5-day fetal livers. In the presence of prolactin alone, only cells infected with PM-R were able to give rise to the whole spectrum of myeloid colonies in both cases (Figure 3A). A significant increase in the number of BFU-Es (early erythroid colonies) and GEMM colonies was observed, but the PM-R receptor did not support mature erythroid colony development efficiently (Figure 2A), suggesting that signals sent by the Mpl cytoplasmic domain may be active dominantly in primitive hematopoietic progenitors. In the same model, the EPO-R cytoplasmic domain can be replaced by the cytoplasmic domain of a nonhematopoietic receptor, PRL-R, with maintenance of the differentiation of primary erythroid progenitors, as previously published.22,34 BFU-E levels for PE-R–infected fetal liver suspensions were lower than those published by Socolovsky et al or Goldsmith et al.22,42 In those studies, the fact that stem cell factor is present in the culture medium can be taken into account for explaining this difference. In the past years, many models have been developed to investigate the signaling specificity of cytokine receptors; all indicated that hematopoietic cell commitment is independent of extracellular signals and that receptor cytoplasmic domains are sufficient to provide survival and/or proliferation signals for progenitors.22,34,42,43 It was concluded that in that respect, these receptor domains are interchangeable. All these investigations involved mostly terminally differentiated hematopoietic cells. Our data indicate that signaling in immature hematopoietic cells is more specific and that the cytoplasmic domain of a receptor active in such cells, like Mpl, cannot be replaced by that of a receptor that usually signals in more committed cells (EPO-R), and vice-versa. This is in total agreement with the recent study of Zeng et al of mouse adult bone marrow, which shows that Mpl signaling can specifically induce the self-renewal and differentiation of primary multipotential hematopoietic progenitor cells, while 2 other receptors also reported to be expressed in hematopoetic stem cells (Flt-3 and G-CSF-R) failed to support self-renewal of these progenitors.44
Introduction of PM-R into ES cells allowed us to study the impact of Mpl signaling on hematopoietic commitment of a totipotent embryonic stem cell. ES cells are maintained in an undifferentiated state in the presence of LIF. When allowed to form 3-dimensional structures known as embryoid bodies (EBs), ES cells are able to differentiate into various cell types, including hematopoietic cells, in the presence of growth factors.45,46 The expression of genes coding for hematopoietic growth factors, their receptors, and other early markers characteristic of hematopoietic cells has been studied by several groups, and EPO-R is one of the earliest receptors expressed during ES cell differentiation (PCR signal detected between D0 and D1).47-49 Mpl expression is detected later, starting on D6 of differentiation.21 The introduction of chimeric receptors into ES cells allowed us to stimulate Mpl, EPO-R, and PRL-R signaling as soon as D0 of differentiation, by addition of large amounts of PRL (500 ng/mL). Under those conditions, D12 EBs generated from PM-R ES cells contained more hematopoietic progenitors of all types compared to D12 P-R or PE-R EBs. This was even more dramatic when EBs were analyzed in the presence of a more restricted growth factor combination that contained PRL (PEIS) (Figure 6, lower panel). Our results indicate that only PM-R expression was able to promote the earliest stages of hematopoiesis. Interestingly, stimulation of Mpl signaling both in fetal liver hematopoietic progenitors and ES cells did not lead to a notable increase in the megakaryocytic compartment. This is in agreement with the report of Goncalves et al, who showed that introduction of Mpl into murine bone marrow stem cells does not result in preferential commitment toward the megakaryocytic lineage.50
However, our results indicated that early Mpl signaling enhanced the hematopoietic differentiation of ES cells. The physiological significance of this effect was strengthened when Mpl antisense RNA was expressed in control CJ7 ES cells. When induced to differentiate toward hematopoiesis in the presence of a rich combination of cytokines (7GF), 3 independent MPLAS CJ7 clones gave rise to nonhemoglobinized embryoid bodies with a marked decrease in the total number of hematopoietic colonies (80% to 100%). These results are somewhat contradictory to the observation that in vitro, c-mpl−/− ES cells give rise to numbers and proportions of hematopoietic progenitors comparable to those derived from control mpl+/+ ES cells.51 Pertinently, in these last experiments, EBs were formed in the presence of MS-5 stromal cells, which enhance ES cell hematopoietic potential by promoting their plating efficiency before hematopoietic determination, and then by supporting hematopoiesis within EBs through TPO.21 52 This might have emphasized the lack of hematopoietic differentiation observed for MPLAS CJ7 ES cells, which were unable to bind TPO. However, an 85% to 90% decrease in hematopoietic colonies was also observed when hematopoietic differentiation was induced in the absence of MS-5 (data not shown). In preliminary experiments, we demonstrated that when full-length EPO-R was introduced in an antisense orientation in ES cells (EPO-RAS), it did not affect total number of D12 EBs. Nevertheless, a 50% to 80% decrease in CFC-E and a 50% decrease in CFU-Mix colonies with erythroid components were observed, while no effect was detected on CFU-GM (data not shown). It seems, therefore, that the dramatic effect of MPLAS on hematopoietic differentiation of ES cells is rather specific.
This contradiction between the effects of an inactivated gene and the antisense approach on the in vitro hematopoietic differentiation of ES cells has been described already for the vavgene.53-55 In a knockout strategy, a gene important but not essential for hematopoietic development can be functionally complemented at the beginning of ES cell differentiation in the embryo, while in an antisense approach, the persistent presence of the antisense might act upon various pathways involved in hematopoietic differentiation. Accordingly, it is possible that persistent presence of MPLAS blocks other pathways involved in hematopoietic differentiation or apoptosis, while inactivation of Mpl can be functionally substituted for other signals. Nevertheless, the 2 approaches may provide complementary insights into the potential functions and roles of a given gene.
In short, our results strongly suggest that Mpl plays a role during the hematopoietic commitment of embryonic cells and is important in the establishment of definitive hematopoiesis in the embryo.
We would like to thank Bruno Peault for his support and critical comments on the manuscript, and Jean-Philippe Rosa for the helpful discussion. We are indebted to Prof Jack Levin for critical reading, useful comments, and constant support.
Supported by the Institut National de la Santé et de la Recherche Médicale and the Association pour la Recherche contre le Cancer (grant 1397). C.C. is supported by the Ministère de l'Education de la recherche et de la Technologie (MERT) and by the Association pour la Recherche contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michèle Souyri, Institut National de la Santé et de la Recherche Médicale, U506, Hôpital Paul Brousse, 14, avenue Paul-Vaillant Couturier, 94807 Villejuif Cedex, France; e-mail: msouyri@infobiogen.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal