Abstract
Black children with acute lymphoblastic leukemia (ALL) have poor outcomes, but limited information is available for children from other racial and ethnic backgrounds, such as Hispanic and Asian. We undertook a retrospective cohort study of children with ALL treated on Children's Cancer Group therapeutic protocols to determine outcomes by racial and ethnic backgrounds of patients treated with contemporary risk-based therapy. In total, 8447 children (white, n = 6703; Hispanic, n = 1071; black, n = 506; and Asian, n = 167) with newly diagnosed ALL between 1983 and 1995 were observed for a median of 6.5 years. Analysis of disease outcome was measured as overall survival (OS) and event-free survival (EFS) and was adjusted for known predictors of outcome including clinical features, disease biology, socioeconomic status, and treatment era (1983-1989 vs 1989-1995). There was a statistically significant difference in survival by ethnicity (P < .001). Five-year EFS rates were: Asian, 75.1% ± 3.5%; white, 72.8% ± 0.6%; Hispanic, 65.9% ± 1.5%; and black, 61.5% ± 2.2%. Multivariate analysis revealed that when compared with white children, black and Hispanic children had worse outcomes and Asian children had better outcomes after adjusting for known risk factors. The poorer outcomes among black children were most apparent among patients with standard-risk features (relative risk [RR], 2.0; 95% confidence interval [CI], 1.6-2.5), whereas poorer outcomes in Hispanic children (RR, 1.4; 95% CI, 1.2-1.6) were most evident among patients with high-risk features. Asian children had better outcomes than all racial and ethnic groups among high-risk patients, particularly in the recent era (5-year EFS, 90.9% ± 6.1%). Racial and ethnic differences in OS and EFS persist among children with ALL who receive contemporary risk-based therapy. Future studies should focus on reasons—perhaps compliance or pharmacogenetics—for those differences.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy, with an annual incidence rate of 3 to 4 cases per 100 000 children.1 Population-based data indicate that children with ALL treated with contemporary therapy have a 5-year survival rate of 80%.2,3 Racial and ethnic differences in survival after childhood ALL are reported in various studies,4-6 with poorer outcomes reported for black children than for white children.7-13 The exception is one report that demonstrates no difference in survival between black children and white children treated with contemporary multimodality therapy.14 However, most studies have focused on survival differences between white and black patients, and there are insufficient data on children from other ethnic backgrounds, such as Hispanic and Asian. In the few studies that have reported treatment outcomes among other racial and ethnic groups,4 6 it has been difficult to obtain precise statistical estimates of the true differences in treatment outcomes because of small samples. We studied the racial and ethnic differences in survival of 8762 children with newly diagnosed ALL who were registered and treated according to Children's Cancer Group (CCG) therapeutic protocols from 1983 to 1995 and observed through 1999, a median of 6.5 years. This study was able to categorize children according to 5 primary racial and ethnic groups—white, black, Hispanic, Asian, and mixed or others—based on data collected on patient registration in CCG clinical trials. In addition, the analyses also considered the potential impact of risk categorization based on other features at presentation.
Patients, materials, and methods
The CCG has conducted clinical trials in cooperation with member institutions throughout the United States and Canada. At the time of analysis, the CCG included 122 institutions. These institutions were required to register all patients with newly diagnosed cancer with the Operations Office; after registration, eligible patients were entered in active therapeutic clinical trials. The Operations Office was responsible for determining patient eligibility, randomization to the appropriate therapeutic arm (if necessary), and follow-up of patients for all potential outcomes. Member institutions were required to submit follow-up reports on all patients enrolled in therapeutic protocols, which included information regarding survival status, disease status, and development of second malignancies in addition to the development of other adverse events, as required by individual therapeutic studies. These follow-up reports are submitted annually for as long as patients live.
Study population
From 1983 through 1995, 14 117 children and adolescents diagnosed with ALL were registered with CCG. Among those, 8762 (62%) were enrolled in 1 of 12 therapeutic protocols conducted by CCG that were open for enrollment between 1983 and 1995 for untreated ALL. Those 12 protocols were CCG-104, -105, -106, -107, -123, -139, -1881, -1882, -1883, -1891, -1901, and -1922. Informed consent forms were signed by patients, parents, or guardians at enrollment. Patients were randomly assigned to 1 of 2 or more therapeutic schedules of chemotherapy and radiation. Therapy duration ranged from 2 to 3 years. Clinical results of many of those trials, with the therapeutic plans, have been published.15-25
Racial and ethnic distributions for patients not placed in therapeutic studies (38% of the cohort) differed significantly from children with ALL placed in therapeutic studies. Sixty-four percent of the black children and 49% of Asian children were not enrolled in therapeutic studies, compared with 40% of white and 26% of Hispanic children (P < .001). No follow-up information was required for patients not placed in CCG therapeutic studies by the CCG Operations Office; hence, that group of patients not placed in CCG therapeutic studies could not be included in this analysis.
Thus, the study population (n = 8762) included 6703 white children, 1071 Hispanic children, 506 black children, 167 Asian children, and 315 children of mixed or other racial origin. Children of mixed or other racial origin were excluded from further statistical analysis because no further information on specifics of their ethnic backgrounds was available to us. Therefore, this report focuses on outcomes of patients with known racial and ethnic backgrounds who were placed in therapeutic studies and for whom we had follow-up data (n = 8447).
Subset analysis
Socioeconomic status.
In addition, 1596 children with ALL were enrolled in an epidemiologic study conducted between 1989 and 1993.26 Data for that study were collected through telephone interviews with children's parents. Interviews included questions regarding family income and highest educational levels attained by both parents. Socioeconomic status was assessed using parental education (categorized as high school or less compared with some college or more) and annual household income (categorized as less than or equal to $30 000 compared with more than $30 000).
Lineage and chromosomal abnormalities.
Information on the lineage of lymphoblasts (B or T cell) was available for 4722 patients, whereas information on ploidy and structural chromosomal abnormalities was available for 1872 patients. Therefore, subset analysis was conducted to adjust for influences of all those factors on survival.
Statistical analysis
Analysis of disease outcome was examined as overall survival (OS) and event-free survival (EFS). OS was measured from date of initial diagnosis of ALL to date of death from any cause or date of last contact using the Kaplan-Meier method.27 EFS was defined as the time to first induction failure, relapse at any site (for those who achieved remission), or death, whichever occurred first. For those who did not experience events, OS and EFS were the time to last contact. Associated standard errors were calculated by the method of Peto et al.28 Analysis of EFS gives more events for the life table analyses than survival comparisons (hence, more statistical power to detect differences), particularly for patients entered in the more recent periods. OS and EFS distributions were compared using log-rank global χ2 analysis.29Pvalues are for 2-sided tests.
Patients were categorized as at standard risk or high risk using standardized National Cancer Institute (NCI) risk categorization.30 Standard risk was defined as age at diagnosis between 1 and 9 years and initial white blood cell (WBC) count less than 50 000/μL. High risk was defined as age at diagnosis either younger than 1 year or 10 years and olderor initial white blood cell count greater than 50 000/μL.
Patients also were categorized into early and recent treatment eras. Early treatment era corresponded to patients who were treated according to 1 of 6 therapeutic protocols open between 1983 and 1989 (CCG-104, -105, -106, -107, -123, -139). Recent treatment era corresponded to patients who were treated according to the remaining 6 therapeutic protocols open between 1989 and 1995 (CCG-1881, -1882, -1883, -1891, -1901, -1922).
Cox regression analysis was used for calculating estimates of relative risk for OS and EFS and significance levels after adjustment for prognostic influence of other factors.31 Variables included in the regression model were ethnicity, age at diagnosis, WBC count at diagnosis, platelet count at diagnosis, sex, splenomegaly, hepatomegaly, lineage, structural and numerical chromosomal abnormalities, socioeconomic status, and treatment era.
Results
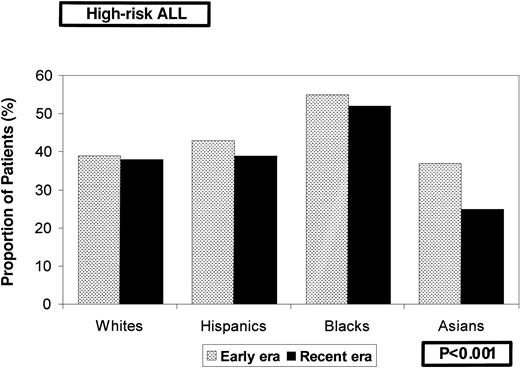
Table 1 summarizes the characteristics of this cohort. Median length of follow-up of the entire cohort was 6.5 years (range, 0.1 to 15.1 years); it was 9.4 years for patients diagnosed and treated during the early era and 5.1 years for those diagnosed in the recent era. The date of last contact was within 2 years of the study analysis for 75% of the white, 69% of the Asian, 67% of the Hispanic, and 61% of the black children. Racial distribution of patients across various risk categories varied significantly (Table 1). High leukocyte counts and age at presentation resulted in an overrepresentation of black children in the high-risk group. Racial distribution in the 2 risk groups remained fairly constant across treatment eras and is summarized in Figure1, again depicting the overrepresentation of blacks among the high-risk group overall and in both treatment eras (P < .001). There was no difference in the incidence of central nervous system leukemia at diagnosis by ethnic group (white, 1.6%; black, 1.7%; Hispanic, 0.9%; Asian, 1.7%;P = .7). Remission induction rates ranged from 97% to 99% in the 4 racial and ethnic groups studied (P = .3). A comparison of the day 7 marrow results by ethnicity revealed that significantly more Asian children (60%) achieved M1 marrow than black (56%), white (51%), or Hispanic (50.5%) children (P = .004). M3 marrow on day 7 was least likely among Asian children (19.1%), followed by white (24.7%), black (25.8%), and Hispanic (30.8%) children. Pairwise comparison of the day 7 marrow results revealed that statistically significant differences were obvious only between Hispanic children and white children.
Patient characteristics of 8 447 children with ALL
| . | Total cohort (N = 8 447) . | Whites (n = 6 703) . | Blacks (n = 506) . | Hispanics (n = 1 071) . | Asians (n = 167) . | P . |
|---|---|---|---|---|---|---|
| Sex (%) | ||||||
| Female | 3 642 (43) | 2 908 (43) | 218 (43) | 443 (41) | 73 (44) | — |
| Male | 4 805 (57) | 3 795 (57) | 288 (57) | 628 (59) | 94 (56) | .7 |
| WBC count/mL (%) | ||||||
| Lower than 50 000 | 6 606 (78) | 5 280 (79) | 353 (70) | 834 (78) | 139 (83) | — |
| 50 000 or higher | 1 841 (22) | 1 423 (21) | 153 (30) | 237 (22) | 28 (17) | < .001 |
| Age at diagnosis, y (%) | ||||||
| Younger than 1 | 224 (3) | 168 (2.5) | 18 (4) | 31 (3) | 7 (4) | — |
| 1 to 10 | 6 359 (75) | 5 104 (76) | 329 (65) | 792 (74) | 134 (80) | — |
| Older than 10 | 1 864 (22) | 1 431 (21.5) | 159 (31) | 248 (23) | 26 (16) | < .001 |
| NCI risk group (%) | ||||||
| Good risk | 5 127 (71) | 4 137 (62) | 236 (47) | 639 (60) | 115 (69) | — |
| Poor risk | 3 320 (29) | 2 566 (38) | 270 (53) | 432 (40) | 52 (31) | < .001 |
| Events (%) | ||||||
| Adverse event* | 2 482 (29) | 1 892 (28) | 197 (39) | 352 (33) | 41 (25) | < .001 |
| Death | 1 661 (20) | 1 255 (19) | 139 (28) | 247 (23) | 20 (12) | < .001 |
| Hemoglobin level, g/dL (%) | ||||||
| Lower than 10 | 6 628 (78) | 5 221 (78) | 399 (79) | 868 (81) | 140 (84) | .05 |
| 10 or higher | 1 819 (22) | 1 482 (22) | 107 (21) | 203 (19) | 27 (16) | — |
| Platelet count/mL (%) | ||||||
| Lower than 50 000 | 4 016 (48) | 3 213 (48) | 222 (44) | 511 (48) | 70 (42) | — |
| 50 000 or higher | 4 431 (52) | 3 490 (52) | 284 (56) | 560 (52) | 97 (58) | .2 |
| Liver size (%) | ||||||
| Normal | 3 889 (46) | 3 070 (46) | 250 (49) | 496 (46) | 73 (44) | — |
| Enlarged | 4 558 (54) | 3 633 (54) | 256 (51) | 575 (54) | 94 (56) | .4 |
| Spleen size (%) | ||||||
| Normal | 3 880 (46) | 2 991 (45) | 254 (50) | 545 (51) | 90 (54) | — |
| Enlarged | 4 567 (54) | 3 712 (55) | 252 (50) | 526 (49) | 77 (46) | < .001 |
| Chromosomal abnormalities (%) (N = 1 872)† | (n = 1 577) | (n = 118) | (n = 153) | (n = 24) | — | — |
| Normal | 579 (31) | 481 (31) | 30 (25) | 57 (37) | 11 (46) | — |
| Structural abnormalities‡ | 972 (52) | 822 (52) | 73 (62) | 69 (45) | 8 (33) | — |
| Numerical abnormalities | 321 (17) | 274 (17) | 15 (13) | 27 (18) | 5 (21) | .07 |
| Ploidy groups† | (n = 1 872) | (n = 1 577) | (n = 118) | (n = 153) | (n = 24) | — |
| Normal | 579 (31) | 481 (31) | 30 (25) | 57 (37) | 11 (46) | — |
| Hypodiploid | 111 (6) | 98 (6) | 5 (4) | 8 (5) | 0 (0) | — |
| Pseudodiploid | 510 (27) | 425 (27) | 44 (37) | 36 (24) | 5 (21) | — |
| Hyperdiploid (47-50) | 198 (11) | 164 (10) | 16 (14) | 15 (10) | 3 (13) | — |
| Hyperdiploid (> 50) | 474 (25) | 409 (26) | 23 (20) | 37 (24) | 5 (21) | .2 |
| Maternal education (%)1-153 | (n = 1 596) | (n = 1 381) | (n = 85) | (n = 103) | (n = 27) | — |
| High school or less | 694 (43.5) | 573 (42) | 46 (54) | 63 (61) | 12 (44) | — |
| More than high school | 902 (56.5) | 808 (58) | 39 (46) | 40 (39) | 15 (56) | < .001 |
| Paternal education (%)1-153 | (n = 1 491) | (n = 1 319) | (n = 61) | (n = 85) | (n = 26) | — |
| High school or less | 614 (41) | 510 (37) | 46 (75) | 52 (61) | 6 (23) | — |
| More than high school | 877 (59) | 809 (63) | 15 (25) | 33 (39) | 20 (77) | < .001 |
| Annual household income (%)1-153 | (n = 1 585) | (n = 1 373) | (n = 83) | (n = 103) | (n = 26) | — |
| $30 000 or less | 889 (56) | 724 (53) | 62 (75) | 86 (84) | 17 (65) | — |
| More than $30 000 | 696 (44) | 649 (47) | 21 (25) | 17 (16) | 9 (35) | < .001 |
| . | Total cohort (N = 8 447) . | Whites (n = 6 703) . | Blacks (n = 506) . | Hispanics (n = 1 071) . | Asians (n = 167) . | P . |
|---|---|---|---|---|---|---|
| Sex (%) | ||||||
| Female | 3 642 (43) | 2 908 (43) | 218 (43) | 443 (41) | 73 (44) | — |
| Male | 4 805 (57) | 3 795 (57) | 288 (57) | 628 (59) | 94 (56) | .7 |
| WBC count/mL (%) | ||||||
| Lower than 50 000 | 6 606 (78) | 5 280 (79) | 353 (70) | 834 (78) | 139 (83) | — |
| 50 000 or higher | 1 841 (22) | 1 423 (21) | 153 (30) | 237 (22) | 28 (17) | < .001 |
| Age at diagnosis, y (%) | ||||||
| Younger than 1 | 224 (3) | 168 (2.5) | 18 (4) | 31 (3) | 7 (4) | — |
| 1 to 10 | 6 359 (75) | 5 104 (76) | 329 (65) | 792 (74) | 134 (80) | — |
| Older than 10 | 1 864 (22) | 1 431 (21.5) | 159 (31) | 248 (23) | 26 (16) | < .001 |
| NCI risk group (%) | ||||||
| Good risk | 5 127 (71) | 4 137 (62) | 236 (47) | 639 (60) | 115 (69) | — |
| Poor risk | 3 320 (29) | 2 566 (38) | 270 (53) | 432 (40) | 52 (31) | < .001 |
| Events (%) | ||||||
| Adverse event* | 2 482 (29) | 1 892 (28) | 197 (39) | 352 (33) | 41 (25) | < .001 |
| Death | 1 661 (20) | 1 255 (19) | 139 (28) | 247 (23) | 20 (12) | < .001 |
| Hemoglobin level, g/dL (%) | ||||||
| Lower than 10 | 6 628 (78) | 5 221 (78) | 399 (79) | 868 (81) | 140 (84) | .05 |
| 10 or higher | 1 819 (22) | 1 482 (22) | 107 (21) | 203 (19) | 27 (16) | — |
| Platelet count/mL (%) | ||||||
| Lower than 50 000 | 4 016 (48) | 3 213 (48) | 222 (44) | 511 (48) | 70 (42) | — |
| 50 000 or higher | 4 431 (52) | 3 490 (52) | 284 (56) | 560 (52) | 97 (58) | .2 |
| Liver size (%) | ||||||
| Normal | 3 889 (46) | 3 070 (46) | 250 (49) | 496 (46) | 73 (44) | — |
| Enlarged | 4 558 (54) | 3 633 (54) | 256 (51) | 575 (54) | 94 (56) | .4 |
| Spleen size (%) | ||||||
| Normal | 3 880 (46) | 2 991 (45) | 254 (50) | 545 (51) | 90 (54) | — |
| Enlarged | 4 567 (54) | 3 712 (55) | 252 (50) | 526 (49) | 77 (46) | < .001 |
| Chromosomal abnormalities (%) (N = 1 872)† | (n = 1 577) | (n = 118) | (n = 153) | (n = 24) | — | — |
| Normal | 579 (31) | 481 (31) | 30 (25) | 57 (37) | 11 (46) | — |
| Structural abnormalities‡ | 972 (52) | 822 (52) | 73 (62) | 69 (45) | 8 (33) | — |
| Numerical abnormalities | 321 (17) | 274 (17) | 15 (13) | 27 (18) | 5 (21) | .07 |
| Ploidy groups† | (n = 1 872) | (n = 1 577) | (n = 118) | (n = 153) | (n = 24) | — |
| Normal | 579 (31) | 481 (31) | 30 (25) | 57 (37) | 11 (46) | — |
| Hypodiploid | 111 (6) | 98 (6) | 5 (4) | 8 (5) | 0 (0) | — |
| Pseudodiploid | 510 (27) | 425 (27) | 44 (37) | 36 (24) | 5 (21) | — |
| Hyperdiploid (47-50) | 198 (11) | 164 (10) | 16 (14) | 15 (10) | 3 (13) | — |
| Hyperdiploid (> 50) | 474 (25) | 409 (26) | 23 (20) | 37 (24) | 5 (21) | .2 |
| Maternal education (%)1-153 | (n = 1 596) | (n = 1 381) | (n = 85) | (n = 103) | (n = 27) | — |
| High school or less | 694 (43.5) | 573 (42) | 46 (54) | 63 (61) | 12 (44) | — |
| More than high school | 902 (56.5) | 808 (58) | 39 (46) | 40 (39) | 15 (56) | < .001 |
| Paternal education (%)1-153 | (n = 1 491) | (n = 1 319) | (n = 61) | (n = 85) | (n = 26) | — |
| High school or less | 614 (41) | 510 (37) | 46 (75) | 52 (61) | 6 (23) | — |
| More than high school | 877 (59) | 809 (63) | 15 (25) | 33 (39) | 20 (77) | < .001 |
| Annual household income (%)1-153 | (n = 1 585) | (n = 1 373) | (n = 83) | (n = 103) | (n = 26) | — |
| $30 000 or less | 889 (56) | 724 (53) | 62 (75) | 86 (84) | 17 (65) | — |
| More than $30 000 | 696 (44) | 649 (47) | 21 (25) | 17 (16) | 9 (35) | < .001 |
Total number of children in the study (N = 8447) excludes children of mixed or other racial origin.
Adverse event denotes induction failure, relapse at any site, or death from any cause as the first event.
Chromosomal abnormalities were based on a subset of 1 872 cases (see “Materials and methods”).
Structural abnormalities include t(4;11), t(8;14), t(9;22), t(1;19), and others.
Parental education and household income were based on a subset of 1 596 cases (see “Materials and methods”).
Distribution of a cohort of 8447 children diagnosed with ALL, by racial and ethnic groups, according to treatment era and NCI risk groups.
Distribution of a cohort of 8447 children diagnosed with ALL, by racial and ethnic groups, according to treatment era and NCI risk groups.
Entire cohort
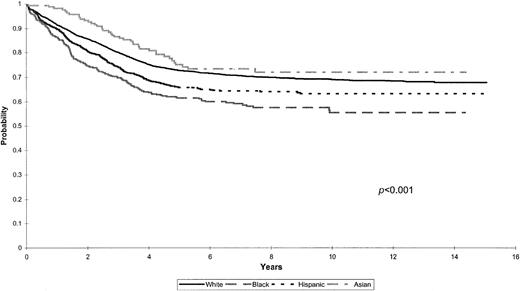
There was a statistically significant difference in OS among the 4 groups (P < .001). OS rates at 5 years were 89.3% ± 2.5% for Asian, 83.6% ± 0.5% for white, 78.1% ± 1.3% for Hispanic, and 74.4% ± 2.0% for black children.
Figure 2 shows the EFS for the entire cohort by racial and ethnic group, again revealing significant differences among the 4 groups studied (P < .001). EFS rates at 5 years were 75.1% ± 3.5% for Asian, 72.8% ± 0.6% for white, 65.9% ± 1.5% for Hispanic, and 61.5% ± 2.2% for black children.
Kaplan-Meier estimates of EFS for a cohort of 8447 children with ALL according to racial and ethnic distribution.
Kaplan-Meier estimates of EFS for a cohort of 8447 children with ALL according to racial and ethnic distribution.
Multivariate analysis revealed racial and ethnic background to be independently associated with EFS, after adjusting for known risk factors such as age at diagnosis, high initial leukocyte count, splenomegaly, hepatomegaly, male sex, and treatment era (Table2). Outcomes were worse for black and Hispanic children and better for Asian children than for white children.
Risk factors for OS and EFS: multivariate analysis
| Risk factors . | Overall survival RR (95% CI) . | Event-free survival RR (95% CI) . |
|---|---|---|
| Age at diagnosis, y | ||
| 1 to 10 | 1.0 | 1.0 |
| Younger than 1 | 3.8 (3.1-4.6)* | 3.3 (2.8-4.0)* |
| Older than 10 | 2.3 (2.1-2.6)* | 1.8 (1.6-1.9)* |
| Initial WBC count | ||
| Lower than 50 000/mL | 1.0 | 1.0 |
| 50 000/mL or higher | 1.7 (1.5-1.9)* | 1.4 (1.2-1.5)* |
| Initial platelet count | ||
| 50 000/mL or higher | 1.0 | 1.0 |
| Less than 50 000/mL | 1.0 (0.9-1.1)2-153 | 1.1 (1.0-1.2)‡ |
| Sex | ||
| Female | 1.0 | 1.0 |
| Male | 1.1 (1.0-1.3)† | 1.2 (1.1-1.3)* |
| Liver size | ||
| Normal | 1.0 | 1.0 |
| Enlarged | 1.1 (0.9-1.2)2-153 | 1.2 (1.0-1.3)† |
| Spleen size | ||
| Normal | 1.0 | 1.0 |
| Enlarged | 1.3 (1.2-1.4)* | 1.1 (1.0-1.3)‡ |
| Era | ||
| Recent | 1.0 | 1.0 |
| Early | 1.4 (1.3-1.6)* | 1.5 (1.4-1.6)* |
| Ethnicity | ||
| White | 1.0 | 1.0 |
| Black | 1.4 (1.1-1.6)* | 1.4 (1.2-1.7)* |
| Hispanic | 1.4 (1.2-1.6)* | 1.3 (1.2-1.5)* |
| Asian | 0.6 (0.4-0.9)‡ | 0.8 (0.6-1.1)2-153 |
| Risk factors . | Overall survival RR (95% CI) . | Event-free survival RR (95% CI) . |
|---|---|---|
| Age at diagnosis, y | ||
| 1 to 10 | 1.0 | 1.0 |
| Younger than 1 | 3.8 (3.1-4.6)* | 3.3 (2.8-4.0)* |
| Older than 10 | 2.3 (2.1-2.6)* | 1.8 (1.6-1.9)* |
| Initial WBC count | ||
| Lower than 50 000/mL | 1.0 | 1.0 |
| 50 000/mL or higher | 1.7 (1.5-1.9)* | 1.4 (1.2-1.5)* |
| Initial platelet count | ||
| 50 000/mL or higher | 1.0 | 1.0 |
| Less than 50 000/mL | 1.0 (0.9-1.1)2-153 | 1.1 (1.0-1.2)‡ |
| Sex | ||
| Female | 1.0 | 1.0 |
| Male | 1.1 (1.0-1.3)† | 1.2 (1.1-1.3)* |
| Liver size | ||
| Normal | 1.0 | 1.0 |
| Enlarged | 1.1 (0.9-1.2)2-153 | 1.2 (1.0-1.3)† |
| Spleen size | ||
| Normal | 1.0 | 1.0 |
| Enlarged | 1.3 (1.2-1.4)* | 1.1 (1.0-1.3)‡ |
| Era | ||
| Recent | 1.0 | 1.0 |
| Early | 1.4 (1.3-1.6)* | 1.5 (1.4-1.6)* |
| Ethnicity | ||
| White | 1.0 | 1.0 |
| Black | 1.4 (1.1-1.6)* | 1.4 (1.2-1.7)* |
| Hispanic | 1.4 (1.2-1.6)* | 1.3 (1.2-1.5)* |
| Asian | 0.6 (0.4-0.9)‡ | 0.8 (0.6-1.1)2-153 |
P .001.
.001 < P .01.
.01 < P < .05.
P = NS.
Stratified analysis
Risk groups.
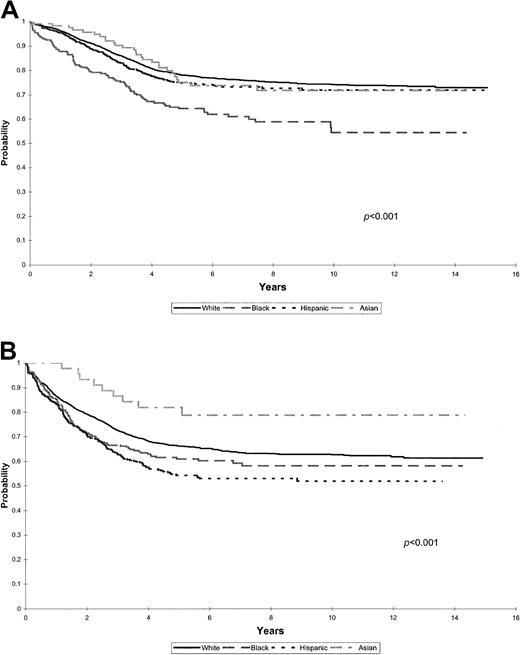
The cohort was stratified into standard- and high-risk groups to further assess the impact of racial and ethnic groups on EFS in the 2 risk categories (Figure 3A-B; Table3).
Risk groups.
(A) Kaplan-Meier estimates of EFS for a cohort of 5127 children with ALL with standard-risk features at presentation, according to racial and ethnic distribution. (B) Kaplan-Meier estimates of EFS for a cohort of 3320 children with ALL with high-risk features at presentation according to racial and ethnic distribution.
Risk groups.
(A) Kaplan-Meier estimates of EFS for a cohort of 5127 children with ALL with standard-risk features at presentation, according to racial and ethnic distribution. (B) Kaplan-Meier estimates of EFS for a cohort of 3320 children with ALL with high-risk features at presentation according to racial and ethnic distribution.
Risk factors for EFS according to risk group: multivariate analysis
| Risk factors . | Standard risk RR (95% CI) . | High risk RR (95% CI) . |
|---|---|---|
| Age at diagnosis, y | ||
| 1-10 | 1.0 | 1.0 |
| Younger than 1 | — | 3.3 (2.7-4.1)3-150 |
| Older than 10 | — | 1.8 (1.5-2.1)3-150 |
| Initial WBC count | ||
| Lower than 50 000/mL | 1.0 | 1.0 |
| 50 000/mL or higher | — | 1.4 (1.2-1.7)3-150 |
| Initial platelet count | ||
| Higher than 50 000/mL | 1.0 | 1.0 |
| Lower than 50 000/mL | 1.2 (1.0-1.3)3-152 | 1.1 (0.9-1.2)3-153 |
| Sex | ||
| Females | 1.0 | 1.0 |
| Males | 1.2 (1.0-1.3)3-151 | 1.2 (1.0-1.3)3-151 |
| Liver size | ||
| Normal | 1.0 | 1.0 |
| Enlarged | 1.2 (1.0-1.3)3-152 | 1.2 (1.1-1.4)3-151 |
| Spleen size | ||
| Normal | 1.0 | 1.0 |
| Enlarged | 1.1 (1.0-1.3)3-152 | 1.1 (0.9-1.3)3-153 |
| Ethnicity | ||
| White | 1.0 | 1.0 |
| Black | 2.0 (1.6-2.5)3-150 | 1.2 (0.9-1.4)3-153 |
| Hispanic | 1.2 (0.9-1.4)3-153 | 1.4 (1.2-1.6)3-150 |
| Asian | 1.0 (0.7-1.5)3-153 | 0.6 (0.4-1.1)3-153 |
| Era | ||
| Recent | 1.0 | 1.0 |
| Early | 1.6 (1.4-1.7)3-150 | 1.4 (1.3-1.6)3-150 |
| Risk factors . | Standard risk RR (95% CI) . | High risk RR (95% CI) . |
|---|---|---|
| Age at diagnosis, y | ||
| 1-10 | 1.0 | 1.0 |
| Younger than 1 | — | 3.3 (2.7-4.1)3-150 |
| Older than 10 | — | 1.8 (1.5-2.1)3-150 |
| Initial WBC count | ||
| Lower than 50 000/mL | 1.0 | 1.0 |
| 50 000/mL or higher | — | 1.4 (1.2-1.7)3-150 |
| Initial platelet count | ||
| Higher than 50 000/mL | 1.0 | 1.0 |
| Lower than 50 000/mL | 1.2 (1.0-1.3)3-152 | 1.1 (0.9-1.2)3-153 |
| Sex | ||
| Females | 1.0 | 1.0 |
| Males | 1.2 (1.0-1.3)3-151 | 1.2 (1.0-1.3)3-151 |
| Liver size | ||
| Normal | 1.0 | 1.0 |
| Enlarged | 1.2 (1.0-1.3)3-152 | 1.2 (1.1-1.4)3-151 |
| Spleen size | ||
| Normal | 1.0 | 1.0 |
| Enlarged | 1.1 (1.0-1.3)3-152 | 1.1 (0.9-1.3)3-153 |
| Ethnicity | ||
| White | 1.0 | 1.0 |
| Black | 2.0 (1.6-2.5)3-150 | 1.2 (0.9-1.4)3-153 |
| Hispanic | 1.2 (0.9-1.4)3-153 | 1.4 (1.2-1.6)3-150 |
| Asian | 1.0 (0.7-1.5)3-153 | 0.6 (0.4-1.1)3-153 |
| Era | ||
| Recent | 1.0 | 1.0 |
| Early | 1.6 (1.4-1.7)3-150 | 1.4 (1.3-1.6)3-150 |
P < .001.
.001 < P .01.
.01 < P < .05.
P = NS.
Standard-risk group.
Figure 3A shows EFS among patients who met the criteria for standard-risk group categorization at presentation of ALL (n = 5127), showing a significant difference by race and ethnicity (P < .001). Five-year EFS rates for white, Asian, and Hispanic children ranged between 75% and 78%, with significantly lower EFS rates for black children at 64%. Multivariate analysis adjusted for treatment era revealed that black children had significantly worse outcomes than white children (relative risk [RR], 2.0; 95% confidence interval [CI], 1.6-2.5) (Table 3). Outcomes for Hispanic (RR, 1.2; 95% CI, 0.9-1.4) and Asian (RR, 1.0; 95% CI, 0.7-1.5) children with standard-risk features did not differ significantly from those for white children.
High-risk group.
Figure 3B shows EFS for patients with high-risk features at presentation (n = 3320). Asian children had significantly better outcomes, with estimated EFS at 5 years of 75% compared with 64% for white, 59% for black, and 53% for Hispanic children (P < .001). Multivariate analysis adjusted for treatment era found that among patients with high-risk features, Hispanic children had significantly worse outcomes than white children (RR, 1.4; 95% CI, 1.2-1.6). Asian children had the best outcomes (RR, 0.6; 95% CI, 0.4-1.1), whereas outcomes for black children did not differ significantly from those for white children (RR, 1.2; 95% CI, 0.9-1.4) (Table 3).
Treatment era.
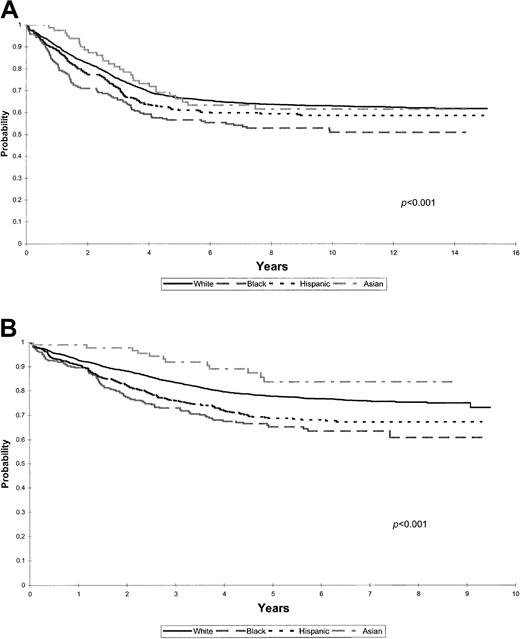
The cohort was stratified into early and recent treatment eras to further assess the effect of racial and ethnic groups on EFS among patients treated with contemporary risk-based therapy. (Figure4A-B).
Treatment era.
(A) Kaplan-Meier estimates of EFS for a cohort of 3545 children with ALL treated between 1983 and 1989 (early era) according to racial and ethnic distribution. (B) Kaplan-Meier estimates of EFS for a cohort of 4902 children with ALL treated between 1989 and 1995 (recent era) according to racial and ethnic distribution.
Treatment era.
(A) Kaplan-Meier estimates of EFS for a cohort of 3545 children with ALL treated between 1983 and 1989 (early era) according to racial and ethnic distribution. (B) Kaplan-Meier estimates of EFS for a cohort of 4902 children with ALL treated between 1989 and 1995 (recent era) according to racial and ethnic distribution.
Early era.
There was a significant difference in EFS (P < .001) among various racial and ethnic groups of patients treated before 1989 (Figure 4A). The 5-year EFS rates were 67% for white and Asian, 61% for Hispanic, and 57% for black children.
Recent era. Patients treated after 1989 (Figure 4B) continue to have significant differences in EFS rate by ethnicity (P < .001). Five-year EFS rates are 84% for Asian, 78% for white, 69% for Hispanic, and 65% for black children.
Risk group and treatment era
Table 4 summarizes EFS by NCI risk groups (standard vs high risk) and treatment era (early vs recent) among the 4 racial and ethnic groups studied. Among patients with standard-risk features across both treatment eras, white children had the best and the black children had the worse outcomes. On the other hand, among patients with high-risk features, Asian children had the best outcomes and Hispanic children had the worse outcomes. The most marked differences in EFS were evident among patients with high-risk ALL treated on recent therapeutic protocols, with 5-year EFS rates of 91% for Asian, 70% for white, 62% for black, and 54% for Hispanic (P < .001) children.
EFS by race and ethnicity, according to risk group and treatment era
| Race, ethnicity . | Event-free survival ± SE (5 y) . | |||
|---|---|---|---|---|
| Standard risk, % . | High risk, % . | |||
| Early era (n = 2118) . | Recent era (n = 3009) . | Early era (n = 1426) . | Recent era (n = 1894) . | |
| White | 72.5 ± 1.1 | 82.6 ± 0.8 | 57.2 ± 1.5 | 69.5 ± 1.2 |
| Black | 57.9 ± 5.1 | 68.9 ± 4.3 | 55.6 ± 4.7 | 61.5 ± 4.1 |
| Hispanic | 69.1 ± 3.2 | 77.9 ± 2.2 | 50.7 ± 3.9 | 53.8 ± 3.3 |
| Asian | 68.5 ± 6.8 | 80.8 ± 5.7 | 62.6 ± 8.9 | 90.9 ± 6.1 |
| P | .001 | < .001 | .4 | < .001 |
| Race, ethnicity . | Event-free survival ± SE (5 y) . | |||
|---|---|---|---|---|
| Standard risk, % . | High risk, % . | |||
| Early era (n = 2118) . | Recent era (n = 3009) . | Early era (n = 1426) . | Recent era (n = 1894) . | |
| White | 72.5 ± 1.1 | 82.6 ± 0.8 | 57.2 ± 1.5 | 69.5 ± 1.2 |
| Black | 57.9 ± 5.1 | 68.9 ± 4.3 | 55.6 ± 4.7 | 61.5 ± 4.1 |
| Hispanic | 69.1 ± 3.2 | 77.9 ± 2.2 | 50.7 ± 3.9 | 53.8 ± 3.3 |
| Asian | 68.5 ± 6.8 | 80.8 ± 5.7 | 62.6 ± 8.9 | 90.9 ± 6.1 |
| P | .001 | < .001 | .4 | < .001 |
Pairwise comparisons
Pairwise comparisons of EFS rates by ethnicity across risk groups and treatment eras are summarized in Table5.
Pair-wise comparison of EFS by ethnicity, according to treatment era, risk group
| . | Event-free survival ± SE (5 y) . | ||||
|---|---|---|---|---|---|
| Entire cohort . | Early era . | Recent era . | Standard risk . | High risk . | |
| Black, % | 62 ± 2.2 | 57 ± 3.5 | 65 ± 2.9 | 64 ± 3.3 | 61 ± 3.2 |
| White, % | 73 ± 0.6 | 67 ± 0.9 | 78 ± 0.7 | 78 ± 0.7 | 66 ± 1 |
| RR | 1.65-150 | 1.55-150 | 1.75-150 | 1.95-150 | 1.25-153 |
| Hispanic, % | 66 ± 1.5 | 61 ± 2.7 | 69 ± 1.9 | 75 ± 1.8 | 54 ± 2.6 |
| White, % | 73 ± 0.6 | 67 ± 0.9 | 78 ± 0.7 | 78 ± 0.7 | 66 ± 1 |
| RR | 1.35-153 | 1.25-153 | 1.55-150 | 1.25-154 | 1.45-150 |
| Asian, % | 75 ± 3.6 | 66 ± 5.4 | 84 ± 4.4 | 75 ± 4.4 | 82 ± 5.8 |
| White, % | 73 ± 0.6 | 67 ± 0.9 | 78 ± 0.7 | 78 ± 0.7 | 66 ± 1 |
| RR | 0.85-153 | 1.05-154 | 0.65-153 | 1.05-154 | 0.55-152 |
| Black, % | 62 ± 2.2 | 57 ± 3.5 | 65 ± 2.9 | 64 ± 3.3 | 61 ± 3.2 |
| Hispanic, % | 66 ± 1.5 | 61 ± 2.5 | 69 ± 1.9 | 75 ± 1.8 | 54 ± 2.6 |
| RR | 1.25-152 | 1.35-154 | 1.25-154 | 1.75-150 | 0.95-154 |
| Black, % | 62 ± 2.2 | 57 ± 3.5 | 65 ± 2.9 | 64 ± 3.3 | 61 ± 3.2 |
| Asian, % | 75 ± 3.6 | 66 ± 5.4 | 84 ± 4.4 | 75 ± 4.4 | 82 ± 5.8 |
| RR | 1.95-150 | 1.55-152 | 3.05-150 | 1.95-151 | 2.45-151 |
| Hispanic, % | 66 ± 1.5 | 62 ± 2.5 | 69 ± 1.9 | 75 ± 1.8 | 54 ± 2.6 |
| Asian, % | 75 ± 3.6 | 66 ± 5.4 | 84 ± 4.4 | 75 ± 4.4 | 82 ± 5.8 |
| RR | 1.65-151 | 1.25-154 | 2.55-150 | 1.15-154 | 2.95-150 |
| . | Event-free survival ± SE (5 y) . | ||||
|---|---|---|---|---|---|
| Entire cohort . | Early era . | Recent era . | Standard risk . | High risk . | |
| Black, % | 62 ± 2.2 | 57 ± 3.5 | 65 ± 2.9 | 64 ± 3.3 | 61 ± 3.2 |
| White, % | 73 ± 0.6 | 67 ± 0.9 | 78 ± 0.7 | 78 ± 0.7 | 66 ± 1 |
| RR | 1.65-150 | 1.55-150 | 1.75-150 | 1.95-150 | 1.25-153 |
| Hispanic, % | 66 ± 1.5 | 61 ± 2.7 | 69 ± 1.9 | 75 ± 1.8 | 54 ± 2.6 |
| White, % | 73 ± 0.6 | 67 ± 0.9 | 78 ± 0.7 | 78 ± 0.7 | 66 ± 1 |
| RR | 1.35-153 | 1.25-153 | 1.55-150 | 1.25-154 | 1.45-150 |
| Asian, % | 75 ± 3.6 | 66 ± 5.4 | 84 ± 4.4 | 75 ± 4.4 | 82 ± 5.8 |
| White, % | 73 ± 0.6 | 67 ± 0.9 | 78 ± 0.7 | 78 ± 0.7 | 66 ± 1 |
| RR | 0.85-153 | 1.05-154 | 0.65-153 | 1.05-154 | 0.55-152 |
| Black, % | 62 ± 2.2 | 57 ± 3.5 | 65 ± 2.9 | 64 ± 3.3 | 61 ± 3.2 |
| Hispanic, % | 66 ± 1.5 | 61 ± 2.5 | 69 ± 1.9 | 75 ± 1.8 | 54 ± 2.6 |
| RR | 1.25-152 | 1.35-154 | 1.25-154 | 1.75-150 | 0.95-154 |
| Black, % | 62 ± 2.2 | 57 ± 3.5 | 65 ± 2.9 | 64 ± 3.3 | 61 ± 3.2 |
| Asian, % | 75 ± 3.6 | 66 ± 5.4 | 84 ± 4.4 | 75 ± 4.4 | 82 ± 5.8 |
| RR | 1.95-150 | 1.55-152 | 3.05-150 | 1.95-151 | 2.45-151 |
| Hispanic, % | 66 ± 1.5 | 62 ± 2.5 | 69 ± 1.9 | 75 ± 1.8 | 54 ± 2.6 |
| Asian, % | 75 ± 3.6 | 66 ± 5.4 | 84 ± 4.4 | 75 ± 4.4 | 82 ± 5.8 |
| RR | 1.65-151 | 1.25-154 | 2.55-150 | 1.15-154 | 2.95-150 |
P ≤ .001.
.001 < P .01.
0.01 < P .05.
.05 < P < .1.
P = NS.
Overall, black children had poorer EFS rates than Asian (RR, 1.9;P < .001), white (RR, 1.6; P < .001), and Hispanic (RR, 1.2; P = .02) children. The worse outcome for black children was most apparent among patients with standard-risk features.
Pairwise comparison of Hispanic and white children (RR, 1.3;P < .001) and Asian children (RR, 1.6;P = .007) showed worse outcomes for Hispanic children that were still better than those for black children (RR, 0.8;P = .02). The worse outcomes for Hispanic children compared individually with those for white and Asian children were most apparent among patients with high-risk features.
Asian children had significantly better outcomes than black (RR, 0.5;P < .001) and Hispanic (RR, 0.6; P = .007) children. There was no statistically significant difference in EFS rate between Asian and white children overall, but Asian children with high-risk features did significantly better than white children (RR, 0.5; P = .03) with high-risk features.
Subset analysis
Lineage.
Information on the lineage of lymphoblasts (B or T cell) was available for 4722 patients. There was overrepresentation of black children (25%) with T-cell phenotypes compared with Asian (19%), white (15%), and Hispanic (13%) children (P < .001). Examination of single-antigen expression (CD10, CD19, CD24, CD2, CD3, CD5, and CD7) found no difference in distribution by ethnicity.
Multivariate analysis revealed race and ethnicity to be independently associated with EFS (blacks: RR, 1.3, 95% CI, 1.0-1.6; Hispanics: RR, 1.3, 95% CI, 1.2-1.6; Asians: RR, 0.9, 95% CI, 0.6-1.3) after adjusting for lineage (T-cell leukemia: RR, 1.0; 95% CI, 0.9-1.2), high initial leukocyte count (RR, 1.3; 95% CI, 1.2-1.5), and age at diagnosis (younger than 1 year: RR, 3.2, 95% CI, 2.5-4.1; older than 10 years: RR, 1.8, 95% CI, 1.5-2.0).
Chromosomal abnormalities.
Information on ploidy was available for 1872 patients. Multivariate analysis revealed ethnicity (blacks: RR, 1.5, 95% CI, 1.2-1.8; Hispanics: RR, 1.5, 95% CI, 1.2-1.8; Asians: RR, 0.6, 95% CI, 0.4-1.1) to be independently associated with EFS, even after adjusting for ploidy (hypodiploidy: RR, 2.1, 95% CI, 1.6-2.8; pseudodiploidy: RR, 1.2, 95% CI, 1.0-1.5; low hyperdiploidy [47-50 chromosomes]: RR, 1.5, 95% CI, 1.1-1.9; high initial leukocyte count: RR, 1.6, 95% CI, 1.4-1.8) and age at diagnosis (younger than 1 year: RR, 3.8, 95% CI, 3.0-4.9; older than 10 years: RR, 1.7, 95% CI, 1.5-2.0).
Information on structural chromosomal abnormality was available for 1872 patients. Multivariate analysis again revealed that ethnicity was independently associated with EFS (blacks: RR, 1.4, 95% CI, 1.2-1.8; Hispanics: RR, 1.5, 95% CI, 1.3-1.7; Asians: RR, 0.6, 95% CI, 0.4-1.1) even after adjusting for structural chromosomal abnormalities (t(4;11): RR, 3.1, 95% CI, 2.1-4.6; t(9;22): RR, 4.5, 95% CI, 3.2-6.5), high initial leukocyte count (RR, 1.6, 95% CI 1.4-1.8), and age at diagnosis (younger than 1 year: RR, 3.3, 95% CI, 2.6-4.3; older than 10 years: RR, 1.8, 95% CI, 1.6-2.0).
Socioeconomic status.
Information on parental education and income was available for 1596 patients. Multivariate analysis revealed the following association between ethnicity and EFS (blacks: RR, 1.7, 95% CI, 1.2-2.4; Hispanics: RR, 1.0, 95% CI, 0.7-1.6; Asians: RR, 0.5, 95% CI, 0.2-1.5) even after adjusting for maternal education (high school education or less: RR, 0.9, 95% CI, 0.8-1.2), paternal education (high school education or less: RR, 1.2, 95% CI, 0.9-1.5), and annual household income ($30 000 or less: RR, 1.0, 95% CI, 0.8-1.3).
Discussion
Overall, childhood ALL is associated with excellent outcomes.2,3 The improvement in survival achieved in the last 3 decades is attributed partially to the identification of risk factors that predict poor outcomes and risk-stratified treatment of patients placed on well-designed therapeutic trials.2Accordingly, it is important to continue to identify patient subgroups with differences in outcomes to focus efforts to improve overall survival. Black children historically have been reported to have poorer survival rates than white children, but limited information is available for children from other racial and ethnic backgrounds.7-13
We studied OS and EFS rates by ethnic and racial groups for all children with newly diagnosed ALL treated since 1983 according to therapeutic protocols developed by CCG. Analysis of this cohort of 8447 children took into consideration secular changes in outcome for ALL and the potential impact of predictors of outcome, including clinical features and disease biology.
Our results indicated that black children had poorer outcomes than white children. Outcomes for Hispanic children were intermediate between those of white and black children, whereas Asian children had outcomes better than all other racial and ethnic groups. Among patients with standard-risk features, black children had significantly worse outcomes, whereas outcomes for Hispanic and Asian children did not differ significantly from those for white children. Among patients with high-risk features, Hispanic children had significantly worse outcomes than white children and Asian children had the best outcomes, whereas outcomes for blacks did not differ significantly from those for white children. Thus, our report suggests that there are ethnic differences in outcome, but the ethnic groups responded differently in the 2 risk groups, thus raising issues regarding cause(s) underlying differences in survival.
Our results were consistent with those of a recent report that showed inferior survival of black and Hispanic children in a cohort of 5086 children with ALL treated on Pediatric Oncology Group (POG) therapeutic protocols.32 The disparity in survival by ethnicity in the POG cohort could not be explained by differences in clinical presentation, tumor biology, or surrogate measures of compliance, and the authors of the POG study speculated that the differences possibly were related to variations in response to therapy.32However, several aspects of that report differ from the current study. First, the POG cohort did not assess survival among Asians as a separate ethnic group. Second, data on socioeconomic status was not collected; therefore, the authors could not assess the effect of socioeconomic status on survival by ethnicity.
To consider improved survival rates over time for ALL, we stratified our cohort into early and recent treatment eras. In addition, in contrast to a recent report from St Jude Hospital,14 there continue to be significant survival differences by race and ethnicity for patients treated in both eras. The median follow-up of patients treated in the recent era in our study was 5.1 years. Recurrence after 5 years is rare among patients who have received contemporary therapy.33 Thus, we believe that additional follow-up of the recent era is unlikely to alter this finding significantly. However, a possible reason for the difference in results of the current study from that reported by St Jude Hospital could be the difference in treatment protocols used in the 2 study populations. The current study reports a nonsignificant difference in EFS between black and white children among the high-risk patients, similar to that reported by St Jude Hospital in the recent era. This suggests that more intense therapy (given to high-risk patients in the current study) could possibly overcome the ethnic or racial differences in bioavailability or intrinsic resistance of the leukemic cells.
Age at diagnosis of ALL and leukocyte count at presentation have been identified as the most important predictors of disease-free survival.30 Abnormalities in chromosomal number and structure also have been reported as prognostically significant.34-37 Other presenting variables have been of little value as treatment has improved.38 Our study confirms previous reports9-11 that black children are more likely to be diagnosed with poor-risk features such as high WBC counts and older age at diagnosis. However, within our large study population, multivariate analysis found that, after adjusting for all known adverse factors, including lineage and chromosomal abnormalities, race and ethnicity remained significant independent predictors of outcome. Comparison of the day 7 marrow results by ethnicity revealed statistically significant differences in the results, with significantly more Asian children achieving M1 marrow than black, white, or Hispanic children. However, pairwise comparison of the day 7 marrow results revealed that statistically significant differences were obvious only between Hispanic and white children. Thus, it seems that if the day 7 marrow results were used as surrogate markers of differences in disease biology by ethnicity and race, the results might have indicated that Hispanic children had intrinsically more aggressive disease.
The influence of race and ethnicity on survival is closely linked with socioeconomic status. Early diagnosis, ready access to quality health care, and sufficient time and energy to maintain compliance with treatment all are linked closely with socioeconomic status and ethnicity—hence survival—as shown in studies of adult mortality for major disease categories.39 Information on certain socioeconomic indices such as parental education and annual household income was available for 1596 patients. There was an overrepresentation of black and Hispanic patients among the low parental education and low-income categories. However, inclusion of those variables in the multivariate model did not alter the worse outcomes for black compared with white children. There was no significant difference in outcomes of Hispanic and Asian children compared with whites after adjusting for socioeconomic status. All patients in the current study were treated in the context of clinical trials from the time of diagnosis, thus ensuring uniform therapeutic guidelines across all ethnic or racial groups, dictated by risk categorization of patients with ALL.
A comparison of compliance with long-term follow-up by ethnicity revealed significant differences in the completeness of follow-up. Date of last contact was within 2 years of the study analysis for 75% of the white, 69% of the Asian, 67% of the Hispanic, and 61% of the black children. These differences in compliance with long-term follow-up may serve as a surrogate marker for differences in compliance with therapy, hence outcome, by ethnicity and race.
Unlike solid tumors or acute myeloid leukemia, treatment of ALL includes a maintenance phase composed of oral administration of antimetabolites (6-mercaptopurine [6MP] and methotrexate) for prolonged periods. Previous studies have shown that low systemic exposure to oral 6MP during maintenance therapy adversely affects prognosis.40 It is speculated that genetic differences in metabolism and bioavailability of those drugs might explain the ethnic influence on treatment outcome. Genetic differences in metabolism of 6MP and methotrexate have been shown. For example, methotrexate effects are greater in children with trisomy 21.41 6MP is more active in patients with genetic deficiency of thiopurine methyl transferase (TPMT), an enzyme involved in its detoxification, and lower activity is associated with better outcome.42,43 The frequency and distribution of mutant alleles in different ethnic populations have been described, with differences reported between white, black, and Asian populations.44,45 These ethnic differences may be important in the clinical use of thiopurine drugs and the differences observed in outcome. Alternatively, ethnic differences in other enzymes, such as CYP3A4, responsible for the metabolism of drugs such as alkylating agents and vinca alkaloids, could account for the observed differences in survival.46
This study had a number of strengths. The large cohort gave us the opportunity to explore OS and EFS in children with ALL among various racial and ethnic groups, in addition to confirming previously described differences between whites and blacks. Treatment of patients according to CCG therapeutic protocols allowed us access to data on clinical and biologic factors that could influence outcome and centralized follow-up of the large cohort.
Several limitations to our report must be discussed. We relied on self-report by parents at the time of admission to the primary treating institution for information on their racial and ethnic backgrounds. The data were abstracted from the medical records and reported to the CCG Operations Office at the time of registration on therapeutic protocols. This method of collecting information on racial and ethnic backgrounds, albeit not perfect, was not unlike that used by other sources that report incidence and outcome, such as Surveillance, Epidemiology and End Results,13 and regional and state cancer registries.
An attempt was made to adjust for the influence of socioeconomic status on outcome by race and ethnicity. However, the analysis was limited by the fact that data on socioeconomic status were available for a small proportion of the study population studied and might not have been generalizable to the entire population.
Although all our patients were treated according to therapeutic protocols based on their eligibility criteria, we do not have information on the exact drug doses received by the patients while on study, which could have influenced their outcomes. Moreover, though the large cohort facilitated discerning differences in outcomes among ethnic groups, highly significant statistical differences for modest increases in risk were driven to some degree by the large cohort.
There was a disproportionate representation of patients from different ethnic groups treated on CCG therapeutic protocols in our cohort. Thirty-six percent of the black children with newly diagnosed ALL were enrolled on therapeutic studies compared with 60% of white children. On the other hand, 74% of Hispanic children and 51% of Asian children were enrolled on therapeutic studies. The proportion of patients enrolled by ethnicity does not necessarily reflect overall performance—that is, a larger proportion of enrollment among Hispanics did not result in better outcomes. On the contrary, a smaller proportion of enrollment by Asians (51%) was not reflected by inferior outcome among them. There could be many reasons for this disproportionate distribution of patients, among them ethnic beliefs and socioeconomic status. The reasons for disproportionate entry in therapeutic protocols are not maintained at treating institutions. Therefore, it is difficult to control for those factors because there is no centralized follow-up available for patients not on study. Our on-study cohort did reflect racial and ethnic differences in risk group distribution reported by others,9-11 suggesting that our on-study cohort possibly represents an unbiased sample. Moreover, among patients placed on study, adjustment for all known prognostic factors did not alter the effect of ethnicity on EFS. Therefore, we believe that differential participation in therapeutic trials did not necessarily explain differences in survival.
It is clear that survival rates have improved across all ethnic groups with recent therapy, but racial and ethnic differences continue in OS and EFS among children with ALL who received contemporary risk-based therapy. Improvement has been most dramatic for Asian children, especially among patients with high-risk prognostic features treated in the recent era. The poorer outcomes in black children are most apparent among patients with standard-risk features. Poorer outcomes in Hispanic children are most evident among patients with high-risk features. Future studies must focus on reasons for these differences, including racial and ethnic differences in compliance with therapeutic protocols, and for ethnic differences in drug metabolism and bioavailability of agents commonly used in ALL so that drug administration can be modified if needed.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2002-02-0395.
Supported in part by National Institutes of Health grants 5U10 CA13539-2652.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Smita Bhatia, Children's Oncology Group, City of Hope National Medical Center, PO Box 60012, Arcadia, CA 91006-6012; e-mail: sbhatia@coh.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal