We read with great interest the report of Latagliata et al1 on therapy-related myelodysplastic syndrome (t-MDS) and acute myelogenous leukemia (t-AML) following treatment of acute promyelocytic leukemia (APL). The authors observed 5 out of 77 patients with APL, who after intensive chemotherapy including all-trans retinoic acid (ATRA) relapsed with MDS or AML while in molecular remission of APL. Before relapse progressive cytopenia was observed in all 5 patients and at relapse, a phase of MDS was verified in 4. Three of the patients presented new, cytogenetically unrelated clones. The authors conclude that all 5 cases are induced by idarubicin, mitoxantrone, and etoposide used as induction therapy or by alkylating agents used before autologous stem cell transplantation in 2 patients.
The chemotherapy regimen administered as frontline therapy for APL included 3 courses of idarubicin with a total cumulative dose of 80 mg/m2 and one single course of mitoxantrone and etoposide, all topoisomerase II inhibitors, plus cytosine-arabinoside, plus ATRA. Maintenance treatment was methotrexate plus 6-mercaptopurine, and in some instances ATRA. This regimen is not suspected to be extremely leukemogenic. Cases of relapsing APL were reinduced with mitoxantrone and cytosine-arabinoside, were conditioned with alkylating agents, and received an autologous stem cell transplantation. Following such a procedure, the risk of t-MDS and t-AML has been shown mainly to depend on the cumulative dose of alkylating agents and topoisomerase II inhibitors administered as primary therapy before transplantation.2 The 2 patients with t-MDS or t-AML receiving autologous stem cell transplantation for APL were apparently not heavily pretreated.
Previously, close associations have been observed between therapy with alkylating agents and development of t-MDS with deletions or loss of 5q or 7q; and between therapy with topoisomerase II inhibitors and development of t-AML with the recurrent balanced translocations, most often to chromosome bands 11q23 and 21q22. Results from our studies of 49 consecutive cases of t-MDS or t-AML diagnosed in 7 closely followed cohorts of patients receiving intensive chemotherapy3 are shown in Table 1.
Type of chromosome aberration and type of previously administered chemotherapy in 49 consecutive cases of t-MDS and t-AML from the Copenhagen series3
| . | Alkylating agents without topoisomerase II inhibitors . | Topoisomerase II inhibitors + alkylating agents or cisplatin . | Significance by χ2 test . |
|---|---|---|---|
| Deletion or loss of 5q or 7q, monosomy 5 or monosomy 7 | 20/24 | 12/25 | .02 |
| Balanced translocations to chromosome band 11q23 or 21q22 | 0/24 | 12/25 | .0001 |
| . | Alkylating agents without topoisomerase II inhibitors . | Topoisomerase II inhibitors + alkylating agents or cisplatin . | Significance by χ2 test . |
|---|---|---|---|
| Deletion or loss of 5q or 7q, monosomy 5 or monosomy 7 | 20/24 | 12/25 | .02 |
| Balanced translocations to chromosome band 11q23 or 21q22 | 0/24 | 12/25 | .0001 |
In the study by Latagliata et al1 none of the 5 relapsing patients presented any of the recurrent balanced chromosome abnormalities characteristic of t-AML following therapy with topoisomerase II inhibitors. Furthermore, the patient relapsing with monosomy 7 had not received alkylating agents. This raises doubt about the origin of the relapses of MDS or AML, at least in 4 of the 5 patients discussed, and questions the postulated extremely high risk of t-MDS and t-AML after therapy of APL.
Relapse of leukemia, for instance AML, with a cytogenetically unrelated clone is a well-described phenomenon.4,5 Previously, authors have been very cautious to classify such cases as therapy-induced. Rather, a shift of karyotype with the same underlying stem cell disease has been considered. This possibility was emphasized in one of the reports on MDS following successful therapy of APL.6 A similar phenomenon with development of unrelated clones, sometimes also with loss of 5q or 7q, has been observed following therapy of CML with interferon alpha7 or imatinib mesylate8 without any previous chemotherapy. Gene-based therapy such as ATRA or imatinib mesylate may facilitate a complete shift of karyotype in myeloid malignancies with a common underlying stem cell abnormality.
In the future, it is recommended that cases of MDS and AML, as described by Latagliata et al, are considered as therapy-induced, only if molecular studies have ascertained an independent origin.
Independent clonal origin of therapy-related MDS-AML developing after treatment of acute promyelocytic leukemia
We thank Drs Andersen and Pedersen-Bjergaard for their interesting comments about our recently reported article on secondary (therapy-related) myelodysplastic syndrome–acute myelogenous leukemia (sMDS-AML) developing after treatment for acute promyelocytic leukemia (APL).1-1 The authors outlined some presumed discrepancies between cytogenetic lesions and previous antileukemic drug exposure in our cases, as compared to those reported in the literature and observed in their own experience in postalkylating and topoisomerase II inhibitor–induced sMDS-AML. In particular, Andersen and Pedersen-Bjergaard remarked the inconsistency in our series of specific cytogenetic aberrations that should have been expected based on the previously received antileukemic agents. Based on these considerations, they raised doubts about the true nature of our MDS-AML cases and hypothesized the possible development, rather than sMDS-AML, of leukemia relapse with karyotypic shift toward a more immature phenotype, yet originating from a common stem cell with respect to the APL clone.
For a number of reasons, we believe that our cases were true sMDS-AML. First, the existence of an MDS phase preceding sAML (in 4 of 5 cases), coupled with the extremely poor response to anti-AML therapy, was rather characteristic of sAML, at least from a clinical viewpoint.1-1 In this respect, we remark that patient 2 described in our series as alive with sMDS1(Tab2)subsequently developed sAML and died recently of resistant leukemia. As for the patient with no MDS preceding sAML, at the time of sAML development this patient showed a balanced chromosome translocation t(10;11), although not apparently involving the MLLgene, as it would be predicted for topoisomerase II inhibitor–induced leukemia. We would also like to comment that, in spite of the reported statistically significant associations between sMDS-AML karyotype lesions and previous chemotherapeutic agent type, such correlations are not absolute, as clearly shown in the table reported by Andersen and Pedersen-Bjergaard.
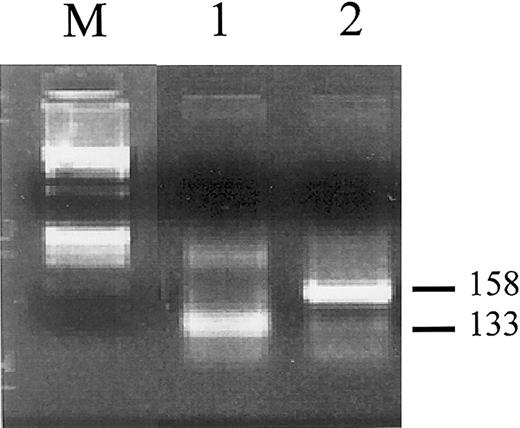
Besides these considerations, we had the possibility to better investigate the clonal relationships between the APL and the subsequent AML phase in one of our patients. In fact, marrow RNA samples from both the initial APL and subsequent AML phases (previously used forPML/RARα testing) were still available in 2 females, 1 of which (patient 2 in our series1-1) was informative (ie, heterozygous) at the X-linked iduronate-2-sulfatase (IDS)locus.1-2 After reverse transcription using random examers as primers, cDNA were amplified and the resulting fragments digested with the methylation-sensitive HpaII restriction enzyme and run on an agarose gel. As shown in Figure1-1, 2 clearly distinct fragments were amplified, indicating the existence of 2 distinct clones. Significantly, this patient was the one in our series who had not received alkylating agents for APL and yet developed sAML with monosomy 7, that is, one of the more questionable cases according to Andersen and Pedersen-Bjergaard.
Clonality analysis of leukemic cells obtained from the initial APL phase (lane 1) and subsequently developed AML phase (lane 2) in patient 2.1-1
RNA was reverse transcribed using random primers and cDNA were amplified with primers specific for the iduronate-2-sulfatase(IDS) gene as described.1-2 Amplified fragments were digested with the methylation-sensitive HpaII restriction endonuclease prior to agarose gel electrophoresis. Two differently sized bands of 133 and 158 bp are visible in lanes 1 and 2, which correspond to distinct allelic methylation patterns. M indicates molecular weight marker (ΦX 174 DNA/BsuRI).
Clonality analysis of leukemic cells obtained from the initial APL phase (lane 1) and subsequently developed AML phase (lane 2) in patient 2.1-1
RNA was reverse transcribed using random primers and cDNA were amplified with primers specific for the iduronate-2-sulfatase(IDS) gene as described.1-2 Amplified fragments were digested with the methylation-sensitive HpaII restriction endonuclease prior to agarose gel electrophoresis. Two differently sized bands of 133 and 158 bp are visible in lanes 1 and 2, which correspond to distinct allelic methylation patterns. M indicates molecular weight marker (ΦX 174 DNA/BsuRI).
On the other hand, we agree with Andersen and Pedersen-Bjergaard's comments regarding several still unsolved issues on sMDS-AML after APL, which may be the subject of future investigation. For example, the true incidence of this severe complication is not assessed in our study and deserves to be established through tailored studies analyzing the cumulative incidence of this event taking into account all competing factors (death, APL relapse, and so forth).
Finally, it remains to be clarified why sMDS-AML with alkylating agent–induced karyotypic features might occur in patients who did not receive such agents. We are prone to believe that, because anthracyclines and mitoxantrone (heavily employed in APL therapy) not only act as topoisomerase II inhibitors but are also known to intercalate the DNA strand, these drugs may result in similar DNA damage as produced by alkylating agents, thus inducing in some cases alkylating agent–type karyotypic aberrations.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal