The dendritic cell (DC)–specific molecule DC-SIGN is a receptor for the HIV-1 envelope glycoprotein gp120 and is essential for the dissemination of HIV-1. DC-SIGN is expressed by DCs, both monocyte-derived DCs and DCs in several tissues, including mucosa and lymph nodes. To identify a DC-SIGN+ DC in blood that may be involved in HIV-1 infection through blood, we have analyzed the expression of DC-SIGN in human blood cells. Here we describe the characterization of a subset of DCs in human blood, isolated from T-/NK-/B-cell–depleted peripheral blood mononuclear cells (PBMCs) on the basis of expression of DC-SIGN. This subset coexpresses CD14, CD16, and CD33 and is thus of myeloid origin. In contrast to CD14+ monocytes, DC-SIGN+ blood cells display a DC-like morphology and express markers of antigen-presenting cells, including CD1c, CD11b, CD11c, CD86, and high levels of major histocompatibility complex (MHC) class I and II molecules. This DC population differs from other described CD14−blood DC subsets. Functionally, DC-SIGN+ blood DCs are able to stimulate proliferation of allogeneic T cells and can produce tumor necrosis factor–α (TNF-α) and interleukin-6 (IL-6) upon activation with lipopolysaccharide (LPS). When they encounter HIV-1, low amounts of these blood DC-SIGN+ DCs enhance infection of T lymphocytes in trans, whereas blood monocytes and CD14−blood DCs are not capable of transmitting HIV-1. Therefore DC-SIGN+ blood DCs can be the first target for HIV-1 upon transmission via blood; they can capture minute amounts of HIV-1 through DC-SIGN and transfer HIV-1 to infect target T cells in trans.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) that can prime naive T cells. DCs in tissues capture antigens during local inflammations and migrate to lymph nodes for presentation of these antigens to T cells.1 DC-SIGN is a C-type lectin exclusively expressed by DCs that facilitates DC migration through endothelium by binding the vascular ligand ICAM-2.2 DC-SIGN also mediates interactions between DCs and resting T cells, by binding ICAM-3.3 DC-SIGN is expressed by in vitro–generated monocyte-derived DCs and by DCs in peripheral tissues and lymph nodes.3 Besides cellular ligands ICAM-2 and ICAM-3, DC-SIGN has been shown to bind to the HIV-1 envelope glycoprotein gp120.4,5 We have demonstrated that DC-SIGN plays a crucial role in the dissemination of HIV-1 from the mucosal site of entry in the periphery to T-cell areas in lymphoid tissues.5 When HIV-1 is sexually transmitted, DC-SIGN+ DCs in mucosal tissues capture HIV-1 through DC-SIGN–gp120 interactions. After migration to lymphoid organs, DCs promote efficient transinfection of T cells through DC-SIGN, resulting in a vigorous viral replication. DC-SIGN as a viral attachment receptor for primary R5, X4, and R5X4 HIV-1, HIV-2, and SIV strains has been demonstrated to enhance infection of T cells in situations where low amounts of HIV-1 do not adequately infect T cells directly.5-7 The cells that are the first target for HIV-1 when the infection is transmitted through blood have not been identified yet. We have recently identified a cell population in blood that expresses DC-SIGN2 and may play a significant role in the dissemination of low amounts of virus that enter the blood and that do not directly infect T cells.
Human peripheral blood contains several distinct subsets of precursor DCs at low frequencies (< 1%) that are en route to tissues and lymphoid organs.8-10 These subsets are of either lymphoid or myeloid origin and are thought to give rise to functionally different subsets of DCs at different anatomical sites. Lymphoid blood DCs, also called plasmacytoid DCs because of their lymphoplasmacytoid morphology, enter lymph nodes directly from the bloodstream upon inflammation and produce high amounts of interferon-α on contact with pathogens.11,12 Plasmacytoid DCs express the interleukin-3R (IL-3R) and the novel markers BDCA-2 and BDCA-4.13,14 In contrast, myeloid blood DCs are precursors of antigen-capturing tissue DCs and can be subdivided into epidermal Langerhans cell precursors, which express CD1c and CD11c, and precursors of other tissue DCs, which lack CD1c expression but express the novel markers BDCA-3 and CMRF58.9,14-17 In addition to these blood DC subsets, CD14+ blood monocytes, which constitute 10% to 20% of the peripheral blood mononuclear cells (PBMCs), can be differentiated into DCs by culturing in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4.18 Interestingly, reverse transmigration of monocytes through an endothelial cell layer resulted in a rapid differentiation into DCs, indicating that in vivo circulating blood monocytes can represent a substantial population of precursor tissue DCs.19
In recent studies, it was demonstrated that DC-SIGN is not expressed by plasmacytoid DCs, by myeloid blood precursor DC subsets, or by monocytes.3,20,21 However, these DC subsets were purified by depletion of CD14+ cells, which included monocytes. We observed that upon in vitro culture, DC-SIGN expression is rapidly induced in CD14+ monocytes, indicating that a DC-SIGN+ precursor DC might coexpress CD14.3To identify DC-SIGN+ cells in human blood as potential first targets for HIV-1 infection, an isolation method was designed that was based on previously described methods of isolating precursor DC subsets, but with the inclusion of CD14+cells.2 Using this new isolation protocol, we demonstrate here that a DC-SIGN+ cell population is indeed present at low frequency in peripheral blood. This DC-SIGN+ blood cell population represents a subset of precursor DCs that readily obtain typical DC morphology and are able to stimulate proliferation of T lymphocytes. Upon incubation with HIV-1, these blood DC-SIGN+ DCs capture HIV-1 through binding of gp120 to DC-SIGN and subsequently infect T lymphocytes in trans. Our data suggest that immature DC-SIGN+ DCs at mucosal sites facilitate HIV-1 dissemination upon sexual transmission of HIV-1, whereas blood DC-SIGN+ DCs play a key role in HIV-1 infection transmitted through blood.
Methods
Antibodies
The following antibodies were used: anti–DC-SIGN (AZN-D1), anti-CD3 (T3b), anti-CD11a (NKI-L15), anti-CD56 (NBL-1), anti–major histocompatibility complex class I (MHC-I) (W6/32), anti-CD11b (CBRM1/1) from the 5th International Leukocyte Workshop (1993), anti-CD86, and anti-CD16 (Pharmingen, San Diego, CA). IgG1 isotype control, anti-CD2 (MT910), and anti-CD33 (WM-54) were obtained from DAKO (Glostrup, Denmark); anti–HLA-DR (B8.12.2), anti-CD14 (RMO52), anti-CD20 (B9E9), and anti-CD83 (HB15A) from Immunotech (Marseille, France); anti-CD11c (leu-M5) and anti-CD80 (L307.4) from Becton Dickinson (San Jose, CA); and anti-CD1c (BDCA-1, -2, -3, and -4) from CLB (Amsterdam, The Netherlands). Antibodies were used unconjugated or conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or PE-Cy5.
Isolation of monocytes, T cells, and blood DCs and generation of immature DCs
Monocytes were isolated from PBMCs by means of MACS CD14 microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany). The negative fraction contained the peripheral blood lymphocytes (PBLs) and was further depleted of B cells and natural killer (NK) cells (by means of anti-CD20 and anti-CD56 antibodies, followed by MACS antimouse microbeads) to obtain purified T cells. Blood DCs were isolated as lineage− CD4+ cells by means of a 2-step MACS isolation kit (Miltenyi).
Immature DCs were generated by culturing monocytes in RPMI 1640/10% fetal calf serum (FCS) in the presence of IL-4 (500 U/mL) and GM-CSF (800 U/mL) for 7 days.3
Isolation of DC-SIGN+ blood cells
PBMCs were isolated from buffy coats from healthy donors (Blood Transfusion Service, Nijmegen, or Central Laboratory for Blood Transfusion, Amsterdam, The Netherlands) by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density-gradient centrifugation. T, B, and NK cells were removed by immunomagnetic depletion with anti-CD3, anti-CD20, and anti-CD56 antibodies, followed by sheep antimouse magnetic beads (Dynal, Hamburg, Germany). After staining with FITC-labeled DC-SIGN antibodies (AZN-D1), DC-SIGN–expressing cells were isolated with a Coulter EPICS Elite cell sorter (Coulter, Hialeh, FL) or FACS vantage (BD Biosciences, Franklin Lakes, NJ).
Expression levels of cell surface markers were assessed by direct immunofluorescence. Briefly, DC-SIGN+ blood cells (0.5-1.104) were incubated in phosphate-buffered saline containing 0.5% bovine serum albumin and 0.02% sodium azide for 30 minutes at 4°C with PE-labeled and PE-Cy5–labeled antibodies. The relative fluorescence intensity was measured by FACScan analysis (BD Biosciences) and analyzed with CellQuest software (BD Biosciences).
To analyze the morphology, we spun down cells on slides, using a cytocentrifuge, and stained them with hematoxylin-eosin.
T-cell proliferation assay
APCs were added to autologous or allogeneic T cells in different ratios and cultured in RPMI 1640/10% FCS. After 4 to 6 days, 0.0185 Bq (0.5 μCi) of (methyl-3H)-thymidine (Amersham Pharmacia Biotech, Uppsala, Sweden) was added to each well. Thymidine incorporation was quantified after an 18-hour pulse. As a control, T cells were cultured without APCs.
Stimulation-induced cytokine production
APCs (10 000) were cultured in 200 μL RPMI 1640/10% FCS in the presence of lipopolysaccharide (LPS, 2 μg/mL) or with autologous T cells (100 000) and purified protein derivative (PPD, 5 μg/mL, Sanbio, Uden, The Netherlands) or Candida albicans(2.5 μg/mL, ARTU Biologicals, Lelystad, The Netherlands). Supernatant was harvested at day 1 and day 4, pooled, and analyzed for the presence of TNF-α, IL-6, IL-10 (Biosource International, Camarillo, CA), and IL-12 (kind gift of Dr M. Kapsenberg) by enzyme-linked immunosorbent assay (ELISA).
HIV-1 infection assay
Cells (25 000) were incubated with 800 T-cell infectious dose (TCID50) of the M-tropic HIV-1JRCSFstrain at 37°C for 2 hours to allow binding by DC-SIGN. Specificity was determined by preincubating the cells with 20 μg/mL blocking antibodies against DC-SIGN (AZN-D1) for 20 minutes at room temperature. HIV-1–pulsed cells were cocultured with activated CD4+ T cells for several days. Culture supernatants were collected at different days and p24 antigen levels, as a measure of HIV-1 infection, were determined by an antibody-based ELISA, using anti-p24 D7320 (Aalto Bio Reagents, Dublin, Ireland) and BC1071 (Aalto). Recombinant p24 (ABL) was used to determine the concentrations of p24. PBMCs were activated by culturing in the presence of IL-2 (100 U/mL) and phytohemagglutinin (PHA) (1 μg/mL) for 2 days and CD4+ T cells were isolated with anti-CD4 magnetic Dynalbeads. As a control, CD4+ T cells alone were incubated with HIV-1.
Results
Isolation and characterization of DC-SIGN–expressing cells in human blood
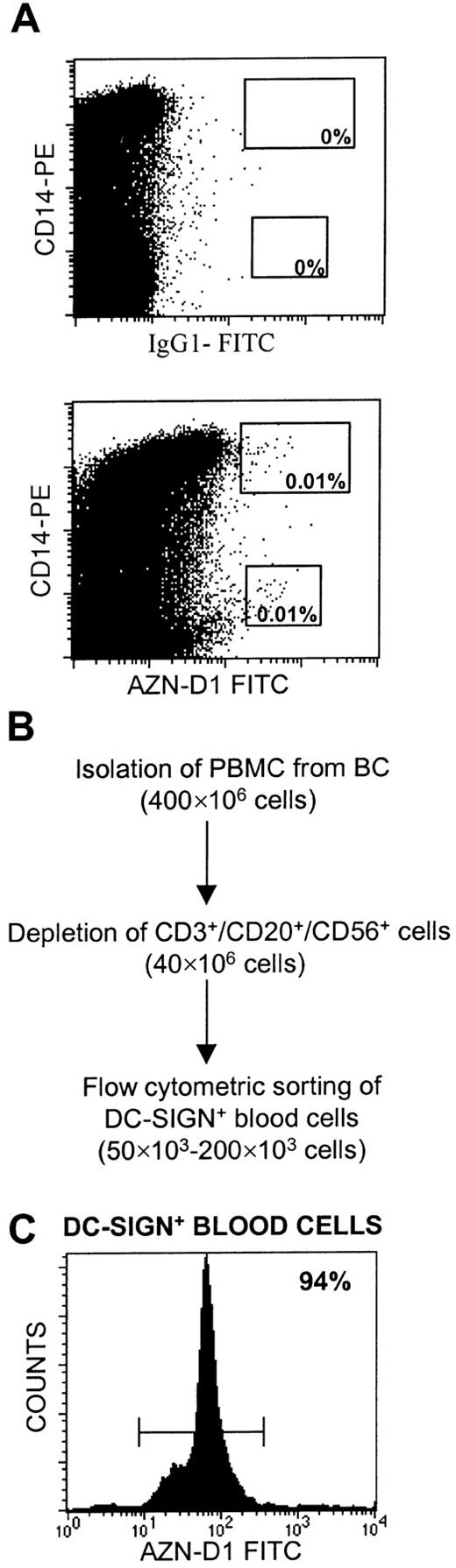
By virtue of its ability to capture HIV-1 and infect T cells in trans, DC-SIGN could be an important factor in the dissemination of HIV-1 upon infection via blood. However, recent studies demonstrated that myeloid and lymphoid precursor DC subsets in blood lack DC-SIGN expression.20,21 We set out to investigate the expression of DC-SIGN by human blood mononuclear cells. Initial experiments showed the presence of a small population of cells (0.02% of total PBMCs) that expressed DC-SIGN and that could be visualized by direct staining in human blood (Figure 1A).2Part of the DC-SIGN+ cells coexpressed CD14 (Figure 1A). An isolation method was set up to obtain larger quantities of these DC-SIGN+ blood cells, starting from PBMCs (Figure 1B). First, T, B, and NK cells were depleted. Different from earlier isolation protocols, but based on a possible coexpression of CD14 and DC-SIGN, CD14+ cells (monocytes) were not removed in this depletion step. DC-SIGN+ blood cells were obtained by purification by positive flow cytometric sorting, resulting in a cell fraction that contained at least 90% DC-SIGN+ blood cells (Figure 1C). The yield of DC-SIGN+ blood cells was between 50 000 and 200 000 cells, corresponding to 0.01% to 0.04% of the total PBMC fraction and in agreement with the percentage stained directly in total blood.
Isolation of DC-SIGN+ blood cells from PBMCs.
(A) Total blood was stained with anti-CD14–PE and anti–DC-SIGN–FITC antibodies or with IgG1 isotype control antibodies coupled to FITC. (B) PBMCs were immunomagnetically depleted of T, B, and NK cells, using anti-CD3, anti-CD20, and anti-CD56, respectively, followed by positive flow cytometric sorting of DC-SIGN+ cells stained with FITC-conjugated anti–DC-SIGN (AZN-D1) antibody. The number of cells at each isolation step is shown. (C) DC-SIGN+ blood cells were obtained with more than 90% purity, as shown by re-examination of the cell population isolated as described in panel B. A representative experiment is shown.
Isolation of DC-SIGN+ blood cells from PBMCs.
(A) Total blood was stained with anti-CD14–PE and anti–DC-SIGN–FITC antibodies or with IgG1 isotype control antibodies coupled to FITC. (B) PBMCs were immunomagnetically depleted of T, B, and NK cells, using anti-CD3, anti-CD20, and anti-CD56, respectively, followed by positive flow cytometric sorting of DC-SIGN+ cells stained with FITC-conjugated anti–DC-SIGN (AZN-D1) antibody. The number of cells at each isolation step is shown. (C) DC-SIGN+ blood cells were obtained with more than 90% purity, as shown by re-examination of the cell population isolated as described in panel B. A representative experiment is shown.
Strikingly, freshly isolated DC-SIGN+ blood cells immediately adopted a DC-like morphology with eminent cell protrusions when cells were kept at 37°C without the addition of any cytokines (Figure 2A); in contrast, monocytes adhered during this time period (not shown). We compared the morphology of DC-SIGN+ blood cells with that of monocytes and immature DCs, using hematoxylin-eosin staining of cytocentrifuge slides. Monocytes had a typical morphology, with round cells and either oval or indented nuclei (Figure 2C). In contrast, the DC-SIGN+cells were larger and frequently contained hyperlobulated nuclei (Figure 2B). Immature DCs had round nuclei and an even more extended cytoplasm (Figure 2D). Taken together, these findings establish the existence of a small but homogeneous population of DC-SIGN+cells in human blood.
DC-SIGN+ blood cells display a unique morphology.
(A) After the isolation procedure, DC-SIGN+ blood cells were allowed to recover for 2 hours at 37°C without addition of any cytokines. Note the veiledlike cytoplasmic extensions. Cytospins of DC-SIGN+ blood cells (B), monocytes (C), and immature DCs (D) were stained with hematoxylin-eosin to compare morphology. Inset shows a higher magnification. Representative cells are shown. Original magnification A, ×60; B-D, ×20; insets, ×40.
DC-SIGN+ blood cells display a unique morphology.
(A) After the isolation procedure, DC-SIGN+ blood cells were allowed to recover for 2 hours at 37°C without addition of any cytokines. Note the veiledlike cytoplasmic extensions. Cytospins of DC-SIGN+ blood cells (B), monocytes (C), and immature DCs (D) were stained with hematoxylin-eosin to compare morphology. Inset shows a higher magnification. Representative cells are shown. Original magnification A, ×60; B-D, ×20; insets, ×40.
Phenotypic analysis of DC-SIGN+ blood cells
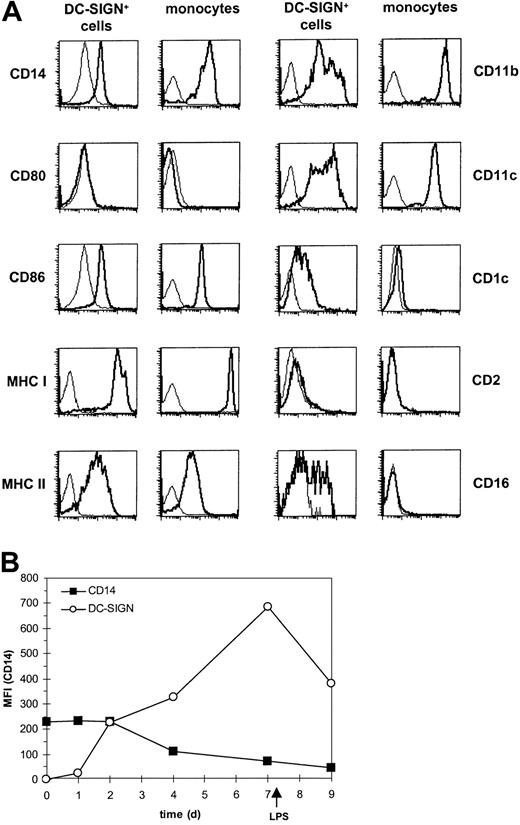
On the basis of expression of DC-SIGN and the readily adopted DC morphology, the DC-SIGN+ cells were phenotypically analyzed for other DC and APC markers and compared with blood monocytes and immature monocyte-derived DCs (Figure 3A and Table 1). As expected, the DC-SIGN+ blood cells coexpressed CD14; however, a small percentage of the DC-SIGN+ cells (with a variation between donors ranging from 1% to 10%) were negative for CD14. No major differences were observed between the CD14-positive and -negative cells in expression levels of all cell surface markers that we tested. DC-SIGN+ blood cells expressed both MHC class I and II molecules; the adhesion molecules CD11a, CD11b, and CD11c; and the costimulatory molecule CD86, whereas no expression of CD80 could be detected (Figure 3A and Table 1). The DC marker CD1c was present at low levels on the DC-SIGN+ cells but absent from blood monocytes. Other recently described markers for blood DC subsets, such as CD2 and BDCA-2, -3, and -4, were not expressed by DC-SIGN+ blood cells (Table 1). Interestingly, about 50% of DC-SIGN+ blood cells stained positive for CD16, which is expressed on a subset of monocytes with APC function.22Phenotypic comparison with both monocytes and monocyte-derived immature DCs showed that DC-SIGN+ blood cells display a unique expression pattern that seems like an intermediate cell type between monocyte and immature DC (Figure 3A and Table 1).
DC-SIGN+ blood cells express CD14, MHC, adhesion, and costimulatory molecules and differ from monocytes.
(A) DC-SIGN+ blood cells and monocytes were stained with PE-conjugated monoclonal antibodies as indicated (bold line). The thin line represents the isotype-matched control. (B) Blood monocytes were cultured in the presence of IL-4 and GM-CSF for 7 days and matured by addition of LPS for 48 hours. The expression of DC-SIGN and CD14 was analyzed at different days as indicated. Results are representative for at least 3 independent experiments.
DC-SIGN+ blood cells express CD14, MHC, adhesion, and costimulatory molecules and differ from monocytes.
(A) DC-SIGN+ blood cells and monocytes were stained with PE-conjugated monoclonal antibodies as indicated (bold line). The thin line represents the isotype-matched control. (B) Blood monocytes were cultured in the presence of IL-4 and GM-CSF for 7 days and matured by addition of LPS for 48 hours. The expression of DC-SIGN and CD14 was analyzed at different days as indicated. Results are representative for at least 3 independent experiments.
Surface phenotype of DC-SIGN+ blood cells, monocytes, and immature DCs
| Markers . | CD14+monocytes . | DC-SIGN+ blood cells . | Immature DCs . |
|---|---|---|---|
| Lineage | |||
| CD2 | 4 | 10 | 22 |
| CD14 | 105 | 81 | 15 |
| CD16 | 4 | 85* | 6 |
| CD33 | 126 | 145 | 50 |
| APCs | |||
| CD80 | 2 | 5 | 48 |
| CD86 | 104 | 154 | 404 |
| MHC I | 3308 | 1182† | 742 |
| MHC II | 41 | 121* | 1104 |
| DCs | |||
| CD83 | 3 | 2 | 34 |
| CD1c | 7 | 15* | 687 |
| BDCA-2 | 6 | 7 | 9 |
| BDCA-3 | 7 | 8 | 10 |
| BDCA-4 | 5 | 9 | 2184 |
| Adhesion | |||
| CD11a (LFA-1) | 452 | 391 | 296 |
| CD11b (MAC-1) | 1186 | 371† | 2231 |
| CD11c (P150,95) | 425 | 318 | 593 |
| HIV receptors | |||
| DC-SIGN | 4 | 73* | 708 |
| CD4 | 51 | 65 | 75 |
| CCR5 | 36 | 24 | 43 |
| CXCR4 | 68 | 84 | 54 |
| Markers . | CD14+monocytes . | DC-SIGN+ blood cells . | Immature DCs . |
|---|---|---|---|
| Lineage | |||
| CD2 | 4 | 10 | 22 |
| CD14 | 105 | 81 | 15 |
| CD16 | 4 | 85* | 6 |
| CD33 | 126 | 145 | 50 |
| APCs | |||
| CD80 | 2 | 5 | 48 |
| CD86 | 104 | 154 | 404 |
| MHC I | 3308 | 1182† | 742 |
| MHC II | 41 | 121* | 1104 |
| DCs | |||
| CD83 | 3 | 2 | 34 |
| CD1c | 7 | 15* | 687 |
| BDCA-2 | 6 | 7 | 9 |
| BDCA-3 | 7 | 8 | 10 |
| BDCA-4 | 5 | 9 | 2184 |
| Adhesion | |||
| CD11a (LFA-1) | 452 | 391 | 296 |
| CD11b (MAC-1) | 1186 | 371† | 2231 |
| CD11c (P150,95) | 425 | 318 | 593 |
| HIV receptors | |||
| DC-SIGN | 4 | 73* | 708 |
| CD4 | 51 | 65 | 75 |
| CCR5 | 36 | 24 | 43 |
| CXCR4 | 68 | 84 | 54 |
Higher expression on DC-SIGN+ blood cells as compared to CD14+ monocytes.
Lower expression on DC-SIGN+ blood cells as compared to CD14+ monocytes.
Since DC-SIGN+ blood cells express high levels of CD14 and coexpress DC-SIGN, we investigated whether, during differentiation of CD14high monocytes into DC-SIGNhigh immature DCs, these cells coexpress both molecules. Indeed, after 2 days of differentiation in the presence of IL-4 and GM-CSF, developing DCs start to express DC-SIGN while still expressing high levels of CD14 (Figure 3B). These data support the presence of DC-SIGN+CD14high cells in blood and indicate that these cells are myeloid precursors of DCs.
In conclusion, on the basis of morphology and cell surface expression, DC-SIGN identifies a unique population of precursor DCs in blood.
Functional analysis of DC-SIGN+ blood DCs
To test whether DC-SIGN+ blood DCs could function as APCs, we determined the capacity of these cells to stimulate T-cell proliferation and to produce cytokines upon activation. We compared the functional capacities of DC-SIGN+ blood DCs with those of previously described blood DC subsets, which were isolated as lineage−CD4+ cells that contain myeloid blood DCs (CD11c+, 15%-25%) and plasmacytoid blood DCs (BDCA-2+ and BDCA-4+, 75%-85%). Both DC-SIGN+ blood DCs and CD4+ blood DCs were isolated from the same donor to enable direct comparison and to exclude interdonor variabilities. Monocyte-derived immature DCs, which are known to function as potent APCs, were included.
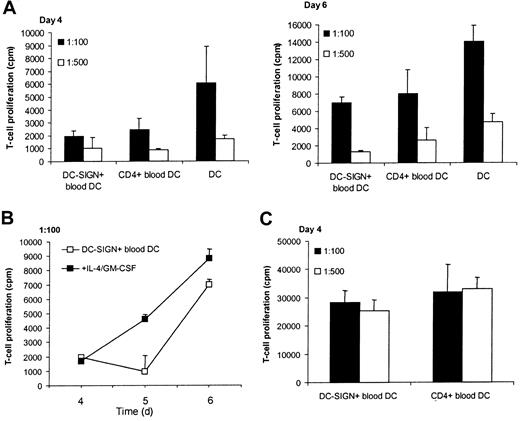
When APCs were added to naive allogeneic responder T cells in a 1:100 or 1:500 APC:T cell ratio, DC-SIGN+ blood DCs displayed a stimulatory capacity similar to that of CD4+ blood DCs after 4 and 6 days of coculture (Figure4A). Moreover, DC-SIGN+ blood DCs induced proliferation of autologous T lymphocytes in the presence of the mycobacteria-derived recall antigen PPD (Figure 4C), whereas CD4+ blood DCs were slightly more potent. As expected, immature DCs could induce a strong T-cell proliferation (Figure 4A). Interestingly, addition of IL-4 and GM-CSF to DC-SIGN+blood cells during coculture with allogeneic T cells resulted in APCs almost as potent as immature DCs (Figure 4B). These results indicate that DC-SIGN+ DCs in blood can stimulate T-cell proliferation like other blood DC subsets, and this capacity is increased upon differentiation of the DC-SIGN+ DC by IL-4 and GM-CSF.
DC-SIGN+ blood cells stimulate proliferation of T cells.
(A) DC-SIGN+ blood cells, CD4+ blood DCs (from the same donor), and immature DCs were cocultured with allogeneic T cells at ratios of 1:100 and 1:500 for 4 to 6 days, after which (methyl-3H)-thymidine incorporation was measured. (B) DC-SIGN+ blood DCs were cocultured with allogeneic T cells as in panel A, in the absence or presence of IL-4 and GM-CSF. (C) DC-SIGN+ blood cells and CD4+ blood DC (from the same donor) were cocultured with autologous T cells as in panel A in the presence of PPD (5 μg/mL). The results are expressed as mean counts per minute (cpm) from triplicate wells (error bars represent SD). Background proliferation of allogeneic T cells: 815 ± 405 cpm at day 4, 652 ± 336 cpm at day 5, 3500 ± 462 cpm at day 6. Background proliferation of autologous T cells in the presence of PPD: 12 216 ± 405 cpm.
DC-SIGN+ blood cells stimulate proliferation of T cells.
(A) DC-SIGN+ blood cells, CD4+ blood DCs (from the same donor), and immature DCs were cocultured with allogeneic T cells at ratios of 1:100 and 1:500 for 4 to 6 days, after which (methyl-3H)-thymidine incorporation was measured. (B) DC-SIGN+ blood DCs were cocultured with allogeneic T cells as in panel A, in the absence or presence of IL-4 and GM-CSF. (C) DC-SIGN+ blood cells and CD4+ blood DC (from the same donor) were cocultured with autologous T cells as in panel A in the presence of PPD (5 μg/mL). The results are expressed as mean counts per minute (cpm) from triplicate wells (error bars represent SD). Background proliferation of allogeneic T cells: 815 ± 405 cpm at day 4, 652 ± 336 cpm at day 5, 3500 ± 462 cpm at day 6. Background proliferation of autologous T cells in the presence of PPD: 12 216 ± 405 cpm.
The functionality of DC-SIGN+ blood DC was further analyzed by measuring the production of cytokines upon activation with various stimuli. DC-SIGN+ blood DCs, CD4+ blood DCs, and monocyte-derived immature DCs were incubated with LPS or cocultured with autologous T cells in the presence of the recall antigen PPD or an antigen from C albicans. The amount of IL-6, IL-10, IL-12, and TNF-α produced was measured by ELISA (Table2). As expected, upon stimulation with LPS, immature DCs could produce all cytokines that we analyzed. Whereas LPS stimulation of CD4+ blood DC did not induce any detectable levels of cytokines, the DC-SIGN+ blood DCs produced the proinflammatory cytokines TNF-α and IL-6. In addition, both blood DC subsets produced IL-10 upon coculture with autologous T cells in the presence of C albicans (Table 2), but not with PPD (not shown).
Cytokine production upon activation of DC-SIGN+blood cells, CD4+ blood DCs, and immature DCs
| . | Stimulus . | TNF-α . | IL-6 . | IL-10 . | IL-12 . |
|---|---|---|---|---|---|
| DC-SIGN+ | — | < 4 | < 4 | < 1.2 | < 2.7 |
| Blood DCs | LPS | 26 | 145 | < 1.2 | < 2.7 |
| CA | < 4 | < 4 | 36 | < 2.7 | |
| CD4+ | — | < 4 | < 4 | < 1.2 | < 2.7 |
| Blood DCs | LPS | < 4 | < 4 | < 1.2 | < 2.7 |
| CA | < 4 | < 4 | 42 | < 2.7 | |
| Immature DCs | — | < 4 | < 4 | < 1.2 | < 2.7 |
| LPS | 536 | 445 | 29 | 11 |
| . | Stimulus . | TNF-α . | IL-6 . | IL-10 . | IL-12 . |
|---|---|---|---|---|---|
| DC-SIGN+ | — | < 4 | < 4 | < 1.2 | < 2.7 |
| Blood DCs | LPS | 26 | 145 | < 1.2 | < 2.7 |
| CA | < 4 | < 4 | 36 | < 2.7 | |
| CD4+ | — | < 4 | < 4 | < 1.2 | < 2.7 |
| Blood DCs | LPS | < 4 | < 4 | < 1.2 | < 2.7 |
| CA | < 4 | < 4 | 42 | < 2.7 | |
| Immature DCs | — | < 4 | < 4 | < 1.2 | < 2.7 |
| LPS | 536 | 445 | 29 | 11 |
As determined by ELISA, in picograms per milliliter.
CA indicates C albicans.
These findings indicate that the DC-SIGN+ blood DCs have the potency to induce T-cell proliferation and to produce cytokines. On the basis of expression profiles and cytokine secretion, it can be concluded that the DC-SIGN+ blood DCs are distinct from the CD4+ blood DCs.
DC-SIGN+ blood DCs efficiently capture HIV-1 and enhance infection of T cells in trans
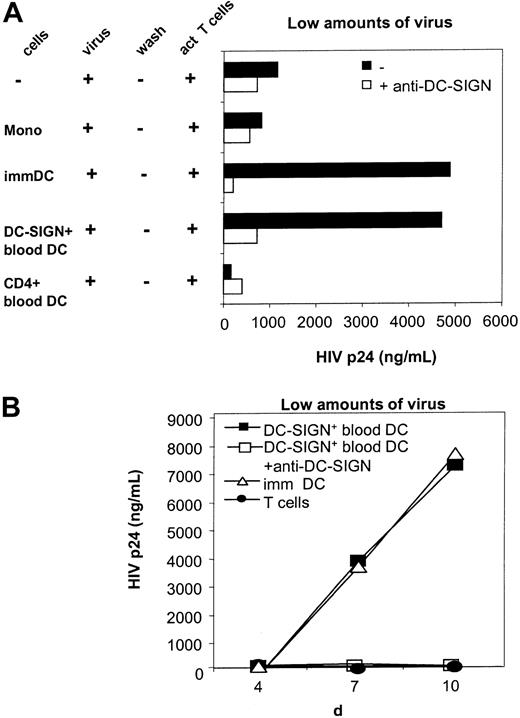
The dissemination pathway of HIV-1 during drug abuse and hemophilia occurs via infection through blood. Vertical transmission of HIV-1 is likely to require a means for small amounts of virus to infect cells that are permissive for viral replication. This may be achieved because of the ability of virus to interact with DC-SIGN+blood DCs, which capture HIV-1 and infect T cells in trans. To mimic in vivo conditions in which HIV-1 levels are likely to be limiting, we challenged DC-SIGN+ blood DCs with low titers of HIV-1 and subsequently cocultured these cells with HIV-1–permissive T cells. Thus, DC-SIGN+ blood DCs were pulsed with low amounts of R5-tropic HIV-1 (JRCSF strain) and were subsequently cocultured with CD4+ T cells. Under these conditions T cells alone were not infected, owing to the low amounts of virus (Figure5).5 As previously described under these low virus multiplicity of infection (MOI) conditions, coculture with immature DCs leads to enhanced virus infection of T cells (Figure 5). Also, surprisingly, the DC-SIGN+blood DCs capture HIV and enhance T-cell infection. DC-SIGN on blood DCs potently captures HIV-1 and efficiently transmits the virus to CD4-, CCR5-expressing T cells, thereby enhancing T-cell infection in trans and thus facilitating a vigorous HIV-1 infection in T cells (Figure 5). The capture of HIV-1 by the DC-SIGN+blood DCs is mediated by DC-SIGN, since blocking antibodies against DC-SIGN completely inhibited HIV-1 transmission (Figure 5). Thus, despite the expression of CD4 and the chemokine receptors CXCR4 and CCR5 on the DC-SIGN+ blood DCs (Table 1), DC-SIGN is both sufficient and essential to capture HIV-1 and infect T lymphocytes in trans. Monocytes were not able to transmit HIV-1 (Figure 5A). Similarly, CD4+ blood DCs, which lack expression of DC-SIGN, did not transmit HIV-1 to T cells, indicating that DC-SIGN+ blood DCs are the only cells in blood that can capture HIV-1 and efficiently infect T cells (Figure 5). At higher HIV-1 titers, T-cell infection was detected with all stimulator cells and to the same extent as with T cells alone, indicating that direct infection of T cells occurs with high amounts of virus (not shown).
DC-SIGN+ blood DCs transmit HIV-1 to responder T cells.
Monocytes (mono), DC-SIGN+ blood DCs, CD4+blood DCs (all from the same donor), and immature (imm) monocyte-derived DCs were incubated with low amounts of HIV-1 in the presence or absence of blocking antibodies to DC-SIGN (AZN-D1, 20 μg/mL) and subsequently cocultured with PHA/IL-2–activated CD4+ T cells for 7 days (A) or for the time points indicated (B). As a control, T cells alone were incubated with HIV-1. Levels of p24 were determined by ELISA in the supernatants. A representative experiment (of 2 experiments) is shown.
DC-SIGN+ blood DCs transmit HIV-1 to responder T cells.
Monocytes (mono), DC-SIGN+ blood DCs, CD4+blood DCs (all from the same donor), and immature (imm) monocyte-derived DCs were incubated with low amounts of HIV-1 in the presence or absence of blocking antibodies to DC-SIGN (AZN-D1, 20 μg/mL) and subsequently cocultured with PHA/IL-2–activated CD4+ T cells for 7 days (A) or for the time points indicated (B). As a control, T cells alone were incubated with HIV-1. Levels of p24 were determined by ELISA in the supernatants. A representative experiment (of 2 experiments) is shown.
Despite the lower level of DC-SIGN expression on blood DCs compared with immature monocyte-derived DCs, the HIV-1 transmission by DC-SIGN+ blood DCs is similar to that of monocyte-derived immature DCs (Figure 5A), indicating that both DC-SIGN–expressing DCs enhance HIV infection of T cells equally well. The time kinetics of HIV-1 transmission are also similar between DC-SIGN+immature DCs and blood DCs (Figure 5B), indicating that DC-SIGN+ blood DCs are highly efficient in HIV-1 transmission and can thus play a role in HIV-1 infection through contaminated blood.
Discussion
The presence of the C-type lectin DC-SIGN on mucosal DCs and its ability to efficiently bind and transmit HIV-1 to T cells are important for viral dissemination upon sexual transmission of HIV-1.5 DC-SIGN does not mediate viral infection of DCs, despite coexpression of CD4 and CCR5,5 indicating that its function as an HIV-1 receptor is distinct from that of the HIV-1 receptor CD4 and the coreceptor CCR5.23 Recently, it was shown that the interaction of DC-SIGN with ligand induces internalization of the complex into lysosomal compartments.24 In addition, internalization of HIV-1 by DC-SIGN turned out to be required for efficient in trans infection of T cells.25 It is still unclear how virus returns to the cell surface for transmission to T cells; however, upon infection of DC at high virus levels, the Nef protein of HIV induces increased surface expression of DC-SIGN and a concomitant increased viral transfer to T cells.26 Although other C-type lectin receptors for HIV-1 have been postulated,21 so far only DC-SIGN can mediate efficient capture and enhancement of HIV-1 infection. DC-SIGN may play an important role in sexual transmission of HIV-1; however, it is not known whether DC-SIGN might also play a role during HIV-1 transmission after contact with blood, as observed during drug abuse and hemophilia. Here we demonstrate that a DC-SIGN+ DC precursor is present in blood. DC-SIGN on blood DCs is functionally active and can very efficiently capture minute amounts of HIV-1 and enhance T-cell infection in trans with efficiency similar to that of immature DCs. Thus, during HIV-1 infection through blood, DC-SIGN+ blood DCs may play a crucial role in the dissemination of HIV-1, disease progression, and clinical outcome.
DC-SIGN+ blood cells represent a unique subset of blood precursor DCs. They share expression of MHC, adhesion, and costimulatory molecules with other blood DC subsets and expression of CD14 with monocytes. Others have shown the presence of CD14+ DC-like subsets within the population of blood monocytes that coexpress CD33 and CD16.27-30 The population of CD14+ DC-SIGN+ blood cells described here expressed CD33, while about 50% of the cells coexpressed CD16, indicating an overlap with the previously described DC-like monocyte subsets. Interestingly, monocytes can differentiate into DCs either during in vitro culture in the presence of IL-4 and GM-CSF or upon reverse transendothelial migration18,19 Here we show that during in vitro differentiation of monocytes in DCs, DC-SIGN is rapidly induced on these cells, while CD14 is still highly expressed. We therefore propose that the CD14+DC-SIGN+ blood DCs are derived from monocytes and are early precursors of tissue DCs. In support of this proposition, in a recent report a population of CD14+ cells in skin dermis were identified as precursors of skin Langerhans cells; these CD14+ cells were located around blood vessels.31 Interestingly, the presence of IL-4 and GM-CSF during coculture with allogeneic T lymphocytes resulted in differentiation of DC-SIGN+ blood cells into potent stimulatory cells (Figure 4B). These results support our hypothesis that DC-SIGN+ blood cells are precursors of immature tissue DCs.
DC-SIGN is a DC-specific adhesion molecule and interacts with ICAM-2, expressed on endothelial cells, and allows transendothelial migration of DC.2 The adhesion between DC-SIGN and ICAM-2 is shear-stress resistant, as described for selectins, which is a requirement to function as rolling receptor. Rolling on endothelial cells is the first step in transendothelial migration. The expression of DC-SIGN on a CD14+ subset of precursor DCs in blood indicates that DC-SIGN/ICAM-2 interactions may be essential for these blood precursor DCs to migrate into tissues at specific anatomical sites. Also, chemokines are involved in regulating transendothelial migration of DC-SIGN+ blood cells at sites of inflammation. DC-SIGN+ blood DCs express CXCR4 and CCR5 (Table 1), indicating that these cells can react to chemokines. DC-SIGN expression would enable close contact of blood DCs with endothelial linings of blood vessels to react on chemokines presented at sites of inflammation. In addition, inflammatory chemokines presented on high endothelial venules of lymph nodes could recruit DC-SIGN+blood DCs directly into lymph nodes, as was recently demonstrated for blood monocytes.32-34 Rapid influx into peripheral tissues or lymph nodes through DC-SIGN/ICAM-2 interactions would explain the fact that DC-SIGN+ blood DCs are present in very low numbers in peripheral blood (maximally, 0.05% of PBMCs). The finding that DC-SIGN+ blood cells acquire a DC morphology within 2 hours after isolation is in agreement with this model.
As reported previously, we confirmed that myeloid and plasmacytoid blood DC subsets, isolated as lineage−/CD4+cells, lack expression of DC-SIGN.21 This is in contrast to a recent report showing that DC-SIGN was expressed on a small percentage of plasmacytoid blood DCs using polyclonal antibodies.35 Both myeloid and plasmacytoid DC subsets were infectable with HIV-1 in vitro when high amounts of HIV-1 were used, through CD4 and the chemokine receptors CCR5 and CXCR4.20 36 However, here we show that CD4+blood DC were not able to transmit HIV-1 virus to T lymphocytes at low concentrations of virus, as opposed to DC-SIGN+ blood DCs, which potently captured and transmitted low amounts of HIV-1 to T cells through DC-SIGN. It is possible that capture of HIV by DC-SIGN+ blood DCs could be responsible for the infection of other blood DC subsets or T cells in blood. This indicates that upon HIV-1 infection through blood, either by drug use or blood transfusion, the DC-SIGN+ blood DCs will be the first target to efficiently capture HIV-1 when low amounts of virus are present. These blood DCs can subsequently migrate to tissues or T-cell areas in lymph nodes, to facilitate infection of T cells in trans.
Although the expression level of DC-SIGN on blood DCs is much lower than on immature monocyte-derived DCs, these cells had a similar efficiency to enhance infection of T cells (Figure 5). Studies by others have demonstrated that relative high levels of DC-SIGN expression are required for efficient virus binding and transmission to T cells.6 However, these experiments were performed with DC-SIGN transfectants. It may well be that the potency of DC-SIGN+ blood DCs for HIV-1 transmission is dependent on the cell type or on expression of adhesion and costimulatory molecules that may facilitate cellular interactions with T cells. The dissemination of HIV-1 upon infection through blood may depend not only on the amount of virus present, but also on the number of DC-SIGN+ blood cells and the amount of DC-SIGN expressed per cell. We found differences between healthy individuals in the number of DC-SIGN+ blood DCs, ranging from 0.01% to 0.05% of total PBMCs. Certainly, differences in DC-SIGN expression levels may also exist, which we unfortunately could not study because of the currently used isolation protocol. It will be important to determine whether variability in DC-SIGN expression levels between individuals exists. Expression levels of CCR5 on blood T cells were shown to vary considerably between individuals and were correlated with HIV-1 infectability of the T cells.37 Polymorphism within the promotor region of DC-SIGN may regulate expression levels of DC-SIGN and determine viral transmission, disease progression, and clinical outcome of the disease. It is hypothesized that if such polymorphisms exist they may influence the capacity of HIV-1 transmission through blood by affecting DC-SIGN expression levels of the here identified DC-SIGN+ blood DCs.
We thank G. Vierwinden and A. Pennings of the Central Hematology Laboratory, Nijmegen, The Netherlands; W. Jansen for technical assistance; M. Chalaby and B. Paxton of the Human Retroviral Laboratory, AMC, Amsterdam, The Netherlands, for technical and practical support; and M. Kapsenberg, AMC, Amsterdam, The Netherlands, for reagents. This work was initiated in the Department of Tumor Immunology, Nijmegen, The Netherlands, headed by C. Figdor, whom we thank for support and discussions.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2001-12-0179.
Supported by the Heart Foundation (grant 97.078 to A.E.) and the AIDS Foundation (grant 5008 to T.B.H.G.).
A.E. and S.J.v.V. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yvette van Kooyk, Department of Molecular Cell Biology, Vrÿe Universiteit Medical Center, van der Boechorststraat 7, 1081 BT Amsterdam, The Netherlands; e-mail: y.van_kooyk.cell@med.vu.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal